Abstract
Introduction
Cardiac resynchronization therapy combined with an implantable cardioverter defibrillator (CRT-D) is widely applied in heart failure patients. Sufficient data on arrhythmia and defibrillator therapies during long-term follow-up of more than 4 years are lacking and data on mortality are conflicting. We aimed to characterize the occurrence of ventricular arrhythmia, respective defibrillator therapies and mortality for several years following CRT-D implantation or upgrade.
Material and methods
Eighty-eight patients with ischemic (ICM) or non-ischemic dilated cardiomyopathy (DCM) and at least one CRT-D replacement were included in this study and analyzed for incidence of non-sustained ventricular tachycardia (NSVT), defibrillator shocks, anti-tachycardia pacing (ATP) and mortality.
Results
ICM was the underlying disease in 59%, DCM in 41% of patients. During a mean follow-up of 76.4 ±24.8 months the incidence of appropriate defibrillator therapies (shock or ATP) was 46.6% and was elevated in ICM compared to DCM patients (57.7% vs. 30.6%, respectively; p = 0.017). Kaplan-Meier analysis revealed significantly higher ICD therapy-free survival rates in DCM patients (p = 0.031). Left ventricular ejection fraction, NSVT per year and ICM (vs. DCM) were independent predictors of device intervention. The ICM patients showed increased mortality compared to DCM patients, with cumulative all-cause mortality at 9 years of follow-up of 45.4% and 10.6%, respectively. Chronic renal failure, peripheral artery disease and chronic obstructive pulmonary disease were independent predictors of mortality.
Conclusions
The clinical course of patients with ICM and DCM treated with CRT-D differs significantly during long-term follow-up, with increased mortality and incidence of ICD therapies in ICM patients.
Keywords: cardiac resynchronization therapy, implantable cardioverter defibrillator, heart failure, ischemic cardiomyopathy, non-ischemic dilated cardiomyopathy
Introduction
Cardiac resynchronization therapy (CRT) has evolved into a broadly used treatment for patients suffering from chronic heart failure with severely reduced left ventricular ejection fraction (LVEF) and left bundle branch block to reduce morbidity and mortality [1, 2]. Furthermore, it is known that the risk of sudden cardiac death (SCD) due to ventricular tachycardia (VT) or ventricular fibrillation (VF) can be reduced by an implantable cardioverter defibrillator (ICD) in these patients [2, 3]. Up to now there is insufficient clinical data from randomized clinical trials to prove superiority of a combined CRT and ICD implantation (CRT-D) over CRT pacemakers (CRT-P) [1, 4]. Besides improvement of LVEF there is evidence for anti-arrhythmic effects following CRT implantation [5]. A recent meta-analysis of more than 12,000 patients demonstrated a reduction in the risk of all-cause mortality for CRT-D, which was more pronounced in patients suffering from ischemic cardiomyopathy (ICM) compared to non-ischemic dilated cardiomyopathy (DCM) [4]. Detailed data on arrhythmic events and respective ICD therapies in CRT-D patients during long-term follow-up are scarce. Analysis of the German DEVICE-registry did neither show a mortality difference nor a disparity in first ICD shock delivery according to heart failure etiology in CRT-D patients [6]. McLeod et al. observed no significant difference in appropriate or inappropriate shocks but reported a greater improvement of left ventricular systolic function (also shown by Barsheshet et al. [7] and in the MADIT-CRT study [8]) and a significant difference in survival favoring DCM patients [9]. In ICD patients as well, most studies reported similar shock delivery rates for ICM and DCM [10, 11].
Follow-up in the above-mentioned studies rarely exceeds 4 years and we therefore aimed to delineate differences between ICM and DCM patients treated with CRT-D in incidence of ventricular arrhythmia and ICD therapies as well as mortality during long-term follow-up of at least 5 years.
Material and methods
Study design
The present study is a retrospective single-center study at a German tertiary care university hospital. The study was approved by the local ethics committee. All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration. To ensure long-term follow-up of more than 5 years, only patients with CRT-D replacement due to end of the battery lifespan between February 2007 and November 2015 were included. This selection resulted in 88 patients with first CRT-D implantation between 2002 and 2011 that were routinely (3–6 months intervals) followed up as outpatients at our department.
CRT-D devices were either implanted primarily or patients were upgraded from pre-existing pacemaker, ICD or CRT-P devices. Both directly implanted and upgraded patients were only included following at least one replacement of CRT-D due to end of the battery lifespan.
The primary endpoints were mortality and a composite of appropriate ATP or shock by ICD. The secondary outcomes were appropriate ATP or shock by ICD, respectively.
The LVEF was calculated via transthoracic echocardiography by biplane Simpson method. Change in LVEF was defined as difference between the LVEF prior to CRT-D implantation/upgrade and the last documented LVEF. To account for differences due to varying follow-up durations the change in LVEF, total numbers of shocks for VT/VF, ATP for VT/VF and non-sustained VT/VF (NSVT/NSVF) are presented per year.
Arrhythmia events and device therapies
Each individual follow-up at our center was analyzed for occurrence of ventricular arrhythmia and ICD therapies (shock or ATP) starting with the first visit after initial CRT-D implantation or upgrade. In patients having received an upgrade from ICD to CRT-D, arrhythmia and device therapies before the upgrade were not included. All arrhythmia episodes and device therapies were analyzed by two different physicians with long-standing experience in device therapy. If in doubt, electrograms were sent to the respective company. Appropriate therapy was defined as shock or ATP for real VT or VF following analysis of the intracardiac electrograms. NSVT and NSVF were defined as true arrhythmia episodes < 30 s in the respective programmed VT or VF zones.
Device programming
CRT-D devices were programmed individually according to standard clinical care at our center. The pre-set parameters were almost never used. In general, two or three therapy zones (mainly one VT zone, one VF zone and possibly an additional fast VT (FVT) zone) were programmed. VT was primarily treated with ATP and possibly consecutive ICD shocks. VF was primarily treated with ICD shock with ATP during charging if available.
