Abstract
Off-target activities of drug candidates observed during in vitro pharmacological profiling frequently do not translate to adverse events (AEs) in human. This could be because off-target activities do not have functional consequences, are not observed at exposures achieved during clinical testing, or may not translate into clinical outcomes. We report clinical consequences of an off-target activity observed during profiling of AMG 337, a selective inhibitor of the mesenchymal-epithelial transition factor being evaluated for treatment of solid tumors. In our screen of 151 potential off-targets, AMG 337 inhibited only adenosine transporter (AT). During clinical trials, headache emerged as the dose-limiting AE in the first-in-human trial. It was thought that headache was caused by extracellular accumulation of adenosine from inhibition of AT by AMG 337 and subsequent adenosine-mediated vasodilation through adenosine receptors (ARs). Further nonclinical studies were performed to evaluate this hypothesis. AMG 337 inhibited AT function in dog and human cells in vitro and dog and human arteries ex vivo. In a dog telemetry study, AMG 337 caused hypotension, which was reduced by pretreatment with theophylline, an AR antagonist. Overall, nonclinical and clinical data suggested that headache was due to cerebral vasorelaxation caused by AMG 337-mediated inhibition of AT. When subjects were advised to drink coffee, an AR antagonist, prior to AMG 337, the severity of headaches was reduced, allowing them to continue treatment. These findings demonstrate the importance of carefully evaluating clinical observations during early drug development and the value of translational nonclinical studies to investigate the mechanism of action driving clinical observations.
Introduction
An initial step in conventional medicinal chemistry-driven drug discovery is the identification of molecules that bind to a specific target and affect its function with the intended goal of modulating a key disease-related biological event. During this process, a large number of molecules are screened to select those with optimal potency and selectivity for the target receptor. To profile pharmacologic activities, promising molecules are evaluated for off-target effects using proprietary and commercially available assays. The pharmacological approaches used to screen and identify the off-target profile of a drug candidates (secondary pharmacology) are generally consistent across the pharmaceutical industry and typically includes in vitro binding assays for well-known receptors, ion channels, transporters, and enzymes. Positive “hits” for binding activity can be further characterized by determination of potency and functional consequences such as agonism or antagonism [1], [2], [3].
Characterizing the pharmacologic profile early in drug discovery has several advantages. For example, highly selective molecules will presumably have fewer off-target liabilities, and identification of off-target “hits” allows better prediction of potential clinical effects.
Additional steps such as in vitro, ex vivo, or in vivo functional assays may be needed to fully characterize off-target activities and to determine its relevance to humans. Moreover, the concentration at which functional impact is observed should be compared to that achieved during clinical trials to evaluate the relevance of in vitro finding to human.
Across industry, the panels used for pharmacological characterization of molecules are customized to capture off-target effects that have been observed in the chemical space of the company or are based on the established association of an off-target effect with a clinical adverse event [2]. In addition, off-targets relevant to a particular therapeutic area should be included in the panel. For example, in developing therapeutics for respiratory diseases, equilibrative nucleoside transporter 1 (ENT1) should be included in the panel because in patients with respiratory diseases, in contrast to patient without respiratory diseases, substantial inhibition of ENT1 was associated with increased incidence of bronchospasm and/or severe dyspnea [4].
Secondary pharmacology data are typically included in the application that is submitted to the regulatory authorities in support of first-in-human (FIH) studies. Papoian et al. [5] has recently provided the regulatory perspective on pharmacological profiling or secondary pharmacology evaluation of new molecular entities. The authors include the attributes of these data that are useful for regulatory review such as selection of targets, assay methods, and discussion of the relevance of finding to the proposed FIH study. In addition, examples of cases are provided where a regulatory action could be recommended based on the review of the secondary pharmacology data.
AMG 337 is a highly selective small molecule inhibitor of the mesenchymal-epithelial transition factor (MET), a receptor tyrosine kinase. AMG 337 potently inhibited the activity of wild-type MET with a half-maximal inhibitory concentration (IC50) of 0.001 μM [6]. MET is important in cancer cell proliferation, migration, and survival, and its overexpression, amplification, and mutation lead to receptor activation in many cancer types [7]. AMG 337 is in development for treatment of solid tumors. Here, we report the pharmacological profiling of AMG 337 and show how a single “hit” translated into a dose-limiting adverse event in humans during clinical trials.
Materials and Methods
In Vitro Kinase Assays
The effect of AMG 337 on kinase activity was tested in a panel containing 402 human kinases based on a proprietary method [8], [9]. Details of the assay are described elsewhere (https://www.discoverx.com/products-applications/kinase-solutions). AMG 337 was screened at 1 μM, and the results were expressed as percent control where a lower number indicates a stronger effect (inhibition).
In Vitro Pharmacological Profiling
Pharmacological properties of AMG 337 were evaluated in a custom panel containing 151 receptors, ion channels (except hERG), selected enzymes, and transporters using radioligand binding assays (at 10 μM in duplicate) based on established methods at a contract research organization [10]. The specific ligand binding to the receptors was defined as the difference between the total binding and the nonspecific binding determined in the presence of an excess of unlabeled ligand. The results were expressed as percent of control specific binding [(measured specific binding/control specific binding) × 100] and as a percent inhibition of control specific binding [100 − (measured specific binding/control specific binding) × 100] obtained in the presence the test compound. Effect on enzyme function was assayed on established methods [10]. The results were expressed as a percent of control specific activity [(measured specific activity/control specific activity) × 100] and as percent inhibition of control specific activity [100 − (measured specific activity/control specific activity) × 100] obtained in the presence of the test compound. A ≥50 inhibition of controlled specific binding or enzyme activity was considered a “hit” and was flagged for further evaluation. In each experiment, the respective reference compound was tested concurrently with the test compound, and the data were compared with historical values determined at the testing laboratory.
