Abstract
Aims
Non-ischaemic cardiomyopathies (NICM) can cause heart failure and death. Cardiac magnetic resonance (CMR) detects myocardial scar/fibrosis associated with myocardial infarction (MI) and NICM with late gadolinium enhancement (LGE). The aim of this study was to determine the prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in a community-based sample of older adults.
Methods and results
The ICELAND-MI cohort, a substudy of the Age, Gene/Environment Susceptibility Reykjavik (AGES-Reykjavik) study, provided a well-characterized population of 900 subjects after excluding subjects with pre-existing heart failure. Late gadolinium enhancement CMR divided subjects into four groups: MI (n = 211), major (n = 54) non-ischaemic fibrosis (well-established, classic patterns, associated with myocarditis, infiltrative cardiomyopathies, or pathological hypertrophy), minor (n = 238) non-ischaemic fibrosis (remaining localized patterns not meeting major criteria), and a no LGE (n = 397) reference group. The primary outcome was time to death or first heart failure hospitalization. During a median follow-up of 5.8 years, 192 composite events occurred (115 deaths and 77 hospitalizations for incident heart failure). After inverse probability weighting, major non-ischaemic fibrosis [hazard ratio (HR) 3.2, P < 0.001] remained independently associated with the primary endpoint, while MI (HR 1.4, P = 0.10) and minor non-ischaemic LGE (HR 1.2, P = 0.39) did not. Major non-ischaemic fibrosis was associated with a poorer outcome than MI (HR = 2.3, P = 0.001) in the adjusted analysis.
Conclusion
Major non-ischaemic patterns of myocardial fibrosis portended worse prognosis than no fibrosis/scar in an older community-based cohort. Traditional risk factors largely accounted for the effect of MI and minor non-ischaemic LGE.
Keywords: Fibrosis, Prognosis, Gadolinium, Myocardial infarction, Non-ischaemic cardiomyopathy
Introduction
Non-ischaemic cardiomyopathies (NICM) refer to a diverse group of diseases which can cause heart failure, arrhythmias, and death.1,2 Late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) is well validated for detecting scar/fibrosis within the myocardium.3 Specific patterns of scar/fibrosis corresponding to myocardial infarction (MI) and various NICM are diagnostically useful.3,4 Both non-ischaemic scar/fibrosis and MI as detected by CMR have prognostic value, and recent guidelines highlight the importance of imaging myocardial fibrosis by CMR5. However, most prior studies have focused on tertiary care referral populations or those with pre-existing heart failure.6–9
In the ICELAND-MI cohort,10 we have previously shown that participants with CMR evidence of unrecognized MI had similar mortality to participants with clinically recognized MI.11 However, the prevalence and prognosis of scar/fibrosis from non-ischaemic aetiologies was not known in this cohort or in the general population.
Thus, the primary aim of this study was to use the ICELAND-MI cohort to determine the prevalence and prognosis of myocardial scar/fibrosis consistent with NICM as detected by LGE. Specifically, we hypothesized that major patterns of scar/fibrosis associated with NICM would portend higher risk of heart failure and death when compared with other participants.
Methods
Study participants were recruited from January 2004 to January 2007 into ICELAND-MI (n = 936) from the AGES-Reykjavik study (n = 5764), a community-based cohort of survivors from the original Reykjavik Study born between 1907 and 1935 who were followed by the Icelandic Heart Association in Iceland since 1967.10 Further details regarding recruitment for ICELAND-MI have been described.11 AGES-Reykjavik and ICELAND-MI were approved by both the National Institutes on Aging intramural institutional review board and the National Bioethics Committee in Iceland which serves as the institutional review board for the Iceland Heart Association.
AGES-Reykjavik subjects who could provide written and informed consent and were deemed eligible for recruitment and were enrolled in two phases. Participants were randomly recruited for ICELAND-MI in Phase I, while Phase II preferentially recruited diabetic participants due to an under-representation in Phase I. Subjects were excluded if they could not safely undergo magnetic resonance imaging (e.g. implantable devices) or receive gadolinium contrast (e.g. severe renal disease). For this study, participants were excluded if they had missing CMR images or antecedent heart failure as incident heart failure was part of the study outcome. The AGES-Reykjavik study, which encompassed the full cohort of ICELAND-MI, recorded a large spectrum of demographics, comorbidities, clinical variables, and biochemical measurements over three clinic visits performed within a 4–6 week time window.10,11
Endpoint definitions
The primary study outcome was a composite of time to hospitalization for heart failure or death. All-cause mortality was determined by the national mortality index with validation performed using death certificates. Heart failure outcomes were adjudicated by a cardiologist (blinded to CMR data) using hospital discharge ICD 10 codes, which were then verified by a review of hospital records. Details of the heart failure endpoint definition have been published.12 The analysis of the heart failure endpoint was restricted by the last available date of adjudication (31 December 2010 at time of submission). Thus, the duration of follow-up for the composite endpoint of incident heart failure hospitalization or death was limited to this date.
Cardiac magnetic resonance imaging and late gadolinium enhancement classification
Cardiac magnetic resonance exams were performed on a 1.5 T scanner (GE Healthcare) with an 8-element cardiac phased array coil. Steady-state free precession cine imaging was performed in long- and short-axis views using pixel dimensions of 1.8 × 2.1 mm, a slice thickness of 8 mm, and 30 images per cycle.
