Abstract
Resveratrol (3,5,4′-trihydroxystilbene) belongs to a family of polyphenolic compounds known as stilbenes, particularly concentrated in grape and red wine. The aim of our review was to critically review the available evidence of resveratrol effects on brain function and its potential impact on therapy. In preclinical models of cognitive decline, resveratrol displays potent antioxidant activity by scavenging free radicals, reducing quinone reductase 2 activity and upregulating endogenous enzymes. Resveratrol also inhibits pro-inflammatory enzyme expression, reduces nuclear factor-κB activation and cytokine release. Treatment with resveratrol can affect multiple signaling pathway effectors involved in cell survival, programmed cell death and synaptic plasticity. Direct and/or indirect activation of the deacetylase sirtuins by resveratrol has also been suggested. In humans, clinical evidence derived from randomized clinical trials suggests that resveratrol is able to improve cerebral blood flow, cerebral vasodilator responsiveness to hypercapnia, some cognitive tests, perceived performances, and the Aβ40 plasma and cerebrospinal fluid level.
Keywords: clinical trials, cognitive function, neuroprotection, preclinical models, resveratrol
Introduction
Resveratrol (3,5,4′-trihydroxystilbene) belongs to a family of polyphenolic compounds known as stilbenes, particularly concentrated in grape and red wine [1, 2]. Resveratrol is also one of the phytoalexins, a group of low-molecular-mass substances with antimicrobial activity, produced by plants as a defense response to some exogenous stimuli, such as UV radiation, chemical stressors, and, particularly, microbial infections [3]. The compound exists in two isomeric forms. The trans isomer occurs in the berry skins of most grape cultivars, and its synthesis is stimulated by UV light, injury, and fungal infection. The cis isomer is produced by UV irradiation of the trans isomer; it is generally absent or only slightly detectable in grapes but is originated during vinification [4]. Most research on resveratrol concerns the trans isomer, owing to its natural presence in grapes and its greater stability [5].
A huge pre-clinical literature and some clinical evidence recently demonstrated a potentially relevant impact of resveratrol on central nervous system functionality [6].
The aim of our review was to critically resume the available evidence of resveratrol effects on brain function and its potential impact on therapy.
Neuroprotection in preclinical models
Postoperative cognitive dysfunction represents a severe neurological complication in approximately 25% of elderly people. Indeed, in the hippocampus, by inducing over-production of pro-inflammatory cytokines (i.e., tumor necrosis factor (TNF)-α and interleukin (IL)-1β), isoflurane anesthesia impairs synaptic plasticity leading to neurodegeneration with a consequent deficit in the cognitive function [7, 8]. In this context, in aged mice, the intraperitoneal injection of 100 mg/kg resveratrol for 7 days attenuates the isoflurane anesthesia hippocampus-dependent cognitive impairment through anti-inflammatory and anti-apoptosis effects [9]. Among the possible molecular mechanisms beyond these effects, the involvement of sirtuin (SIRT) 1 has raised particular interest. It belongs to the sirtuin family of NAD-dependent protein deacetylases and it is a deacetylase for several metabolic and signaling proteins involved in the stress response, apoptosis, mitochondrial function, self-renewal, and neuroprotection [10]. In particular, in neurons, SIRT1 is able to deacetylate p53 at the Lys residues (Lys373 and/or Lys382) and protect various cells from DNA damage-induced apoptosis [11]. As a further confirmation, studies in cells, primary neurons, and mouse models described SIRT1 to be a neuroprotective mediator as well as a central player in counteracting neurodegeneration [12]. Resveratrol is one of the polyphenols able to modulate SIRT1 expression and activity. By inducing SIRT1, resveratrol may promote neurite outgrowth and enhance neural plasticity in the hippocampal region [13]. The importance of this pathway has also been described in a rat model of induced neuropathological Alzheimer disease (AD)-like pattern; the activation of SIRT1 by resveratrol (30 mg/kg/day per 8 weeks) attenuated the hyperphosphorylation of tau protein induced by the intracerebroventricular injection of streptozotocin, confirming the role of resveratrol in protecting hippocampus neurons from tau hyperphosphorylation and cognitive impairment [14].