Determination of IC50 of the Hit
The IC50 value (concentration causing a half-maximal inhibition of control specific binding) and Hill coefficients (nH) for the hits from in vitro radioligand binding assay were determined in vitro using a concentration range of the test compound based on an established method [10], [11]. IC50 was determined by nonlinear regression analysis of the competition curves generated with mean replicate values using Hill equation curve fitting (Y = D + [(A − D)/(1 + (C/C50)nH)], where Y = specific binding, D = minimum specific binding, A = maximum specific binding, C = compound concentration, C50 = IC50, and nH = slope factor).
In Vitro Adenosine Uptake Assay in Human Cells
This in vitro assay was performed to determine the inhibitory effect of AMG 337 on the uptake of adenosine into human Hela cells based on an established method [10], [12]. Briefly, HeLa cells were incubated for 60 minutes at room temperature with [3H]2-8 adenosine (0.1 μM), and adenosine uptake into the cells was measured using scintillation counting. AMG 337 was used at 0.0003, 0.003, 0.01, 0.03, 0.1, 0.3, 1, and 10 μM. Nitrobenzylthioinosine (NBTI) was used as the reference compound. The results are expressed as a percent of control specific activity [(measured specific activity / control specific activity) × 100] and as 100 − [(measured specific activity / controlled specific activity) × 100] obtained in the presence of AMG 337. The IC50 values (concentration causing a half-maximal inhibition of control specific activity), EC50 values (concentration producing a half-maximal increase in control basal activity), and Hill coefficients (nH) were determined by nonlinear regression analysis of the inhibition/concentration-response curves generated with mean replicate values using Hill equation curve fitting [Y = D + (A − D) / (1 + C/C50)nh], where Y = specific activity, A = left asymptote of the curve, D = right asymptote of the curve, C = compound concentration, C50 = IC50 or EC50, and nH = slope factor.
In Vitro Adenosine Uptake Assay in Dog Cells
This in vitro assay was performed to determine the inhibitory effect of AMG 337 on the uptake of adenosine into Madin-Darby Canine Kidney II (MDCK) cells based on an established method [10], [12]. Briefly, MDCK cells were incubated for 2 hours at 22°C with [3H]2-8 adenosine (0.1 μM), and adenosine uptake into the cell was measured using scintillation counting. AMG 337 was used at 0.001, 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 μM. NBTI was used as the reference compound. The results were expressed using the same values as those used in the in vitro adenosine uptake assay in human cells (above).
Ex Vivo Assays Using Human and Dog Arteries
Effects of AMG 337 on arteries from human and dog were evaluated in vitro based on established methods (Biopta, Glasgow, United Kingdom). Subcutaneous (SC) arteries were dissected from fresh human tissue samples. Vascular tissues were obtained from human volunteers (normal healthy or patients) who consented to tissue donation following surgical procedures. Fresh canine heart, brain, and skin specimens were removed following euthanasia with pentobarbitone at a dose of 1 ml/kg of body weight. Canine basilar cerebral arteries, anterior descending coronary arteries, and SC arteries were dissected from the brain, heart, and skin samples. All test arteries were cut into ring segments of approximately 2-mm length. All basilar and coronary artery segments were obtained from the same length of artery. SC arteries were usually obtained from one or more different lengths of artery. Arteries were then attached to 40-μm diameter wire running through the lumen of the artery to stainless steel heads in 5-ml myograph baths containing PSS (11,900 μM NaCl, 4700 μM KCl, 1200 μM MgS04, 24,900 μM NaHCO3, 1200 μM KH2PO4, 2500 μM CaCl2 and 11,000 μM glucose), aerated with 95% O2 and 5% CO2, and maintained at a temperature of 37°C. Changes in tension were recorded using an isometric force transducer. The artery segments were allowed to equilibrate in PSS for at least 30 minutes. Artery segments were then processed through a standardization procedure [13] to reduce signal variability prior to pharmacological intervention.
Arteries were exposed sequentially three times to high potassium physiological saline solution (KPSS; 62,500 μM) to maximally constrict the arteries, with washing in between exposures.
The relaxant ability of the arteries was assessed by exposing arteries to a thromboxane A2 mimetic (U46619) at 0.03 μM in canine coronary arteries and 0.01 μM in all other arteries. Following the plateau of the constriction response, endothelium-dependent relaxation was assessed by adding a known endothelium-dependent dilator. Arteries were then exposed to 100 μM sodium nitroprusside (SNP) to assess maximal endothelial-independent relaxation. Arteries were then rinsed, and tension was allowed to return to baseline levels before proceeding to the assay with the test compound (0.01, 0.1, 0.3, 1, 3, and 10 μM), or positive (adenosine) or negative (DMSO and deionized water) controls. Incubation time for each concentration was 5 minutes or until maximum response was achieved.