Late gadolinium enhancement images were analysed by consensus read of two cardiologists with expertise in CMR who were blinded to participant outcomes. Scar/fibrosis from MI or non-ischaemic scar was assessed using prospective, electrocardiogram-gated, segmented, phase-sensitive gradient echo inversion recovery sequence performed approximately 6–25 min after 0.1 mmol/kg intravenous administration of gadolinium (Magnevist, Berlex) contrast.
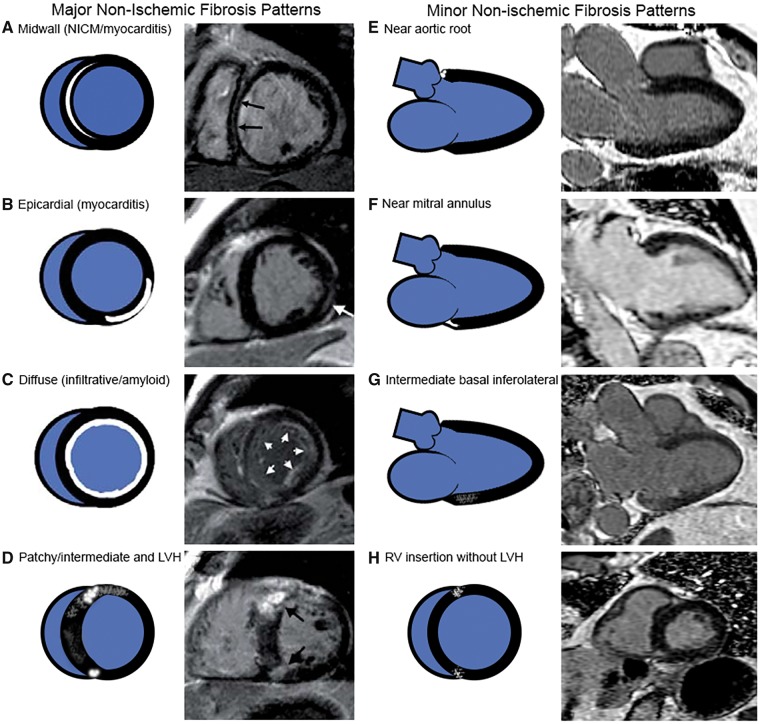
Cardiac magnetic resonance images had already been analysed for MI.11 A new analysis assessed for non-ischaemic myocardial scar/fibrosis in all participants. No reading of MI was changed from the original analysis but presence of non-ischaemic patterns of LGE in addition to the MI was noted in the re-reading. Using standard established clinical definitions (Figure 1),4 each subject was then re-categorized into one of the three following groups in a hierarchical order: (i) MI by LGE; (ii) non-ischaemic pattern of fibrosis (Figure 1); and no LGE as (iii) the reference group. On predefined basis of scar localization, subjects with non-ischaemic scar were further classified as having major or minor ischaemic scar (see Supplementary material online, CMR methods material and Figure 2 for flowchart of CMR image scoring).
Figure 1.
Categorization of non-ischaemic myocardial scar/fibrosis type.
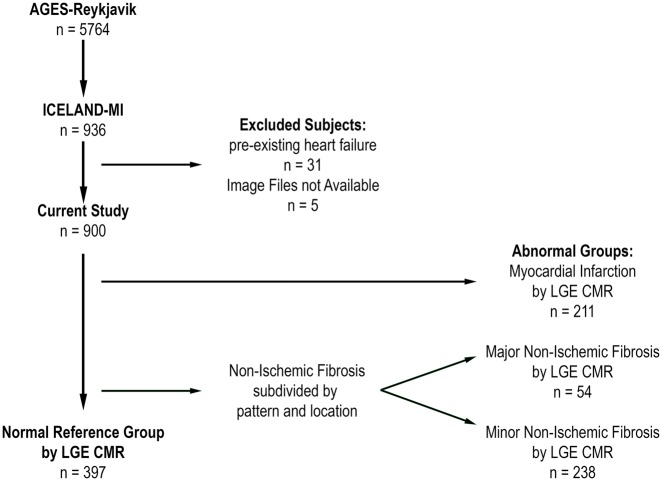
Figure 2.
Study flowchart.
Extent of fibrosis/scar in the MI and major non-ischaemic cohort was further quantified using automated feature analysis and combined thresholding algorithm (FACT) which has been previously clinically validated.13
Statistical analysis
Analysis was performed using SAS9.4 (Cary, NC, USA). Categorical data were presented as percentages and continuous data as median with interquartile ranges. After inspection for normality, between group differences according to LGE pattern were calculated by χ2, one-way ANOVA, and Wilcoxon as appropriate. Pairwise comparisons to the reference group were evaluated by least square means corrected with Dunnett adjustment. The Kaplan–Meier was used to assess univariate differences in event free survival according to CMR classification. To account for baseline differences between LGE groups, a propensity score model was developed. Due to the multinomial structure (reference group, non-ischaemic LGE and MI LGE) a generalized logit model was used to calculate the probability of the observed LGE pattern. Specifics of the model development and fit statistics were supplied as Supplementary material online.
After checking assumptions, a Cox proportional hazard and Kaplan–Meier model were weighted with the inverse probability of observed LGE pattern to obtain independent risk ratio estimates and predicted survival curves for the primary endpoint according to LGE pattern. Sensitivity analyses were performed to evaluate model dependency by: (i) standard multivariable Cox regression adjusted by covariates with a priori prognostic presumed importance; (ii) by including extent of LGE as a linear predictor with and without an interaction term for MI LGE and major non-ischaemic LGE; and (iii) by performing a test of interaction between LGE pattern and left ventricular ejection fraction (LVEF) to evaluate if the prognostic value of LGE varied as a function of LVEF. The incremental predictive information contained in LGE category was evaluated by likelihood ratio statistics and changes in time dependent C-index when adding CMR findings to the other predictors in the multivariable Cox model. A two-tailed P-value <0.05 was required for statistical significance.
