Abstract
Aims: How mitochondrial reactive oxygen species (ROS) impact physiological function may depend on the quantity of ROS generated or removed, and the subcellular microdomain in which this occurs. However, pharmacological tools currently available to alter ROS production in vivo lack precise spatial and temporal control.
Results: We used CRISPR/Cas9 to fuse the light-sensitive ROS-generating protein, SuperNova to the C-terminus of mitochondrial complex II succinate dehydrogenase subunits B (SDHB-1::SuperNova) and C (SDHC-1::SuperNova) in Caenorhabditis elegans to localize SuperNova to the matrix-side of the inner mitochondrial membrane, and to the intermembrane space (IMS), respectively. The presence of the SuperNova protein did not impact complex II activity, mitochondrial respiration, or C. elegans development rate under dark conditions. ROS production by SuperNova protein in vitro in the form of superoxide (O2˙−) was both specific and proportional to total light irradiance in the 540–590 nm spectra, and was unaffected by varying the buffer pH to resemble the mitochondrial matrix or IMS environments. We then determined using SuperNova whether stoichiometric ROS generation in the mitochondrial matrix or IMS had distinct effects on redox signaling in vivo. Phosphorylation of PMK-1 (a p38 MAPK homolog) and transcriptional activity of SKN-1 (an Nrf2 homolog) were each dependent on both the site and duration of ROS production, with matrix-generated ROS having more prominent effects. Furthermore, matrix- but not IMS-generated ROS attenuated susceptibility to simulated ischemia reperfusion injury in C. elegans.
Innovation and Conclusion: Overall, these data demonstrate that the physiological output of ROS depends on the microdomain in which it is produced. Antioxid. Redox Signal. 31, 594–607.
Keywords: reactive oxygen species, mitohormesis, optogenetics, superoxide, SuperNova, photosensitizer, ischemia reperfusion injury
Introduction
Mitochondria are subcellular organelles responsible for vital cellular processes, including the synthesis of ATP, and are an important source of reactive oxygen species (ROS) generation. The primary form of ROS, the superoxide anion (O2˙−), has been shown to be generated from at least 11 sites associated with the mitochondrial electron transport chain (ETC), with differing contributions from each site depending on the metabolic conditions and/or pathological state (28, 30, 56). Since these sites of O2˙− generation are located at either side of the inner mitochondrial membrane, distinct levels of O2˙− and their subsequent radical species may be expected to occur in the matrix compartment relative to the intermembrane space (IMS) under different conditions. Moreover, different landscapes of ROS removal systems exist in each compartment. For example, there are multiple isoforms of the superoxide dismutase (SOD) enzyme that are differentially distributed throughout the cytosol and mitochondrial compartments, which remove O2˙− by dismutation into the membrane-permeable hydrogen peroxide (H2O2). Notably, the IMS contains an abundance of cytochrome c, which has a high affinity for O2˙−, but this scavenging mechanism does not result in the formation of H2O2 (35). This is notable because moderate levels of localized H2O2 accumulation (i.e., oxidative eustress) in specific microdomains can initiate redox signaling cascades through mechanisms such as thiol oxidation (5). These redox signals can lead to hormesis via stress signaling responses, yet excessive or prolonged O2˙−/H2O2 accumulation may lead to cellular damage via irreversible oxidative modifications (i.e., oxidative distress) (44). Therefore, the precise location of O2˙−/H2O2 generation at a submitochondrial microdomain may be a key factor in eliciting a subsequent physiological effect, as per the third principle of the redox code (22). To date, however, our understanding of the effects of localized ROS generation in defined subcellular compartments (i.e., microdomains) is limited, since precise experimental control over O2˙−/H2O2 generation at specific microdomains in vivo has remained a significant technical challenge.
Innovation
Mitochondrial redox biology is a rapidly expanding field, but suffers from lack of precise spatial and temporal experimental control, especially in physiological contexts. Optogenetics offers a platform to overcome these barriers, and using this approach we demonstrate differing physiological responses to equal amounts of reactive oxygen species (ROS) generated on either side of the inner mitochondrial membrane at the mitochondrial complex-II microdomain. These findings show that spatial and temporal discrimination can be achieved in vivo, and this discrimination may reveal novel mechanisms of mitochondrial redox physiology.
Optogenetics involves the use of light to activate genetically encoded photosensitive proteins to modify cellular function or elicit a signaling response. Optogenetics has typically been used to alter neuronal function via light-sensitive ion channels, but more recently, photosensitive ROS-generating proteins have been developed [reviewed elsewhere; (49, 52)]. One such example is SuperNova, a monomeric ∼29 kDa fluorescent protein capable of generating oxidants when the chromophore absorbs ∼580 nm photons through a process called photosensitization (46). SuperNova is thought to primarily generate O2˙−, although its precise ROS-generating capabilities are uncharacterized (49). A key feature of photosensitive proteins such as SuperNova, is that they can be targeted to specific microdomains in vivo by expressing in fusion with an endogenous protein, such as the nuclear-encoded mitochondrial ETC subunits (55).