Typically, defibrillator settings (for primary prevention) were programmed as follows: VT zone: cycle length: 350–400 ms; detection: 20–24; redetection: 12; VF zone: cycle length: 300–320 ms; detection: 18 of 24; re-detection: 12 of 16.
After emergence of the MADIT-RIT study [12] ICD programming was adapted to: VT zone: cycle length: 330–400 ms; detection: 24–28; re-detection: 12; VF zone: cycle length: 270–315 ms; detection: 30 of 40; re-detection: 12 of 16.
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Normally distributed continuous variables are expressed as mean ± standard deviation (SD). Non-normally distributed continuous variables are shown as median with interquartile range (25th–75th percentile). The unpaired two-tailed Student’s t-test or one-way ANOVA with Tukey post-hoc test were performed for normally distributed continuous variables, whereas the Mann-Whitney U test was chosen for non-normally distributed continuous variables. The homogeneity of variance was assessed using the Levene test. Categorical variables are displayed as frequencies and percentages and the χ2 test was used for data analysis. The cumulative survival plots (free of appropriate therapy/shock/ATP; survival) were estimated according to the Kaplan-Meier method. Survival in groups was compared with the log rank test. Univariate Cox regression analysis was performed to identify significant independent predictors of outcome. For multivariate analysis (backward stepwise selection method) significant predictors from the univariate analysis were included. Results are reported as the adjusted hazard ratio (HR) with 95% confidence interval (CI). A two-sided p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software version 23.0 (SPSS, Chicago, IL).
Results
Study population
The study population consisted of 88 patients who received a CRT-D according to guideline recommendations for the treatment of chronic heart failure as either de novo implantation or an upgrade from a previously implanted ICD, pacemaker or CRT-P. Mean follow-up was 76.4 ±24.8 months. ICM was the underlying etiology in 52 (59%) patients and DCM in 36 (41%) patients. The mean age of our study population at first CRT-D implantation/upgrade was 68.0 ±9.1 years and the mean LVEF prior to CRT-D was 25 ±7%. Baseline characteristics and medication of all patients and the ICM and DCM subgroups are presented in Table I. ICM patients were older than DCM patients (p = 0.011) and more often male (p = 0.032). DCM patients had a higher body mass index (p = 0.009). Of note, there was no difference in BMI between patients without sleep apnea and those with central or obstructive sleep apnea (27.0 ±5.1 kg/m2, 27.4 ±4.1 kg/m2 and 28.9 ±4.1 kg/m2, respectively). The use of statins was more frequent in ICM patients (p = 0.002). 76.1% of the whole study population were treated with an ACE inhibitor or AT-1 blocker, 92.0% received a β-blocker and 45.5% were additionally treated with an aldosterone antagonist. Functional NYHA class was only numerically higher in ICM patients (p = 0.095).
Table I.
Baseline characteristics of study population
| Parameter | All patients (n = 88) | ICM (n = 52) | DCM (n = 36) | P-value |
|---|---|---|---|---|
| Age at 1st CRT-D [years] | 68.0 ±9.1 | 70.0 ±8.2 | 65.1 ±9.6 | 0.011 |
| LVEF (%) | 25 ±7 | 25 ±6 | 26 ±7 | 0.197 |
| Male (%) | 71 (80.7) | 46 (88.5) | 25 (69.4) | 0.032 |
| Diabetes (%) | 33 (37.5) | 20 (38.5) | 13 (36.1) | 1.000 |
| Hypertension (%) | 62 (70.5) | 41 (78.8) | 21 (58.3) | 0.057 |
| Dyslipidemia (%) | 29 (33.0) | 21 (40.4) | 8 (22.2) | 0.106 |
| BMI [kg/m2] | 27.4 ±4.8 | 26.2 ±4.5 | 29.0 ±4.8 | 0.009 |
| Obesity (%) | 28 (33.7) | 13 (26.5) | 15 (44.1) | 0.106 |
| Peripheral artery disease (%) | 16 (17) | 12 (23.1) | 3 (8.3) | 0.088 |
| Coronary artery disease (%) | 55 (62.5) | 49 (94.2) | 6 (16.7) | < 0.0001 |
| Previous MI (%) | 29 (33.0) | 27 (51.9) | 2 (5.6) | < 0.0001 |
| Previous CABG (%) | 15 (17.0) | 15 (28.8) | 0 (0.0) | < 0.0001 |
| Previous myocarditis (%) | 7 (8.0) | 2 (3.8) | 5 (13.9) | 0.117 |
| Previous CPR (%) | 10 (11.4) | 8 (15.4) | 2 (5.6) | 0.189 |
| Previous stroke (%) | 8 (9.1) | 7 (13.5) | 1 (2.8) | 0.134 |
| Chronic renal failure (%) | 39 (44.3) | 27 (51.9) | 12 (33.3) | 0.126 |
| COPD (%) | 16 (18.2) | 12 (23.1) | 4 (11.1) | 0.173 |
| Obstructive sleep apnea (%) | 14 (16.3) | 8 (15.7) | 6 (17.1) | 1.000 |
| Central sleep apnea (%) | 10 (11.6) | 8 (15.7) | 2 (5.7) | 0.190 |
| Paroxysmal AF (%) | 21 (23.9) | 12 (23.1) | 9 (25.0) | 1.00 |
| Persistent AF (%) | 18 (20.5) | 14 (26.9) | 4 (11.1) | 0.106 |
| Permanent AF (%) | 16 (18.2) | 9 (17.3) | 7 (19.4) | 0.787 |
| Medication at last follow-up: | ||||
| ACE inhibitor/AT-1 blocker (%) | 67 (76.1) | 37 (71.2) | 30 (83.3) | 0.214 |
| Aldosterone antagonist (%) | 40 (45.5) | 25 (48.1) | 15 (41.7) | 0.664 |
| β-Blocker (%) | 81 (92.0) | 48 (92.3) | 33 (91.7) | 1.00 |
| Diuretic (%) | 81 (92.0) | 48 (92.3) | 33 (91.7) | 1.00 |
| Acetylsalicylic acid (%) | 41 (46.6) | 28 (53.8) | 13 (36.1) | 0.130 |
| Statin (%) | 67 (76.1) | 46 (88.5) | 21 (58.3) | 0.002 |
| Digoxin/digitoxin (%) | 23 (26.1) | 15 (28.8) | 8 (22.2) | 0.623 |
| Amiodarone (%) | 31 (35.2) | 20 (38.5) | 11 (30.6) | 0.501 |
| Phenprocoumon (%) | 45 (51.1) | 31 (59.6) | 14 (38.9) | 0.082 |
| Direct oral anticoagulant (%) | 11 (12.5) | 6 (11.5) | 5 (13.9) | 0.754 |
| NYHA at last follow-up | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.095 |
Values are n (%), mean ± standard deviation or median (25th–75th percentile). ICM – ischemic cardiomyopathy, DCM – non-ischemic dilated cardiomyopathy, LVEF – left ventricular ejection fraction, BMI – body mass index. Obesity was defined as BMI ≥ 30 kg/m². MI – myocardial infarction, CABG – coronary artery bypass grafting, CPR – cardiopulmonary resuscitation, COPD – chronic obstructive pulmonary disease, AF – atrial fibrillation.