At the end of the relaxation experiments, the nitric oxide donor SNP (100 μM) was added to all baths to assess maximal endothelium-independent relaxation. The concentration-response effects of AMG 337 were measured and compared to the associated vehicle responses. The raw data values, measured in millinewtons (mN), were converted to a percentage of the preceding U46619 response. Responses were then plotted against the log molar concentration of agonist. Arteries that were deemed by the analyst to have had an insufficient response to U46619, leading to a drop-off in the preconstriction tone, or displayed instability during the cumulative concentration response curve were excluded from further analysis. Resulting response curves were then further evaluated by two-way analysis of variance (ANOVA) to determine statistically significant differences between test compound and its associated vehicle. Two-way ANOVA was not carried out where there were less than three replicates in a group (DMSO vehicle; canine and human SC arteries).
In Vivo Assessment of Hemodynamic Function in Dogs
In this study, the effects of AMG 377 on the in vivo hemodynamic function were evaluated in conscious freely moving dogs. Female beagle dogs (n = 6) instrumented with telemetry devices were treated initially with oral doses of vehicle for 7 consecutive days followed by AMG 337 (30 mg/kg/day) for 10 consecutive days and vehicle for an additional 14 consecutive days. Systolic, diastolic, and mean arterial blood pressure (BP) and heart rate (HR) were monitored continuously from at least 2 hours prior to dosing until at least 24 hours postdose on days 1, 7, 8, 13, 17, 18, 24, and 31. Prior to the first treatment on study, untreated animals were continuously monitored for BP and HR for at least 24 hours to verify the telemetry signal quality and to establish baseline values.
In Vivo Cardiovascular Evaluation of the Interaction between AMG 337 and an Adenosine Receptor Antagonist in Dogs
Previous studies have shown that AMG 337 caused a reduction in arterial pressure in dogs (data not shown). The purpose of this study was to evaluate whether pretreatment with theophylline (40 mg/kg), an adenosine receptor antagonist, could blunt or prevent the putative adenosine-mediated hypotensive effect of AMG 337 (30 mg/kg) in conscious freely moving dogs fitted with a telemetry device. The dosage was based on literature information on the effects of aminophylline and theophylline on cardiovascular parameters in the dog [14], [15]. Assessments of cardiovascular changes and general toxicity were based on clinical observations, body weight, body temperature, BP (systolic, diastolic, and mean arterial BP), HR, and electrocardiogram (ECG) parameters (QRS duration and RR, PR, and QT intervals).
Cardiovascular Effects of AMG 337 in Human
Evaluation of cardiovascular effects of AMG 337 was based on the observation of inhibition of adenosine transporters (AT) at concentrations relevant to human exposure. It was hypothesized that AMG 337-mediated inhibition of AT leads to the extracellular accumulation of adenosine and secondary adenosine receptor–mediated hemodynamic changes such as hypotension or hypertension.
The clinical trial database was searched for the subject incidences of relevant cardiovascular effects (changes in BP and HR). The BP and HR were measured in the supine position for 3 to 5 minutes predose and at several time points postdose at each dosing cycle (0.5, 1.5, 3, 6, and 24 hours postdose at each cycle).
To determine whether there were any dose-dependent changes in systolic BP, diastolic BP, or HR, the change from baseline of systolic BP, diastolic BP, and HR was analyzed using a repeated-measures analysis of variance within day 1 and within cycle 1. The model included dose level and visit as the nominal fixed effects. Correlations between measurements from different visits were accommodated by fitting subjects as a random effect. The type III tests of fixed effects were reported to test the dose effect. The analysis was considered exploratory and hypothesis generating. P values generated from analyses were used mainly as a descriptive measure rather than to test hypotheses.
Due to large number of the subjects (∼50%) who had preexisting hypertension, similar analysis was also carried out on the subpopulations of subjects with and without preexisting hypertension to determine whether preexisting hypertension affected the systolic BP, diastolic BP, and HR changes after administration of AMG 337. The baseline measurements of systolic BP, diastolic BP, and HR were also compared between these two subpopulations using two-sample t test.
In addition, the relationships between systolic and diastolic BP with exposure (plasma concentration) were explored by plotting the changes of these values from baseline against log plasma concentration of AMG 337. BP changes from baseline were analyzed using repeated-measures analysis of variance within cycle 1. The model included dose level, visit, and indicator of whether the patient had preexisting hypertension as the nominal fixed effects and BP baseline measurement as the continuous fixed effect. Correlations between measurements from different visits were accommodated by fitting patients as a random effect. The type III tests of fixed effects were reported to test the exposure effect. Again, the analysis was considered exploratory and hypothesis generating. P values generated from analyses were used mainly as a descriptive measure rather than to test hypotheses.
Incidence of Headache Events in FIH Clinical Trial
The clinical trial database for the FIH trial (NCT01253707) was searched for the subject incidences of all treatment-emergent adverse events, and the subject incidences of headache events were determined.
Review of the Regulatory Information for Drugs Affecting Adenosine Pharmacology
PHARMAPENDIUM (PHARMAPENDIUM.com) was used to extract relevant information from the approval package of drugs that have been marketed to inhibit AT or affect adenosine receptors [16], [17]. The search was based on the chemical names. “All these sources” category was searched which included European Medicine Agency approval documents, United States Food and Drug Administration (US FDA) approval packages, FDA Advisory Committee documents, Mosby’s Drug Consult, and Meyler’s. Also, we collected the “Adverse Events/Tox data” and FDA Adverse Event Reporting System. Nonclinical, clinical, and postmarketing data were reviewed with emphasis on events related to nervous system and vascular disorders.