Mitochondrial complex-II (succinate dehydrogenase [SDH]) consists of four nuclear-encoded subunits: A, B, C, and D. Subunits A and B comprise the catalytic core (flavin binding and iron–sulfur clusters, respectively) located in the mitochondrial matrix, while C and D are integral membrane proteins that span the inner membrane, thus being in contact with the IMS. Complex-II has been shown to generate ROS under both physiological and pathological conditions (39), and is also involved in oxygen sensing (54). Complex-II is a central player in the succinate-driven reverse electron transfer (RET) toward complex-I under conditions of a highly reduced ubiquinone (Q) pool, which leads to bulk O2˙− generation that is a major contributor to the etiology of ischemia reperfusion injury (8, 26). Collectively, due to its physiological relevance and its all-nuclear-encoded subunits, mitochondrial complex-II/SDH is germane to investigating the intricate effects of ROS in vivo. To achieve this, an ideal model organism for optogenetic studies is the nematode Caenorhabditis elegans, due to its transparent tissues that allow for light transmission to photosensitive proteins, amenability to genetic editing, and well-conserved mitochondrial stress responses.
Here, we demonstrate that SuperNova generates O2˙− in response to light using high-performance liquid chromatography (HPLC) separation of dihydroethidium (DHE)-oxidation products. We then use clustered regularly interspaced short palindromic repeats/Cas9 endonuclease (CRISPR/Cas9) to insert the SuperNova coding sequence at the C-termini of the B and C subunits of mitochondrial complex-II (Cx-II) of C. elegans. This novel model not only provides unique insight into complex II ROS but also into stoichiometric amounts of ROS generated from different mitochondrial compartments. We aimed to determine whether specific ROS microdomains within mitochondria elicit distinct redox signals and protection against simulated ischemia reperfusion injury in the model organism C. elegans.
Results
Purified SuperNova generates O2˙− in a light-dependent manner in vitro
We first cloned and purified SuperNova protein to assess its photosensitization properties. SuperNova migrated via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at the expected molecular weight of ∼29 kDa, and immunoblotting revealed specificity with a commercially available antibody against KillerRed, the dimeric version of SuperNova (Supplementary Fig. S1A). Purified SuperNova protein had an extinction coefficient of ɛ = 31,238 M−1·cm−1 and exhibited fluorescence excitation/emission peaks at 584/610 nm, respectively (Supplementary Fig. S1B), as expected (46). We then evaluated the ability of SuperNova to generate O2˙− in a light-dependent manner as depicted in Figure 1A. To detect O2˙−, we used DHE, a probe that yields fluorescent reaction products in the presence of oxidants. The O2˙− selective fluorescent product is 2-hydroxyethidium (2-OH-E+), which is separated from the nonselective oxidation product ethidium (E+) using HPLC (59, 60). We found that the formation of 2-OH-E+ by SuperNova was light dependent and specific to O2˙−, since this was attenuated in the presence of SOD enzyme (Fig. 1B). Formation of 2-OH-E+ by SuperNova was also proportional to total light irradiance in the 540–600 nm spectra (Fig. 1C). Since pH is known to influence fluorescent protein function (25) and because the mitochondrial matrix and IMS compartments differ by ∼1 pH unit (37), we measured 2-OH-E+ generation by SuperNova under buffer pH conditions resembling that of the mitochondrial matrix (pH 7.8), IMS (pH 6.8), or the cytosolic (pH 7.4) environments (36). Notably, we found no impact of buffer pH on 2-OH-E+ formation by SuperNova protein in response to light (Fig. 1D). Together, these data demonstrate that SuperNova protein generates O2˙− in a light-dependent manner regardless of the different physiological pH levels tested, in vitro.
FIG. 1.
Purified SuperNova protein generates superoxide in response to light, in vitro. (A) Schematic depicting ∼580 nm light-dependent superoxide (O2˙−) generation by purified SuperNova protein in vitro that is detected with the probe DHE, with HPLC used to separate and measure the superoxide-specific reaction product, 2-OH-E+ that can be prevented by SOD. (B) SuperNova (0.25 mg/mL) induces 2-OH-E+ formation from DHE in response to light (5 min ∼580 nm irradiation, 6.7 mW/mm2), is attenuated in the presence of SOD, occurs in a (C) time-dependent manner (y = 448x + 636; r2 = 0.999; p < 0.001); and is (D) unaffected by buffer pH levels corresponding to either the mitochondrial IMS (pH ∼6.8), cytosol (pH ∼7.3), or mitochondrial matrix (pH ∼7.8), as depicted by wedge shape from left to right. Values are expressed as mean ± SEM for n = 3 independent experiments; *p < 0.05 versus all others; n.s., not significant. 2-OH-E+, 2-hydroxyethidium; DHE, dihydroethidium; HPLC, high-performance liquid chromatography; IMS, intermembrane space; SOD, superoxide dismutase. Color images are available online.