Results
Baseline characteristics and prevalence of myocardial scar/fibrosis
A total of 900 individuals were included in this study after excluding individuals with pre-existing heart failure (n = 31) or missing CMR images (n = 5). Using the defined LGE categories, the prevalence of MI was 23.4% (n = 211), 6.0% (n = 54) had a major non-ischaemic pattern of fibrosis, 26.4% (n = 238) had only a minor non-ischaemic pattern of fibrosis, leaving 44.1% (n = 397) with no LGE, constituting the normal reference group.
Most subjects in the major non-ischaemic group (88.7%) had a normal LVEF, compared with 54.5% of the participants with MI (P < 0.001). Severely reduced LVEF (LVEF < 35%) was found in 9% of the subjects with MI, whereas all subjects with major non-ischaemic fibrosis had LVEF >35% (P = 0.02).
Compared with the reference group, the non-ischaemic fibrosis group had more men, Type 2 diabetes mellitus and had higher left ventricular mass and coronary calcium scores (Table 1). As expected, the MI group had a risk profile consistent with ischaemic heart disease. After inverse propensity adjustment, all baseline variables were balanced across LGE categories (Table 1).
Table 1.
Baseline characteristics according to ischaemic and non-ischaemic LGE pattern: the Iceland-MI study
| Parameters | Unadjusted |
P-value | Inverse propensity adjusted |
P-value | ||||
|---|---|---|---|---|---|---|---|---|
| No LGE (n = 397) | MI LGE (n = 211) | Non-ischaemic LGEa (n = 292) | No LGE (n = 397) | MI LGE (n = 211) | Non-ischaemic LGEa (n = 292) | |||
| Age (years) | 75.0 (72.0–80.0) | 77.0 (74.0–82.0)b | 77.0 (72.0–81.0) | <0.001 | 76.0 (72.0–81.0) | 76.0 (73.0–82.0) | 77.0 (72.0–81.0) | 0.60 |
| Males (%) | 35.5 | 63.5b | 53.1b | <0.001 | 46.9 | 50.2 | 46.5 | 0.69 |
| Weight (kg) | 75.1 (66.7–84.8) | 80.5 (71.4–89.3)b | 78.2 (68.4–87.8) | 0.004 | 75.6 (67.7–85.8) | 79.3 (69.7–89.1) | 76.1 (67.8–86.9) | 0.10 |
| Smokerc (%) | 10.6 | 13.7 | 10.6 | 0.45 | 10.6 | 11.0 | 10.5 | 0.98 |
| Type 2 DM (%) | 25.4 | 46.5b | 40.4b | <0.001 | 33.7 | 37.1 | 33.2 | 0.64 |
| HTN (%)d | 77.3 | 92.4b | 83.2 | <0.001 | 83.0 | 84.9 | 82.1 | 0.70 |
| HTN med (%) | 60.0 | 82.5b | 63.4 | <0.001 | 65.1 | 72.3 | 63.5 | 0.11 |
| CACe | 5.2 (3.6–6.3) | 6.8 (6.0–7.5)b | 5.9 (4.5–6.7)b | <0.001 | 5.6 (4.2–6.7) | 6.1 (4.8–7.2) | 5.7 (4.2–6.7) | 0.05 |
| MI before (%)f | 8.8 | 51.7b | 10.6 | <0.001 | 14.0 | 19.3 | 14.0 | 0.21 |
| Stroke/TIA (%) | 5.5 | 5.2 | 5.1 | 0.97 | 6.4 | 7.5 | 5.4 | 0.64 |
| Aspirin (%) | 28.2 | 61.6b | 35.3 | <0.001 | 36.2 | 43.7 | 34.6 | 0.11 |
| BetaB (%) | 34.5 | 56.4b | 35.6 | <0.001 | 40.1 | 43.0 | 38.3 | 0.58 |
| Statins (%) | 20.0 | 49.3b | 25.0 | <0.001 | 25.3 | 28.9 | 24.3 | 0.50 |
| LDL (mg/dL) | 134.7 (112.4–162.2) | 109 (86.1–146.7)b | 132.0 (103.7–161.4) | <0.001 | 130.5 (105.8–159.8) | 136.3 (95.4–165.3) | 133.6 (103.9–162.2) | 0.91 |
| Creatinine | 0.94 (0.79–1.09) | 1.02 (0.88–1.20)b | 0.96 (0.82–1.11) | <0.001 | 0.97 (0.83–1.14) | 0.96 (0.83–1.19) | 0.94 (0.78–1.10) | 0.71 |
| LVEF (%) | 63.7 (59.0–67.5) | 56.7 (48.1–63.4)b | 62.6 (58.3–66.9) | <0.001 | 62.7 (57.2–70.0) | 62.4 (57.0–67.2) | 62.8 (58.2–70.0) | 0.95 |
| LVSV (mL) | 83.1 (72.1–95.8) | 81.5 (72.5–99.8) | 83.1 (73.6–97.6) | 0.62 | 83.1 (71.2–97.4) | 83.7 (74–104.6) | 82.9 (73.4–96.1) | 0.29 |
| LV mass (g) | 90.1 (76–107.5) | 111.7 (92.4–138.2)b | 111.7 (92.4–138.2)b | <0.001 | 97.6 (82.2–116.9) | 101.6 (85.3–120) | 94.2 (78.1–119.5) | 0.05 |
BetaB, beta blocker; CAC, coronary calcium; DM, diabetes mellitus; HTN, hypertension; LDL, low density lipoprotein; LGE, late gadolinium enhancement; LV mass, left ventricular mass; LVEF, left ventricular ejection fraction; LVSV, left ventricular stroke volume; TIA, transient ischaemic attack.
