Abstract
Federal investment in survivorship science has grown markedly since the National Cancer Institute’s creation of the Office of Cancer Survivorship in 1996. To describe the nature of this research, provide a benchmark, and map new directions for the future, a portfolio analysis of National Institutes of Health-wide survivorship grants was undertaken for fiscal year 2016. Applying survivorship-relevant terms, a search was conducted using the National Institutes of Health Information for Management, Planning, Analysis and Coordination grants database. Grants identified were reviewed for inclusion and categorized by grant mechanism used, funding agency, and principal investigator characteristics. Trained pairs of coders classified each grant by focus and design (observational vs interventional), population studied, and outcomes examined. A total of 215 survivorship grants were identified; 7 were excluded for lack of fit and 2 for nonresearch focus. Forty-one (19.7%) representing training grants (n = 38) or conference grants (n = 3) were not coded. Of the remaining 165 grants, most (88.5%) were funded by the National Cancer Institute; used the large, investigator-initiated (R01) mechanism (66.7%); focused on adult survivors alone (84.2%), often breast cancer survivors (47.3%); were observational in nature (57.3%); and addressed a broad array of topics, including psychosocial and physiologic outcomes, health behaviors, patterns of care, and economic/employment outcomes. Grants were led by investigators from diverse backgrounds, 28.4% of whom were early in their career. Present funding patterns, many stable since 2006, point to the need to expand research to include different cancer sites, greater ethnoculturally diverse samples, and older (>65 years) as well as longer-term (>5 years) survivors and address effects of newer therapies.
It is now over two decades since the National Cancer Institute (NCI) established the Office of Cancer Survivorship (OCS), heralding the intent of NCI leadership to invest in research to better understand and address the long-term consequences of surviving a cancer diagnosis (1). The unique purview of the OCS was—and remains—to support and direct research designed to enhance quality of life, and not simply length of survival, for all those diagnosed with cancer, and to champion studies that examine, as well as intervene, to improve the health and function of those post definitive treatment for cancer. In the years since OCS was created, five national reports have been released, two produced by the Institute of Medicine (2,3), two led by the President’s Cancer Panel (4,5), and one sponsored by the Centers for Disease Control and Prevention, in partnership with the Lance Armstrong Foundation (6), highlighting the gaps in our knowledge regarding the challenges of cancer survivorship and necessary steps to address these. Among the handful of key recommendations cited in each of these documents was the need for more research.
Considerable progress has been made since publication of the Lost in Transition report in 2006, considered by many as the benchmark review of the state of survivorship science and care (7). This has included steady growth in survivorship research funding and publications (8,9). As the field matures into young adulthood, however, it is imperative to understand the direction that current research is taking, to evaluate this in the context of current trends in epidemiology and care, and to use this information to inform an agenda for the future. The drive to understand how best to reduce the burden of cancer is fueled by the growing appreciation that the number of cancer survivors not only continues to grow both in the United States and globally (10–13) but will continue to do so for the foreseeable future due in no small measure to the aging of the world’s citizens (14,15).
Two requests for applications (RFAs) to foster research on cancer survivors living five or more years post-treatment were generated by the OCS following its launch (CA-97–018 Long-Term Cancer Survivors: Research Initiatives; reissued as CA-04–003). Survivorship was also identified as an Extraordinary Opportunity for investment in the NCI’s FY2004 and FY2005 budget proposals to Congress. Beyond this early stimulation with set-aside funds, the field has grown rapidly, driven primarily by the investigator community, with subtopics of special interest shaped by the release of Program Announcements (eg, Examination of Survivorship Care Planning Efficacy and Impact, PA-18–002 [R01] and PA-18–012 [R21]; Physical Activity and Weight Control Interventions Among Cancer Survivors: Effects on Biomarkers of Prognosis and Survival, PAR-18–006 [R01] and PAR-18–016 [R21]). With survivorship advancing into its young adulthood, it seemed a fruitful time to take stock of where the science stands now and how it may be moving over time, and to use this information to guide future directions for pursuit.
The purpose of the current project was to identify National Institutes of Health (NIH)-wide survivorship grants funded in fiscal year (FY) 2016 (October 1, 2015 through September 30, 2016)—the year that marked the 20th anniversary of the OCS—and to examine the portfolio with respect to topics being pursued, mechanisms used, types of studies conducted, and information regarding who is leading these grants, with an eye toward 1) identifying gap areas for potential pursuit and 2) providing a benchmark against which future changes in research focus and funding at the federal level could be tracked.
Methods
Search Strategy
This analysis examined all extramural NIH research grants, including cooperative agreements and training awards, focused on cancer survivorship active in FY2016. Grants that were active but in no-cost extension status (ie, did not receive additional funding in FY2016) were not counted in this review. Grants were identified through the internal NIH Information for Management, Planning, Analysis, and Coordination grants database. The OCS definition of survivorship research (Supplementary Box 1) was used to guide the selection of search terms. Abstracts and titles of grant applications were queried for multiple combinations of the following terms: survivor, survivorship, follow-up, late effects, chronic, quality of life, and long-term effects. Grants were included if the study aims assessed outcomes in one or more of the following areas: physiologic, behavioral, psychosocial, patterns of care or care delivery, and/or economics. Studies that examined or intervened with survivors and/or their family members during active treatment were included only when individuals were followed a minimum of 6 months post-treatment. Studies of survivors with a local recurrence who are followed post-treatment were also included. Because this analysis focused on the effects of cancer and cancer treatment on individuals and their families, studies that focused exclusively on animal models, survival only (eg, no data on quality of life, or other aspects of health or function), active treatment with no post treatment endpoints, and bereavement were excluded.