Results
Only one “hit” was identified. There were no other “hits” as defined by >50% inhibition of binding or activity, and the values for other target did not exceed 32%. AMG 337 inhibited the binding of a reference ligand to the AT (NBMPR-sensitive equilibrative nucleoside transporter subtype, ENT) by 97% and affected its function. In vitro results are summarized in Table 1 and Figure 1.
Table 1.
Summary of Results from In Vitro Assays
| Assay | Findings |
|---|---|
| In Vitro kinase | AMG 337 inhibited only MET among the kinases in the competition-binding assay (3% control). |
| In vitro pharmacological profiling | AMG 337 inhibited the binding of the reference compound to the guinea pig cerebellum AT by 97%. |
| Determination of IC50 of the hit | The IC50 value of AMG 337 for guinea pig cerebral cortex AT in the binding assay was 2.8 × 10−7 M (0.28 μM). |
| In vitro adenosine uptake by human cells | AMG 337 showed an IC50 value of 2.8 × 10−7 M (0.28 μM). |
| In vitro adenosine uptake by dog cells | AMG 337 showed an IC50 value of 1.7 × 10−7 M (0.17 μM). |
Figure 1.
Dose-dependent inhibition of AT by AMG 337 in guinea-pig cerebral cortex (binding, blue) and human HeLa cells (functional, black) and in vivo exposure in humans and dogs. The maximum concentration of free AMG 337 at the 400-mg QD dose in human is estimated to be 3.4 μM. This level of exposure is approximately 12 times higher than the IC50 of the AT in the functional assay with human cells (0.28 μM) and 20 times higher than the IC50 of the AT in the functional assay with dog cells (0.17 μM). In the dog study with theophylline, the free concentration of AMG 337 at 8 hours postdose (30 mg/kg) was 5.4 μM, which is similar to the free Cmax of 5.8 μM on day 1 in a previous 1-month study in dogs with AMG 337 at 30 mg/kg/day.
Ex Vivo Assays Using Human and Dog Arteries
A relaxation response to AMG 337 (0.01 to 10 μM) was tested in precontracted vessels with U46619 (0.01 μM) in the dog cerebral and coronary arteries (data not shown), and dog and human SC arteries (Figure 2). At 10 μM, AMG 337 substantially decreased the contraction induced by U46619 in all four arteries. The responses of the isolated arteries to the positive and negative controls, adenosine, were as expected.
Figure 2.
Relaxation responses of AMG 337 in precontracted dog and human vessels (A) dog subcutaneous artery and (B) human subcutaneous artery. Black = vehicle and blue = AMG 337.
In Vivo Assessment of Hemodynamic Function in Dogs
AMG 337–related changes in BP were variable in magnitude and direction over the course of the study (Figure 3). On day 1 of AMG 337 administration, decreases in mean BP (maximal mean decreases of approximately 25% to 34%) were noted. In contrast, increases in mean BP (maximal mean increases of approximately 20% to 25%) were noted on the sixth day of AMG 337 administration. AMG 337–related increases in BP were limited to a single animal on the final day of AMG 337 administration. Consistent increases in HR were noted on days 1, 6, and 10 (maximal mean increases of approximately 95%, 105%, and 74%, respectively; Figure 4). No remarkable changes in BP or HR were noted during the recovery period when compared to baseline values.
Figure 3.
Mean arterial pressure in dogs administered AMG 337.
Figure 4.
Mean heart rate in dogs administered AMG 337.
In Vivo Cardiovascular Evaluation of the Interaction between AMG 337 and an Adenosine Receptor Antagonist in Dogs
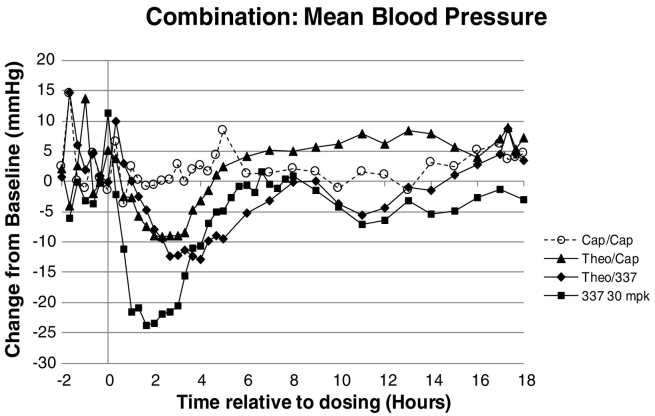
Decreased BP (Figure 5) and increased HR (Figure 6) were noted at 40 mg/kg theophylline followed by 0 mg/kg AMG 337 and 40 mg/kg theophylline followed by 30 mg/kg AMG 337. The mean BP and HR changes were comparable in magnitude and duration for both dose groups. A single dose of 30 mg/kg AMG 337, administered to conscious telemetered female dogs, produced a decrease in BP of 25% to 34% and an increase in HR of approximately 95%. Administration of 40 mg/kg theophylline followed by 30 mg/kg AMG 337 produced a decrease in BP of only 10% to 15%, indicating that theophylline pretreatment reduced the magnitude of the cardiovascular responses elicited by AMG 337. At 30 mg/kg, the concentration measured at 8 hours postdose was 6.37 μg/ml (13.8 μM, 5.4 μM free). In a previous 1-month study in dogs with AMG 337 at 30 mg/kg/day, the average Cmax on day 1 was 6.83 μg/ml (14.7 μM, 5.8 μM free).