Resveratrol also induces neurogenesis and mitochondrial biogenesis by enhancing AMP-activated protein kinase (AMPK), which is known to stimulate neuronal differentiation and mitochondrial biogenesis in neurons. These effects are independent of SIRT1 involvement, evidence supported by the use of SIRT1 inhibitors or studies conducted in the brain of SIRT1 knock-out mice [15]. It is important to highlight that a tight interplay between SIRT1 and AMPK does exist [16]. In a mouse model of familial AD, it has been demonstrated that an equilibrium among SIRT1 and AMPK signaling, mitochondrial status and inflammatory changes is mandatory for the effect of resveratrol in the protection against Aβ plaque formation and cognitive loss [17]. The evidence of a neuroprotective effect of resveratrol has been further corroborated in in vitro rat hippocampal H19-7 neuronal cells in which a 2-h pretreatment with resveratrol (75 μM) attenuated the Aβ-induced oxidative damage and the decrement of proteins essential for synaptic maturity and plasticity [18]. Another feature impaired in AD and that may be rescued by resveratrol is neurovascular coupling. In a mouse model of aging recapitulating cerebrovascular deficits of cortical function, treatment with resveratrol rescued cortical neurovascular coupling responses, an effect mediated by the down-regulation of cortical expression of NADPH and the reduction of oxidase-derived ROS production [19]. Considering that in the rat aorta SIRT1 inhibits NADPH oxidase activation and protects endothelial function, this pathway cannot be excluded in understanding the link between resveratrol and cerebromicrovascular endothelial function [20].
Besides the involvement of SIRT1 and AMPK, a genome-wide gene expression analysis showed how, in streptozotocin-induced diabetic rats, resveratrol normalized the hippocampal expression of genes implicated in neurogenesis and synaptic plasticity (Hdac4, Hat1, Wnt7a, ApoE) and reduced that of Jak-Stat pro-inflammatory signaling (IL-15, IL-22, Socs2 and Socs5) [21]. In a rodent model of vascular dementia, resveratrol supplementation induced hippocampal nerve growth factor expression, attenuating pyramidal cell death in the CA1 hippocampal sub-region and improving spatial working memory [22]. Further evidence on the neuroprotective effect of resveratrol on vascular dementia came from a study reporting how in a rat model of permanent bilateral common carotid artery occlusion, the daily administration of resveratrol improved learning and memory ability as evaluated by the Morris water maze test; the escape latency and escape distances were significantly shorter in the animals treated with resveratrol. Moreover, upon resveratrol administration, levels of malonyldialdehyde, a marker of oxidative stress in neurodegenerative disease, were decreased in cerebral cortex and hippocampus; conversely, resveratrol resulted in increased superoxide dismutase activity and glutathione levels [23].
Even if the effect of resveratrol on cognitive function is magnified in extreme models, it is interesting to highlight that resveratrol appears effective also in models of mild stress. In rats exposed to chronic unpredictable mild stress, able to induce cognitive deficits, the chronic administration of resveratrol (20 mg/kg/day) significantly attenuates the deficit in emotional learning and spatial memory. Analysis of the hippocampus showed that, compared to stressed rats, resveratrol prevented the decrement of brain-derived neurotrophic factor [24], a key molecule of hippocampal plasticity [25].
In the context of insufficient sleep, a model of cognitive decline, the effects of polyphenols (including resveratrol) on memory deficits have been described. Resveratrol significantly improves sleep deprivation-induced contextual memory deficits, through the activation of two independent but interrelated pathways, namely, cAMP-response element-binding protein and mammalian target of rapamycin (mTOR) signaling pathways [26]. The positive impact of resveratrol on cognitive function in old rats (20 months) could be related to direct modulation of neurotransmission. Chronic administration of resveratrol (20 mg/kg/day for 4 weeks) leads to an increase of serotonin levels, in the pineal gland, in the hippocampus, and in the striatum, and of noradrenaline and dopamine, in the hippocampus and in striatum, respectively. These changes are to be ascribed to increased activity of tryptophan hydroxylase-1 (463% in the pineal gland), tryptophan hydroxylase-2 (70 and 51% in the hippocampus and striatum, respectively), and tyrosine hydroxylase (150 and 36% in the hippocampus and striatum, respectively), all enzymes involved in monoamine syntheses [27].