Non-ischaemic LGE patterns consist of major non-ischaemic LGE (n = 54) and minor non-ischaemic LGE (n = 238) (see Supplementary material online, Table S2 for pairwise comparisons).
P < 0.05 in pairwise comparison with the reference group after Dunnett’s adjustment.
Smoker, active smoker.
HTN, treated HTN or SBP ≥140 mmHg untreated.
Natural logarithm of coronary calcium, Agatston score.
MI before, past history of heart attack or myocardial infarction.
The most important predictors for non-ischaemic fibrosis pattern derived from the propensity model in order from strongest to weakest were left ventricular mass, diabetes mellitus Type 2, left ventricular stroke volume, coronary calcium score, and left ventricular end-systolic volume (Supplementary material online, Table S1). Pairwise comparisons of baseline characteristics in individuals with minor- vs. major non-ischaemic LGE are given in Supplementary material online, Table S2.
Prognosis of types of myocardial fibrosis as characterized by cardiac magnetic resonance
There were a total of 192 (21.3%) primary events which included 115 deaths and 77 hospitalizations for incident heart failure (details in Table 2). Of the subjects with heart failure, 32 (41.6%) ultimately died.
Table 2.
Breakdown of death and hospitalization for incident heart failure by study groupa
| Outcome types | Non-ischaemic LGE |
Ischaemic LGE |
Total | ||
|---|---|---|---|---|---|
| No LGE | Minor non-ischaemic LGE | Major non-ischaemic LGE | Myocardial infarction LGE | ||
| Death | 45/397 | 30/238 | 8/54 | 32/211 | 115/900 |
| 11.3% | 12.6% | 14.8% | 15.2% | 12.8% | |
| Hospitalization for incident heart failure | 15/397 | 17/238 | 13/54 | 32/211 | 77/900 |
| 3.8% | 7.1% | 24.1% | 15.2% | 8.6% | |
| Primary study outcome | 60/397 | 47/238 | 21/54 | 64/211 | 192/900 |
| 15.1% | 19.8% | 38.9% | 30.3% | 21.3% | |
LGE, late gadolinium enhancement.
Results are listed as number of events in study group/study group size and percentage. Note that events summarized in this table represent the first event. Of the 77 participants with heart failure as their endpoint, 32 eventually died. These 32 deaths are not in the table since the Kaplan–Meier method censors a patient from further analysis after meeting the study endpoint. However, the high mortality of the adjudicated heart failure endpoint is an indication that this was a relevant metric of adverse subject outcome.
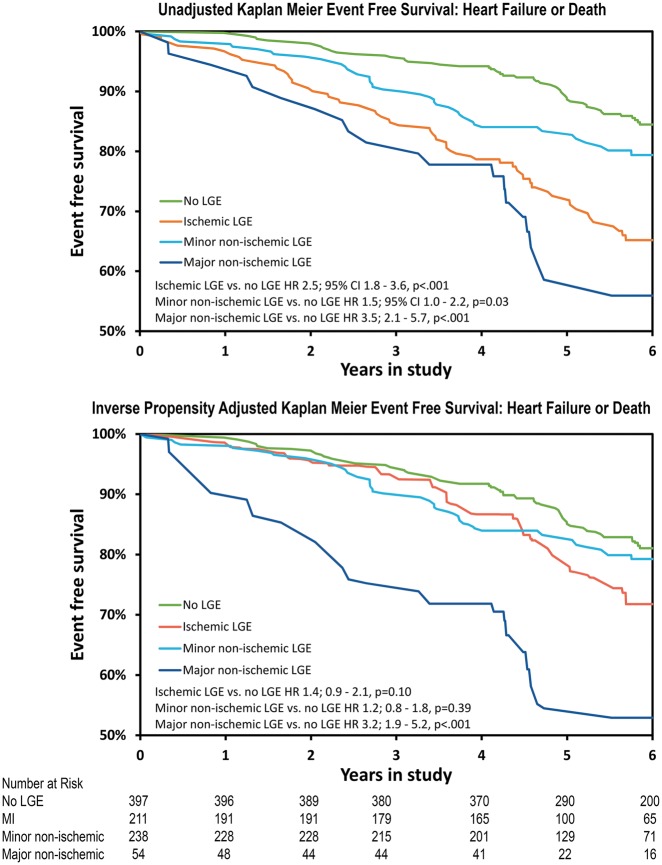
Unadjusted and inverse-propensity adjusted Kaplan–Meier curves for each of the groups were plotted with a median follow-up of 5.8 years in Figure 3. On univariate Cox analysis (Table 3), the major non-ischaemic scar/fibrosis group was at the highest risk of the primary endpoint [hazard ratio (HR) 3.5, P < 0.001] when compared with no LGE. The adverse event rate in subjects with MI was also significantly higher than the reference group (HR 2.5, P < 0.001) and minor non-ischaemic LGE (HR 1.5, P = 0.03). The difference between the major non-ischaemic group and subjects with MI was not statistically different (P = 0.07) in univariate analysis. A sensitivity analysis did not detect any significant dependency of the prognostic impact of LGE on LVEF (P = 0.82 for interaction).