Figure 5.
Mean arterial pressure in dogs treated with empty capsules, theophylline, AMG 337, or a combination of theophylline and AMG 337.
Figure 6.
Heart rate in dogs treated with empty capsules, theophylline, AMG 337, or a combination of theophylline and AMG 337.
Cardiovascular Effects of AMG 337 in Humans
The subject incidence of selected cardiovascular effects (hypertension, hypotension, and HR changes) was 15% among 80 subject who were evaluated. Five subjects had hypertension, five subjects had hypotension, one (2%) subject had increased HR, and one subject had tachycardia (Table 2).
Table 2.
Incidence of Selected Cardiovascular Treatment-Emergent Adverse Events in Subjects Treated with AMG 337
| Preferred Term | Subject Incidence N (%) |
AMG 337 N (mg, Frequency) |
|---|---|---|
| Hypertension | 5 (6) | 3 (100, QD), 2 (200, QD) |
| Hypotension | 5 (6) | 1 (100, QD), 2 (200, QD), 1 (300, QD), 1 (150, BID) |
| HR increased | 1 (1) | 1 (200, QD) |
| Tachycardia | 1 (1) | 1 (150, QD) |
N, number of subjects; QD, once daily; BID, twice daily.
Overall, statistical analyses of systolic BP, diastolic BP, and HR data indicated that there were no dose-dependent changes in these parameters with administration of various doses of AMG 337 on either a QD or a BID schedule (data not shown). Subjects with preexisting hypertension had a relatively higher baseline BP as compared to nonhypertensive subpopulation, especially systolic BP, which was significantly higher (P < .05). The baseline HR profiles were similar between subjects with/without hypertension. Exposure analysis on changes from baseline of systolic BP (entire population) did not show any exposure-dependent pattern (data not shown). The maximum plasma concentration (Cmax) of AMG 337 on day 1 ranged from 0.499 μg/ml (1.1 μM, 0.37 μM free) at 25 mg QD to 4.58 μg/ml (9.9 μM, 3.4 μM free) at 400 mg QD (Figure 1).
Clinical Safety Data
AMG 337 was evaluated at doses from 25 mg to 400 mg (PO daily). Of the 90 subjects who received AMG 337, 89 (99%) reported at least 1 treatment-emergent adverse event. Adverse events occurring in at least 10% of subjects are shown in Table 3.
Table 3.
Incidence of Treatment-Emergent Adverse Event Occurring in ≥10% of Subjects Treated with AMG 337
| Preferred Term | Subject Incidence N (%) |
|---|---|
| Headache | 58 (64) |
| Nausea | 39 (43) |
| Fatigue | 36 (40) |
| Vomiting | 24 (27) |
| Constipation | 21 (23) |
| Anemia | 19 (21) |
| Hypoalbuminemia | 18 (20) |
| Peripheral edema | 17 (19) |
| Decreased appetite | 15 (17) |
| Diarrhea | 14 (16) |
| Dyspnea | 14 (16) |
| Back pain | 14 (16) |
| Myalgia | 11 (12) |
| Dry skin | 11 (12) |
| Rash | 10 (11) |
| Dizziness | 9 (10) |
| Aspartate aminotransferase increased | 9 (10) |
N, number of subjects.
Incidence of headache among subjects treated with AMG 337 is shown in Table 4. The severity of headache, described as throbbing and migraine-like, did not exceed grade 3 in any subject treated with AMG 337. At 400 mg, all subjects developed headache which was reported as serious among 33% of the subjects. AMG 337 at 400 mg QD was deemed not tolerated. The incidence of headache appeared to be dose-dependent.
Table 4.
Incidence of Headache Among Subjects Treated with AMG 337
| Dose (mg) |
Number of Subject Treated |
Number of Subjects with Headache |
Subject Incidence % |
|---|---|---|---|
| 25 QD | 3 | 0 | 0 |
| 50 QD | 4 | 2 | 50 |
| 100 QD | 14 | 6 | 43 |
| 150 QD | 9 | 6 | 67 |
| 100 BID | 5 | 3 | 60 |
| 200 QD | 15 | 10 | 67 |
| 150 BID | 9 | 6 | 67 |
| 300 QD | 18 | 12 | 67 |
| 200 BID | 7 | 7 | 100 |
| 400 QD | 6 | 6 | 100 |
| Total | 90 | 58 | 64 |
BID, twice daily; QD, once daily.
Review of the Regulatory Information for Drugs Affecting Adenosine Pharmacology
The results are summarized in Table 5. Dipyridamole (PERSANTINE) is used in combination with blood thinners to keep clots from forming after heart valve replacements. Cilastazole (PLETAL) is indicated for the reduction of symptoms of intermittent claudication. Adenosine (ADENOSCAN) is used as an adjunct to thallium-201 myocardial perfusion scintigraphy in patients unable to exercise adequately. Regadenoson (LEXISCAN) is used for radionuclide myocardial perfusion imaging in patients unable to undergo adequate exercise stress.