Coding Procedure
Coding procedures were adapted from prior NIH-wide survivorship portfolio analyses (unpublished) and other NCI-specific analytic projects (16–18). In addition to general grant characteristics (eg, IC or institutional funder, mechanism and funding opportunity utilized, principal investigator (PI) training/degree and location), information on the focus of and approach used in the grant was drawn from the abstract, specific aims, and human subjects sections of each application. A codebook of the grant features of interest was developed, with coding criteria and decision rules. Coding categories allowed for description of: 1) the population studied (adult vs childhood cancer survivors, or both); cancer site(s); age of the sample at time of study; time since diagnosis (<2 years, 2–5 years, 5+ years); and whether special populations were involved, such as LGBT, rural, or caregivers; 2) the primary, mutually exclusive survivorship research area of interest (economics/employment/finances, establishment of a cohort, health behaviors and adherence, patterns/quality of care, physiologic and psychosocial, psychosocial sequelae only, physiologic sequelae only, psychometric/data mining tools); 3) whether the study was observational (and, if so, whether the analysis was cross-sectional or longitudinal) or interventional (and, if so, if it was a randomized controlled trial design or not, and how the intervention was delivered: self, other, both); and 4) major factors addressed in the specific aims.
Ten grants were coded by all six authors. Observed discrepancies in coding were discussed and the codebook refined to further clarify criteria going forward. Remaining grants were assigned to two paired coders. In cases where a coding difference occurred, pairs came to an agreement about how the grant should be coded, and those final responses were used in the data analysis. In cases where questions remained, the grant was discussed by all six coders and coding assignments made by group consensus.
Data Analysis
By their nature, portfolio analyses are exploratory exercises. There are rarely a priori hypotheses to be tested, multiple comparisons are often conducted, and sample or category sizes can be small. Because we were interested in shifts made in funding patterns over time, we include here some comparisons to data captured in a FY2006 analysis of the NIH-wide survivorship grant portfolio that also helped to inform the approach to the current analysis.
Results
A total of 215 grants were identified as representing survivorship science. Seven were excluded after review of the abstract, because they were not survivorship research studies or human subject studies. Among the remaining studies, two infrastructure development grants (P20, U54), as well as 41 grants (19.7%) representing training (N = 38) or conference awards (R13 = 2, U13 = 1), were excluded from further analysis. Among the training grants, a range of mechanisms was used, including: 17 K07s, 9 R25s, 4 F31s, 2 each of the K01, K05, and K23 mechanisms, and 1 each of the K24 and F32 mechanisms. Because both the training grants and the conference awards do not lend themselves to content coding, they were excluded from the content analysis performed on more standard research mechanisms. Discussion of these 41 excluded grants will be provided in a separate paper.
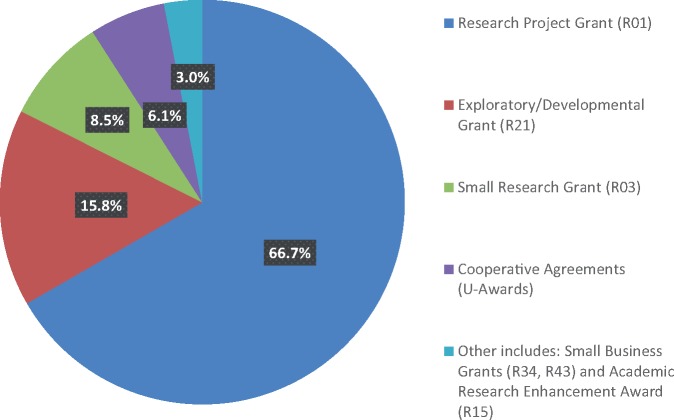
The pattern of funding by mechanism among the research project grants identified is shown in Figure 1. The majority of grants represented large, investigator-initiated R01 funded projects (n = 110, 66.7%). A total 15.8% (n = 26) were exploratory R21 funded grants, and 8.5% (n = 14) represented small R03 grants. Grants within the current analysis were received under a variety of funding opportunities, most reflecting “omnibus” or generic funding for large R01, small grant (R21, R03, R15), or Small Business Award initiatives (n = 102; 61.8%). The remaining grants were in response to specific program announcements with or without separate review (n = 55; 33.3%), with only eight responding to a targeted RFA (five funded by NCI and one each by other institutes [National Institute of Nursing Research, National Institute of Minority Health and Health Disparities, National Institute of Dental and Craniofacial Research]). Information on findings from the in-depth coding of the 165 research project grants are the focus of the remaining descriptive analyses.
Figure 1.
Distribution of fiscal year 2016 National Institutes of Health-wide survivorship research grants by funding mechanism (N = 165). Other includes: R34, R43 (Small Business Grants), and R15 (Academic Research Enhancement Award).
Pattern of Funding
As seen in Table 1, the NCI is the lead institute supporting survivorship science NIH-wide (88.5%); within the NCI, the Division of Cancer Control and Population Sciences (DCCPS), home to the OCS, manages the largest share of these applications (85.6%). Up until 2014, the OCS managed most of the survivorship grants referred to DCCPS. However, beginning in January of 2014, survivorship grants were actively distributed across the division, reflecting the fact that resident topical expertise had become more broadly embedded in other programs division-wide. As of the publication of this review, no grants are currently assigned to OCS.
Table 1.