Device-related characteristics
The majority of devices (89.8%) were implanted for primary prevention of SCD. The mean time to first CRT-D replacement due to end of the battery lifespan was 45.6 ±10.3 months and to second replacement 101.1 ±14.3 months. There was no significant difference between ICM and DCM patients. Atrial pacing (AP) and bi-ventricular pacing (biVP) rates at last follow-up were comparable between the groups. Both ICM and DCM patients had excellent biVP rates with a median of 99% each. The median change in LVEF per year was 0.4% (0.0, 1.5) for ICM and 1.5% (0.0, 3.3) for DCM patients (p = 0.112). All essential device-related characteristics are shown in Table II.
Table II.
Device-related characteristics of study population
| Parameter | All patients (n = 88) | ICM (n = 52) | DCM (n = 36) | P-value |
|---|---|---|---|---|
| Upgrade to CRT-D (%): | 25 (28.4) | 21 (40.4) | 4 (11.1) | 0.004 |
| Upgrade from ICD | 12 (13.6) | 11 (21.2) | 1 (2.8) | |
| Upgrade from CRT-P | 2 (2.3) | 2 (3.8) | 0 (0.0) | |
| Upgrade from PM | 11 (12.5) | 8 (15.4) | 3 (8.3) | |
| Time to 1st CRT-D replacement [months] | 45.6 ±10.3 | 49.8 ±8.8 | 49.2 ±12.1 | 0.813 |
| Time to 2nd CRT-D replacement [months] | 101.1 ±14.3 | 109.0 ±18.2 | 97.2 ±11.0 | 0.137 |
| Follow-up [months] | 76.4 ±24.8 | 73.7 ±23.7 | 80.2 ±26.1 | 0.228 |
| Primary prevention (%) | 79 (89.8) | 46 (88.5) | 33 (91.7) | 0.732 |
| AP rate at last FU [%] | 11.5 (0.3, 75.7) | 13.0 (0.3, 70.0) | 6.8 (0.2, 75.7) | 0.715 |
| biVP rate at last FU [%] | 99.0 (97.8, 99.7) | 99.0 (97.5, 99.6) | 99.0 (98.2, 99.7) | 0.341 |
| Change in LVEF per year [%] | 0.7 (0.0, 2.6) | 0.4 (0.0, 1.5) | 1.5 (0.0, 3.3) | 0.112 |
| Appropriate shock (%) | 29 (33.0) | 21 (40.4) | 8 (22.2) | 0.106 |
| Appropriate shock for VT (%) | 23 (26.1) | 16 (30.8) | 7 (19.4) | 0.325 |
| Appropriate shock for VF (%) | 18 (20.5) | 14 (26.9) | 4 (11.1) | 0.106 |
| Inappropriate shock (%) | 6 (6.8) | 2 (3.8) | 4 (11.1) | 0.221 |
| ATP in total (%) | 36 (40.9) | 26 (50.0) | 10 (27.8) | 0.048 |
| ATP for VT (%) | 36 (40.9) | 26 (50.0) | 10 (27.8) | 0.048 |
| ATP for VF (%) | 9 (10.2) | 5 (9.6) | 4 (11.1) | 1.000 |
| Appropriate shock or ATP (%) | 41 (46.6) | 30 (57.7) | 11 (30.6) | 0.017 |
| NSVT (%) | 68 (77.3) | 37 (71.2) | 31 (86.1) | 0.125 |
| NSVF (%) | 3 (3.4) | 2 (5.6) | 1 (1.9) | 0.565 |
| Shocks for VT per year | 0.23 ±0.62 | 0.22 ±0.49 | 0.23 ±0.78 | 0.483 |
| Shocks for VF per year | 0.16 ±0.61 | 0.18 ±0.72 | 0.14 ±0.42 | 0.309 |
| ATP for VT per year | 2.83 ±14.64 | 4.34 ±18.90 | 0.65 ±1.96 | 0.049 |
| ATP for VF per year | 0.53 ±3.88 | 0.73 ±5.01 | 0.24 ±0.82 | 0.771 |
| NSVT per year | 4.47 ±7.95 | 3.96 ±6.43 | 5.21 ±9.78 | 0.396 |
| NSVF per year | 0.02 ±0.14 | 0.003 ±0.02 | 0.04 ±0.22 | 0.345 |
Values are n (%), mean ± standard deviation or median (25th–75th percentile). ICM – ischemic cardiomyopathy, DCM – non-ischemic dilated cardiomyopathy, PM – pacemaker, FU – follow-up, AP – atrial pacing rate, biVP – bi-ventricular pacing rate, LVEF – left ventricular ejection fraction, VT – ventricular tachycardia, VF – ventricular fibrillation, ATP – anti-tachycardia pacing, NSVT/NSVF – non-sustained ventricular tachycardia/fibrillation.
