Abstract
The cell maintains a balance between the production and removal of reactive oxygen species (ROS). Changes in ROS levels can impact many cellular functions, and dysregulation contributes to pathologies. How a specific cellular environment or microdomain influences the ROS-generating systems and biological impact of ROS remains an active area of research. This Forum highlights the complexity of ROS microdomains and their contributions to health and disease. Novel technologies to measure or generate ROS in defined regions are important developments in the spatial control of ROS. Using these advances, the articles herein demonstrate how site-specific redox environments influence cellular function and pathology. Antioxid. Redox Signal. 31, 591–593.
Keywords: reactive oxygen species, optogenetics, ischemia reperfusion injury, mitochondria, hydrogen peroxide gradients
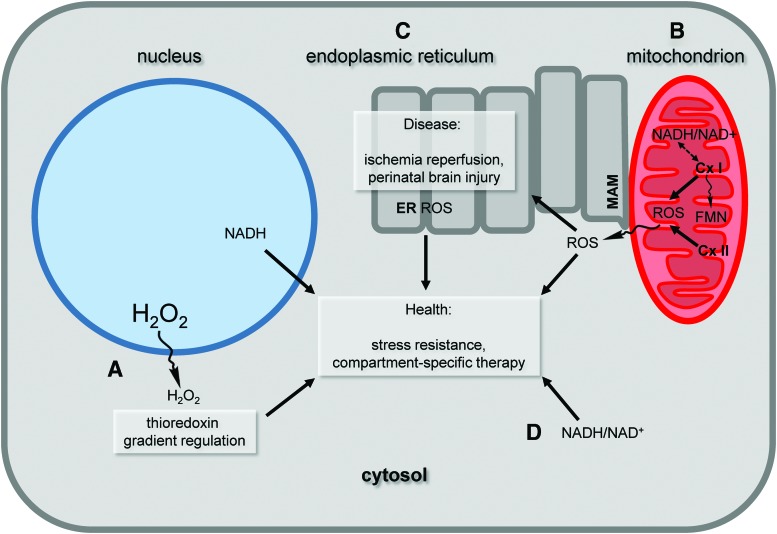
The redox state of the cell is highly regulated, and the dysregulation of reactive oxygen species (ROS) production or ROS removal contributes to disease. However, using antioxidants to treat diseases has been largely unsuccessful and suggests that the role of ROS in the cell is complex. Moreover, ROS have a beneficial role in the cell and can participate in signaling pathways. Determining how ROS contribute to diverse cellular outputs is an active area of research, and this Forum highlights the role of compartmentalization in redox-mediated events (Fig. 1).
FIG. 1.
Compartmentalization of cellular ROS affects health and disease. The cell maintains a balance of ROS production and removal that is regulated. (A) ROS gradients in cells can be regulated by specific antioxidant systems. In addition to removing ROS for spatial regulation, site-specific ROS production also leads to different outputs. For example, microdomains within one organelle type, such as (B) mitochondria or (C) ER, can have different effects that impact cellular health and function. (D) Overall redox status governed by the redox couple NAD+/NADH can affect cellular compartments differently. This Forum demonstrates how compartmentalized cellular events can impact important functional outputs that are relevant to human health. Cx I, mitochondrial complex I; Cx II, mitochondrial complex II; ER, endoplasmic reticulum; FMN, flavin mononucleotide; MAM, mitochondrial associated ER membrane; H2O2, hydrogen peroxide; ROS, reactive oxygen species. Color images are available online.
The concepts of microdomains and compartmentalization are recognized for second messengers such as calcium (1). Classical redox signaling approaches rely on global application of oxidants or antioxidants; however, in vivo signaling is more complicated and compartmentalized. As in the field of calcium signaling, better understanding of the spatial and temporal organization of ROS production and removal in cells will advance the field of redox signaling. More recently, strategies to target and understand ROS microdomains have been developed and tested (2). Learning where, when, and how ROS are made and metabolized will reveal new avenues of investigation for the diverse pathologies associated with oxidative stress. Progress in these areas will result in a more targeted approach to compartment-specific treatment of ROS dysfunction in disease.