Figure 3.
Unadjusted and inverse probability adjusted Kaplan–Meier estimates for the composite endpoint of heart failure or death: during a mean follow-up of 5.8 years.
Table 3.
Univariate and adjusted hazard ratios for first of heart failure or death according to LGE patterna
| Variables | Univariates |
Multivariates |
Inverse propensity adjustment |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | χ2 | P-value | HR (95% CI) | χ2 | P-value | HR (95% CI) | χ2 | P-value | |
| Ischaemic or non-ischaemic LGE | 2.1 (1.6–2.9) | 23.0 | <0.001 | 1.6 (1.1–2.2) | 7.6 | 0.006 | 1.5 (1.0–2.0) | 4.7 | 0.03 |
| Ischaemic LGE | 2.5 (1.8–3.6) | 26.2 | <0.001 | 1.4 (1.0–2.2) | 3.0 | 0.08 | 1.4 (0.9–2.1) | 2.7 | 0.10 |
| Non-ischaemic LGE | 1.8 (1.3–2.6) | 11.8 | <0.001 | 1.7 (1.2–2.4) | 7.9 | 0.005 | 1.5 (1.0–2.2) | 4.5 | 0.03 |
| Minor non-ischaemic LGE | 1.5 (1.0–2.2) | 4.7 | 0.03 | 1.5 (1.0–2.2) | 3.9 | 0.05 | 1.2 (0.8–1.8) | 0.8 | 0.39 |
| Major non-ischaemic LGE | 3.5 (2.1–5.7) | 23.8 | <0.001 | 2.3 (1.4–3.9) | 10.7 | 0.001 | 3.2 (1.9–5.2) | 20.4 | <0.001 |
| Age (per 10-years older) | 2.9 (2.2–3.7) | 64.9 | <0.001 | 2.9 (2.2–3.8) | 56.2 | <0.001 | NA | NA | NA |
| Current smoker | 1.8 (1.2–2.6) | 9.1 | 0.003 | 2.2 (1.5–3.2) | 16.7 | <0.001 | NA | NA | NA |
| Male gender | 1.5 (1.1–2.0) | 7.3 | 0.007 | 1.1 (0.8–1.6) | 0.7 | 0.41 | NA | NA | NA |
| HTN | 2.3 (1.4–3.7) | 11.3 | <0.001 | 1.8 (1.1–3.0) | 5.6 | 0.02 | NA | NA | NA |
| LVEF (per 10% lower) | 1.3 (1.2–1.5) | 15.8 | <0.001 | 1.2 (1.1–1.4) | 6.9 | 0.009 | NA | NA | NA |
| MI beforeb | 1.7 (1.2–2.3) | 9.9 | 0.002 | 1.1 (0.8–1.6) | 0.2 | 0.62 | NA | NA | NA |
| Log-coronary calcium | 1.2 (1.1–1.3) | 25.9 | <0.001 | 1.1 (1.0–1.2) | 3.2 | 0.07 | NA | NA | NA |
CI, confidence interval; HR, hazard ratio; HTN, hypertension; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; NA, not applicable.
Multivariable associations of non-LGE covariates reflects adjustment by LGE as ischaemic, minor- or major non-ischaemic.
MI before, in the past 5 years: has a doctor or other health professional told you that you had a heart attack or myocardial infarction?
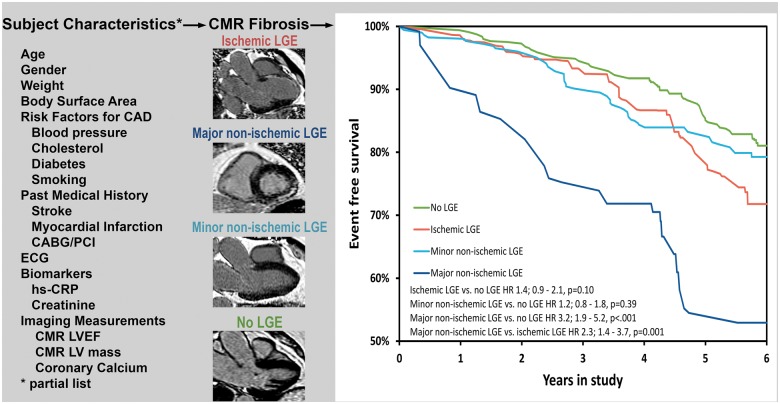
In multivariable Cox modelling and after adjustment using inverse probability weighting (Table 3), major non-ischaemic fibrosis remained a strong predictor of heart failure and death (HR 3.2, P = 0.001) compared with no LGE. Conversely, inverse propensity adjustment accounted for the risk associated with MI (HR 1.4, P = 0.10) and minor non-ischaemic LGE (Take home figure) (HR 1.2, P = 0.39). Of note, major non-ischaemic fibrosis was associated with worse outcome than the MI subgroup (HR 2.3, P = 0.001, Figure 3) and minor non-ischaemic LGE (HR 2.7, P < 0.001) in the inverse propensity adjusted analysis.
Take home figure.
Inverse propensity adjusted prognosis of ischaemic and non-ischaemic myocardial fibrosis.
In a separate sensitivity analysis among individuals with major non-ischaemic fibrosis or MI by LGE (n = 253), the amount of LGE, as a percentage of the left ventricle, was higher among patients with MI (median 10.6% of the left ventricle with interquartile range 5.6–17.6%) vs. major non-ischaemic fibrosis (median 3.0% of the left ventricle with interquartile range 3.0–5.9%, P < 0.001). Notwithstanding, the risk ratio for the composite outcome was comparable for individuals with non-ischaemic LGE (HR 1.13) vs. MI LGE (HR 1.01) for each 1% increase in the amount of LGE (P = 0.14 for interaction).