ICD therapies in ICM and DCM patients
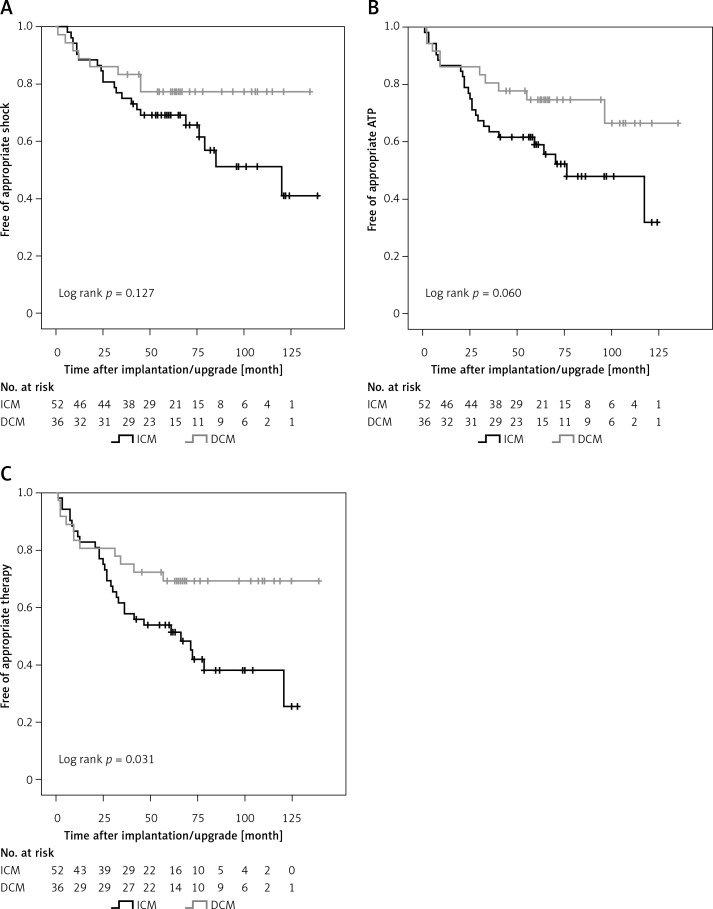
Appropriate shocks were delivered in 29 (33.0%) and ATP in 35 (39.8%) patients. Forty-one (46.6%) patients had any device therapy (appropriate shock or ATP), with significantly higher therapy rates in patients with ICM (57.7%) compared to DCM patients (30.6%) (p = 0.017). A detailed overview of ICD therapies is displayed in Table II. A total of 6 (6.8%) patients received inappropriate shocks, which did not differ between the groups (p = 0.221). ICM patients had significantly more ATP for VT treatment per year (p = 0.049). There was no difference in the incidence or amount of NSVT/NSVF between the groups. The secondary endpoints, shock-free (Figure 1 A) and ATP-free (Figure 1 B) survival, were only numerically higher in DCM patients. For the combined primary endpoint, DCM patients had a significantly higher therapy-free survival compared to ICM patients (Figure 1 C). The cumulative incidence of ICD therapies at 3, 6 and 9 years of follow-up was 42.3%, 58.2% and 62.0% for ICM patients and 25.0%, 29.8% and 29.8% for DCM patients, respectively. Time to first therapy was numerically longer in DCM patients (ICM: 49.3 ±32.2 months, DCM: 61.4 ±37.0 months; p = 0.107).
Figure 1.
ICD therapy-free survival in ICM and DCM patients. A – Kaplan-Meier estimates for survival free of appropriate shock delivered by CRT-D. B – Kaplan-Meier estimates for survival free of appropriate ATP delivered by CRT-D. C – Kaplan-Meier estimates for survival free of appropriate therapy (shock or ATP) delivered by CRT-D
ICM – ischemic cardiomyopathy, DCM – non-ischemic dilated cardiomyopathy, CRT-D – cardiac resynchronization therapy combined with an implantable cardioverter defibrillator (ICD), ATP – anti-tachycardia pacing.
Predictors of ICD intervention
Univariate Cox proportional analyses for appropriate device intervention revealed a significant influence of male gender, LVEF (per 5%), ICM (versus DCM) and NSVT (Table III). Subsequently these were fitted as independent variables in a multivariate Cox proportional hazards model with ICD therapy as the dependent variable. In this model, ICM patients had a 150% higher risk for defibrillator therapies when compared to DCM patients (HR = 2.529). Each NSVT per year resulted in a 6% increase in the risk for ICD intervention (HR = 1.056), whereas each 5% of LVEF reduced the risk for ICD therapy by 30% (HR = 0.703) (Table III).
Table III.
Univariate and multivariate Cox proportional hazard ratios (HR) for appropriate CRT-D intervention (shock or anti-tachycardia pacing)
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, per 5 years | 1.068 (0.898–1.270) | 0.459 | ||
| Male | 3.989 (1.229–12.948) | 0.021 | 1.778 (0.518–6.107) | 0.360 |
| ICM | 2.100 (1.051–4.197) | 0.036 | 2.529 (1.201–5.325) | 0.015 |
| Diabetes | 0.521 (0.261–1.040) | 0.064 | ||
| Hypertension | 1.083 (0.542–2.163) | 0.822 | ||
| BMI, per 1 kg/m2 | 0.968 (0.904–1.035) | 0.342 | ||
| Obesity | 0.565 (0.267–1.198) | 0.137 | ||
| Dyslipidemia | 1.450 (0.778–2.702) | 0.242 | ||
| Peripheral artery disease | 1.256 (0.579–2.729) | 0.564 | ||
| Previous stroke | 1.603 (0.627–4.100) | 0.325 | ||
| COPD | 1.423 (0.679–2.983) | 0.350 | ||
| Chronic renal failure | 1.084 (0.585–2.010) | 0.797 | ||
| Obstructive sleep apnea | 0.489 (0.173–1.378) | 0.176 | ||
| Central sleep apnea | 0.955 (0.338–2.694) | 0.930 | ||
| Previous CPR | 0.639 (0.197–2.074) | 0.456 | ||
| NSVT per year | 1.047 (1.018–1.077) | 0.001 | 1.056 (1.022–1.091) | 0.001 |
| LVEF, per 5% | 0.704 (0.562–0.882) | 0.002 | 0.703 (0.550–0.899) | 0.005 |
ICM – ischemic cardiomyopathy, BMI – body mass index, COPD – chronic obstructive pulmonary disease, CPR – cardiopulmonary resuscitation, NSVT – non-sustained ventricular tachycardia, LVEF – left ventricular ejection fraction.