Complex-II—SuperNova fusion proteins are expressed with correct localization and topology in C. elegans mitochondria
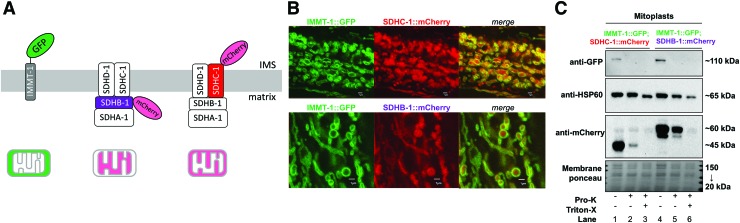
Next, we used CRISPR/Cas9 to generate fusion proteins with endogenous nuclear-encoded mitochondrial complex-II subunits succinate dehydrogenase subunit B (SDHB) and subunit C (SDHC) in C. elegans. At the C-terminus of each of these, we inserted the SuperNova construct (SDHB-1::SuperNova and SDHC-1::SuperNova). We also generated separate strains containing the red fluorescent protein mCherry (SDHB-1::mCherry and SDHC-1::mCherry) in place of SuperNova as an additional approach to confirm appropriate expression. Next, we generated a strain expressing green fluorescent protein (GFP) fused to mitofilin (IMMT, inner mitochondrial membrane protein 1, or C. elegans ortholog of mitofilin) (IMMT-1::GFP), a 76 kDa protein that is part of the mitochondrial contact site and cristae organizing system complex, which tethers the outer/inner mitochondrial membrane, but does not localize to the interior cristae regions (21). We confirmed with confocal microscopy that the IMMT-1::GFP signal colocalized with MitoTracker Red dye in live anesthetized C. elegans (Supplementary Fig. S2A). Next, to confirm the anticipated mitochondrial expression pattern of the complex-II fusion constructs depicted in Figure 2A, we crossed the IMMT-1::GFP strain with each of the other (mCherry and SuperNova) fluorescent protein strains. We observed the expected mitochondrial expression pattern across all tissues with colocalization between IMMT-1::GFP and each of SuperNova and mCherry fusion proteins for both the SDHB-1 and SDHC-1 constructs (Fig. 2B and Supplementary Fig. S2A).
FIG. 2.
Compartmentalized localization of mitochondrial complex-II fusion proteins in Caenorhabditis elegans. (A) Predicted fusion protein construct mitochondrial complex-II localization, demonstrated via (B) confocal microscopy of C. elegans expressing mitofilin GFP fusion protein (IMMT-1::GFP) to indicate the IMS with either of SDHB-1::mCherry or SDHC-1::mCherry (scale bars = 1 μm). (C) pro-K-protection assay on isolated mitochondrial mitoplasts demonstrates correct inner mitochondrial membrane-sidedness, with the matrix facing SDHB-1::mCherry construct protected from degradation by proteinase-K (lane 5) similar to the matrix marker protein (HSP60), relative to the IMS-facing SDHC-1::mCherry (lane 2), which shows the same susceptibility to pro-K as the IMS marker (IMMT-1::GFP). Triton-X dissolves inner membranes to allow access of pro-K to the matrix compartment. Images are representative of n = 3 independent experiments; uncropped images are shown in Supplementary Figure S2C. GFP, green fluorescent protein; IMMT, inner mitochondrial membrane protein 1 (C. elegans ortholog of mitofilin); pro-K, proteinase-K; SDHB, succinate dehydrogenase subunit B; SHDC, succinate dehydrogenase subunit C. Color images are available online.
To confirm the inner mitochondrial membrane topology (i.e., sidedness) of the fusion protein construct, we made mitoplasts and performed a protease-protection assay using the serine protease, proteinase-K (pro-K). Mitoplasts are isolated mitochondria whose outer membrane is ruptured, leaving the intact inner membrane exposed. IMMT-1::GFP was used as a marker of the IMS, and was readily cleaved with exposure to pro-K as anticipated (Fig. 2C; lanes 2 and 5). Heat shock protein of 60 kDa (HSP60) was used as a marker of the mitochondrial matrix, which was protected from pro-K until the inner membrane was dissolved with detergent. Note that only partial digestion of the overall HSP60 protein pool is to be expected in the 10-min digestion time (indicated by a small downward shift in molecular weight and abundance in lanes 3 and 6), whereas the fusion proteins only require cleavage of the linking peptide sequence between the fluorescent protein and the endogenous protein to result in a dramatic decrease in molecular weight. Consistent with the predicted topology, the SDHC-1 fusion protein (mCherry) was susceptible to pro-K digestion, suggesting that it was exposed to the IMS (lane 2), whereas the SDHB-1 construct was protected from degradation (lane 5) until the inner membrane was dissolved (lane 6), indicating its location within the matrix compartment. Profile plot analyses of confocal images were also consistent with these pro-K findings, which demonstrate that both mCherry and SuperNova fused to both SDHB-1 and SDHC-1 subunits were correctly localized to the mitochondrial matrix and IMS compartments, respectively (Supplementary Fig. S2A, B). Finally, we also confirmed by immunoblot that the overall protein expression levels of SDHB-1::SuperNova and SDHC-1::SuperNova were equal in whole-worm lysates (Supplementary Fig. S2D).