Distribution of FY2016 survivorship research grants by funding institute and NCI division*
| Funding institute | No. (%) |
|---|---|
| NIH institute/center (n = 165) | |
| NCI | 146 (88.5) |
| NINR | 5 (3.0) |
| NIH OD | 4 (2.4) |
| NICHD | 3 (1.8) |
| NIA | 2 (1.2) |
| NHLBI | 2 (1.2) |
| NIMHD | 2 (1.2) |
| NIDCR | 1 (0.6) |
| NCI division/funding locus (n = 146) | |
| DCCPS | 125 (85.6) |
| DCP | 16 (11.0) |
| DCTD | 3 (2.1) |
| NCI Office of the Director | 2 (1.3) |
DCCPS = Division of Cancer Control and Population Sciences; DCP = Division of Cancer Prevention; DCTD = Division of Cancer Treatment and Diagnosis; FY = fiscal year; NCI = National Cancer Institute; NHLBI = National Heart Lung and Blood Institute; NIA = National Institute of Aging; NICHD = National Institute of Child Health and Human Development; NIDCR = National Institute of Dental and Craniofacial Research; NIH = National Institutes of Health; NIH OD = NIH Office of the Director; NIMHD = National Institute of Minority Health and Health Disparities; NINR = National Institute of Nursing Research.
Grant Focus and Design
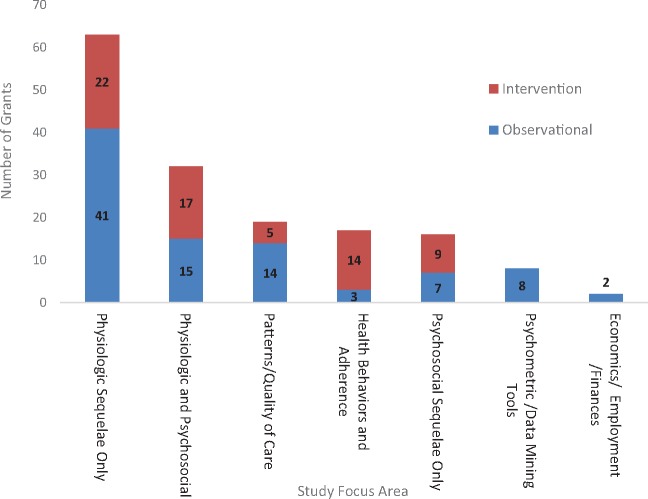
Figure 2 shows the distribution of applications by the primary focus of the research and whether the study approach was observational only or included an intervention component. Overall, 90 (57.3%) projects (exclusive of the 8 cohort infrastructure studies that do not lend themselves to grant focus or design coding) were observational in nature, and the majority (60.5%; including both observational and intervention designs) targeted physiologic sequelae alone (n = 63) or in combination with psychosocial outcomes (n = 32). In 45 cases, grants were examining the effects of a specific cancer treatment. Grants examining psychosocial outcomes alone (n = 16) represented only 10.2% of the portfolio. Grants that included a focus on psychosocial outcomes, either alone or in combination with physiologic outcomes (n = 48), were more likely to include an intervention (54.2%) than studies examining physiologic outcomes alone (34.9%). Inclusion of biomarkers in the specific aims was identified in 64 grants (40.7%). A total of 41 grants (26.1%) highlighted use of technology in their specific aims (eg, incorporation of social media in some aspect of the study, electronic delivery of intervention, and medical applications such as fMRI).
Figure 2.
Distribution of fiscal year 2016 National Institutes of Health-wide survivorship research grants by study focus and design (N = 157). The eight grants coded as establishment of a cohort are excluded from this figure, because these applications did not lend themselves to coding by a major area of focus.
Among the 90 observational studies, 15 (16.7%) employed a cross-sectional and 75 (83.3%) used a longitudinal design. These 75 longitudinal studies do not include the 8 funded as infrastructure grants to establish a survivor cohort; scientific-specific aims for these grants were not required in the application. With respect to the intervention studies (n = 67), the majority (86.5%) were randomized clinical trials. Interventions were administered by another person in 34 cases (50.7%), self-administered in 12 cases (17.9%) (ie, required an individual to engage in some activity or behavior over time after brief instruction), and used a combination of both in 21 cases (31.3%).
Target Population
The majority (84.2%) of studies examined outcomes for survivors of adult onset cancer alone, whereas 13.9% examined survivors of childhood cancer alone or in combination with adult onset cancer survivors (1.8%). Although a range of cancers were represented among the populations studied, breast cancer survivors predominated as the target of funded research (N = 78, 47.3%) (Table 2). Reflecting the dominance of breast cancer research, more research is being conducted among populations of women than men. Most studies examine outcomes for survivors less than 2 years post-diagnosis (64.2%), with the percent of those studied between 2 and 5 years or more than 5 years post-diagnosis being approximately evenly distributed (18.8% and 16.4%, respectively). Distribution by age at assessment reflects a focus primarily on those in middle to late middle age. Approximately 7% of studies examined an adolescent/young adult population, and 4.8% had a specific focus on older adults.
Table 2.