An additional sensitivity analysis was performed classifying near aortic root and mitral annular fibrosis as the midwall/epicardial pattern of fibrosis (classified under major non-ischaemic patterns of fibrosis) to address concerns that these patterns may overlap. Despite the small sample size of this group, the event-free survival of the midwall/subepicardial pattern of fibrosis differed significantly from that of the aortic and mitral annular fibrosis group and explained most of the effect on the composite group. This annular fibrosis group alone performed similarly to the other minor non-ischaemic patterns, and overall did not change the main results (see Supplementary material online, Figures S9 and S10).
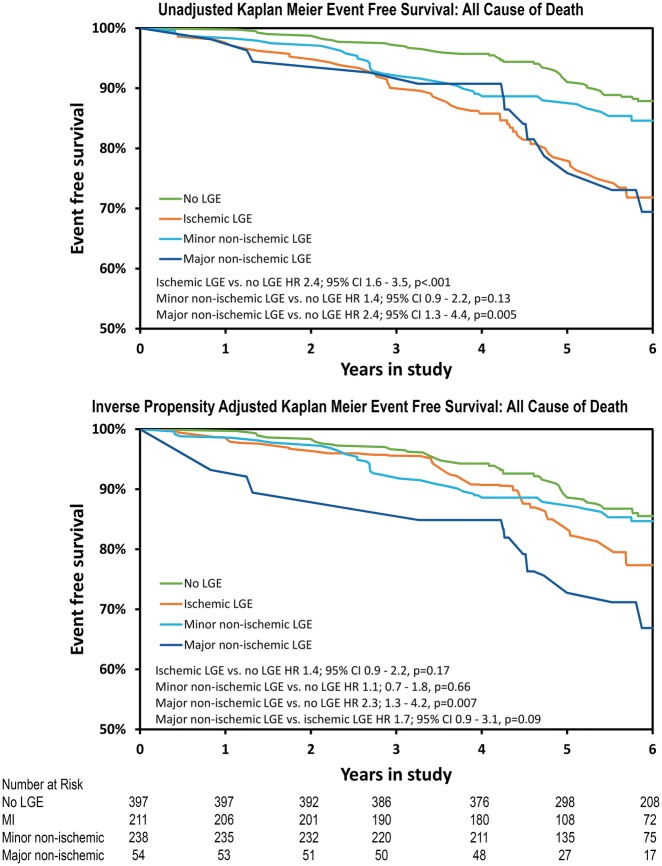
In subgroup analysis of all-cause mortality, both MI by LGE (HR 2.4, P < 0.001) and major non-ischaemic scar by LGE (HR 2.4, P = 0.005) were associated with higher mortality compared with the reference group on univariate analysis while minor non-ischaemic LGE was not (HR 1.4, P = 0.13, Figure 4). However, in the inverse propensity-adjusted analysis only major non-ischaemic LGE (HR 2.3, P = 0.007) portended an increased risk of mortality (Figure 4).
Figure 4.
Unadjusted and inverse probability adjusted Kaplan–Meier estimates for all-cause mortality: during a mean follow-up of 5.8 years.
Ranked by χ2, LGE pattern was the third strongest predictor of the composite endpoint after age and current smoking in a multivariate Cox model (Table 3). The addition of LGE pattern to standard risk factors in the multivariable Cox model resulted in a significant increase in likelihood ratio (Δχ2 11.7, P = 0.009) and time-dependent area under curve (AUC) (Δ∼0.02, P < 0.001, Supplementary material online, Figures S6 and S7).
Discussion
This is the first population-based study to demonstrate that individuals with either non-ischaemic or ischaemic myocardial fibrosis/scar have worse prognosis than those without myocardial scar in older adults without pre-existing heart failure. Equally important, the risk for heart failure and death associated with non-ischaemic scar as detected by LGE CMR was worse than that of MI after propensity score adjusting for baseline characteristics including LVEF, left ventricular mass, and traditional cardiovascular risk factors. This further suggests that the pathophysiological process resulting in non-ischaemic scar is distinctly different from ischaemic scar and is associated with poor prognosis which is incompletely unaccounted for by traditional cardiac risk factors for coronary artery disease. As such, non-ischaemic scar added incremental predictive information of the primary outcome and had the highest χ2 value in multivariable models aside from age and current smoking status. Finally, this prospective cohort also allowed determinations of the prevalence of myocardial fibrosis in the community. Thus, this work is an important and complementary step beyond prior referral centre studies of non-ischaemic myocardial scar.
It is important to note that increased left ventricular mass was the single strongest predictor of major non-ischaemic LGE. Whether preventing left ventricular hypertrophy would improve the prognosis in these patients with non-ischaemic LGE is untested since a high percentage of this population was already on treatment for hypertension. Also of note, non-ischaemic scar was a stronger predictor of death or heart failure than LVEF. This may be partially explained by the observation that LVEF tended to be low in patients with MI but most patients with non-ischaemic fibrosis had preserved systolic function.
Newer T1 mapping methods may be more sensitive in detecting diffuse interstitial fibrosis than LGE imaging.14 Thus, LGE likely represents only a fraction of the extent and severity of the underlying fibrosis in these NICM. These techniques have the potential to offer further insight into non-ischaemic aetiologies. A few studies demonstrate prognostic significance associated with changes in native myocardial T1 or extracellular volume fraction.15,16 These methods were not available at the time of the ICELAND-MI study. Furthermore, these T1 mapping techniques are not yet widely available. The referral centre studies quoted earlier6–8 used simple LGE to detect myocardial fibrosis, a methodology that is widely distributed that has approximately 15 years of clinical experience.