Photoactivated SuperNova at Cx-II does not impair basal complex-II function or cause oxidative stress in C. elegans
To test whether the physical presence of the SuperNova fusion protein alone impacted mitochondrial bioenergetics, we isolated mitochondria and assessed succinate/rotenone-supported state-2 and adenosine diphosphate (ADP)-stimulated state-3 oxygen consumption rates under dark conditions. There were no intrinsic defects to succinate-linked respiratory function due to the presence of the fusion proteins at complex-II compared with N2 wild type (Fig. 3A). Consistent with the lack of impact on basal mitochondrial bioenergetics, the presence of the fusion proteins also had no effect on whole organism development rate compared with C. elegans wild-type strain (N2) wild-type worms grown under dark conditions (Fig. 3B). We then sought to determine whether SuperNova affects complex-II enzyme activity after photoactivation. There were no changes in succinate-supported, malonate-sensitive complex-II enzyme activity of mitochondria isolated from SDHB-1::SuperNova or SDHC-1::SuperNova animals after photoactivation compared with dark-control conditions (Fig. 3C). Thus, altered mitochondrial bioenergetics can be excluded as a possibility for any downstream physiological effects of SuperNova photoactivation. We then asked whether photoactivated SuperNova-induced O2˙− leads to oxidative distress in whole worms. For this, we used Western blotting to probe for protein carbonylation, an irreversible oxidative posttranslational protein modification (12). We found no change in protein carbonyl levels as a result of 1 h light exposure relative to dark-controls for either of the SuperNova strains or N2 worms (Fig. 3D). In addition, there was no change in reduced/oxidized glutathione ratio (GSH:GSSG) after 1 h of light exposure (Supplementary Fig. S3D).
FIG. 3.
Complex-II SuperNova fusion constructs do not impair mitochondrial function under dark or light conditions or cause cellular oxidative stress in C. elegans. (A) The presence of either complex-II SuperNova fusion protein does not impact isolated mitochondria respiratory flux normalized to a marker of mitochondrial content (citrate synthase enzyme activity) relative to N2 (wild-type) under dark conditions. Succinate+rotenone (S/Rot)-supported state-2 leak state respiratory flux was followed by addition of ADP (0.2 mM) to stimulate state-3 oxidative phosphorylation, which was inhibited by malonate (Malo); n = 3 individual mitochondrial isolation experiments. (B) Body length of individual C. elegans measured at intervals from eggs through to adulthood to estimate animal development rate is unaffected by the presence of either SuperNova fusion construct under dark conditions; n = 6 worms/strain. (C) Succinate-linked, malonate-sensitive complex-II enzyme activity normalized to citrate synthase enzyme activity is unaffected by light-activated SuperNova (10 min ∼580 nm, 1 mW/mm2); n = 4 independent mitochondrial preparations analyzed in duplicate. (D) Protein carbonylation, indicating cellular oxidative stress, was unchanged by SuperNova immediately after whole worms were exposed to 1 h ∼580 nm light (+, 1 mW/mm2) or kept in the dark (-). Bars represent mean ± SEM, with individual responses overlaid for n = 3 independent experiments; uncropped images are shown in Supplementary Figure S3C. No statistically significant differences observed. ADP, adenosine diphosphate; N2, C. elegans wild-type strain. Color images are available online.
Taken together, these data suggest that SuperNova may generate ROS at rates that do not lead to oxidative distress. Instead, single-copy expression of SuperNova may permit low levels of compartmentalized ROS generation localized to either side of the inner mitochondrial membrane in response to light—characteristics that may be suitable for redox signaling.
Photoactivated SuperNova O2˙− generation at Cx-II induces redox signaling
Having established that photoactivated SuperNova expressed at single-copy levels does not cause oxidative distress, we sought to investigate whether this induces redox signaling in vivo and whether these responses are distinct when O2˙− is generated stoichiometrically at either side of the inner mitochondrial membrane. To this end, we focused our attention on SKN-1, the C. elegans functional ortholog of mammalian Nrf2 encoded by NFE2L2, a transcription factor responsible for orchestrating the cellular antioxidant response (1). Upon activation, SKN-1 can be released from WD40 repeat protein, DDB1- and CUL4- associated factor 11 homolog (WDR-23), a cytosolic-localized protein, allowing it to translocate to the nucleus and bind to and promote transcription from regions of DNA known as antioxidant response elements common to the promotor regions of phase II detoxification genes (6). To assess this, we used a transgenic reporter strain expressing the glutathione S-transferase-4 gene promoter sequence fused to GFP (gst-4p::GFP). We first confirmed that the expression of gst-4p::GFP increased in response to exposure to a chemical oxidant (paraquat [PQ]) in wild-type background reporter worms, and that this increase was completely prevented by RNA interference (RNAi) against skn-1, which demonstrates both the efficacy of the knockdown and the specificity of the response (Supplementary Fig. S4A, B). Then, in wild-type background reporter strain worms (lacking SuperNova), there was no change in gst-4p::GFP expression levels 24 h after exposure to 10 or 30 min of light (Fig. 4A, B). On the contrary, in worms also expressing SDHB-1::SuperNova, the level of gst-4p::GFP expression was significantly greater 24 h after exposure to 10 min of light (Fig. 4A, B). In the reporter strain worms crossed with SDHC-1::SuperNova, level of gst-4p::GFP expression was also greater 24 h after the light treatment, but this required a longer duration of exposure (30 min) to achieve an equivalent response (Fig. 4A, B). These SuperNova+light-dependent increases in gst-4p::GFP expression were absent in the presence of skn-1 RNAi (Supplementary Fig. S4C).