Cells have enzymatic antioxidant systems to remove ROS, such as thioredoxins and glutathione (5). These systems are present at different levels throughout the cell. Mishina et al. investigated how ROS removal systems regulate hydrogen peroxide (H2O2) gradients. Their chemogenic approach targeted d-amino acid oxidase to the nucleus to generate H2O2 upon the addition of d-alanine (4). The production of H2O2 was visualized using the yellow fluorescent protein-based biosensor, HyPer-3. They found that forming H2O2 gradients are determined primarily by the thioredoxin system, such that thioredoxin prevented the diffusion of H2O2 throughout the cytoplasm. Interestingly, experiments showed that catalase and glutathione did not play a role in compartmentalizing the H2O2 in the nucleus. This approach probes redox gradients in vivo, and the results provide evidence for preferential roles of the diverse antioxidant systems that exist in cells. Future directions can apply this method to investigate the antioxidant systems in other intracellular H2O2 gradients.
In contrast to compartmentalized ROS removal, site-specific ROS production can alter redox microenvironments and lead to either damage or protective signaling. Genetically encoded photosensitizers are optogenetic tools that generate oxidants such as superoxide and singlet oxygen in response to light exposure. One genetically encoded photosensitizer is SuperNova, which can be used to generate ROS in a site-specific manner (9). There are many sites of ROS production within a cell, one of which is the mitochondrion. However, how each mitochondrial ROS production site contributes to signaling or oxidative damage is unclear. Trewin et al. targeted SuperNova to the mitochondrial matrix or the intermembrane space. Using this optogenetic approach, they demonstrated that distinct ROS microdomains in mitochondria lead to differential cell signaling responses (8). They found that matrix-generated superoxide resulted in activation of ROS signaling cascades. In addition, they showed that matrix superoxide production results in protective signaling in a model of ischemia/reperfusion (I/R) injury. These results suggest that matrix superoxide production may be more important for stress resistance than intermembrane space ROS production.
Mitochondrial complex I of the electron transport chain both contributes to ROS and is a target of oxidative damage during I/R injury. Stepanova et al. used a model of brain I/R injury and proposed a novel cause of mitochondrial energy failure centered on complex I inhibition due to the dissociation of the enzyme cofactor flavin (7). The authors observed a loss of the complex I flavin mononucleotide (FMN) in parallel with loss of complex I activity after I/R injury. The administration of FMN precursor riboflavin in vivo or exogenous FMN in vitro prevented the flavin loss, preserving complex I activity. Interestingly, in vitro data showed that released FMN is not compartmentalized only within mitochondrial matrix and potentially can act as a pro-oxidant in cytoplasm. These results suggest a mechanism of energetic failure in ischemia reperfusion, linking impairment of mitochondrial function and oxidant-mediated damage.
In addition to mitochondria, endoplasmic reticulum (ER)-mediated oxidative stress also contributes to brain I/R injury. Singh-Mallah et al. reviewed the role of mitochondrial and ER ROS sources of perinatal brain I/R injury (6). The authors highlight that findings in older tissue cannot be translated directly to neonates. They further discussed how compartment-specific inhibitors of ROS production are being developed, and how combinatorial therapy to alleviate damage from both mitochondrial and ER stress could be a therapeutic way forward. This Forum serves as an introduction to oxidative brain injury, and provides useful insight to bridging mechanistic discovery to more targeted therapies to maintain health.