Specific patterns of scar/fibrosis can distinguish between different aetiologies of cardiomyopathy and thus are clinically useful (Figure 1).4,17 LGE closely correlates with collagen scar in chronic MI.3,18 Fibrosis caused by MI follows a coronary artery territory and spreads from the subendocardial layer outward towards the epicardium, a pattern recognizable on LGE CMR scans.19 In NICM, multiple mechanisms lead to LGE in patterns distinguishable from MI (Figure 1). These mechanisms include replacement fibrosis, myocardial necrosis, myocyte apoptosis, and expansion of the extracellular space as a result of infiltrative disorders. Strictly speaking, LGE in patients with amyloidosis should not be equated with fibrosis. This distinction did not affect the study as no cases of amyloidosis were encountered.
Beyond the well-established major patterns of non-ischaemic fibrosis, we encountered patterns of localized scar, which were relatively prevalent in this older population. LGE findings near the mitral and aortic valve may be explained by fibrosis in myocardium adjacent to a calcified mitral annulus or calcified aortic valve.20–26 Extension of calcification, inflammation and fibrosis from aortic and mitral annular calcification has been demonstrated on necropsy which in severe cases has resulted in heart block secondary to disruption of the bundle of His.27,28 The prevalence of aortic sclerosis in population studies is estimated to be 25% in those >65 years and climbs to 50% after 85 years of age.29 Mitral annular calcification has been demonstrated to be as high as 55%30 it is not surprising that the most frequently encountered patterns of fibrosis were localized along the valve planes. We also observed fibrosis at the right ventricular (RV) insertion points which has been previously described predominantly in the setting of pulmonary hypertension, hypertrophic cardiomyopathy, and RV hypertrophy.31,32 LGE near the RV insertion points may also be linked to other conditions associated with advanced age since most of our participants did not have signs of pulmonary hypertension or pathological hypertrophy. Focal regions of fibrosis have been described in multiple autopsy series, and the prevalence increases with age.33 Although their aetiology is uncertain, they are unrelated to coronary stenosis and may be due to metabolic processes or rheumatic diseases.34 Thus, there are many possible pathologic correlates to the minor patterns of fibrosis seen in this study.
Study limitations
The results of this study are most applicable to Caucasian subjects and may need testing in other ethnicities. The generalizability is also limited by the advanced age of this cohort which inherently introduces survivor bias. The sensitivity of detecting fibrosis may be reduced as a result of the dose of gadolinium contrast (0.1 mmol/L/kg) used. This effect was compensated by the use of phase-sensitive inversion recovery imaging which provided better signal-to-noise ratios than what was commercially available at the time of study recruitment. The study was underpowered to study differences between groups so one should not over-interpret statistically non-significant comparisons. Additionally, this study was underpowered to evaluate differences between the individual patterns of non-ischaemic scar. Adjustment for some MI-associated risk factor covariates (e.g. smoking, which is strongly associated with MI) may have led to an overfitted model, making direct comparisons of adjusted HRs for ischaemic and non-ischaemic scar groups difficult.
Conclusion
Major non-ischaemic fibrosis/scar, as detected by LGE on CMR, was associated with a significantly greater risk for death or heart failure hospitalization than no fibrosis/scar in older community-dwelling persons without pre-existing heart failure. Major non-ischaemic fibrosis/scar portended worse prognosis than ischaemic scar in adjusted analyses.
Funding
This work was supported by the National Heart, Lung, and Blood Institute Intramural Research Program (Z01 HL004607-08 CE and 1 ZIA HL006136-06), the National Institute on Aging Intramural Research Program (N01-AG-12100), Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). This study was approved by the Icelandic National Bioethics Committee (VSN: 00-063) and the Medstar Research Institute (Project 2003-145).
Assurances
The study was approved by the IRB for the NIA/NIH and for the National Bioethics Committee in Iceland, which serves as the IRB for the Iceland Heart Association (Methods, Paragraph 1).
Data analysis was performed by the following authors: Shanbhag, Greve, Aspelund, Schelbert, Cao, Danielsen, Gudnason, and Arai.
ClinicalTrials.gov Identifier: NCT01322568 (refer to archived version).
This aspect of the study does not involve microarrays and therefore is not associated with a specific accession number or repository.
Conflict of interest: none declared.