Table 5.
Summary of Approval Packages for Drugs Affecting Adenosine Pharmacology
| Compound Trade Name Class |
Adenosine Uptake Inhibition (IC50 M) |
Nonclinical Data (Adverse Effect/Toxicity) |
Clinical Data (Adverse Effect/Toxicity) |
Postmarketing Reports (Adverse Events) |
|---|---|---|---|---|
| Dipyridamole PERSANTINE AT inhibitor |
10−7 | • Coronary arteriospasm (dog) • Hypotension (dog) • Coronary artery dilatation (dog) • Decreased systemic vascular resistance (dog) |
• Headache • Hypertension • Hypotension |
• Headache • Hypotension • Hypertension |
| Cilostazol PLETAL AT inhibitor |
10−6 | • Arterial hemorrhage (monkey) • Vasodilation (dog) |
• Headache • Vasodilatation |
• Headache • Hypotension • Hypertension • Vasodilation |
| Adenosine ADENOSCAN Receptor agonist |
NA | • No relevant data | • Flushing • Headache • Hypotension • Vasodilation |
• Hypotension • Headache • Flushing |
| Regadenoson LEXISCAN Receptor agonist |
NA | • No relevant data | • Feeling hot • Flushing • Headache • Hypertension • Hypotension |
• Headache • Hypertension • Flushing • Decreased BP |
AT, adenosine transporter; BP, blood pressure; NA, not available.
Discussion
AMG 337 is in development for treatment of various solid tumors. Headache was the most commonly reported treatment-emergent adverse event in the FIH clinical trial. The incidence of headaches appeared to be dose-related, and the severity of headaches at the 400-mg dose became serious and dose-limiting. To address the impact of this adverse clinical event, a team of drug development scientists evaluated the available data and conducted a series of nonclinical studies to elucidate the mechanism of action (MOA) of AMG 337–related headaches.
The initial step in investigating the MOA of AMG 337 was to review the available nonclinical safety data. In an in vitro pharmacological profiling assay containing 151 potential targets, only one “hit” (>50% inhibition of specific binding or activity) was identified.
AMG 337 inhibited the binding of a reference ligands to the AT by 97% and affected its function (IC50 = 0.28 μM). Effects on other targets were minimal, with the next highest inhibition being binding of reference compound to muscarinic receptor 1 (M1).
Prior to the FIH trial, the significance of the inhibition of AT by AMG 337 was uncertain. Based on the remarkable lack to promiscuity of AMG 337 in its in vitro pharmacologic profile, it was anticipated that AMG 337 would not have off-target pharmacologic activities in the clinic. However, the emergence of headaches during the FIH study prompted the team to evaluate the relevance of the AT inhibition as the only significant finding from the in vitro pharmacological profiling.
Based on the available data and a review of the literature on AT and adenosine biology, it was hypothesized that the headache observed in clinical trial subjects could be caused by excessive adenosine resulting from inhibition of AT by AMG 337.
Inhibition of adenosine transporter has been reported to lead to extracellular accumulation of adenosine and subsequent adenosine-mediated effects such as vasodilation through adenosine receptors [18], [19]. Adenosine is an endogenous molecule that has numerous physiological effects (including neuronal, cardiac, metabolic, and renal effects) and is implicated in many pathological conditions (neurological, cardiovascular, inflammatory, and autoimmune disease and cancer). Adenosine exerts its effects through four receptor subtypes which are widely distributed in the body. Negative inotropy, chronotropy, and dromotropy, ischemic preconditioning, vasodilation, and inhibition of platelet aggregation are among the cardiovascular effect of adenosine [20]. Headache is among the side effects of adenosine administered intravenously for stress testing [18]. In addition, headache is the most common adverse reaction of drugs that exert their pharmacological effects through adenosine receptor agonism or AT inhibition and is treated with aminophylline ([21] Lexiscan (regadenoson), prescribing information [22]). These drugs include AT inhibitors such as dipyridamole and cilestazole and adenosine receptor agonists such as adenosine and regadenososn ([23], [24] Lexiscan (regadenoson), prescribing information [22]).
The hypothesis that the headache observed in clinical trial subjects could be caused by excessive adenosine resulting from inhibition of AT by AMG 337 was tested in a series of in vitro, ex vivo, and in vivo studies. Initially, it was determined that in vitro binding of AMG 337 to AT transporter has functional consequences by demonstrating that AMG 337 inhibited the function of AT in dog and human cells in vitro. AMG 337 also caused vasorelaxation of vascular tissues from dog and human ex vivo. Further, in dog telemetry studies, it was demonstrated that cardiovascular effects of AMG 337 could be dampened by treatment with theophylline, an antagonist of adenosine receptors.
Collectively, results from these studies suggest that inhibition of AT by AMG 337 leads to accumulation of adenosine which causes vasorelaxation through activation of adenosine receptor and results in headache. However, effects of AMG 337 on the function of AT in human would only be is possible if free AMG 337 reaches high enough levels in the blood of subjects to inhibit AT. Considering the plasma protein binding of AMG 337 in human (34.7%), the maximum concentration of free AMG 337 at the 400-mg QD dose in human is estimated to be 3.4 μM. This level of exposure is approximately 12 times higher than the IC50 of the AT in the functional assay with human cells (0.28 μM), indicating that there was adequate free AMG 337 in the blood of subjects participating in the FIH clinical trial to inhibit AT and lead to headache.
