Abstract
Objectives
To ensure the accuracy of susceptibility testing methods for ceftazidime/avibactam.
Methods
The performances of the Etest (bioMérieux), 30/20 μg disc (Hardy diagnostics) and 10/4 μg disc (Mast Group) were evaluated against the reference broth microdilution (BMD) method for 102 clinically relevant Gram-negative organisms: 69 ceftazidime- and meropenem-resistant Klebsiella pneumoniae and 33 MDR non-K. pneumoniae. Essential and categorical agreement along with major and very major error rates were determined according to CLSI guidelines.
Results
A total of 78% of isolates were susceptible to ceftazidime/avibactam. None of the three methods met the defined equivalency threshold against all 102 organisms. The Etest performed the best, with categorical agreement of 95% and major errors of 6.3%. Against the 69 ceftazidime- and meropenem-resistant K. pneumoniae, only the Etest and the 10/4 μg disc met the equivalency threshold. None of the three methods met equivalency for the 33 MDR isolates. There were no very major errors observed in any analysis. These results were pooled with those from a previous study of 74 carbapenem-resistant Enterobacteriaceae and data from the ceftazidime/avibactam new drug application to define optimal 30/20 μg disc thresholds using the error-rate bound model-based approaches of the diffusion breakpoint estimation testing software. This analysis identified a susceptibility threshold of ≤19 mm as optimal.
Conclusions
Our data indicate that the Etest is a suitable alternative to BMD for testing ceftazidime/avibactam against ceftazidime- and meropenem-resistant K. pneumoniae. The 30/20 μg discs overestimate resistance and may lead to the use of treatment regimens that are more toxic and less effective.
Introduction
The adoption of ceftazidime/avibactam for the treatment of carbapenem-resistant Gram-negative infections has been swift and is supported by clinical reports demonstrating efficacy superior to that of traditional treatment regimens.1,2 However, the availability of accurate susceptibility testing methods against these target pathogens has lagged behind this rapid adoption. Given the difficulties and delays in implementing FDA-cleared automated commercial antimicrobial susceptibility (cAST) devices, laboratories often turn to manual methods such as gradient strips and discs to provide susceptibility information. Consequently, there is an urgent need to evaluate the accuracy of available susceptibility testing methods for ceftazidime/avibactam against the target pathogens for which they are used in clinical practice.
Materials and methods
Bacterial isolates
One hundred and two Gram-negative isolates were included: 69 ceftazidime- and meropenem-resistant Klebsiella pneumoniae and 33 MDR non-K. pneumoniae Gram-negative isolates (Table 1). These were clinical isolates from our in-house biorepository and the FDA-CDC Antimicrobial Resistance Bank.3K. pneumoniae ATCC 700603 was used for quality control on each day of testing. Isolates were maintained at −80°C in CAMHB with 20% glycerol and subcultured twice prior to use on tryptic soy agar with 5% sheep’s blood.
Table 1.
Performance of Etest and discs compared with BMD for evaluated Gram-negative isolates
| Method | EA (%)a | CA (%) | MEs (%) | VMEs (%) | CLSI threshold |
|---|---|---|---|---|---|
| All isolates tested (n = 102) | |||||
| Etest (error-rate bound method) | 77.1a | 95.1 | 6.3 | 0 | no |
| BMD MIC 16 or 32 mg/L | – | – | – | 0b | yes |
| BMD MIC 8 or 16 mg/L | – | – | 36.4c | 0 | no |
| BMD MIC 4 or 8 mg/L | – | – | 46.2d | 0 | no |
| 30/20 μg disc (error–rate bound method) | – | 80.4 | 25 | 0 | no |
| BMD MIC 16 or 32 mg/L | – | – | – | 0b | yes |
| BMD MIC 8 or 16 mg/L | – | – | 63.6c | 0 | no |
| BMD MIC 4 or 8 mg/L | – | – | 76.9d | 0 | no |
| 10/4 μg disc (error-rate bound method) | – | 87.3 | 16.3 | 0 | no |
| BMD MIC 16 or 32 mg/L | – | – | – | 0b | yes |
| BMD MIC 8 or 16 mg/L | – | – | 72.7d | 0 | no |
| BMD MIC 4 or 8 mg/L | – | – | 84.6d | 0 | no |
| Meropenem and ceftazidime non-susceptible K. pneumoniae isolates (n = 69) | |||||
| Etest | 82.4e | 100 | 0 | 0 | yes |
| 30/20 μg disc | – | 82.6 | 18.5 | 0 | no |
| 10/4 μg disc | – | 98.5 | 1.5 | 0 | no |
| MDR non-K. pneumoniae isolates (n = 33)f | |||||
| Etest | 64.3g | 84.8 | 33.3 | 0 | no |
| 30/20 μg disc | – | 75.8 | 53.3 | 0 | no |
| 10/4 μg disc | – | 63.6 | 80 | 0 | no |
Six isolates could not be assessed for EA as the Etest MIC exceed the highest MIC reading on the strip (256 mg/L).
n = 9 isolates.
n = 11 isolates.
n = 13 isolates.