Information on the target population characteristics of FY2016 survivorship research grants (n = 165)*
| Characteristic | No. (%) |
|---|---|
| Cancer type† | |
| Breast | 78 (47.3) |
| Colorectal | 25 (15.2) |
| Prostate | 23 (13.9) |
| Hematologic‡ | 20 (12.1) |
| Gynecological§ | 13 (7.9) |
| Lung | 10 (6.1) |
| Head and neck | 9 (5.5) |
| Bladder | 6 (3.6) |
| Other‖ | 59 (35.8) |
| Pediatric or adult cancer survivors (at time of diagnosis) | |
| Pediatric survivors | 23 (13.9) |
| Adult survivors | 139 (84.2) |
| Both | 3 (1.8) |
| Sex | |
| Male | 9 (5.5) |
| Female | 71 (43.0) |
| Both | 85 (51.5) |
| Time since diagnosis | |
| Specified (categories below not mutually exclusive) | 127 (77.0) |
| <2 years of diagnosis | 106 (64.2) |
| 2 to 5 years after diagnosis | 31 (18.8) |
| >5 years after diagnosis | 27 (16.4) |
| Recurrent cancer survivors | 2 (1.2) |
| Not specified | 38 (23.0) |
| Special populations | |
| Adolescents and young adults | 11 (6.7) |
| Older adults (65 y or older) | 8 (4.8) |
| Rural populations | 5 (3.0) |
| Families (couples/dyads, parents/siblings) | 15 (9.1) |
FY = fiscal year.
Percent does not add up to 100% for cancer type, because some grants included multiple types of cancer.
Includes lymphomas, leukemias, myeloma.
Includes cervical, endometrial, ovarian.
Includes adult not otherwise specified, pediatric not otherwise specified, testicular, renal, bone, soft tissue, Wilms’ tumor, basal cell carcinoma, melanoma, brain, retinoblastoma, and gastrointestinal.
Outcome Categories
Over 75.8% of grants (n = 119) identified at least one physiologic issue as a mediator/moderator or outcome of the study (Table 3). Among physiologic issues identified, mortality/progression/recurrence/survival (n = 26/119; 21.8%), cardiovascular function (n = 28/119; 23.5%), and fatigue (n = 18/119; 15.1%) were the most commonly studied physiologic endpoints. However, the most common physiologic outcomes varied based on whether adult or pediatric survivors were the population of focus; for example, among studies examining physiologic sequelae, a higher percentage of pediatric only (46.7%) compared with adult only (19.8%) survivor grants included cardiovascular function as an endpoint, and no pediatric survivor grant was identified as having mortality/progression/recurrence/survival as an outcome. Physiologic issues also varied by the four most prevalent cancer sites (female breast, colorectal, lung, and prostate) (data not shown). In addition to the mortality/progression/recurrence/survival endpoint, which was relatively common across all four cancer types, the most common outcomes under study among the four most prevalent cancer types were: for breast cancer, cardiovascular function (19.2%) and fatigue (17.9%); for colorectal cancer, sleep disturbance (16.0%); for lung cancer, neurocognitive function (20.0%); and for prostate cancer, sexual functioning (23.1%).
Table 3.
Physiologic sequelae as a mediator, moderator, or outcome of study, by study population type*
| Adult-onset cancer only(n = 133) |
Pediatric-onset cancer only(n = 21) |
Both adult andPediatric-onset cancers(n = 3) |
|
|---|---|---|---|
| Physiologic sequelae | No. (%)† | No. (%)† | No. (%)† |
| Any | 101 (75.9) | 15 (71.4) | 3 (100.0) |
| Mortality/progression/recurrence/survival | 26 (25.7) | 0 (0.0) | 0 (0.0) |
| Cardiovascular function | 20 (19.8) | 7 (46.7) | 1 (33.3) |
| Fatigue | 18 (17.8) | 0 (0.0) | 0 (0.0) |
| Pain/neuropathy | 14 (13.9) | 1 (6.7) | 0 (0.0) |
| Neurocognitive function | 12 (11.9) | 3 (20.0) | 0 (0.0) |
| Sleep disturbances | 10 (9.9) | 0 (0.0) | 0 (0.0) |
| Physiologic sequelae NOS‡ | 10 (9.9) | 2 (13.3) | 1 (33.3) |
| Musculoskeletal function/osteoporosis/bone density§ | 9 (8.9) | 2 (13.3) | 0 (0.0) |
| Body composition/obesity/weight control | 8 (7.9) | 1 (6.7) | 0 (0.0) |
| Sexual functioning/fertility | 7 (6.9) | 3 (20.0) | 1 (33.3) |
| Diabetes/other comorbidity NOS | 6 (5.9) | 4 (26.7) | 0 (0.0) |
| Gastrointestinal/urinary function | 5 (5.0) | 1 (6.7) | 0 (0.0) |
| Immune function/inflammation | 5 (5.0) | 1 (6.7) | 0 (0.0) |
| Second cancers | 3 (3.0) | 2 (13.3) | 0 (0.0) |
| Other‖ | 10 (9.9) | 3 (20.0) | 0 (0.0) |
N = 157; excludes eight cohort studies not coded. NOS = not otherwise specified.
Percent for specific sequelae is calculated out of all grants within study population type with a physiologic sequelae mediator/moderator/outcome.
Includes physical functioning NOS, complications NOS, physical symptoms and disability.
Includes falls/fractures/frailty.
Includes physiologic sequelae with three grants or less identified: birth defects, dry mouth, kidney function, lymphedema, respiratory function, premature menopause, menopausal symptoms, speech impairment, and skin problems.