In addition to presence of adequate free drug in the blood, the incidence and severity of adverse events among patients depend on number of factors such as density, expression, and functionality of targets/off-targets, as well as the status of the disease at the time of therapy. Pennycooke et al. [25] has reported that the expression of nucleotide transporters (NT) in normal and tumor tissues is highly variable and is lower in tumor tissues. In gynecological cancers, the expression of concentrative nucleoside transporters (CNT1) and ENT1 in human ovarian, endometrial and uterine cervix carcinomas was highly variable. The expression of CNT1 was low (33%-39% of tumors), and in contrast, the expression of ENT1 was high (91%-98%) [26]. Rosenbrier-Ribeiro and Storer [4] have reported a positive correlation between the level of inhibition of ENT1 and severity of respiratory disorder.
Based on results of our comprehensive investigation of AT inhibition by AMG 337, the investigators were instructed to manage the headache by administration of acetaminophen, caffeine, or triptans, depending on the severity. It was reported that the use of caffeine in the FIH study prior to dosing or during the onset of headache reduced the severity of headaches, allowing the subjects to continue their treatment.
This study demonstrates the utility of the approaches outlined in the recent reports [2], [3], [5]. The diligent evaluation of the nonclinical and clinical data as these are being generated during clinical development can inform the development of strategies to mitigate the risks to the patients and ensure the continuation of the development of safe and effective therapeutics.
Conclusions
Our findings describe the clinical implications of an in vitro pharmacology-profiling hit. They demonstrate the significance of carefully evaluating early observations during drug development with regard to translation of nonclinical observation to the clinic.
Acknowledgments
Acknowledgement
This work was supported by Amgen Inc.
Animal Welfare Statement
Animals were housed in an AAALAC, international accredited facility. Animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition. All research protocols were reviewed and approved by the Amgen Institutional Animal Care and Use Committee.
Canines (Canis familiaris, beagle, female, 7 to 11 kg (In Vivo Assessment of Hemodynamic Function in Dogs) or 1 to 4 years old (In Vivo Cardiovascular Evaluation of the Interaction between AMG 337 and an Adenosine Receptor Antagonist in Dogs)) were obtained from a commercial vendor (Covance Research Products, Kalamazoo, MI) and were housed individually in single- or double-sized stainless steel cages with plastic coated flooring. Canines were allowed to acclimate for a minimum of 7 days prior to experimentation. Lighting in animal holding rooms was maintained on 12:12-hour light:dark cycle, and the ambient temperature and humidity range was at 64 F to 84 F and 30% to 70%, respectively. Dogs were fed Lab Diet (Certified Canine Diet #5007, PMI Nutritional International, Inc) ad libitum and had continuous access to clean water (tap water monitored for contaminants) via an automatic watering system.
Data Sharing Statements
A data sharing statement must be included in all manuscripts reporting the results of Amgen-sponsored clinical trials and observational studies. Review GDE-000503 Section 7.1 for additional information.
Full-length Data Sharing Statement:
There is a plan to share data. This may include de-identified individual patient data for variables necessary to address the specific research question in an approved data-sharing request; also related data dictionaries, study protocol, statistical analysis plan, informed consent form, and/or clinical study report. Data sharing requests relating to data in this manuscript will be considered
after the publication date and 1) this product and indication (or other new use) have been granted marketing authorization in both the US and Europe, or 2) clinical development discontinues and the data will not be submitted to regulatory authorities. There is no end date for eligibility to submit a data sharing request for these data. Qualified researchers may submit a request containing the research objectives, the Amgen product(s) and Amgen study/studies in scope, endpoints/outcomes of interest, statistical analysis plan, data requirements, publication plan, and qualifications of the researcher(s). In general, Amgen does not grant external requests for individual patient data for the purpose of re-evaluating safety and efficacy issues already addressed in the product labeling. A committee of internal advisors reviews requests. If not approved, a Data Sharing Independent Review Panel will arbitrate and make the final decision. Upon approval, information necessary to address the research question will be provided under the terms of a data sharing agreement. This may include anonymized individual patient data and/or available supporting documents, containing fragments of analysis code where provided in analysis specifications. Further details are available at the following: http://www.amgen.com/datasharing
Abbreviated Data Sharing Statement:
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing
Journals with drop-down menus or questions:
Refer to GDE-000503 Section 7.1 for guidance when answering the questions. Submit for internal review to datasharing@amgen.com with “ICMJE statement consultation” in the subject line.
For publications that include data from Amgen biosimilar products:
Please email datasharing@amgen.com with a request to for guidance on the appropriate data sharing statement to use for your publication. Include “ICMJE statement consultation” in the subject line.