Finally, recent data on rats in late middle-age highlighted how the effect of resveratrol in improving memory and mood function is mainly due to the modulation of hippocampus plasticity and the suppression of chronic low-level inflammation, steps improving several age-related changes [28].
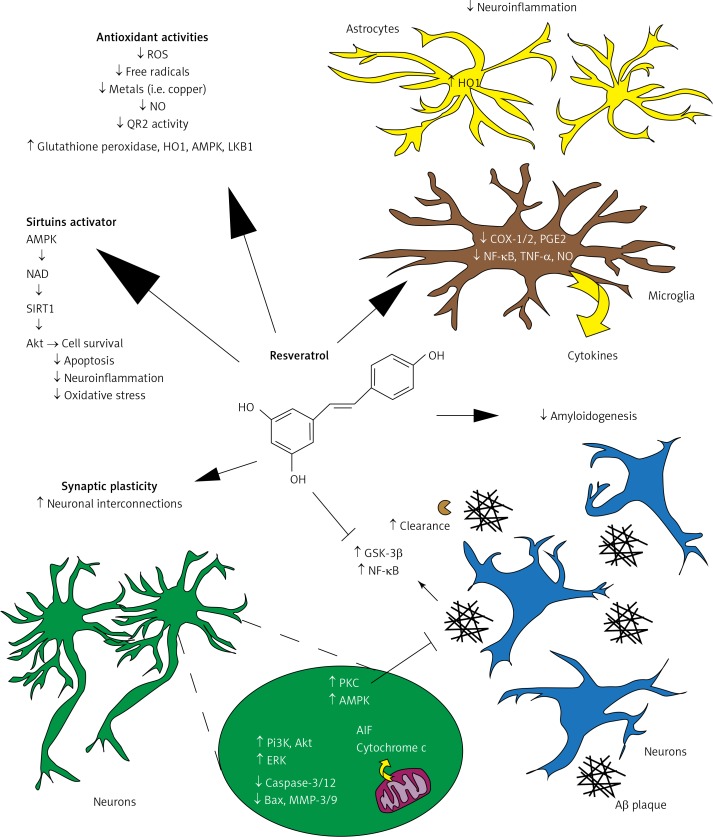
These neuroprotective actions of resveratrol have been summarized in Figure 1 [29].
Figure 1.
Summary of the neuroprotective action of resveratrol. Resveratrol displays potent antioxidant activity by scavenging free radicals and metals, protecting against NO toxicity, reducing QR2 activity and upregulating endogenous enzymes such as glutathione peroxidase, AMPK and LKB1. Resveratrol also inhibits pro-inflammatory enzyme (i.e., COX-1 and -2) expression, reduces NF-κB activation as well as PGE2, NO, and TNF-α production, and cytokine release. It may promote Aβ clearance. Treatment with resveratrol can affect multiple signaling pathway effectors involved in cell survival (AMPK, PI3-k and AkT), programmed cell death (caspase-3/12, Bax, and cytochrome c) and synaptic plasticity (ERK1/2). Direct and/or indirect activation of the deacetylase sirtuins by resveratrol has also been suggested.
AMPK – AMP-activated protein kinase, COX – cyclooxygenase, ERK – extracellular signal-regulated kinase-1 and -2 (ERK1/2), LKB1 – liver kinase B1, NF-κB – nuclear factor κB, NO – nitric oxide, PGE2 – prostaglandin E2, PI3-k – phosphoinositide 3-kinase, QR2 – quinone reductase 2. Reproduced with permission of Elsevier [29].
Effects on cognitive decline in humans
Cognitive deficits were shown to be associated with higher levels of reactive oxygen (ROS) and nitrogen species: indeed, oxidative stress seems to precede the formation of senile plaques [30]. Resveratrol has potent antioxidant activity that could have a role in preventing neurodegeneration in Alzheimer’s disease [31]. Particularly, it scavenges free radicals, protects neurons and microglia [32, 33] and attenuates Aβ-induced intracellular ROS accumulation [34]. The treatment of a hippocampal cell line with resveratrol attenuated ROS production and mitochondrial membrane-potential disruption; moreover, it restored the normal levels of glutathione (GSH) depleted by Aβ1-42 [35].