Over one-third of grants identified at least one psychosocial issue as a mediator/moderator or outcome of the study (n = 59; 37.6%), with the most prevalent issues being adaptation/stress (n = 28/59; 47.5% of psychosocial grants), depression (n = 23/59; 39.0%), and anxiety (n = 13/59; 22.0%) (Table 4). Common psychosocial issues studied did not differ substantially across the most prevalent cancer sites, with adaptation/stress and depression represented among the issues examined in all four cancer sites (data not shown). Over one-third of grants (n = 56; 35.7% of total grants) included at least one health behavior as a mediator/moderator or outcome of the study, with physical activity (n = 32; 57.1% of health behavior grants) and diet (n = 22; 39.3%) being the most common topics of focus (data not shown). Other health behaviors identified as a moderator/mediator or outcome included cancer screening (n = 11; 19.6%) and tobacco use (n = 8; 14.3%). Healthcare delivery factors were identified in 56 grants (35.7% of total grants), with the most common factors being adherence (n = 22; 39.3% of healthcare delivery grants), healthcare utilization (n = 18; 32.1%), and processes of care (n = 13; 23.2%). Other healthcare delivery issues identified included complementary therapies (n = 7; 12.5%) and healthcare satisfaction (n = 7; 12.5%). These factors did not vary among the four most prevalent cancer sites.
Table 4.
Psychological sequelae as a mediator, moderator, or outcome of study, by study population type*
| Adult-onset cancer only(n = 133) |
Pediatric-onset cancer only(n = 21) |
Both adult and pediatric-onset cancers(n = 3) |
|
|---|---|---|---|
| Psychological sequelae | No. (%)† | No. (%)† | No. (%)† |
| Any | 49 (36.8) | 8 (38.1) | 2 (66.7) |
| Adaptation/stress | 22 (44.9) | 5 (62.5) | 1 (50.0) |
| Depression | 21 (35.6) | 2 (25.0) | 0 (0.0) |
| Anxiety | 10 (20.4) | 3 (37.5) | 0 (0.0) |
| Fear of recurrence | 3 (6.1) | 0 (0.0) | 0 (0.0) |
| Psychosocial constructs/cognitions (eg, self-efficacy, motivation) | 3 (6.1) | 1 (12.5) | 0 (0.0) |
| Social support | 3 (6.1) | 0 (0.0) | 0 (0.0) |
| Post-traumatic stress/post-traumatic growth | 1 (2.0) | 1 (12, 5) | 0 (0.0) |
| Other‡ | 12 (24.5) | 4 (50.0) | 1 (50.0) |
N = 157; excludes eight cohort studies not coded.
Percent for specific sequelae is calculated out of all grants within study population type with a psychological sequelae mediator/moderator/outcome.
Includes body image, somatization, stigma/social isolation, and psychological sequelae not otherwise specified.
Individual Investigator and Grant Characteristics
Of the 149 unique PIs leading the science reviewed, most held a PhD alone (63.1%), another 28.8% were trained as physicians, some with a dual PhD degree; the remaining 8.1% of PIs held other degrees (eg, JD, DDS, ScD) (Table 5). Over one-fifth (22.4%) of grants awarded had multiple PIs. Among 102 unique PIs who submitted R01 applications, 28.4% qualified as early-stage investigators. The NIH defines an early-stage investigator (ESI) as “any PI who has completed their terminal research degree or end of post-graduate clinical training, whichever date is later, within the past 10 years and who has not previously competed successfully as PI for a substantial NIH independent research award (eg, prior R01, P01, U01 etc.).” PIs self-identify ESI status upon application submission. A small number of the grants included a foreign component (9.7%), the largest group of collaborators coming from Canada (N = 6).
Table 5.
Principal investigator characteristics of FY2016 survivorship research grants (n = 165)
| Characteristic | No. (%) |
|---|---|
| Early stage investigator grant: R01 grants only† | |
| Yes | 29 (28.4) |
| No | 70 (68.6) |
| Not marked/missing | 3 (2.9) |
| Multiple-principal investigator grant | |
| Yes | 37 (22.4) |
| No | 128 (77.6) |
| Principal investigator terminal degree‡ | |
| MD | 37 (24.8) |
| PhD | 94 (63.1) |
| MD, PhD | 6 (4.0) |
| Other (JD, DDS, ScD) | 12 (8.1) |
| Principal investigator primary discipline‡ | |
| Medicine | 43 (28.9) |
| Psychology | 38 (25.5) |
| Epidemiology | 23 (15.4) |
| Health sciences | 15 (10.1) |
| Biological/biomedical sciences | 9 (6.0) |
| Social sciences | 9 (6.0) |
| Nursing science | 6 (4.0) |
| Other§ | 6 (4.0) |
FY= fiscal year.
n = 102 unique principal investigators with R01 grants.
n = 149 unique principal investigators.
Includes engineering sciences and mathematics.
Trend Analysis
In an effort to determine whether NIH-supported survivorship science has changed over time, comparison was made with previously unreported data from a similar portfolio analysis conducted of survivorship research funded in FY2006. A similar abstraction and coding process was used for capture of these data and helped inform the approach to the current analysis. However, the overall approach differed in three important ways from the current analysis. First, the search was conducted using three platforms: the NCI/DCCPS Portfolio Management Application, NCI Cancer Research Portfolio, and the NIH Computer Retrieval of Information on Scientific Projects System, since replaced by RePORTER. Second, studies that included newly diagnosed patients were included provided patients were followed for at least 2 (instead of the currently required 6) months post-treatment. These two differences may have resulted in the inclusion of a slightly higher number of grants in the earlier 2006 analysis. In addition, content coding categories were slightly different and included biologic/genetic risk markers, as well as family and caregiver outcomes, as possible foci of study. Thus, strict comparison by content area/topic of grants between 2006 and 2016 is not possible. Nevertheless, examination of the relative distribution of grants by funding institution, mechanism used, nature of study (observational vs interventional), cancers studied, and childhood vs adult cancer survivor focus offers insight into some trends over time.
