Abstract
Premature menopause is a serious long-term side effect of chemotherapy. We evaluated long-term pregnancy and disease-related outcomes for patients in S0230/POEMS, a study in premenopausal women with stage I–IIIA estrogen receptor–negative, progesterone receptor–negative breast cancer to be treated with cyclophosphamide-containing chemotherapy. Women were randomly assigned to standard chemotherapy with or without goserelin, a gonadotropin-releasing hormone agonist, and were stratified by age and chemotherapy regimen. All statistical tests were two-sided. Of 257 patients, 218 were eligible and evaluable (105 in the chemotherapy + goserelin arm and 113 in the chemotherapy arm). More patients in the chemotherapy + goserelin arm reported at least one pregnancy vs the chemotherapy arm (5-year cumulative incidence = 23.1%, 95% confidence interval [CI] = 15.3% to 31.9%; and 12.2%, 95% CI = 6.8% to 19.2%, respectively; odds ratio = 2.34; 95% CI = 1.07 to 5.11; P = .03). Randomization to goserelin + chemotherapy was associated with a nonstatistically significant improvement in disease-free survival (hazard ratio [HR] = 0.55; 95% CI = 0.27 to 1.10; P = .09) and overall survival (HR = 0.45; 95% CI = 0.19 to 1.04; P = .06). In this long-term analysis of POEMS/S0230, we found continued evidence that patients randomly assigned to receive goserelin + chemotherapy were not only more likely to avoid premature menopause, but were also more likely to become pregnant without adverse effect on disease-related outcomes.
Ovarian failure is a side effect of chemotherapy that has a high symptom and quality-of- life burden including menopausal, sexual, and cognitive symptoms as well as infertility. S0230/Prevention of Early Menopause Study (POEMS) was a SWOG-coordinated international phase III randomized trial of goserelin administration during chemotherapy to reduce ovarian failure in premenopausal women with early-stage breast cancer. We previously reported a 70% reduction in ovarian failure at two years with the addition of goserelin to standard chemotherapy for breast cancer (1). The initial results led to endorsement of this approach for reducing the risk of chemotherapy-associated ovarian failure by multiple consensus group guidelines (2–4). The American Society of Clinical Oncology Fertility Guidelines have not yet fully endorsed this approach for preventing premature menopause and thereby improving fertility (5). Skepticism remained regarding the safety of this approach in women with breast cancer and its ability to preserve fertility. Here we present the final analysis of pregnancy, disease-free survival (DFS), and overall survival (OS) outcomes with five years of follow-up.
Patient eligibility criteria have been previously described (1). POEMS enrolled premenopausal women age 18–49 years with stage I–IIIA estrogen receptor–negative, progesterone receptor–negative breast cancer to be treated with adjuvant or neoadjuvant cyclophosphamide-containing chemotherapy. The protocol (ClinicalTrials.gov number NCT00068601) was approved by the institutional review boards of participating institutions. All patients provided written informed consent. The study was monitored by an independent data and safety monitoring committee. A stratified 1:1 randomization to standard chemotherapy with or without goserelin, a gonadotropin-releasing hormone agonist, was used. Stratification factors were age and chemotherapy regimen. Goserelin (3.6 mg) was administered subcutaneously every four weeks beginning at least one week prior to the initial chemotherapy dose until within two weeks of the final chemotherapy dose. The study mandated five years of follow-up.