Resveratrol may also attenuate Aβ-induced intracellular ROS accumulation [36]. It induces the up-regulation of cellular antioxidants (i.e., glutathione) and the gene expression of phase 2 enzymes, protects against oxidative and electrophilic injury [37], and potentiates the HO-1 pathway [38]. Another important pathological hallmark of Alzheimer’s disease is represented by brain inflammation that contributes to the neuronal damage and enhanced Aβ formation [39]. As reported above in detail, resveratrol interferes with the neuroinflammatory process [40], and suppresses the activation of astrocytes and microglia [41, 42], and TNF-α and NO production by inhibiting NF-κB activation and p38 mitogen-activated protein kinase phosphorylation [43]. It also blocks the expression of cyclooxygenase (COX)-2 and inducible NO synthase (iNOS) [44]. Resveratrol treatment was also shown to reverse the Aβ-induced iNOS overexpression [45] and exert anti-inflammatory effects as it inhibits TNF-α, interleukin (IL)-1β, and IL-6 expression [46], and signal transducer and activator of transcription 1 (STAT1) and STAT3 phosphorylation [47].
The neuroprotective effect of resveratrol has been tested in some randomized, double-blind, placebo-controlled clinical trials [48]. Kennedy et al. [49] evaluated the effect of a single oral dose of resveratrol on mental function and cerebral blood flow (CBF) in the frontal cortex of young healthy humans. The enrolled subjects received three single dose treatments: placebo, 250 mg trans-resveratrol, and 500 mg trans-resveratrol. Following administration, resveratrol significantly increased CBF in a dose-dependent manner, but cognitive function did not improve acutely. The effect of resveratrol on CBF was improved by the contemporaneous administration of piperine 20 mg (probably because of a direct effect of piperine on CBF, since resveratrol plasma level was just shown not to be affected by piperine co-administration) [50].
The same authors carried out a randomized, double-blind, placebo-controlled, parallel-groups study, a total of sixty adults aged between 18 and 30 years receiving either placebo or resveratrol for 28 days. The results showed that the cognitive effects of resveratrol on day 1 were restricted to more accurate but slower serial subtraction task performance. The only cognitive finding on day 28 was a beneficial effect of resveratrol on the accuracy of the 3-Back task before treatment consumption. Subjective ratings of ‘fatigue’ were significantly lower across the entire 28 days in the resveratrol condition. Resveratrol also resulted in modulation of CBF parameters on day 1, as assessed by near-IR spectroscopy [51].
A further crossover clinical trial was designed to evaluate the effects of resveratrol on circulatory function and cognitive performance in obese adults [52]. Participants were randomized to consume a capsule containing either 75 mg of resveratrol or a color-matched placebo daily for 6 weeks. Then, participants were crossed over to an alternate dose for another 6 weeks. Moreover, following the assessments in weeks 6 and 12, after an hour participants consumed a single additional dose of the supplement. The primary outcome was to measure the degree of change in vasodilator function assessed by flow-mediated dilatation (FMD) in the brachial artery. Resveratrol supplementation was found to be well tolerated and induced a 23% increase in FMD compared with placebo. Moreover, a single dose of resveratrol (75 mg) following chronic resveratrol supplementation resulted in an acute FMD response 35% greater than placebo. However, attention and concentration were unaffected by chronic resveratrol supplementation [53]. Witte et al. [54] carried out a study aiming to assess the ability of resveratrol, given over 26 weeks, to enhance the cognitive performance. Overweight older adults were randomly divided into an active treated group receiving 200 mg/day of resveratrol in a formulation with quercetin 320 mg [53], in order to increase its bioavailability, and a control group, placebo treated. Volume, microstructure, and functional connectivity of the hippocampus were explored by magnetic resonance imaging. Resveratrol supplementation induced retention of memory and improved the functional connectivity between the hippocampus and frontal, parietal, and occipital areas, compared with placebo [55]. The changes in