A total of 251 grants were identified using this approach. Grants were largely funded by the NCI (87.6%), with DCCPS home to most of these (77.7%); represented observational studies (60%); relied heavily on the R01 mechanism (47.4%); and targeted primarily adult survivors (82.8%), many of whom (41.0%) were breast cancer survivors (see Supplementary Table 1 for descriptive characteristics of grants identified). Coding of grants (exclusive of training and conference mechanisms) by focus and design (observational vs interventional) is shown in Supplementary Figure 1.
Discussion
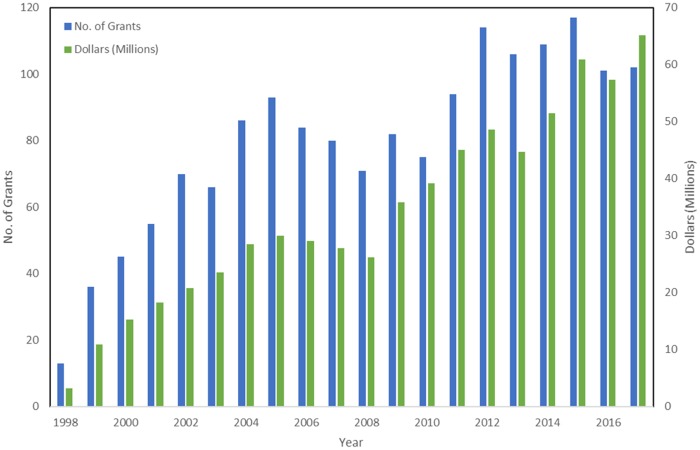
The present analysis provides a valuable benchmark regarding where survivorship science currently stands at the NIH, against which future portfolio and trend analyses can be compared. The number of grants funded is robust and continues to grow over time. As illustrated in Figure 3, the number of survivorship grants held by DCCPS alone has risen steadily over the years since OCS was moved into the division (1998), with some fluctuations reflective of both the availability of additional funds (eg, doubling of the NIH budget, RFA releases, American Recovery and Reinvestment Act monies) as well as more recent flattening of the budget.
Figure 3.
Funding trends for survivorship research grants held in the Division of Cancer Control and Population Sciences from 1998 to 2017.
Investigators from multiple backgrounds are engaged in pursing survivorship research. In cases where this could be identified, more than one-quarter of PIs were considered early stage, reflecting NIH’s commitment to bring along the next generation of scientists. A breadth of topics is covered in this work, with projects serving to chronicle the prevalence of common side effects (eg, fatigue, sleep, pain), identify their patterns, determine the social and health-care burden of such morbidity, and find ways to prevent and treat these common effects. Efforts to encourage the investigator community to incorporate physiologic outcomes when studying psychosocial effects, and the reverse, had an impact in the setting of psychosocial projects, because only 10.2% of studies examined psychosocial outcomes alone. By contrast, 40.1% of studies examined physiologic outcomes alone. The rationale behind a paired approach to studying outcomes is to permit a deeper understanding of the mind-body connection in explaining the mechanism of effect of a specific morbidity (eg, fatigue). Thus, thought should be given as to whether researching a physiologic or psychologic effect should be conducted in isolation of the corollary impact of one on the other. It is important to note that even with time-limited grants, investigators are striving to monitor physiologic outcomes such as mortality/progression/recurrence/survival, historically the purview of longitudinal cohort studies. Absence of emphasis on this as an outcome in the current set of pediatric (vs adult onset) cancer survivor grants may reflect the remarkable progress made in successfully treating this population of young survivors.
Not surprisingly, given the likely overlap in investigators sampled, a number of patterns reflected in this analysis are also reported by others (8,19). In some cases, based on comparison to the FY2006 analysis, the pattern appears consistent over time. Specifically, this includes the large number of studies of breast cancer survivors, over-representation of research among childhood cancer survivors relative to their low (<2.0%) prevalence in the larger population of survivors, and greater use of observational (57.3% across studies) vs interventional study designs.
It is uncertain whether any shift is needed in this latter pattern. Many clinicians and policy makers have argued that if we are to reduce the cancer burden, we need to stop reporting on the problems of survivorship and put more emphasis on either preventing or mitigating these, that is, shift away from descriptive observational studies to promote more intervention research. At the same time, with newer therapies appearing regularly, there is constant pressure to study the impact of these with regard to their latent toxicity to survivors. Ensuring there is ongoing research across both these aspects of survivorship science (observational and interventional) is clearly important, suggesting that the wisdom of dictating any specific allocation of funding between the two may need to be revisited regularly over time.
In addition to the above findings, more in-depth examination of various aspects of this science with regard to the mechanisms used to support it, the populations studied, the designs used, outcomes assessed, and investigators engaged in this research suggest that several modifications may be needed to ensure the findings of this work are broadly applicable to the growing population of survivors and their unique challenges. Potential areas for consideration to advance a robust field of survivorship science going forward are identified in Supplementary Box 2.