One isolate could not be assessed for EA as the Etest MIC exceed the highest MIC reading on the strip (256 mg/L).
20 P. aeruginosa (2 VIM-producing and 2 IMP-producing), 8 Escherichia coli (3 NDM-producing), 3 Enterobacter cloacae (1 NDM-producing), 1 Enterobacter aerogenes and 1 Citrobacter freundii (VIM-producing).
Five isolates could not be assessed for EA as the Etest MIC exceed the highest MIC reading on the strip (256 mg/L).
Susceptibility testing
Ceftazidime and avibactam powder were purchased commercially (Sigma–Aldrich, St Louis, MO, USA). Broth microdilution (BMD) testing was performed utilizing CAMHB (Teknova, Hollister, CA, USA) according to CLSI4 with minor modifications. RUO Etest strips (bioMérieux, Durham, NC, USA) were obtained from International Health Management Associates (IHMA, Schaumburg, IL, USA) and utilized according to the manufacturer’s instructions. We obtained 30/20 μg discs from the manufacturer (Hardy Diagnostics, Santa Maria, CA, USA) and 10/4 μg discs (Mast Group Ltd, UK) were provided by EUCAST. Testing was performed according to CLSI and interpreted according to CLSI M100-S275 and EUCAST6,7 guidelines, respectively. The Etest and disc diffusion were performed on Mueller–Hinton agar (Remel, Lenexa, KS, USA). All four methods were performed in triplicate using the same 0.5 McFarland bacterial suspension and modal values are reported and used for analyses.
Agreement analysis
Using BMD as the reference method, essential agreement (EA), categorical agreement (CA), major errors (MEs) and very major errors (VMEs) were assessed according to standard definitions.8 The primary outcome was equivalency as defined by the CLSI threshold of ME and VME rates of <3%.9,10 The acceptance criterion of >89.9% for EA and CA was also evaluated.8 Additionally, given that the bacterial population evaluated in this study was not binomial (21.6% of the 102 isolates were within 1 log2 dilution of the susceptible/resistant MIC breakpoint), the error-rate bound method was also calculated.8
Additionally, data from the 102 isolates in this study were combined with data from 74 carbapenem-resistant Enterobacteriaceae isolates from Shields et al.11 and 356 Gram-negative isolates from the ceftazidime/avibactam new drug application (320 Enterobacteriaceae, 36 non-fermenting isolates).12 Optimal thresholds were analysed using the error-rate bound and model-based approaches of the diffusion breakpoint estimation testing software package13 according to CLSI M23-A4 guidelines.9
Results
By BMD, 80 (78.4%) of 102 isolates were susceptible to ceftazidime/avibactam whereas 65 (94.2%) of the ceftazidime- and meropenem-resistant K. pneumoniae isolates were susceptible, with MIC50/MIC90 of 1/4 mg/L. Only 15 (45.5%) of the MDR non-K. pneumoniae isolates were susceptible, with MIC50/MIC90 of 32/≥256 mg/L by BMD.
Table 1 displays the performance of the three test methods compared with BMD. None of the three methods met either equivalency threshold. Using the error-rate bound method, all three methods met acceptance criteria for the highest BMD MIC thresholds (16 or 32 mg/L) as there were no VMEs observed. None of the three methods met the acceptance criteria for either of the two lower MIC thresholds.
Against only the ceftazidime- and meropenem-resistant K. pneumoniae isolates, Etest met the equivalency threshold as there were no MEs or VMEs observed. The 30/20 μg disc again did not meet either threshold, although the percentage CA and ME rates were slightly better. The 10/4 μg discs performed better against the ceftazidime- and meropenem-resistant K. pneumoniae isolates and did meet both equivalency thresholds. There were no VMEs observed by any method. Versus the collection of MDR non-K. pneumoniae, none of the three methods met either equivalency threshold. The Etest had the highest CA and the lowest rate of MEs. The 30/20 μg discs outperformed the 10/4 μg discs. There were no VMEs observed by any method.