FIG. 4.
Spatial- and temporal-dependent redox signaling responses to ROS generated by complex-II SuperNova at either the IMS or matrix. (A) L4 stage C. elegans (CL2166) expressing the SKN-1 transcriptional GFP reporter (gst-4p::GFP) alone (wild-type background) or with either of the complex-II SuperNova fusion proteins were exposed to light (∼580 nm, 1 mW/mm2) for 0, 10, or 30 min to induce O2˙− generation, then imaged 24 h later as day-1 adults. (B) Quantification of mean GFP fluorescence per worm from 5 independent experiments, each consisting of 10–15 worms per group. *p < 0.05 strain effect; †p < 0.05 time effect; each point represents an individual worm, bars represent mean ± SD. gst-4p, glutathione S-transferase 4 promoter region; ROS, reactive oxygen species; SKN-1, skinhead-1 (C. elegans ortholog of Nrf2). Color images are available online.
Activation of Nrf2/SKN-1 is largely dependent upon its phosphorylation by PMK-1, the C. elegans ortholog of the mammalian p38 mitogen-activated protein kinase (p38 MAPK) (18). PMK-1 belongs to a highly conserved stress response pathway that transduces signals from stimuli (including oxidants) to promote cellular adaptations (10, 18). Thus, to determine the spatial- and temporal-dependent effects of ROS generation on the activation of this pathway, we measured PMK-1 phosphorylation status in whole worm lysates from N2 wild-type and SDHB-1/C-1::SuperNova-expressing worms immediately after 10, 30, or 60 min exposure to light. There was an overall trend for a time-dependent increase in PMK-1 phosphorylation with light exposure, and this was greater in SDHB-1::SuperNova worms compared with N2 wild-type worms at the 60 min time point (p < 0.05; Fig. 5 and Supplementary Fig S5).
FIG. 5.
PMK-1 phosphorylation in response to complex-II SuperNova-induced ROS generation. (A) Plates of day-1 adult wild-type (N2) C. elegans or those expressing Cx-II SuperNova fusion proteins were kept in the dark (“0”) or exposed to light (580 nm, 1 mW/mm2) for 60, 30, or 10 min (depicted by black wedge from left to right), then immediately snap frozen. Whole worm lysates were separated via SDS-PAGE. Representative immunoblot images are shown from one experiment for phosphorylated PMK-1, SuperNova (fused to complex-II subunits, detected with anti-KillerRed antibody), and mitochondrial ATP synthase F1 subunit alpha (ATP5A). (B) Quantification of phosphorylated PMK-1 expressed relative to ATP5A from n = 3 independent experiments. Values are expressed as mean ± SEM; *p < 0.05 for SDHB-1::SuperNova versus N2. PMK-1, mitogen-activated protein kinase (C. elegans ortholog of p38 MAPK); SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis. Color images are available online.
Taken together, these data indicate that O2˙−/H2O2 generated from the matrix compartment are particularly suited to promoting a SKN-1-mediated antioxidant transcriptional response that may occur in part via PMK-1 phosphorylation (18). The physiological implications of upregulating these stress pathways are the potential cellular adaptations that may provide greater resistance to a subsequent stressor.
Effects of matrix- or IMS-derived Cx-II O2˙− on simulated ischemia reperfusion injury in C. elegans
Organisms, including C. elegans, initiate stress signaling pathways to protect against injury. ROS plays a role in both the protection against and the pathology of ischemia reperfusion injury, which can be modeled in C. elegans using anoxia-reoxygenation (AR) (43). Protection against AR injury can be conferred by anoxic preconditioning, which consists of a short duration of anoxia before subsequent prolonged anoxia, and C. elegans can be preconditioned against AR injury via a number of conserved mechanisms (11). We first confirmed a robust protective effect of anoxic preconditioning against subsequent AR (Fig. 6A). Although the mechanisms underlying this phenomenon are complex and yet to be fully elucidated (53), one proposed mechanism involves upregulation of antioxidant capacity to better cope with the succinate-driven O2˙− burst during RET at reperfusion (7). To assess this, we first used worms with an SDHC-1 mutant allele known as mev-1 [succinate dehydrogenase cytochrome b560 subunit (C. elegans–methyl viologen sensitive mutant), or kn1) (20), which is known to constitutively generate elevated levels of ROS and also be sensitive to hyperoxygenation (19). We found that the mev-1 mutant was endogenously protected against AR injury (Fig. 6A). To begin to better understand whether this protective effect was due to altered ETC bioenergetics or oxidants per se, we tested whether protection against simulated AR injury was afforded by short-term exposure of wild-type N2 worms to PQ. PQ generates O2˙− via redox cycling at mitochondrial complex-I (9), although recent evidence demonstrates that cytochrome P450 oxidoreductase is also a site of PQ-mediated ROS production (41). We found that PQ treatment had a similar protective effect against simulated AR injury (Fig. 6A).