Mortality in CRT-D patients
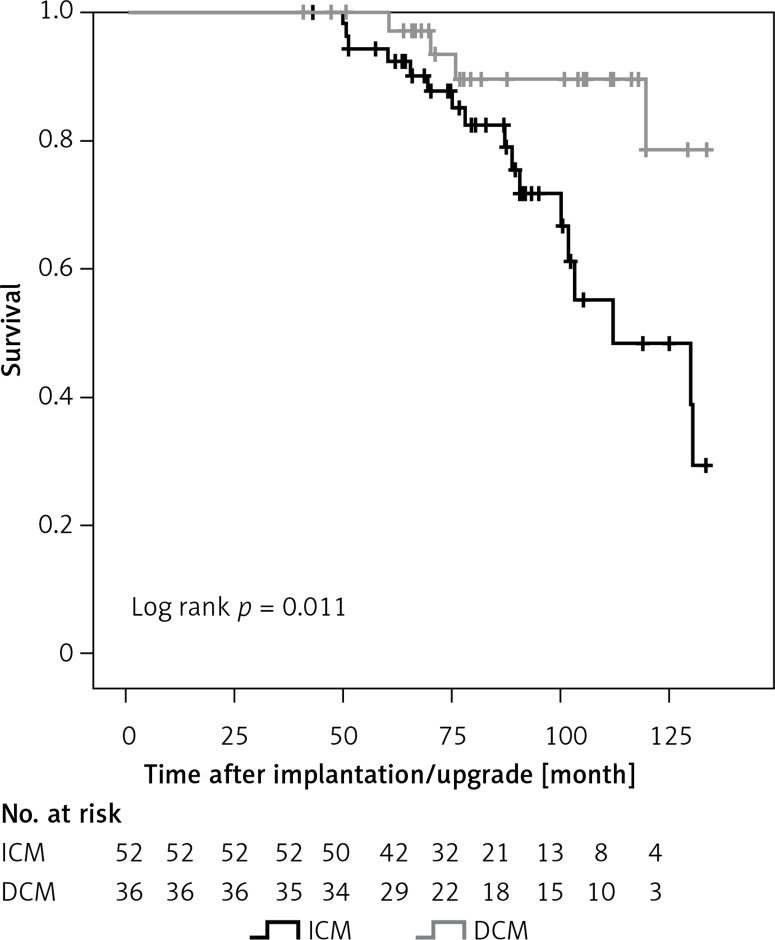
DCM patients had a significantly higher survival rate compared to ICM patients (Figure 2). The cumulative all-cause mortality at 3, 6 and 9 years of follow-up was 0.0%, 12.5% and 45.4% for ICM and 0.0%, 6.8% and 10.6% for DCM patients, respectively. Since only patients with at least one CRT-D replacement were included in this study, there were no deaths < 50 months after implantation.
Figure 2.
Cumulative survival in ICM and DCM patients. Kaplan-Meier estimates for survival in patients treated with cardiac resynchronization therapy combined with an implantable cardioverter defibrillator (CRT-D) according to disease etiology
ICM – ischemic cardiomyopathy, DCM – non-ischemic dilated cardiomyopathy.
Predictors of mortality
Univariate Cox proportional analyses for mortality revealed a significant influence of central sleep apnea, ICM (versus DCM), peripheral artery disease, chronic renal failure and chronic obstructive pulmonary disease (COPD) (Table IV). Subsequently these were fitted as independent variables in a multivariate Cox proportional hazards model with mortality as the dependent variable. In this model, patients suffering from peripheral artery disease had a 279% higher risk for mortality (HR = 3.786), whereas in patients with COPD mortality increased by 289% (HR = 3.889). Chronic renal failure led to a 225% increase of mortality (HR = 3.236) (Table IV).
Table IV.
Univariate and multivariate Cox proportional hazard ratios (HR) for mortality
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, per 5 years | 1.346 (1.000–1.813) | 0.050 | ||
| Male | 4.876 (0.654–36.370) | 0.122 | ||
| ICM | 3.789 (1.266–11.343) | 0.017 | 1.511 (0.421–5.424) | 0.527 |
| Diabetes | 0.790 (0.305–2.045) | 0.627 | ||
| Hypertension | 1.205 (0.465–3.124) | 0.702 | ||
| BMI, per 1 kg/m2 | 0.962 (0.871–1.063) | 0.447 | ||
| Obesity | 0.556 (0.202–1.531) | 0.256 | ||
| Dyslipidemia | 1.108 (0.457–2.686) | 0.820 | ||
| Peripheral artery disease | 4.844 (1.960–11.969) | 0.001 | 3.786 (1.531–9.367) | 0.004 |
| Previous stroke | 1.798 (0.527–6.129) | 0.349 | ||
| COPD | 4.067 (1.704–9.711) | 0.002 | 3.889 (1.598–9.460) | 0.003 |
| Chronic renal failure | 3.164 (1.274–7.860) | 0.013 | 3.236 (1.270–8.243) | 0.014 |
| Obstructive sleep apnea | 1.285 (0.366–4.503) | 0.696 | ||
| Central sleep apnea | 2.927 (1.057–8.100) | 0.039 | 1.289 (0.398–4.180) | 0.672 |
| Previous CPR | 2.448 (0.703–8.521) | 0.159 | ||
| NSVT per year | 0.962 (0.890–1.040) | 0.331 | ||
| LVEF, per 5% | 0.868 (0.645–1.167) | 0.349 | ||
ICM – ischemic cardiomyopathy, BMI – body mass index, COPD – chronic obstructive pulmonary disease, CPR – cardiopulmonary resuscitation, NSVT – non-sustained ventricular tachycardia, LVEF – left ventricular ejection fraction.