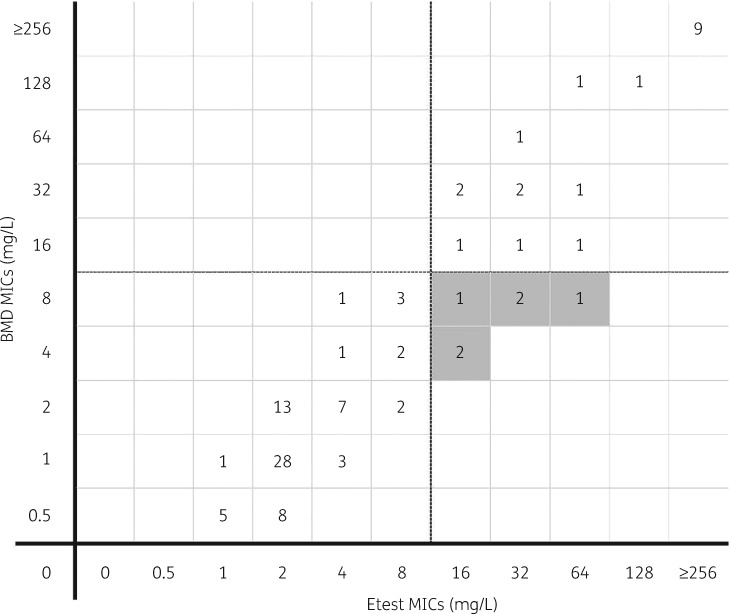
Figure 1 displays the distribution of ceftazidime/avibactam MICs by BMD and the Etest against all 102 isolates. Figures S1 and S2 (available as Supplementary data at JAC Online) display the distribution of ceftazidime/avibactam MICs by BMD and the Etest against the 69 ceftazidime- and meropenem-resistant isolates and the 33 MDR non-K. pneumoniae isolates separately. All MEs were clustered around the susceptibility breakpoint. Two of six (33%) and four of the six (67%) errors occurred at BMD MICs of 4 and 8 mg/L, respectively.
Figure 1.
Distribution of ceftazidime/avibactam MICs by BMD and Etest against all 102 Gram-negative isolates evaluated. The horizontal and vertical dashed lines (between 8 and 16 mg/L on the x- and y-axes) represent the MIC susceptibility breakpoint. Shaded boxes indicate MEs (false resistance) as reported by the Etest compared with BMD.
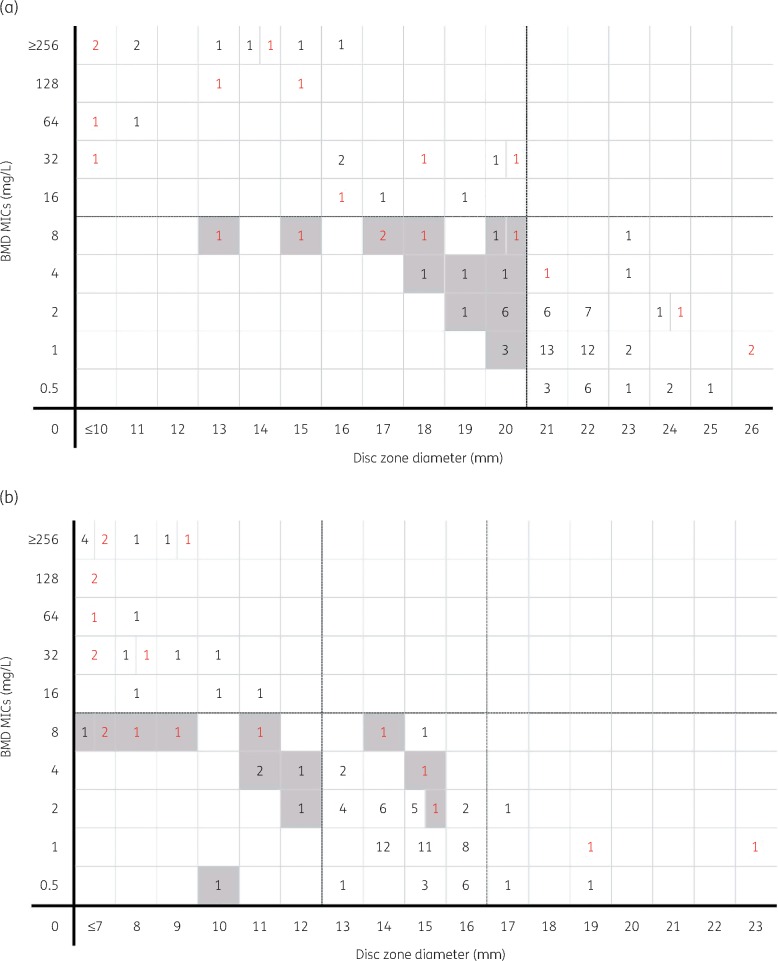
Figure 2(a and b) compare the disc diffusion zone diameters of the 30/20 and 10/4 μg discs, respectively, with the BMD MIC for all 102 isolates. For the 30/20 μg discs, the disc susceptibility breakpoint (≥21 mm) best correlated with a BMD MIC of ≤2 mg/L. Zones within ±3 mm of this susceptible breakpoint (18–24 mm) were demonstrated in 75.5% (77/102) of isolates. For the 10/4 μg discs, the disc susceptibility breakpoint for Enterobacteriaceae (≥13 mm) best correlated with a BMD MIC of ≤2 mg/L. Zones within ±3 mm of susceptibility (10–16 mm) were demonstrated for 84.1% (69/82) of isolates. No correlation with BMD was observed for the 10/4 μg discs against the 20 Pseudomonas aeruginosa isolates and only 2 isolates demonstrated a disc diameter zone ≥17 mm.
Figure 2.
Distribution of ceftazidime/avibactam MICs by BMD versus 30/20 μg disc (a) and 10/4 μg disc (b) diffusion zone diameters against all 102 Gram-negative isolates evaluated. Enterobacteriaceae spp. are shown as black numbers and P. aeruginosa are shown as red numbers. Shaded boxes indicate MEs (false resistance). In (a), dashed lines represent the susceptibility breakpoints for ceftazidime/avibactam. In (b), the horizontal dashed line between 8 and 16 on the y-axis represents the MIC susceptibility breakpoint. The dashed vertical line between 12 and 13 mm on the x-axis represents the disc diffusion diameter for susceptibility to Enterobacteriaceae spp. The dashed vertical line between 16 and 17 mm on the x-axis represents the disc diffusion diameter for susceptibility to P. aeruginosa.