References
- 1.Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov. 2012;11:909–922. doi: 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- 2.Lynch JJ, III, Nan Vleet TR, Mittelstadt SW, Bloome AG. Potential functional and pathological side effects related to off-target pharmacological activity. J Pharmacol Toxicol Meth. 2017;87:108–126. doi: 10.1016/j.vascn.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Valentin JP, Guillon JM, Jenkinsn S, Kadambi V, Ravikumar P, Roberts S, Rosenbrier-Ribeiro L, Schmidt F, Armstrong D. In vitro secondary pharmacological profiling: An IQ-DruSafe industry survey on current practices. J Pharmacol Toxicol Meth. 2018;93:7–14. doi: 10.1016/j.vascn.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbrier-Ribeiro L, Storer RI. A semi-quantitative translational pharmacology analysis to understand the relationship between in vitro ENT1 inhibition and the clinical incidence of dyspnoea and bronchospasm. Toxicol Appl Pharmacol. 2017;317:41–50. doi: 10.1016/j.taap.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Papoian T, Chiu HJ, Ikram E, Jagadeesh G, Khan I, Laniyonu AA, Li CX, Saulnier M, Simpson N, Yang B. Secondary pharmacology data to assess potential off-target activity of new drugs: a regulatory perspective. Nat Rev Drug Discov. 2015;14:294. doi: 10.1038/nrd3845-c1. [DOI] [PubMed] [Google Scholar]
- 6.Hughes PE, Rex K, Caenepeel S, Yang Y, Zhang Y, Broome MA, Kha HT, Burgess TL, Amore B, Kaplan-Lefko PJ. In vitro and in vivo activity of AMG 337, a potent and selective MET kinase inhibitor in MET-dependent cancer models. Mol Cancer Ther. 2016;15(7):1568–1579. doi: 10.1158/1535-7163.MCT-15-0871. [DOI] [PubMed] [Google Scholar]
- 7.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 8.Fabian MA, Biggs WH, III, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 9.Karaman MW, Herrgard S, Treibert DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT. A quantitative analysis of kinase inhibitor selectivity. Nature Biotech. 2008;26(1):127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 10.Eurofins CEREP 2019. https://www.eurofins.com/contact-us/worldwide-interactive-map/france/eurofins-cerep-france/
- 11.Verma A, Marangos PJ. Nitrobenzylthioinosine binding in brain: an interspecies study. Life Sci. 1985;36:286–290. doi: 10.1016/0024-3205(85)90071-2. [DOI] [PubMed] [Google Scholar]
- 12.Harley ER, Patterson AR, Cass CE. Initial rate kinetics of the transport of adenosine and 4-amino-7-(β-D-ribofuranosyl) pyrrolo[2,3-d]pyrimidine (tubercidin) in cultured cells. Cancer Res. 1982;42:1289–1295. [PubMed] [Google Scholar]
- 13.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Afonso S. Inhibition of coronary vasodilating action of dipyridamole and adenosine by aminophylline in the dog. Circ Res. 1970;26:743–752. doi: 10.1161/01.res.26.6.743. [DOI] [PubMed] [Google Scholar]
- 15.Munsiff IJ, Mckiernan BC, Neff-Davis CA, Koritz GD. Determination of the acute oral toxicity of theophylline in conscious dogs. J Vet Pharmacol Thera. 1988;11:381–389. doi: 10.1111/j.1365-2885.1988.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 16.Noji T, Karasawa A, Kusada H. Adenosine uptake inhibitors. Eur J Pharmacol. 2004;495:1–16. doi: 10.1016/j.ejphar.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Sheth S, Brito R, Mukhejea D, Rybak LP, Ramkumar V. Adenosine receptors: expression, function and regulation. Int J Mol Sci. 2014;15:2024–2052. doi: 10.3390/ijms15022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine. physiology, pharmacology, and clinical applications J Am Coll Cardiol Intv. 2014;7:581–591. doi: 10.1016/j.jcin.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Young JD, Yao SYM, Baldwin JM, Cass CE, Baldwin SA. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med. 2013;34:529–547. doi: 10.1016/j.mam.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Borea AP, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 21.Feuerstein TJ, Zeides A. theophylline relieves headache following lumbar puncture — placebo-controlled, double-blind pilot study. Klin Wochenschr. 1986;64:216–218. doi: 10.1007/BF01711650. [DOI] [PubMed] [Google Scholar]
- 22.Lexiscan (regadenoson), prescribing information (2014). Drugs@FDA (http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022161s012lbl.pdf) accessed August 2018.
- 23.Kruuse C, Jacobsen TB, Lassen LH, Thomsen LL, Hasselbalch SG, Dige-Petersen H, Olesen J. Dipyridamole dilates large cerebral arteries concomitant to headache induction in healthy subjects. J of Cereb Bld Flow and Metab. 2000;20:1372–1379. doi: 10.1097/00004647-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Meyers AM, Topham L, Ballow J, Totah D, Wilke R. Adverse reactions to dipyridamole in patients undergoing stress/rest cardiac perfusion testing. J Nucl Med Technol. 2002;30:21–24. [PubMed] [Google Scholar]
- 25.Pennycooke M, Chaudary N, Shuralyova I, Zhang Y, Coe IR. Differential expression of human nucleoside transporters in normal and tumor tissue. Biochem Biophys Res Comm. 2001;280:951–959. doi: 10.1006/bbrc.2000.4205. [DOI] [PubMed] [Google Scholar]
- 26.Farre X, Guillen-Gomez E, Sanchez L, Hardisson D, Plaza Y, Lloberas J, Casado J, Palacios J, Al Pastor-Anglada M. Expression of the nucleoside-derived drug transporters hCNT1, hENT1 and hENT2 in gynecologic tumors. Int J Cancer. 2004;112:959–966. doi: 10.1002/ijc.20524. [DOI] [PubMed] [Google Scholar]