Data on pregnancies and pregnancy attempts were obtained annually. DFS was defined as time from randomization to breast cancer recurrence or death from any cause. OS was defined as time from randomization to death from any cause. The final analysis is based on 218 eligible and evaluable patients, including 113 in the chemotherapy arm and 105 in the goserelin + chemotherapy arm (Figure 1). Differences by arm in the number of patients who reported pregnancy and pregnancy attempt were analyzed using conditional logistic regression stratified by the factors age and chemotherapy regimen. Cumulative incidence of pregnancy was estimated to account for competing risk of death; arms were compared using the Gray test. Kaplan-Meier curves, 5-year rates, and 95% confidence intervals (CIs) for DFS and OS were estimated (6). We used Cox regression, adjusting for the stratification factors and cancer stage, to calculate hazard ratios, 95% CIs, and P values for differences by arm in OS and DFS (7). All statistical tests were two-sided and a P value of less than .05 was considered statistically significant. Median follow-up was 5.1 years for patients who were alive at the end of the study (n = 190; range = 0.13–11.1 years). Analyses are limited to the first five years after registration. For time-to-event analyses, patients who experienced events after this point were censored at five years.
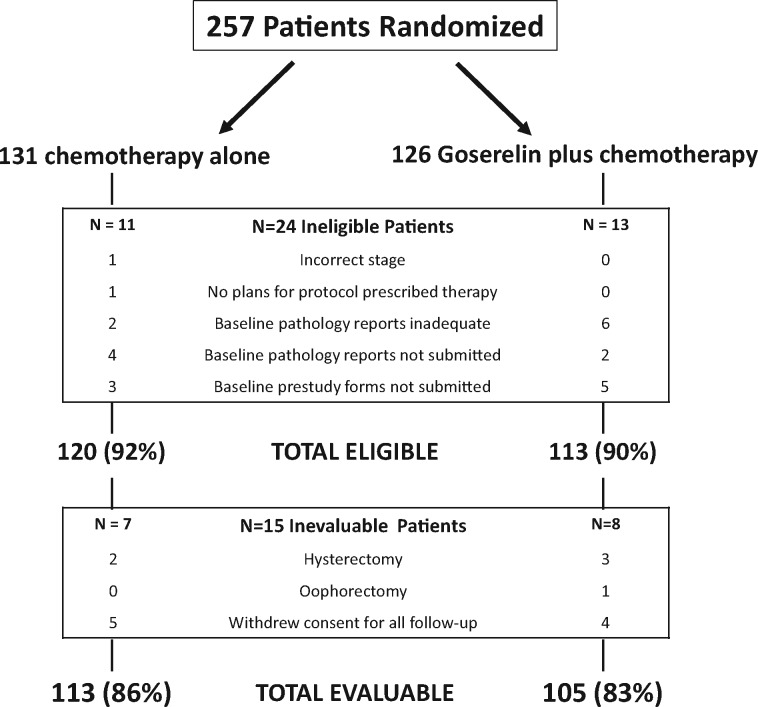
Figure 1.
CONSORT diagram.
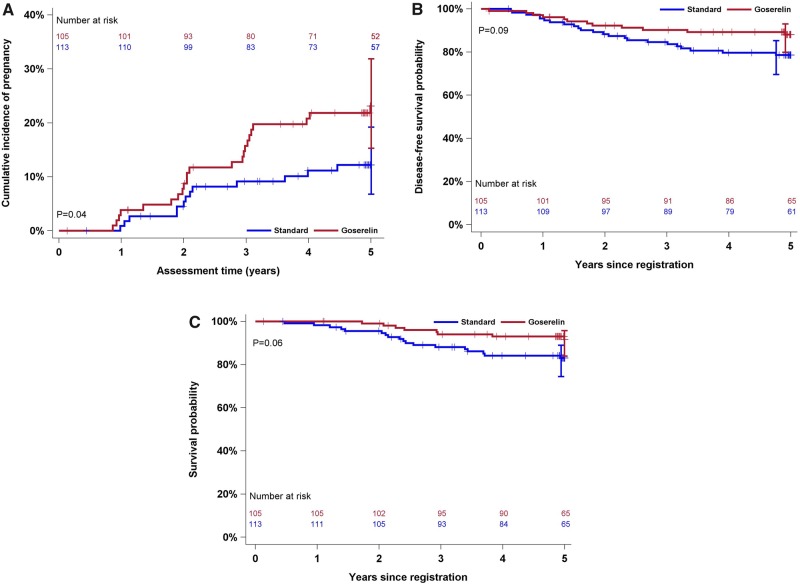
Of 257 patients randomly assigned between February 2004 and May 2011, 218 were eligible and evaluable. Table 1 describes patient characteristics. Five-year cumulative incidence of pregnancy was 23.1% (95% CI = 15.3% to 31.9%) on the goserelin + chemotherapy arm and 12.2% (95% CI = 6.8% to 19.2%) in the chemotherapy arm (Gray P = .04) (Figure 2A). The adjusted OR comparing pregnancy by arm is 2.34 (95% CI = 1.07 to 5.11; P = .03). Nineteen of 113 patients on the chemotherapy arm and 25 of 105 patients randomly assigned to the goserelin + chemotherapy arm reported attempting pregnancy over the 5 years (adjusted OR =1.63; 95% CI = 0.79 to 3.36; P = .18); notably, some pregnancies occurred in patients who did not report attempting pregnancy.