For the 476 Enterobacteriaceae from the three studies included in the pooled analysis, an optimal susceptible/resistant disc diffusion breakpoint of 19 mm was identified by the error-rate bound and non-parametric spline model approach of the optimal threshold analysis. A breakpoint of 18 mm was identified by the logistic model, although the 19 and 18 mm breakpoints had identical probability of producing a correct MIC and disc zone of diffusion over any range of true MIC values and sets of breakpoints (97%). The current CLSI disc diffusion breakpoint of 20 mm was selected by the error-rate bound method only 25.8% of the time among 5000 bootstrapped samples, was not identified by either the logistic or the spline model and had slightly lower probability of producing correct results (95% versus 97%). For the 56 non-fermenting Gram-negative isolates from our study and the ceftazidime/avibactam new drug application, an optimal susceptible/resistant disc diffusion breakpoint of 20 mm was identified by all three methods, with an 88% probability of correct classification.
Discussion
In this study, the FDA-cleared 30/20 μg discs did not meet the primary or secondary thresholds for equivalency against either group of organisms tested. The 10/4 μg discs performed better than the 30/20 μg discs against the ceftazidime- and meropenem-resistant K. pneumoniae isolates and did meet both equivalency thresholds. The 30/20 μg discs performed slightly better than the 10/4 μg discs against the MDR non-K. pneumoniae isolates, although both methods had high rates of MEs and did not meet equivalency standards. Etest consistently had the highest performance and was the most reliable method against all the isolates tested in this analysis. Given that carbapenem-resistant organisms are the target pathogens for which ceftazidime/avibactam is used in clinical practice, the Etest may be preferred over discs by clinical microbiology laboratories until automated cAST devices are available.
By combining our data with multiple other sources, we were able to evaluate optimal 30/20 μg disc diffusion breakpoints for ceftazidime/avibactam against Enterobacteriaceae and non-fermenting Gram-negative isolates. The diffusion Breakpoint Estimation Testing Software model-based approaches used in this study are more precise than traditional error-rate bound methods as they use probability to account for the inherent variability of the tested susceptibility methods. Pooled analysis of 476 Enterobacteriaceae isolates defined 19 mm as the optimal susceptibility breakpoint for the 30/20 μg discs, although this threshold demonstrated a similar classification probability compared with the current CLSI breakpoint of 20 mm (97% versus 95%). Analysis of 56 non-fermenting Gram-negative isolates identified 20 mm as the optimal breakpoint. These results support a single 30/20 μg disc diffusion susceptible/resistant breakpoint of 20 mm, as is currently recommended by CLSI.