resting-state functional connectivity networks of the hippocampus after resveratrol intake were linked with behavioral improvements. Also, glucose metabolism was improved and this may account for some of the beneficial effects of resveratrol on neuronal function. These data have been recently confirmed in another trial by Köbe et al. [55]. A long-term (52-week), larger (n = 119) trial of resveratrol in individuals with mild-to-moderate AD was also conducted [56]. Participants were recruited and randomly divided into a placebo group and a resveratrol group (500 mg orally once daily with dose escalation by 500 mg increments every 13 weeks, ending with 1000 mg twice daily). The levels of Aβ40 in the cerebrospinal fluid and plasma declined more in the placebo group than the resveratrol-treated group with a significant difference at week 52 (note that Aβ40 levels decline as dementia advances). The trial also confirmed that resveratrol was safe and well tolerated. Recently, Wong et al. [57] carried out a balanced crossover clinical trial to evaluate the effect of resveratrol supplementation on cerebrovascular function, in type 2 diabetes adult patients. Participants were randomly allocated to receive placebo or resveratrol at doses of 75 mg, 150 mg, or 300 mg, taken as a single dose during four intervention visits that took place at seven-day intervals over 4 weeks. The main outcome was to determine the most effective dose of resveratrol to improve the cerebral vasodilator responsiveness to hypercapnia in the middle cerebral artery, using transcranial Doppler ultrasound. Resveratrol consumption significantly increased cerebral vasodilator responsiveness in the middle cerebral artery at all tested doses, and the maximum improvement was observed with the lowest one. In a more recent 14-week study carried out on post-menopausal women, the improved cerebral vasodilator responsiveness was also associated with improvement in cognitive function scores [58], which further confirms the possible usefulness of resveratrol in subjects with cognitive decline.
Discussion
Senile dementia is increasing worldwide substantially, paralleling the “graying” of the world’s population [59]. In particular, recent statistics suggest that mild cognitive impairment affects 5.5 to 7.7% of subjects aged more than 60 years old and 22% of those over 70 years old [60], more frequently in those with neuropsychiatric symptoms [61]. Faced with this epidemiology, it is necessary to search for any tools able to slow the progress of this dementia. Several genetic, nutritional and metabolic factors have been suggested to influence the risk of cognitive impairment and dementia in older adults [62, 63]. Both vascular pathology and inflammation have been proposed to explain how these factors can induce brain damage and dementia [64, 65]. Some botanicals seem to counteract these pathogenetic mechanisms [66]. Based on the above summarized evidence, resveratrol seems to be a dietary component with a specific neuroprotective action with some positive effect on human cognitive decline. In fact, beyond its positive effects on the central nervous system, resveratrol seems also to be able to act on multiple cellular targets and therefore have a variety of biological effects, potentially useful in elderly comorbidities, even if it has to be clearly demonstrated in long-term randomized clinical trials [67].
In particular, even if resveratrol seems not to have a specific positive impact on lipid profile [68], it seems to exert an improving effect on a number of metabolic syndrome components such as insulin resistance [69] and blood pressure [70].
The main problems related to the therapeutic or preventive use of resveratrol are linked to its low oral bioavailability and its short half-life in serum [71]. However, pharmaceutical technologies seem to be able to improve the oral bioavailability of resveratrol [72, 73]. On the other hand, the tolerability and safety profile of resveratrol is very high and no clinically significant pharmacological interaction of this nutraceutical with conventional drugs is known. This is of particular interest since the most effective drugs for dementia are usually not well tolerated, thus being not indicated for the treatment of the first disease stages [74], while the pharmacological improvement of cardiovascular risk factors has only a limited efficacy [75, 76].