Table 1.
Characteristics for all eligible patients
| Characteristic | Overall | Chemotherapy | Goserelin + chemotherapy |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Total | 218 (100.0) | 113 (100.0) | 105 (100.0) |
| Age, y | |||
| Median (range) | 37 (25–49) | 38 (25–49) | 37 (26–48) |
| 25–39 | 138 (63.3) | 70 (61.9) | 68 (64.8) |
| 40–49 | 80 (36.7) | 43 (38.1) | 37 (35.2) |
| Median follow-up, y (range) | 5.06 (0.13–11.1) | 5.07 (0.44–10.89) | 5.04 (0.13–11.1) |
| Race | |||
| White | 122 (56.0) | 57 (50.4) | 65 (61.9) |
| Black | 11 (5.0) | 6 (5.3) | 5 (4.8) |
| Asian | 2 (0.9) | 2 (1.8) | 0 (0.0) |
| Native American | 1 (0.5) | 1 (0.9) | 0 (0.0) |
| Unknown | 82 (37.6) | 47 (41.6) | 35 (33.3) |
| Hispanic ethnicity | |||
| Hispanic | 67 (30.7) | 34 (30.1) | 33 (31.4) |
| Non-Hispanic | 59 (27.1) | 26 (23.0) | 33 (31.4) |
| Unknown | 92 (42.2) | 53 (46.9) | 39 (37.1) |
| Planned chemotherapy | |||
| 3–4 cycles of anthracycline-based therapy | 46 (21.1) | 22 (19.5) | 24 (22.9) |
| 3–4 cycles of non-anthracycline-based therapy | 12 (5.5) | 7 (6.2) | 5 (4.8) |
| 6-8 cycles of anthracycline-based therapy | 152 (69.7) | 80 (70.8) | 72 (68.6) |
| 6-8 cycles of non-anthracycline-based therapy | 8 (3.7) | 4 (3.5) | 4 (3.8) |
| AJCC Stage version 6 | |||
| I | 55 (25.2) | 32 (28.3) | 23 (21.9) |
| II | 107 (49.1) | 52 (46.0) | 55 (52.4) |
| IIIA | 54 (24.8) | 29 (25.7) | 25 (23.8) |
| Unknown | 2 (0.9) | 0 (0.0) | 2 (1.9) |
| HER2 Positive status | 32 (14.7) | 19 (16.8) | 13 (12.4) |
Figure 2.
Cumulative incidence of pregnancy (A), disease-free survival probability (B), and overall survival probability (C) by study arm. P values represent the association of the study arm and survival outcomes derived from the corresponding Cox proportional hazards regression (for B and C). P value in (A) is from a Gray test. All statistical tests were two-sided.