As seen in NIH portfolio analyses on other topics, although a diversity of mechanisms is pursued to conduct survivorship research, the R01 mechanism, used in 66.7% of the studies identified, continues to be the main source of funding for this science. When compared with FY2006 funding patterns, there has been a slight shift away from use of the smaller grant mechanisms (R03 and R21), which made up 31.4% in this earlier period vs 24.2% in FY2016. Reflected also in the present data is the fact that a number of teams have successfully collaborated to launch and sustain survivorship cohorts (U or cooperative agreement mechanisms that involve considerable government involvement), an area of interest to the NCI and a rich source for future survivorship studies. See Supplementary Table 2 for a list of these. Of note were the large (N = 41) number of educational and training mechanisms addressing survivorship. It is clearly important that training takes place to ensure a steady pipeline of future researchers to conduct survivorship studies and clinicians to deliver the recommended care. Less certain is what the proportion of training (vs research) mechanisms should be included in any portfolio. At the moment, training applications, as a proportion of all survivorship science funded, appear to be growing over time, from an estimated 14.4% in 2006 to 19.7% in this review. An overall increase in the number of applications by trainees in other areas of science is also reported for this period.
Perhaps not surprising, women treated for breast cancer continue to be the most widely studied group of survivors. As noted by others, breast cancer provides a rich paradigm for examining cancer’s myriad and long-term effects. The disease is the second most commonly diagnosed among women, affects women of all ages, has a genetic basis, the full range of treatment modalities is used to the control the illness (surgery, radiation, chemotherapy, hormonal therapy, biologic/targeted therapies), the vast majority of women treated can expect to be long-term (>10 years) survivors, it involves a body part viewed as having special personal and social meaning, and there has been enormous advocacy for research to address this population. Heartening in the current analysis is that a growing band of other cancers is also being studied as this science matures.
Keeping an eye on the diversity of target populations being researched is important. In this regard, although all grants are required to document the race and ethnicity distribution of study participants, only about 14.0% of the applications reviewed were designed to address (had as a stated focus of the research as stated in the specific aims) the role or relationship of race/ethnicity or sexual orientation to the topic under study. The diversity of target populations included in future research will need to increase over time if we are to better mirror cancer prevalence in these communities and align care to address the potentially unique needs of each (20).
Despite calls for more research among older populations of survivors (21,22), this population does not figure prominently in FY2016 funded research. Only eight of the studies identified focused exclusively on an older sample (survivors aged 65 years or older). Although grants examining adult survivors rarely excluded older adults (unless the focus was specifically on adolescent and young adult [AYA] or young adult samples), this group was not generally oversampled, raising concern about the ability of investigators to run specific subset analyses on outcomes for these individuals. A clear emphasis on funding research looking at those age 70 years and older will be needed if we are to keep pace with the anticipated acceleration in growth of the older population of survivors that is occurring with the aging of the nation (10). Based on the present findings, a case can also be made for increasing research among AYA cancer survivors. Two of the currently funded cohort studies (Childhood Cancer Survivor Study and the St. Jude Life cohort) include adolescent samples. However, studies among young adult groups (6.7%) remain limited. The long post-treatment time horizon anticipated for AYA survivors makes timely identification of and interventions to address adverse sequelae of cancer important targets of study.
In a related vein, more studies will be needed that examine outcomes for the growing population of long-term (10 or more years post-treatment) cancer survivors. With earlier diagnosis, more effective treatments, and improved supportive care both during and after treatment, more survivors can expect to celebrate their 10- and even 20-year anniversary post-cancer. With only 16.4% of the current portfolio capturing outcomes beyond 5 years after diagnosis, we risk being ill-informed and unprepared to manage these individuals’ future care. Studies conducted among survivors of multiple cancers also deserve to be promoted, given the rising incidence of these. It is currently estimated that over 18% of those diagnosed with cancer today will already have a history of the disease (23).
The current analysis also points to a number of specific areas that warrant greater attention. Few studies (N = 11) identified sexual dysfunction as a focus of study, despite the prevalence of concern for this area of well-being among survivors (24). Similarly, financial burden, a growing focus of concern, could be actively targeted for study. The aging of the prevalent population, coupled with the recognition that pre-existing co-morbid conditions increase risk for worse survivorship outcomes, suggests that the investigator community should be encouraged to examine how to better manage these competing conditions across the course of care (25). Finally, given the importance of family caregivers to survivors’ outcomes and the reciprocal effects of response to illness on the well-being of dyads, a call for more research among family caregivers would help enhance our understanding about how to support these critical relationships, with an eye toward improving the health of both parties (26). Of note, this is one area where the funding trend appears to have gone in the wrong direction. In FY2006, 29 studies included a focus on the family, in contrast to only 15 that were identified in FY2016.
Limitations
Limitations to the present portfolio analysis are worth noting. The art of portfolio analysis is constantly evolving, and there were some discrepancies between coders about how to categorize the research examined. Although all grants were dual coded, it is possible that pairs of coders may have differed systematically from other coder pairs in their approach to joint decision making. Although the team did its best to identify all relevant grants, doing this in particular at the NIH level was a challenge, because grants are not typically sorted using the criteria applied here. It is possible that some grants may have been missed in this process. Limitations to the comparison with the earlier 2006 portfolio are as previously noted. Finally, the grants discussed above included only those that actually received funding. It is therefore unknown whether the science proposed but unfunded differed fundamentally from what is described here. It should also be noted here that the NIH is not the only funder of survivorship science (8,19). While the NIH and specifically the NCI are major sponsors of work in this space, the present analysis should not be considered as reflecting the total universe of currently funded survivorship research.