Subgroup of patients with primary preventive CRT-D implantation
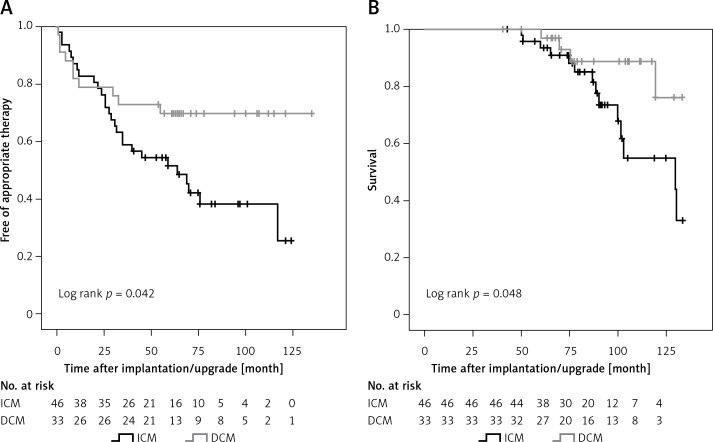
Seventy-nine (89.8%) patients received their CRT-D for primary prevention. This subgroup of our study population revealed reduced therapy-free survival and increased mortality in ICM compared to DCM patients as well (Figures 3 A, B, respectively). ICM, NSVT and LVEF were predictors of appropriate device intervention (Table V), whereas peripheral artery disease and COPD predicted mortality (Table VI).
Figure 3.
ICD therapy-free survival and cumulative survival in patients with CRT-D for primary prevention. A – Kaplan- Meier estimates for survival free of appropriate therapy (shock or anti-tachycardia pacing) delivered by CRT-D. B – Kaplan-Meier estimates for survival in CRT-D patients according to disease etiology
ICM – ischemic cardiomyopathy, DCM – non-ischemic dilated cardiomyopathy, CRT-D – cardiac resynchronization therapy combined with an implantable cardioverter defibrillator (ICD).
Table V.
Univariate and multivariate Cox proportional hazard ratios (HR) for appropriate CRT-D intervention (shock or anti-tachycardia pacing) in patients with CRT-D implantation for primary prevention
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, per 5 years | 1.102 (0.917–1.324) | 0.303 | ||
| Male | 3.781 (1.159–12.337) | 0.028 | 1.507 (0.426–5.327) | 0.524 |
| ICM | 2.088 (1.009–4.319) | 0.047 | 2.500 (1.142–5.476) | 0.022 |
| Diabetes | 0.611 (0.302–1.238) | 0.172 | ||
| Hypertension | 1.048 (0.506–2.167) | 0.900 | ||
| BMI, per 1 kg/m2 | 0.969 (0.905–1.038) | 0.374 | ||
| Obesity | 0.595 (0.276–1.280) | 0.184 | ||
| Dyslipidemia | 1.420 (0.736–2.742) | 0.296 | ||
| Peripheral artery disease | 1.386 (0.607–3.166) | 0.438 | ||
| Previous stroke | 1.381 (0.488–3.914) | 0.543 | ||
| COPD | 1.773 (0.836–3.763) | 0.136 | ||
| Chronic renal failure | 0.954 (0.496–1.834) | 0.887 | ||
| Obstructive sleep apnea | 0.463 (0.141–1.516) | 0.203 | ||
| Central sleep apnea | 0.972 (0.342–2.765) | 0.958 | ||
| NSVT per year | 1.045 (1.015–1.076) | 0.003 | 1.054 (1.018–1.091) | 0.003 |
| LVEF, per 5% | 0.692 (0.547–0.875) | 0.002 | 0.685 (0.529–0.886) | 0.004 |
ICM – ischemic cardiomyopathy, BMI – body mass index, COPD – chronic obstructive pulmonary disease, NSVT – non-sustained ventricular tachycardia, LVEF – left ventricular ejection fraction.
Table VI.
Univariate and multivariate Cox proportional hazard ratios (HR) for mortality in patients with CRT-D implantation for primary prevention
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age, per 5 years | 1.277 (0.945–1.725) | 0.112 | ||
| Male | 4.634 (0.616–34.874) | 0.136 | ||
| ICM | 2.940 (0.962–8.985) | 0.059 | ||
| Diabetes | 0.751 (0.266–2.116) | 0.588 | ||
| Hypertension | 1.415 (0.500–4.004) | 0.512 | ||
| BMI, per 1 kg/m2 | 0.959 (0.863–1.065) | 0.432 | ||
| Obesity | 0.448 (0.146–1.376) | 0.161 | ||
| Dyslipidemia | 1.640 (0.645–4.169) | 0.299 | ||
| Peripheral artery disease | 3.645 (1.341–9.910) | 0.011 | 2.871 (1.028–8.015) | 0.044 |
| Previous stroke | 1.226 (0.281–5.358) | 0.786 | ||
| COPD | 4.072 (1.599–10.371) | 0.003 | 3.412 (1.319–8.829) | 0.011 |
| Chronic renal failure | 2.439 (0.943–6.310) | 0.066 | ||
| Obstructive sleep apnea | 0.520 (0.067–4.018) | 0.531 | ||
| Central sleep apnea | 3.267 (1.145–9.321) | 0.027 | 1.949 (0.549–6.920) | 0.302 |
| NSVT per year | 0.978 (0.909–1.052) | 0.553 | ||
| LVEF, per 5% | 0.856 (0.624–1.173) | 0.332 | ||
ICM – ischemic cardiomyopathy, BMI – body mass index, COPD – chronic obstructive pulmonary disease, NSVT – non-sustained ventricular tachycardia, LVEF – left ventricular ejection fraction.