FIG. 6.
Matrix-localized mitochondrial ROS is protective against AR injury in C. elegans. (A) Survival rates of C. elegans 24 h after 18.5 h anoxia were higher with 4 h anoxia preconditioning performed 24 h before AR, or exposure to the mitochondrial O2˙− generating agent paraquat (PQ, 4 mM for 4 h). An SDHC-1 mutant strain [TK22, mev-1(kn1)] known to generate elevated levels of mitochondrial O2˙− was also endogenously protected from AR-induced death. Data are expressed as mean ± SEM for n = 4 independent experiments of ≥15 worms per plate performed in duplicate; *p < 0.05 versus N2 control. (B) Wild-type (N2) C. elegans or those expressing SuperNova protein in the mitochondrial matrix compartment (SDHB-1::SuperNova) or IMS (SDHC-1::SuperNova) were kept in the dark or pretreated with light for 60 min (∼580 nm, 1 mW/mm2), 24 h later exposed to 17 h of anoxia before being scored for survival 24 h after reoxygenation. Data are expressed as mean ± SEM for n = 8 plates of ≥15 worms per plate over n = 3 independent experiments. AR, anoxia reoxygenation. Color images are available online.
These findings suggest that mitochondrial ROS generation may be involved in protection against AR injury. However, the interpretation of the kn1 mutant experiment could be confounded by compensatory responses across the life span that may be expected from altered mitochondrial bioenergetics due to mutation of SDHC-1, which is a subunit in a key ETC enzyme. This greatly limits the ability to attribute the observed effects exclusively to ROS, per se. Therefore, using our optogenetic model, we sought to determine whether O2˙− acutely generated at complex-II microdomains by photoactivated SuperNova was protective against AR injury. Indeed, we observed that with 60 min of light pretreatment, a significantly greater fraction of SDHB-1::SuperNova worms were alive 24 h after the AR insult compared with the SDHC::SuperNova worms (Fig. 6B). Interestingly, there was no protective effect of O2˙− generated in the IMS against AR, as light-treated SDHC-1::SuperNova worm survival rates were not different from those of N2 wild-type worms (Fig. 6B). Collectively, these data suggest that mitochondria-derived oxidants generated from the matrix compartment may contribute to preconditioning against AR injury.
Discussion
Here, we report the novel findings, in that (i) SuperNova protein generates O2˙− in response to light; (ii) redox signaling but not oxidative distress occurs in response to O2˙− generated using optogenetics at the complex-II microdomain; (iii) this response is more prominent when generated in the mitochondrial matrix compartment relative to the IMS, despite equal opportunity for O2˙− generation in either compartment; and (iv) O2˙− generation in the mitochondrial matrix—but not the IMS—contributes to preconditioning against AR injury in C. elegans.
The primary oxygen-derived radical molecule, the O2˙− anion, is membrane impermeable due to its negative charge. However, it is rapidly dismutated by SOD enzymes at a rate constant of ∼109 M−1 s−1 (24), and the resulting H2O2 can diffuse across lipid membranes and act as a second messenger by interacting with redox-sensitive target molecules (5). In particular, oxidation of thiol groups by H2O2 can lead to conformational changes or post-translational modifications to cysteine and methionine residues contained within kinases, phosphatases, or transcription factors of key regulatory pathways, resulting in the initiation of signal transduction (27). While it could be argued that O2˙− could be made anywhere within the cell, be dismutated into the membrane-permeable H2O2 to then diffuse throughout the cell to exert its effects (34), there is a higher probability of interaction with a target in proximity to its formation (44). Moreover, numerous oxidant removal systems are present in the cell (i.e., glutathione, thioredoxin [TRX] systems), which prevent global levels of oxidants from reaching toxic levels. Therefore, the spatial aspect of ROS formation is an important determinant in the specific redox signal generated, as outlined in the redox code (22).