Supplementary Material
Footnotes
See page 539 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy876)
References
- 1. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 2. Gerber BL, Edvardsen T, Pierard LA, Saraste A, Knuuti J, Maurer G, Habib G, Lancellotti P.. The year 2014 in the European Heart Journal—Cardiovascular Imaging: part II. Eur Heart J Cardiovasc Imaging 2015;16:1180–1184. [DOI] [PubMed] [Google Scholar]
- 3. Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM.. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002. [DOI] [PubMed] [Google Scholar]
- 4. Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ.. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005;26:1461–1474. [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P;. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 6. Kim EK, Chattranukulchai P, Klem I.. Cardiac magnetic resonance scar imaging for sudden cardiac death risk stratification in patients with non-ischemic cardiomyopathy. Korean J Radiol 2015;16:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poyhonen P, Kivisto S, Holmstrom M, Hanninen H.. Quantifying late gadolinium enhancement on CMR provides additional prognostic information in early risk-stratification of nonischemic cardiomyopathy: a cohort study. BMC Cardiovasc Disord 2014;14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Almehmadi F, Joncas SX, Nevis I, Zahrani M, Bokhari M, Stirrat J, Fine NM, Yee R, White JA.. Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy. Circ Cardiovasc Imaging 2014;7:593–600. [DOI] [PubMed] [Google Scholar]
- 9. Wong TC, Piehler KM, Zareba KM, Lin K, Phrampus A, Patel A, Moon JC, Ugander M, Valeti U, Holtz JE, Fu B, Chang CC, Mathier M, Kellman P, Butler J, Gheorghiade M, Schelbert EB.. Myocardial damage detected by late gadolinium enhancement cardiovascular magnetic resonance is associated with subsequent hospitalization for heart failure. J Am Heart Assoc 2013;2:e000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V.. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE.. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012;308:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danielsen R, Thorgeirsson G, Einarsson H, Olafsson O, Aspelund T, Harris TB, Launer L, Gudnason V.. Prevalence of heart failure in the elderly and future projections: the AGES-Reykjavik study. Scand Cardiovasc J 2017;51:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu LY, Ingkanisorn WP, Kellman P, Aletras AH, Arai AE.. Quantitative myocardial infarction on delayed enhancement MRI. Part II: clinical application of an automated feature analysis and combined thresholding infarct sizing algorithm. J Magn Reson Imaging 2006;23:309–314. [DOI] [PubMed] [Google Scholar]
- 14. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB.. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB.. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 2012;126:1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schelbert EB, Piehler KM, Zareba KM, Moon JC, Ugander M, Messroghli DR, Valeti US, Chang CC, Shroff SG, Diez J, Miller CA, Schmitt M, Kellman P, Butler J, Gheorghiade M, Wong TC, Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc 2015;4:e002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts WC, Siegel RJ, McManus BM.. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients. Am J Cardiol 1987;60:1340–1355. [DOI] [PubMed] [Google Scholar]
- 18. Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P, Aletras AH, Arai AE.. Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging 2010;3:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reimer KA, Lowe JE, Rasmussen MM, Jennings RB.. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977;56:786–794. [DOI] [PubMed] [Google Scholar]
- 20. Salisbury AC, Shapiro BP, Martinez MW.. Extensive myocardial and mitral annular calcification leading to mitral regurgitation and restrictive cardiomyopathy: an unusual case of caseous calcification of the mitral annulus. J Cardiovasc Comput Tomogr 2009;3:351–353. [DOI] [PubMed] [Google Scholar]
- 21. Hajsadeghi F, Ahmadi N, Eshaghian S, Budoff M.. Porcelain heart. J Cardiovasc Comput Tomogr 2011;5:183–185. [DOI] [PubMed] [Google Scholar]
- 22. Nance JW Jr, Crane GM, Halushka MK, Fishman EK, Zimmerman SL.. Myocardial calcifications: pathophysiology, etiologies, differential diagnoses, and imaging findings. J Cardiovasc Comput Tomogr 2015;9:58–67. [DOI] [PubMed] [Google Scholar]
- 23. Hamirani YS, Nasir K, Blumenthal RS, Takasu J, Shavelle D, Kronmal R, Budoff M.. Relation of mitral annular calcium and coronary calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2011;107:1291–1294. [DOI] [PubMed] [Google Scholar]
- 24. Revilla A, Sevilla T, Sanchez I, Rodriguez M, San RJA.. Full calcium jacket: massive idiopathic myocardial calcification by cardiovascular magnetic resonance and cardiac CT. Eur Heart J Cardiovasc Imaging 2012;13:627.. [DOI] [PubMed] [Google Scholar]
- 25. Warren BA, Yong JL.. Calcification of the aortic valve: its progression and grading. Pathology 1997;29:360–368. [DOI] [PubMed] [Google Scholar]
- 26. Freeman RV, Otto CM.. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005;111:3316–3326. [DOI] [PubMed] [Google Scholar]
- 27. Yater WM, Cornell VH.. Heart block due to calcareous lesions of the bundle of His—review and report of a case with detailed histopathologic study. Ann Intern Med 1935;8:777–789. [Google Scholar]
- 28. Nestico PF, Depace NL, Morganroth J, Kotler MN, Ross J.. Mitral annular calcification—clinical, patho-physiology, and echocardiographic review. Am Heart J 1984;107:989–996. [DOI] [PubMed] [Google Scholar]
- 29. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P.. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barasch E, Gottdiener JS, Larsen EK, Chaves PH, Newman AB, Manolio TA.. Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am Heart J 2006;151:39–47. [DOI] [PubMed] [Google Scholar]
- 31. Patel AR, Addetia K.. Prediction of prognosis in pulmonary hypertension using CMR: what happens where the right and left ventricles meet? JACC Cardiovasc Imaging 2014;7:1218–1220. [DOI] [PubMed] [Google Scholar]
- 32. Bradlow WM, Assomull R, Kilner PJ, Gibbs JS, Sheppard MN, Mohiaddin RH.. Understanding late gadolinium enhancement in pulmonary hypertension. Circ Cardiovasc Imaging 2010;3:501–503. [DOI] [PubMed] [Google Scholar]
- 33. Lie JT, Hammond PI.. Pathology of the senescent heart: anatomic observations on 237 autopsy studies of patients 90 to 105 years old. Mayo Clin Proc 1988;63:552–564. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz CJ, Mitchell JR.. The relation between myocardial lesions and coronary artery disease. I. An unselected necropsy study. Br Heart J 1962;24:761–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.