Direct implantation or upgrade to CRT-D
In total, 25 (28.4%) patients received an upgrade to CRT-D (Table II). Significantly more ICM patients were upgraded compared to DCM patients (40.4% and 11.1%, respectively; p = 0.004), especially when a previous ICD was upgraded (12 patients in total). In 2 patients a CRT-P and in 11 patients a pacemaker was upgraded to CRT-D. ICD therapy-free survival was similar between directly implanted and upgraded CRT-D devices (Figure 4). Comparing directly implanted and upgraded CRT-D in ICM or DCM patients alone, resulted in comparable event-free survival rates as well (data not shown).
Figure 4.
ICD therapy-free survival in directly implanted versus upgraded CRT-D patients. Kaplan-Meier estimates for survival free of appropriate therapy (shock or anti-tachycardia pacing) delivered by CRT-D in patients with direct implantation of CRT-D or upgrade from previously implanted ICD, pacemaker or CRT-P
ICD – implantable cardioverter defibrillator, CRT-D – cardiac resynchronization therapy combined with an ICD, CRT-P – CRT pacemaker.
Discussion
The present study evaluated the occurrence of ventricular arrhythmia and concomitant ICD therapies in CRT-D patients who survived until first device replacement due to end of the battery lifespan. This pre-selection was chosen to guarantee long-term real-world follow-up for a median of 6–7 years, since previous studies rarely provided information about more than 3–4 years. Data were analyzed for differences between the two major cardiomyopathy etiologies, ischemic and non-ischemic dilated cardiomyopathy, which cause heart failure with reduced LVEF and therefore trigger the implantation of CRT-D or ICD devices.
During the mean follow-up of 76 months, 41 (46.6%) patients had any device therapy (57.7% of ICM patients, 30.6% of DCM patients). Kaplan-Meier analysis revealed a significantly higher rate of any ICD therapy in ICM patients. This was mainly driven by a higher rate of ATP. To date, most studies have reported similar VT/VF or shock rates in CRT-D patients when comparing ICM and DCM [6, 9, 11]. In a post-hoc analysis of the MADIT-CRT study population Kutyifa et al. reported an incidence of first VT/VF of 27% in ICD and 22% in CRT-D patients after a follow-up of 40 months [13]. In our study, we observed at 40 months a higher rate of 38.7% (therapy for VT/VF). In another study including more than 67,000 patients the cumulative shock incidence was 33% (ICD) and 28% (CRT-D) 5 years after device implantation [14]. This is very similar to our shock incidence of 27.5% at 60 months of follow-up. Valles et al. reported an annual shock rate in ICD patients of 10% for ICM and 4% for DCM [15]. Taken together, some previous studies observed at least numerically higher shock rates in ICM patients when compared to DCM patients, but their follow-up duration rarely exceeded 40 months and mainly ICD shocks were analyzed. Our study now adds more than 3 years of extra longitudinal observation and additional data on ATP. Therefore, extended follow-up and the combination of shock and ATP as the endpoint might explain why the presented significant differences between ICM and DCM patients were detected now. Both patient populations have different patterns of myocardial scarring with predominantly endocardial scar formation following ischemia versus more isolated mid-myocardial or epicardial scars in DCM patients. For both etiologies it was shown before that myocardial scarring has an impact on the occurrence of arrhythmia [16, 17]. Therefore, the differing patterns of scar formation might translate into differential arrhythmia and ICD therapy rates.
In addition to the higher rate of ICD interventions, we could also demonstrate a significant increase in mortality for patients suffering from ICM compared to DCM patients. So far there have been conflicting data on mortality when comparing both cardiomyopathies treated with CRT-D, ranging from a reduction in all-cause mortality in ICM patients [4], through no difference in mortality [6], to a survival benefit in DCM patients [9]. The recently published DANISH-Trial reported no reduction in mortality following prophylactic implantation of an ICD in heart failure patients without coronary artery disease [18]. Interestingly, more than 50% of the study population were treated with CRT-D or CRT-P devices. Here we report a higher risk for ICD interventions and increased mortality in ICM patients, likewise in the subgroup of primary prophylactic implanted devices. The excess mortality seems to be at least in part due to a higher rate of co-morbidities in ICM patients. Peripheral artery disease, COPD and chronic renal failure share pathophysiological mechanisms as well as risk factors with coronary artery disease, and rates were numerically higher in patients with ICM. Due to our small patient population these differences were not significant, but multivariate analysis identified these three parameters as strong predictors of mortality. This is in line with previous studies reporting increased mortality in patients with coronary artery disease combined with COPD [19–21], peripheral artery disease [22, 23] or chronic renal failure [24].
Interestingly, univariate analysis for mortality prediction revealed a significant impact of central but not obstructive sleep apnea, which is in line with a recent meta-analysis by Nakamura et al. [25]. Most certainly due to the small patient population with central sleep apnea, no significant predictive value was observed in the multivariate analysis.
We could identify LVEF, NSVT per year and ICM as independent predictors of ICD intervention. For MADIT-CRT patients it has previously been shown that each 5% of LVEF was associated with a 30% reduction in risk for fast VT/VF [13]. In our study we could reproduce this amount of risk reduction (HR = 0.703). For ICD patients it has been demonstrated before that the occurrence of NSVT is accompanied by an increased risk of ICD interventions [26, 27], which we could now also demonstrate in CRT-D patients (6% increase in risk for ICD intervention per occurrence of NSVT per year).
We did not observe any difference in event-free survival between upgraded and directly implanted CRT-D devices. Since significantly more ICM patients were upgraded and patients with ICM showed higher rates of device therapies, this might have influenced the results. Therefore, we analyzed ICM and DCM patients separately and event-free survival remained similar between upgrade and direct implantation.
In addition to the small size of our study population, the retrospective design implies several known limitations. Differences in CRT-D programming for device therapies over time might have influenced the rate of shocks and ATP [12], but similarly in patients with ICM and DCM. We report real-world data from a typical non-selected patient population during standard clinical practice. Throughout the whole study period, the devices were programmed according to current recommendations at the respective point in time. Furthermore, our pre-selection to include only patients who survived until the first CRT-D replacement due to end of the battery lifespan might have induced a certain bias, potentially compromising translation of our results to all patients immediately after CRT-D implantation.