In conclusion, long-term treatment with adequate dosages of resveratrol with improved bioavailability could exert clinically significant protective effects against cognitive decline in humans.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Shi J, He M, Cao J, et al. The comparative analysis of the potential relationship between resveratrol and stilbene synthase gene family in the development stages of grapes (Vitis quinquangularis and Vitis vinifera) Plant Physiol Biochem. 2014;74:24–32. doi: 10.1016/j.plaphy.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Sahebkar A, Serban C, Ursoniu S, et al. Lipid and Blood Pressure Meta-analysis Collaboration Group Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors: results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55. doi: 10.1016/j.ijcard.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Cvejic JM, Djekic SV, Petrovic AV, et al. Determination of trans- and cis-resveratrol in Serbian commercial wines. J Chromatogr Sci. 2010;48:229–34. doi: 10.1093/chromsci/48.3.229. [DOI] [PubMed] [Google Scholar]
- 4.Moreno M, Castro E, Falqué E. Evolution of trans- and cis- resveratrol content in red grapes (Vitis vinifera L. cv Menciá, Albarello and Merenzao) during ripening. Eur Food Res Technol. 2008;227:667–74. [Google Scholar]
- 5.Trela BC, Waterhouse AL. Resveratrol: isomeric molar absorptivities and stability. J Agric Food Chem. 1996;44:1253–7. [Google Scholar]
- 6.Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852:1195–201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–22. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 9.Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ, Yang JJ. Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and -apoptosis actions in aged mice. J Mol Neurosci. 2014;52:286–93. doi: 10.1007/s12031-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 10.Chen B, Zang W, Wang J, et al. The chemical biology of sirtuins. Chem Soc Rev. 2015;44:5246–64. doi: 10.1039/c4cs00373j. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa K, Yoshikawa K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J Neurosci. 2008;28:8772–84. doi: 10.1523/JNEUROSCI.3052-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajami M, Pazoki-Toroudi H, Amani H, et al. Therapeutic role of sirtuins in neurodegenerative disease and their modulation by polyphenols. Neurosci Biobehav Rev. 2017;73:39–47. doi: 10.1016/j.neubiorev.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K, Yoshikawa K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J Neurosci. 2008;28:8772–84. doi: 10.1523/JNEUROSCI.3052-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du LL, Xie JZ, Cheng XS, et al. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age. 2014;36:613–23. doi: 10.1007/s11357-013-9592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porquet D, Griñán-Ferré C, Ferrer I, et al. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J Alzheimers Dis. 2014;42:1209–20. doi: 10.3233/JAD-140444. [DOI] [PubMed] [Google Scholar]
- 18.Rege SD, Geetha T, Broderick TL, Babu JR. Resveratrol protects beta amyloid-induced oxidative damage and memory associated proteins in H19-7 hippocampal neuronal cells. Curr Alzheimer Res. 2015;12:147–56. doi: 10.2174/1567205012666150204130009. [DOI] [PubMed] [Google Scholar]
- 19.Toth P, Tarantini S, Tucsek Z, et al. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarzuelo MJ, López-Sepúlveda R, Sánchez M, et al. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2013;85:1288–96. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Thomas J, Garg ML, Smith DW. Dietary resveratrol supplementation normalizes gene expression in the hippocampus of streptozotocin-induced diabetic C57Bl/6 mice. J Nutr Biochem. 2014;25:313–8. doi: 10.1016/j.jnutbio.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Anastácio JR, Netto CA, Castro CC, et al. Resveratrol treatment has neuroprotective effects and prevents cognitive impairment after chronic cerebral hypoperfusion. Neurol Res. 2014;36:627–33. doi: 10.1179/1743132813Y.0000000293. [DOI] [PubMed] [Google Scholar]
- 23.Ma X, Sun Z, Liu Y, Jia Y, Zhang B, Zhang J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen Res. 2013;8:2050–9. doi: 10.3969/j.issn.1673-5374.2013.22.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yazir Y, Utkan T, Gacar N, Aricioglu F. Resveratrol exerts anti-inflammatory and neuroprotective effects to prevent memory deficits in rats exposed to chronic unpredictable mild stress. Physiol Behav. 2015;138:297–304. doi: 10.1016/j.physbeh.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–8. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Wang J, Bi W, et al. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem Int. 2015;89:191–7. doi: 10.1016/j.neuint.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarubbo F, Ramis MR, Aparicio S, et al. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age. 2015;37:9777. doi: 10.1007/s11357-015-9777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci Rep. 2015;5:8075. doi: 10.1038/srep08075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852:1195–201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Wahlster L, Arimon M, Nasser-Ghodsi N, et al. Presenilin-1 adopts pathogenic conformation in normal aging and in sporadic Alzheimer’s disease. Acta Neuropathol. 2013;125:187–99. doi: 10.1007/s00401-012-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Lee HJ, Lee KW. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J Neurochem. 2010;112:1415–30. doi: 10.1111/j.1471-4159.2009.06562.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang H, Kim YS, Koehler RC, Dore S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann NY Acad Sci. 2003;993:276–86. doi: 10.1111/j.1749-6632.2003.tb07534.x. [DOI] [PubMed] [Google Scholar]
- 33.Candelario-Jalil E, de Oliveira AC, Graf S, et al. Resveratrol potently reduces prostaglandin E2production and free radical formation in lipopolysaccharide-activated primary rat microglia. J Neuroinflam. 2007;4:25. doi: 10.1186/1742-2094-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–10. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 35.Kwon KJ, Kim HJ, Shin CY, Han SH. Melatonin potentiates the neuroprotective properties of resveratrol against beta amyloid-induced neurodegeneration by modulating AMP activated protein kinase pathways. J Clin Neurol. 2010;6:127–37. doi: 10.3988/jcn.2010.6.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koukoulitsa C, Villalonga-Barber C, Csonka R, et al. Biological and computational evaluation of resveratrol inhibitors against Alzheimer’s disease. J Enzyme Inhib Med Chem. 2016;31:67–77. doi: 10.3109/14756366.2014.1003928. [DOI] [PubMed] [Google Scholar]
- 37.Cao Z, Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol. 2004;489:39–48. doi: 10.1016/j.ejphar.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 38.Kwon KJ, Kim JN, Kim MK, et al. Melatonin synergistically increases resveratrol induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: a possible role in neuroprotection. J Pineal Res. 2011;50:110–23. doi: 10.1111/j.1600-079X.2010.00820.x. [DOI] [PubMed] [Google Scholar]
- 39.Sadigh-Eteghad S, Majdi A, Mahmoudi J, Golzari SE, Talebi M. Astrocytic and microglial nicotinic acetylcholine receptors: an overlooked issue in Alzheimer’s disease. J Neural Transm. 2016;123:1359–67. doi: 10.1007/s00702-016-1580-z. [DOI] [PubMed] [Google Scholar]
- 40.Venigalla M, Sonego S, Gyengesi E, Sharman MJ, Münch G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2016;95:63–74. doi: 10.1016/j.neuint.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Xu J, Rottinghaus GE, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–47. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 42.Bi XL, Yang JY, Dong YX, et al. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–93. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Cheng X, Wang Q, Li N, Zhao H. Effects of resveratrol on hippocampal astrocytes and expression of TNF-alpha in Alzheimer’s disease model rate. J Hyg Res. 2015;44:610–4. [PubMed] [Google Scholar]
- 44.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL. Resveratrol protects rats from Abeta-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One. 2011;6:e29102. doi: 10.1371/journal.pone.0029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao Y, Li J, Niu Y, et al. Resveratrol inhibits oligomeric Abeta-induced microglial activation via NADPH oxidase. Mol Med Rep. 2015;12:6133–9. doi: 10.3892/mmr.2015.4199. [DOI] [PubMed] [Google Scholar]
- 47.Capiralla H, Vingtdeux V, Zhao H, et al. Resveratrol mitigates lipopolysaccharide- and Abeta-mediated microglial inflammation by inhibiting the TLR4/NF-kappaB/STAT signaling cascade. J Neurochem. 2012;120:461–72. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Torosyan N, Silverman DH. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: a double-blinded placebo controlled pilot study. Exp Gerontol. 2017;87:121–8. doi: 10.1016/j.exger.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy D, Wightman EL, Reay JL, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010;91:1590–7. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- 50.Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP, Kennedy DO. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. Br J Nutr. 2014;112:203–13. doi: 10.1017/S0007114514000737. [DOI] [PubMed] [Google Scholar]
- 51.Wightman EL, Haskell-Ramsay CF, Reay JL, et al. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br J Nutr. 2015;114:1427–37. doi: 10.1017/S0007114515003037. [DOI] [PubMed] [Google Scholar]
- 52.Wong RH, Berry NM, Coates AM, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819–27. doi: 10.1097/HJH.0b013e328362b9d6. [DOI] [PubMed] [Google Scholar]