Shifting to redox landscape across cellular domains, Kulkarni and Brookes reviewed the redox cofactor nicotinamide adenine dinucleotide (NADH/NAD+ couple), and discussed how its diverse effects are impacted by compartmentalization in cytosol, mitochondria, and nuclei (3). In addition to energy production-related redox conversion, NAD+ and the reduced form, NADH, play a role in many different cellular functions ranging from metabolic transformation to protein post-translational modifications. For example, NADH regulation of lysine deacylases called sirtuins (SIRTs) is discussed. In addition, compartmentalized SIRT signaling and the implications for diseases such as aging and neurodegeneration are discussed. Kulkarni and Brookes critically reviewed how the compartmentalization of NADH can influence these and other processes and contribute to the redox landscape. This Forum is a helpful resource for understanding NADH biology and the complexities of associated cellular systems.
From underlying biology to specific pathologies, these articles demonstrate the importance of precise mechanistic studies and their application to models that foster translation to treat human disease. Importantly, the compartment in which these redox events occur can impact the cellular outcomes. Key advances in both measuring and producing ROS in a site-specific manner have opened new scientific opportunities and will advance the study of compartmentalized ROS. These approaches and ideas will encourage mechanistic research and careful translation to relevant disease models to drive progress in understanding how redox signaling impacts our health.
Acknowledgments
Work in the laboratory of A.P.W. is supported by a grant from National Institutes of Health (R01 NS092558). B.J.B. is supported by an American Heart Association Predoctoral Fellowship (18PRE33990054). Work in the laboratory of A.G. is supported by a grant from the National Institutes of Health (R01 NS100850).
Abbreviations Used
- ER
endoplasmic reticulum
- FMN
flavin mononucleotide
- H2O2
hydrogen peroxide
- I/R
ischemia/reperfusion
- NAD+/NADH
nicotinamide adenine dinucleotide
- ROS
reactive oxygen species
- SIRT
sirtuin
References
- 1. Berridge MJ. Calcium microdomains: organization and function. Cell Calcium 40: 405–412, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Jones DP. and Sies H. The redox code. Antioxid Redox Signal 23: 734–746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kulkarni CA. and Brookes PS. Cellular compartmentation and the redox/nonredox functions of NAD+. Antioxid Redox Signal 2019. [Epub ahead of print]; DOI: 10.1089/ars.2018.7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mishina NM, Bogdanova YA, Ermakova YG, Panova AS, Kotova DA, Bilan DS, Steinhorn B, Arner ESJ, Michel T, and Belousov VV. Which antioxidant system shapes intracellular H2O2 gradients? Antioxid Redox Signal 2019. [Epub ahead of print]; DOI: 10.1089/ars.2018.7697 [DOI] [PMC free article] [PubMed]
- 5. Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal 16: 476–495, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Singh-Mallah G, Nair S, Sandberg M, Mallard C, and Hagberg H. The role of mitochondrial and endoplasmic reticulum ROS production in models of perinatal brain injury. Antioxid Redox Signal 2019. [Epub ahead of print]; DOI: 10.1089/ars.2019.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stepanova A, Sosunov S, Niatsetskaya Z, Konrad C, Starkov AA, Manfredi G, Wittig I, Ten V, and Galkin A. Redox-dependent loss of flavin by mitochondrial complex I in brain ischemia/reperfusion injury. Antioxid Redox Signal 2019. [Epub ahead of print]; DOI: 10.1089/ars.2018.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trewin AJ, Bahr LL, Almast A, Berry BJ, Wei AY, Foster TH, and Wojtovich AP. Mitochondrial reactive oxygen species generated at the complex-II matrix or intermembrane space microdomain have distinct effects on redox signaling and stress sensitivity in Caenorhabditis elegans. Antioxid Redox Signal 2019. [Epub ahead of print]; DOI: 10.1089/ars.2018.7681 [DOI] [PMC free article] [PubMed]
- 9. Trewin AJ, Berry BJ, Wei AY, Bahr LL, Foster TH, and Wojtovich AP. Light-induced oxidant production by fluorescent proteins. Free Radic Biol Med 128: 157–164, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]