Conclusions
Despite some limitations, portfolio analyses provide a unique way to view the state of a given science being funded at the federal level at a specific point in time. The data presented here provide reassurance that survivorship science is alive and well at NCI in particular, and that, although their contribution is modest (11.5% of funded grants), other institutes across the NIH also have an investment in this research. Support for the training of future survivorship scientists and clinicians is also strong. Although there is a reasonable breadth of topics being covered, some gap areas revealed themselves, including the need to expand research on older and longer-term survivors along with their cancer caregivers and those from more richly diverse sociocultural and geographic backgrounds. The field would also benefit from studies designed to address previously neglected challenges post-treatment (eg, sexual dysfunction, financial toxicity, competing comorbid conditions). Because of the diversity of the science being pursued, a broad range of study sections— along with relevant expertise in survivorship research in each—is needed to consider the merits of the proposed research. This is, at times, a source of frustration for the scientific community. One benefit of regular portfolio analysis is to provide a look at what is ultimately supported and whether this matches well the actual challenges faced by today’s and tomorrow’s cancer survivors and the means to address those challenges.
As a final reflection, it is recognized that a portfolio analysis represents only one approach to or necessary component of monitoring the maturation and direction of a field. In addition to understanding what is funded, it is critical to know what impact—if any—findings from the research supported have on the care and outcomes of those treated. Typically, this information lags years beyond a given portfolio analysis because study results generally do not appear in print until following study completion and may appear well beyond termination of active study funding. Although outside the scope of the current project, a future bibliometric analysis of the publications, and their influence on practice, stemming from the grants included here would be a valuable contribution to the field.
Notes
Affiliations of authors: Smith Center for Healing and the Arts, Washington, DC (JHR); Epidemiology and Genomics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD (LG); Healthcare Delivery Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD (MM); Office of the Director, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD (NS, ALF); Behavioral Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, MD (GT).
JR, LG, MM, NS, AF, and GT have no conflicts of interest.
This article was prepared as part of the authors’ official duties as employees of the US Federal Government. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute.
Supplementary Material
References
- 1.National Cancer Institute, Office of Cancer Survivorship website. Definitions. https://cancercontrol.cancer.gov/ocs/statistics/definitions.html. Accessed November 29, 2018.
- 2. Institute of Medicine and National Research Council. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 3. Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 4.National Institutes of Health. Living Beyond Cancer: Finding a New Balance President’s Cancer Panel report, 2003-2004. Bethesda, MD: Department of Health and Human Services, 2004.
- 5.National Institutes of Health. Assessing Progress, Advancing Change President’s Cancer Panel report, 2005-2006 Bethesda, MD: Department of Health and Human Services, 2006.
- 6.Centers for Disease Control and Prevention. A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies Atlanta, GA: US Department of Health and Human Services; 2004.
- 7. Nekhlyudov L, Ganz PA, Arora NK, Rowland JH.. Going beyond being lost in transition: a decade of progress in cancer survivorship. J Clin Oncol. 2017;3518:1978–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrop JP, Dean JA, Paskett ED.. Cancer survivorship research: a review of the literature and summary of current NCI-designated cancer center projects. Cancer Epidemiol Biomarkers Prev. 2011;2010:2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rowland JH, Kent EE, Forsythe LP, et al. Cancer survivorship research in Europe and the United States: where have we been, where are we going, and what can we learn from each other? Cancer. 2013;119(S11):2094–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bluethmann SM, Mariotto AB, Rowland JH.. Anticipating the “Silver Tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;257:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;244:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;664:271–289. [DOI] [PubMed] [Google Scholar]
- 13. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;1099:djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;224:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH.. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;2010:1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perna FM, Dwyer LA, Tesauro G, et al. Research on skin cancer–related behaviors and outcomes in the NIH Grant Portfolio, 2000-2014: skin cancer intervention across the Cancer Control Continuum (SCI-3C). JAMA Dermatol. 2017;1535:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kobrin S, Ferrer R, Meissner H, et al. Use of health behavior theory in funded grant proposals: cancer screening interventions as a case study. Ann Behav Med. 2015;496:809–818. [DOI] [PubMed] [Google Scholar]
- 18. Alfano CM, Bluethmann SM, Tesauro G, et al. NCI funding trends and priorities in physical activity and energy balance research among cancer survivors. J Natl Cancer Inst. 2016;1081:djv285. [DOI] [PubMed] [Google Scholar]
- 19. Jacobsen PB, Rowland JH, Paskett ED, et al. Identification of Key Gaps in Cancer Survivorship Research: Findings from the American Society of Clinical Oncology Survey. J Oncol Pract 2016;123:190–203. [DOI] [PubMed] [Google Scholar]
- 20. Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA.. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;2717:2758–2765. [DOI] [PubMed] [Google Scholar]
- 21. Sedrak MS, Hurria A.. Cancer in the older adult: implications for therapy and future research. Cancer. 2018;1246:1108–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levit LA, Balogh E, Nass SJ, Ganz P.. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 23. Murphy CC, Gerber DE, Pruitt SL.. Prevalence of prior cancer among persons newly diagnosed with cancer: an initial report from the Surveillance, Epidemiology, and End Results Program. JAMA Oncol. 2018;46:832–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schover LR, van der Kaaij M, van Dorst E, Creutzberg C, Huyghe E, Kiserud CE.. Sexual dysfunction and infertility as late effects of cancer treatment. EJC Suppl. 2014;121:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams GR, Mackenzie A, Magnuson A, et al. Comorbidity in older adults with cancer. J Geriatr Oncol. 2016;74:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kent EE, Rowland JH, Northouse L, et al. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;12213:1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.