In conclusion, this present study reveals a different rate of arrhythmic events, subsequent ICD therapies and mortality depending on the underlying etiology of the cardiomyopathy. Patients with ICM had significantly more device interventions during long-term follow-up, an overall 150% higher risk for shock/ATP application and increased mortality. The LVEF prior to device implantation, ICM as heart failure etiology and NSVT were the strongest predictors of device therapy, whereas peripheral artery disease, COPD and chronic renal failure were the strongest mortality predictors.
Acknowledgments
Thomas Beiert and Swanda Straesser contributed equally to this work.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34:2281–329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Barra S, Providencia R, Tang A, Heck P, Virdee M, Agarwal S. Importance of implantable cardioverter-defibrillator back-up in cardiac resynchronization therapy recipients: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saini A, Kannabhiran M, Reddy P, Gopinathannair R, Olshansky B, Dominic P. Cardiac resynchronization therapy may be antiarrhythmic particularly in responders. JACC Clin Electrophysiol. 2016;2:307–16. doi: 10.1016/j.jacep.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Wasmer K, Kobe J, Andresen D, et al. Comparing outcome of patients with coronary artery disease and dilated cardiomyopathy in ICD and CRT recipients: data from the German DEVICE-registry. Clin Res Cardiol. 2013;102:513–21. doi: 10.1007/s00392-013-0559-0. [DOI] [PubMed] [Google Scholar]
- 7.Barsheshet A, Goldenberg I, Moss AJ, et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur Heart J. 2011;32:1622–30. doi: 10.1093/eurheartj/ehq407. [DOI] [PubMed] [Google Scholar]
- 8.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 9.McLeod CJ, Shen W, Rea RF, et al. Differential outcome of cardiac resynchronization therapy in ischemic cardiomyopathy and idiopathic dilated cardiomyopathy. Heart Rhythm. 2011;8:377–82. doi: 10.1016/j.hrthm.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Smith T, Theuns Dominic AMJ, Caliskan K, Jordaens L. Long-term follow-up of prophylactic implantable cardioverter-defibrillator-only therapy: comparison of ischemic and nonischemic heart disease. Clin Cardiol. 2011;34:761–7. doi: 10.1002/clc.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhagen MP, van Boven N, Ruiter JH, Kimman GP, Tahapary GJ, Umans VA. Follow-up of implantable cardioverter-defibrillator therapy: comparison of coronary artery disease and dilated cardiomyopathy. Neth Heart J. 2014;22:431–7. doi: 10.1007/s12471-014-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–83. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 13.Kutyifa V, Moss AJ, Solomon SD, et al. Reduced risk of life-threatening ventricular tachyarrhythmias with cardiac resynchronization therapy: relationship to left ventricular ejection fraction. Eur J Heart Fail. 2015;17:971–8. doi: 10.1002/ejhf.311. [DOI] [PubMed] [Google Scholar]
- 14.Saba S, Adelstein E, Wold N, Stein K, Jones P. Influence of patients’ age at implantation on mortality and defibrillator shocks. Europace. 2017;19:802–7. doi: 10.1093/europace/euw085. [DOI] [PubMed] [Google Scholar]
- 15.Valles AG, Khawaja FJ, Gersh BJ, et al. Implantable cardioverter defibrillators in patients with valvular cardiomyopathy. J Cardiovasc Electrophysiol. 2012;23:1326–32. doi: 10.1111/j.1540-8167.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 16.Kwon DH, Halley CM, Carrigan TP, et al. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging. 2009;2:34–44. doi: 10.1016/j.jcmg.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Neilan TG, Coelho-Filho OR, Danik SB, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944–54. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–30. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 19.Andell P, Koul S, Martinsson A, et al. Impact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarction. Open Heart. 2014;1:e000002. doi: 10.1136/openhrt-2013-000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boschetto P, Beghe B, Fabbri LM, Ceconi C. Link between chronic obstructive pulmonary disease and coronary artery disease: implication for clinical practice. Respirology. 2012;17:422–31. doi: 10.1111/j.1440-1843.2011.02118.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Cheng Y, Zheng W, et al. Impact of chronic obstructive pulmonary disease on long-term outcome in patients with coronary artery disease undergoing percutaneous coronary intervention. Biomed Res Int. 2016;2016:8212459. doi: 10.1155/2016/8212459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subherwal S, Patel MR, Kober L, et al. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. 2015;22:317–25. doi: 10.1177/2047487313519344. [DOI] [PubMed] [Google Scholar]
- 23.Grenon SM, Vittinghoff E, Owens CD, Conte MS, Whooley M, Cohen BE. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the Heart and Soul Study. Vasc Med. 2013;18:176–84. doi: 10.1177/1358863X13493825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Q, Mukku VK, Ahmad M. Coronary artery disease in patients with chronic kidney disease: a clinical update. Curr Cardiol Rev. 2013;9:331–9. doi: 10.2174/1573403X10666140214122234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura S, Asai K, Kubota Y, et al. Impact of sleep-disordered breathing and efficacy of positive airway pressure on mortality in patients with chronic heart failure and sleep-disordered breathing: a meta-analysis. Clin Res Cardiol. 2015;104:208–16. doi: 10.1007/s00392-014-0774-3. [DOI] [PubMed] [Google Scholar]
- 26.Verma A, Sarak B, Kaplan AJ, et al. Predictors of appropriate implantable cardioverter defibrillator (ICD) therapy in primary prevention patients with ischemic and nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2010;33:320–9. doi: 10.1111/j.1540-8159.2009.02566.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Johnson G, Hellkamp AS, et al. Rapid-rate nonsustained ventricular tachycardia found on implantable cardioverter-defibrillator interrogation: relationship to outcomes in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) J Am Coll Cardiol. 2013;61:2161–8. doi: 10.1016/j.jacc.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]