An interesting finding of this study is that the matrix-initiated ROS signaling was more robust, which appears counterintuitive since the IMS is physically closer to the cytosol where redox-sensitive targets such as the SKN-1 transcription factor are located. This may be due to the unique scavenging capacities of each compartment resulting from the amount and localization of different SOD isoforms. The Mn-SOD isoform was shown to be present in rat liver mitochondrial matrix at ∼3.6 × 10–6 M, and the Cu/Zn-SOD (copper/zinc SOD) in the IMS at a similar concentration (31). Yet, the IMS also contains a relatively high concentration of cytochrome c (∼0.7 mM), which is capable of scavenging O2˙− without yielding H2O2 (13). Moreover, it has been shown that IMS-localized CuZnSOD activity is redox dependent and may actually have lower rates of O2˙− dismutation activity (17). In C. elegans, SOD-2/-3 has been shown to localize to mitochondrial ETC supercomplexes (45), where SOD may modulate O2˙− levels generated by the ETC. In addition to scavenging of O2˙− by SODs, H2O2 is removed enzymatically by various peroxidases, including the glutathione peroxidases and peroxiredoxins/TRX systems, depending on the subcellular compartment (2, 29, 42). Peroxiredoxin, such as mitochondrial-localized PRDX-3 in C. elegans, has a low KM for H2O2, and readily undergoes reversible oxidation, allowing for localized H2O2 accumulation, thus promoting the propagation of a redox signal (40, 57). Together, the mitochondrial matrix appears to be a more favorable environment for O2˙− dismutation and localized H2O2 accumulation compared with the IMS for the purposes of redox signaling. Indeed, our data show that the complex-II matrix microenvironment can make a relevant ROS signal, which seems reasonable when considering that 8 of the 11 endogenous O2˙− generation sites in the mitochondrial ETC release O2˙− to the matrix but only three to the IMS compartment (56).
SKN-1 transcriptional activity is regulated by various signals that modulate its expression levels, degradation, and subcellular localization (3). PMK-1 phosphorylation leads to nuclear localization of SKN-1, and this has been shown to occur in response to oxidants (18). ROS-mediated PMK-1 phosphorylation may occur via NSY-1, the C. elegans ortholog of ASK1 (apoptosis signal-regulating kinase 1), a MAP kinase upstream of PMK-1. In mice, ASK1 is negatively regulated under basal conditions by being bound to TRX, a key H2O2 scavenger protein (48). When critical cysteine residues in TRX are oxidized by H2O2, ASK1 can be released allowing its autophosphorylation, leading to downstream phosphorylation of p38 MAPK (15). Phosphorylation of SKN-1 by PMK-1 may prevent it from binding to the WD40 repeat protein WDR-23 in the nucleus, which normally marks it for proteasomal degradation, allowing it to bind to DNA instead (6). Also, SKN-1 has been shown to be bound to PGAM-5 (phosphoglycerate mutase family member 5), an outer mitochondrial membrane scaffold protein (32) whose activity may conceivably impact SKN-1 responses to mitochondria-derived ROS. However, it was recently shown that PMK-1 is sufficient but not necessary for SKN-1 activation in response to oxidative stress (58). Therefore, precisely how PMK-1 phosphorylation is involved in mediating the responses observed in this study requires further investigation.
Previous studies have used KillerRed (from which monomeric SuperNova was derived) in different cell types and subcellular compartments at high expression levels for the purposes of generating a large burst of localized ROS to cause oxidative distress, neuronal ablation, or cell death (16, 50). Expression using plasmid-based systems can result in multicopy and mosaic expression, thereby preventing an equal comparison in different compartments. CRISPR-based expression systems can overcome mosaic and overexpression to allow for stoichiometric comparisons between microdomains. While optogenetics is widely used in the field of neurobiology, these approaches are starting to be applied to ROS and mitochondrial biology (47, 55). Herein, we present an optogenetic approach to generate subtle, physiologically relevant levels of ROS (as opposed to oxidative distress) to study complex redox signaling networks in a living model organism.
It was interesting that the photoactivated SuperNova expressing worms were not as robustly protected from AR as the complex-II mutant (kn1) or PQ-treated worms. We speculate that the small effect for altered resistance to AR injury in photoactivated SDHB-1 relative to SDHC-1::SuperNova worms may be due to the short duration of light treatment, when considered in comparison with the life-long compensatory responses that likely occurs in mutant strains. Similarly, the greater protective effects of PQ treatment may be due to its longer treatment duration (4 h) and because residual PQ likely continued to generate O2˙− in the animals even after they were moved to regular plates, highlighting the lack of temporal control over ROS generation using a pharmacological approach. Among the numerous potential molecular mechanisms that regulate sensitivity to oxygen deprivation in C. elegans (38), it is possible that the greater activation of PMK-1 that we observed in photoactivated SDHB-1::SuperNova worms also played a role in protecting against AR, since it was previously shown in C. elegans that the NSY-1-dual specificity motigen-activated protein kinase-1-PMK-1 pathway modulates sensitivity to anoxia (14). Future studies are required to better understand the precise mechanism(s) that underlie these protective responses.
In summary, this study provides novel in vivo evidence, in the absence of confounding bioenergetic factors, that temporally and spatially distinct patterns of redox signaling occur depending on the mitochondrial site that the O2˙− is generated. In particular, we observed that a lower dose of matrix-generated O2˙− relative to IMS-generated O2˙− is sufficient to elicit redox signaling and stress resistance responses. These data provide important information toward understanding fundamental aspects of mitochondrial biology. Given that these subcellular organelles are responsible for vital cellular processes and physiological function, our novel findings may inform future studies that ultimately lead to improved therapeutic strategies for diseases involving mitochondrial oxidative stress.