DFS events occurred in 12 of 105 patients randomly assigned to goserelin + chemotherapy and 23 of 113 patients in the chemotherapy arm (adjusted HR = 0.55; 95% CI = 0.27 to 1.10; P = .09; Figure 2B). Five-year DFS is 88.1% (95% CI = 79.9% to 93.0%) and 78.6% (95% CI = 69.6% to 85.3%) in the goserelin + chemotherapy and chemotherapy groups, respectively. Eight of 105 patients on the goserelin + chemotherapy arm died compared with 18 of 113 patients on the chemotherapy arm (adjusted HR = 0.45; 95% CI = 0.19 to 1.04; P = .06; Figure 2B). Five-year OS is 91.7% (95% CI = 84.0% to 95.8%) and 83.1% (95% CI = 74.4% to 89.0%) for the goserelin + chemotherapy and chemotherapy groups, respectively. For all 257 randomly assigned patients the adjusted HR for DFS is 0.72 (95% CI = 0.39 to 1.32; P = .29) and the adjusted HR for OS is 0.46 (95% CI = 0.22 to 0.99; P = .05). All deaths were either specifically of cancer, with cancer contributory, or occurred following progression of cancer.
Limitations of POEMS include limited power to determine survival differences by arm, although we can conclude with reasonable confidence that, in this study, goserelin administration did not adversely impact survival. We accounted for age as a model covariate but did not present age-stratified results due to limited sample size. We did not account for the desire to become pregnant and prior pregnancies, which could impact pregnancy outcomes. Because it is desirable to prevent other long-term effects of ovarian failure, POEMS participants were not required to have an interest in future pregnancy; it is unknown how many women in each arm desired future fertility at the time of random assignment. Although participants were asked whether they attempted pregnancy, deliberate pregnancy attempts were likely influenced by the presence of ovarian failure, disease recurrence, and perhaps by the non-blinded random assignment. This report cannot provide a true denominator regarding the number of patients desiring future pregnancy at the time of random assignment. Despite challenges, consistent evidence was found that patients randomly assigned to receive goserelin were less likely to experience ovarian failure and more likely to become pregnant without adverse effect on disease-related outcomes.
Administration of the gonadotropin-releasing hormone agonist during chemotherapy does not prohibit the use of traditional assisted reproductive technology and is the only medical intervention shown to reduce the risk of chemotherapy-associated ovarian failure. Preventing premature menopause should help women avoid unnecessary morbidities and adverse quality-of-life impact. Premenopausal women undergoing curative-intent chemotherapy for hormone receptor–negative breast cancer should be counseled on the availability of this relatively simple option to reduce ovarian failure risk.
Funding
Research support for this study was provided by The National Cancer Institute of the National Institutes of Health, Breast Cancer Trials Australia and New Zealand (BCT-ANZ), and AstraZeneca. The Prevention of Early Menopause Study (POEMS)/S0230 trial was performed in collaboration by investigators from the SWOG Cancer Research Group, the International Breast Cancer Study Group, the ECOG-ACRIN Cancer Research Group, and the Alliance for Clinical Trials in Oncology. This study was supported by the National Cancer Institute of the National Institutes of Health under grant awards CA189974, CA180888, CA180819, CA180821, CA075362, CA180820, CA189808, CA180830, CA180801, CA189872, CA189822, CA189953, CA189858, CA180858, CA189954, CA189957, CA189972, CA04919, CA46368, CA68183, CA46282, CA46113, CA76447, CA58416, CA12644, and CA11083; and by Breast Cancer Trials Australia and New Zealand (BCT-ANZ).