- 53.Serban MC, Sahebkar A, Zanchetti A, et al. Lipid and Blood Pressure Meta‐analysis Collaboration (LBPMC) Group Effects of quercetin on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34:7862–70. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Köbe T, Witte AV, Schnelle A, et al. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front Neurosci. 2017;11:105. doi: 10.3389/fnins.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turner RS, Thomas RG, Craft S, et al. Alzheimer’s disease cooperative study. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–91. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong RH, Nealon RS, Scholey A, Howe PR. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2016;26:393–9. doi: 10.1016/j.numecd.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Evans HM, Howe PR, Wong RH. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients. 2017;9 doi: 10.3390/nu9010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–37. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 60.Apostolo J, Holland C, O’Connell MD, et al. Mild cognitive decline. A position statement of the Cognitive Decline Group of the European Innovation Partnership for Active and Healthy Ageing (EIPAHA) Maturitas. 2016;83:83–93. doi: 10.1016/j.maturitas.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Bidzan M, Bidzan L, Bidzan-Bluma I. Neuropsychiatric symptoms and faster progression of cognitive impairments as predictors of risk of conversion of mild cognitive impairment to dementia. Arch Med Sci. 2017;13:1168–77. doi: 10.5114/aoms.2017.68943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flirski M, Sobow T. Biochemical markers and risk factors of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:47–64. doi: 10.2174/1567205052772704. [DOI] [PubMed] [Google Scholar]
- 63.Lahiri DK, Maloney B, Basha MR, Ge YW, Zawia NH. How and when environmental agents and dietary factors affect the course of Alzheimer’s disease: the ‘LEARn’ model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res. 2007;4:219–28. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- 64.Jiang X, Zhao X, Chen R, Jiang Q, Zhou B. Plasma soluble CD36, carotid intima-media thickness and cognitive function in patients with type 2 diabetes. Arch Med Sci. 2017;13:1031–9. doi: 10.5114/aoms.2016.60821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004;3:219–26. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- 66.Cicero AF, Fogacci F, Banach M. Botanicals and phytochemicals active on cognitive decline: the clinical evidence. Pharmacol Res. 2018;130:204–12. doi: 10.1016/j.phrs.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 67.Erdogan CS, Vang O. Challenges in analyzing the biological effects of resveratrol. Nutrients. 2016;8:E353. doi: 10.3390/nu8060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cicero AF, Colletti A, Bajraktari G, et al. Lipid lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel. Arch Med Sci. 2017;13:965–1005. doi: 10.5114/aoms.2017.69326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patti AM, Al-Rasadi K, Giglio RV, et al. Natural approaches in metabolic syndrome management. Arch Med Sci. 2018;14:422–41. doi: 10.5114/aoms.2017.68717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fogacci F, Tocci G, Presta V, Fratter A, Borghi C, Cicero AF. Effect of resveratrol on blood pressure: a systematic review and meta-analysis of randomized, controlled, clinical trials. Crit Rev Food Sci Nutr. 2018:1–14. doi: 10.1080/10408398.2017.1422480. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 71.Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci. 2014;6:218. doi: 10.3389/fnagi.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonferoni MC, Rossi S, Sandri G, Ferrari F. Nanoparticle formulations to enhance tumor targeting of poorly soluble polyphenols with potential anticancer properties. Semin Cancer Biol. 2017;46:205–14. doi: 10.1016/j.semcancer.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Ethemoglu MS, Seker FB, Akkaya H, et al. Anticonvulsant activity of resveratrol-loaded liposomes in vivo. Neuroscience. 2017;357:12–9. doi: 10.1016/j.neuroscience.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 74.Fisher A, Carney G, Bassett K, Dormuth CR. Tolerability of cholinesterase inhibitors: a population-based study of persistence, adherence, and switching. Drugs Aging. 2017;34:221–31. doi: 10.1007/s40266-017-0438-x. [DOI] [PubMed] [Google Scholar]
- 75.Banach M, Rizzo M, Nikolic D, Howard G, Howard V, Mikhailidis D. Intensive LDL-cholesterol lowering therapy and neurocognitive function. Pharmacol Ther. 2017;170:181–91. doi: 10.1016/j.pharmthera.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Zanchetti A, Liu L, Mancia G, et al. Blood pressure and low-density lipoprotein-cholesterol lowering for prevention of strokes and cognitive decline: a review of available trial evidence. J Hypertens. 2014;32:1741–50. doi: 10.1097/HJH.0000000000000253. [DOI] [PubMed] [Google Scholar]