In this study the probability of error increased for pathogens with an MIC >2 mg/L, and the highest probability of testing errors occurred for isolates with an MIC near the susceptible breakpoint (8 mg/L). Additionally, disc diameter zones of inhibition that grouped around the susceptible breakpoint led to the majority of error classifications. This was particularly true for the 30/20 μg discs (Figure 2a) as opposed to the 10/4 μg discs (Figure 2b). This difference could be due to the different disc potencies and could potentially be resolved by the addition of an intermediate MIC and disc diffusion zone categorization. CLSI is currently considering adding a note regarding uncertainty around disc zones in the range where MEs were observed.
Limitations of this study include the number of isolates tested. Additionally, only one manufacturer and lot of CAMHB and Mueller–Hinton agar were used for this study. Finally, molecular analyses were not performed for all isolates, which precluded assessment of underlying genetic mechanisms of resistance and clonality.
Conclusions
Our data indicate that the Etest is a suitable alternative to BMD for testing ceftazidime/avibactam against ceftazidime- and meropenem-resistant K. pneumoniae, whereas the FDA-cleared 30/20 μg discs are not. The rate of false resistance remains concerning and clinicians should interpret MICs near the breakpoint with caution and consider repeat testing (or validation at a reference laboratory) to avoid unnecessary use of agents that are more toxic and less effective. Clinical microbiology laboratory directors should perform adequate in-house verifications before adopting any of these AST methods for ceftazidime/avibactam.
Supplementary Material
Acknowledgements
The authors thank the members of the Chicago Microbiology Directors Roundtable Organization (CMDRO) for contributing clinical isolates to this study and Erika Matuschek and EUCAST for providing the 10/4 μg discs. These results were presented in part as Abstract 216 at the 2017 American College of Clinical Pharmacy Annual Meeting, Phoenix, AZ, USA.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
References
- 1. van Duin D, Lok JJ, Earley M. et al. Colistin vs. ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66: 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shields RK, Nguyen MH, Chen L. et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017; 61: e00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lutgring JD, Machado M-J, Benahmed FH. et al. FDA-CDC antimicrobial resistance isolate bank: a publicly-available resource to support research, development and regulatory requirements. J Clin Microbiol 2018; 56: e01415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically—Tenth Edition: Approved Standard M07-A10 CLSI, Wayne, PA, USA, 2015.
- 5.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Seventh Informational Supplement M100-S27 CLSI, Wayne, PA, USA, 2017.
- 6.EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 6.0 2016. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf.
- 7. Koeth LM, Matuschek E, Kahlmeter G. et al. Development of EUCAST zone diameter breakpoints and quality control criteria for ceftazidime-avibactam 10-4 µg. Eur J Clin Microbiol Infect Dis 2018; 37: 1047–53. [DOI] [PubMed] [Google Scholar]
- 8. Humphries RM, Ambler J, Mitchell SL. et al. CLSI methods development and standardization working group best practices for evaluation of antimicrobial susceptibility tests. J Clin Microbiol 2018; 56: e01934-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Development of In Vitro Susceptibility Testing Criteria and Quality Control Parameters—Fourth Edition: Approved Standard M23-A4 CLSI, Wayne, PA, USA, 2016.
- 10.Clinical and Laboratory Standards Institute. Verification of Commercial Microbial Identification and Susceptibility Test Systems—First EditionM52 CLSI, Wayne, PA, USA, 2016.
- 11. Shields RK, Clancy CJ, Pasculle AW. et al. Verification of ceftazidime-avibactam and ceftolozane-tazobactam susceptibility testing methods against carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol 2018; 56: e01093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration Center for Drug Evaluation and Research. Application number 206494Orig1s000: Microbiology/Virology Review(s) NDA 206-494/N-000 Ceftazidime-Avibactam for Injection.https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206494Orig1s000MicroR.pdf.
- 13. DePalma G, Turnidge J, Craig BA.. Determination of disk diffusion susceptibility testing interpretive criteria using model-based analysis: development and implementation. Diagn Microbiol Infect Dis 2017; 87: 143–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.