Notes
Affiliations of authors: Cleveland Clinic Foundation, Cleveland, OH (HCFM); SWOG Statistics and Data Management Center, Fred Hutchinson Cancer Research Center, Seattle, WA (JMU, AM, WEB); Peter MacCallum Cancer Centre, University of Melbourne, Melbourne, VIC, Australia (KAP, PAF); Breast Cancer Trials Australia and New Zealand (BCT-ANZ), Newcastle, Australia (KAP, PAF, JFF); International Breast Cancer Study Group (IBCSG), Bern, Switzerland (KAP, PAF); University of Sydney, Sydney, NSW, Australia (FB); National Institute of Oncology, Budapest, Hungary (EH); Auckland Regional Cancer and Blood Service, Auckland, New Zealand (DJP); Fox Chase Cancer Center, Philadelphia, PA (LJG); Instituto Nacional de Enfermedades Neoplasicas, Lima, Peru (HLG); Oncosalud AUNA, Lima, Peru (CSV); Dana Farber Cancer Institute, Boston, MA (AHP); Wichita NCORP, Wichita, KS (SRD); Louisiana State University Health Sciences Center, New Orleans, LA (SRD, AAG); Seattle Cancer Care Alliance, and University of Washington, Seattle, WA (JRG); Calvary Mater Hospital, Newcastle, Australia (JML, JFF); The Angeles Clinic and Research Institute, Santa Monica, CA (SM); University of Kansas Cancer Center, Kansas City, KS (CJF); Division of Cancer Prevention, National Cancer Institute, Bethesda, MD (LMM); University of California at Irvine Chao Family Comprehensive Cancer Center, Orange, CA (FLMJ); IBCSG Statistical Center, Dana-Farber Cancer Institute, Harvard Medical School, Harvard T.H. Chan School of Public Health and Frontier Science and Technology Research Foundation, Boston, MA (RDG); MD Anderson Cancer Center, Houston, TX (GNH); Loyola University Medical Center, Cardinal Bernardin Cancer Center, Maywood, IL (KSA).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
HCFM has received research support to her institution from PUMA and AbbVie. FB has received honoraria from Pfizer and fees for consulting or advising from Pfizer, AstraZeneca, Lilly, Novartis, Eisai, and Roche. PAF has received honoraria from AstraZeneca and gave an overseas lecture for Pfizer. LJG has received honoraria from PUMA, Pfizer, Genentech, Novartis, and Nanostring; fees for consulting or advising for Puma, Pfizer, Genentech, Novartis, and Nanostring; and research funding from Merck and Genetech. JRG has received fees for consulting or advising from Roche, Genentech, Novartis, Pfizer, AstraZeneca, PUMA, Bayer, and Merck. JML has received honoraria from AstraZeneca and fees for consulting or advising from AstraZeneca. SM has received fees for consulting or advising from Cohellus, Astellas, PUMA, Rigel, and Novartis. FLM has stock or other ownership in Cancer Prevention Pharmaceuticals and has patent or intellectual property interest in Chromdex. RDG has received research funding to his institution from AstraZeneca, Novartis, Pfizer, Ipsen, Merck, Celgene, Roche/Genentech, and Ferning. GNH has received consulting fees for Antigen Express, Novartis, Lilly, Pfizer, and Merck. All other authors declare no competing interests.
AstraZeneca provided the study drug. Kelly-Anne Phillips is an Australian National Breast Cancer Foundation Practitioner Fellow. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AstraZeneca.
Our first analysis was presented at the American Society of Clinical Oncology annual meeting 2014 and published in 2015 in the New England Journal of Medicine. Final analysis results were presented, in part, at the 2017 San Antonio Breast Cancer Symposium.
References
- 1. Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;37210:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—improving the management of early breast cancer: St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;268:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. 2017;35:203–217. [DOI] [PubMed] [Google Scholar]
- 4. Gradishar WG, Anderson BO, Balassanian R, et al. Invasive breast cancer version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;143:324–354. [DOI] [PubMed] [Google Scholar]
- 5. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;146:381–385. [DOI] [PubMed] [Google Scholar]
- 6. Kaplan EL, Meier P.. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53282:457–481. [Google Scholar]
- 7. Cox DR. Regression models and life tables. J R Stat Soc Ser B. 1972;342:187–220. [Google Scholar]