Materials and Methods
Protein cloning and purification
SuperNova was purified essentially as described by Takemoto et al. (46). In brief, SuperNova (courtesy of Dr. Takeharu Nagai, Osaka University, Japan; Addgene plasmid No. 53234) was transformed into JM109(DE3) XJ autolysis (Zymo T3031) and grown at 18°C with shaking (80 rpm) for ∼65 h without induction. At optical density (OD) 0.8, the culture was cooled to ∼15°C and induced with 400 μM isopropyl β-D-1-thiogalactopyranoside (IPTG) and shaken at 200 rpm for 16 h at room temperature. Cultures were centrifuged at 3200 g for 10 min, washed with phosphate-buffered saline (PBS), and flash frozen. Cell lysate was incubated with nickel-charged affinity resin (Ni-NTA) agarose beads (Qiagen) for 45 min at 4°C. Bead–protein conjugate was washed three times with PBS (pH 7.4), followed by two washes in TN Buffer (TN: 50 mM Tris, 300 mM NaCl, pH 7.4). The beads were then washed with TN-10 Buffer (TN-10: 50 mM Tris, 300 mM NaCl, 10 mM Imidizole, pH 7.4). SuperNova was eluted with TN-100 Buffer (TN-100: 50 mM Tris, 300 mM NaCl, 100 mM imidizole, pH 7.4), and desalted with a PD-10 column. Protein concentration was determined by the Bradford assay (Pierce). SuperNova was concentrated using Amicon 10 kDa filters (Millipore) and flash frozen for storage at −80°C.
Cas9 protein was expressed and purified as described by Paix et al. (33). In brief, BL21(DE3)lyseS cells (NEB) expressing pHO4d-Cas9 (gift from Michael Nonet; Addgene plasmid No. 67881) were incubated at 37°C with shaking (225 rpm) overnight. Then, 2.5 mL culture was added to 500 mL lysogeny broth, including 0.1% v/v glucose and ampicillin, and grown to OD 0.5 at 37°C. Culture was chilled on ice for 5 min, before 0.4 mM IPTG was added, and the culture grown at room temperature for 24 h with shaking (225 rpm). The culture was pelleted at 2700 g for 8 min, then resuspended in 6 mL/g wet weight Buffer A [20 mM Tris pH 8.0, 250 mM KCl, 20 mM imidazole, 10% glycerol, 1 mM Tris(2-carboxyethyl)phosphine (TCEP)]. Next, 1 mM PMSF, protease inhibitors (Roche), and 1 mg/mL lysozyme were added. Cells were then sonicated with a needle-tip sonicator at 20% duty cycle for 45 min on ice. Lysate was centrifuged at 16,000 g for 30 min, then protein was incubated with Ni-NTA agarose beads (Qiagen) for 45 min at 4°C. Bead–protein conjugate was then transferred to 5 mL columns (Qiagen) and rinsed with 100 mL Buffer B (20 mM Tris 8.0, 800 mM KCl, 20 mM imidazole, 10% glycerol, 1 mM TCEP). Cas9 was then eluted with Buffer C [20 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) pH 8.0, 500 mM KCl, 250 mM imidazole, 10% glycerol]. Protein concentration was determined by Bradford assay (Pierce). DNA was then removed as follows: 1 mL Q sepharose/mL eluate was loaded onto a 5 mL Qiagen column and rinsed with 5 mL 1 M KCl/mL sepharose, then equilibrated with 5 mL Buffer C/mL sepharose. Eluate was then run through sepharose, and flow through discarded. Protein was then eluted with 20 mL Buffer D (20 mM HEPES pH 7.5, 500 mM KCl, 20% glycerol). Buffer exchange was then performed via dialysis in cassettes (Slide-a-lyzer) in Buffer D at 4°C. Cas9 was concentrated using Amicon 100 kDa filters (Millipore) to 25 mg/mL and flash frozen for storage at −80°C.
Optogenetic photoactivation
Photoactivation was performed using a light-emitting diode (LED) system emitting 540–600 nm wavelength through a liquid light guide (GYX module, X-Cite LED1; Excelitas, Waltham MA). For in vitro experiments, irradiance was 6.7 mW/mm2, and for in vivo experiments irradiance was 1 mW/mm2 on a single 35-mm diameter nematode growth media (NGM) plate for the indicated duration. Irradiance was determined using a calibrated thermopile detector (818P-010-12; Newport Corporation, Irvine, CA) and optical power meter (1916-R, Newport Corporation).
In vitro detection of superoxide via HPLC
SuperNova protein was irradiated in poly(methyl methacrylate) plastic cuvettes in the presence of the ROS probe DHE (0.1 mM, No. D11347; Invitrogen) and 0.1 mM diethylenetriaminepentacetic acid (DTPA) in mitochondrial respiration buffer (MRB, refer to Mitochondrial respiration and isolated enzyme activity assay section). To these samples, an equal volume of 200 mM perchloric acid/methanol was added, centrifuged at 17,000 g, and the supernatant transferred to an equal volume of 1 M potassium phosphate K+PO4− at pH 2.6. Samples were then separated via HPLC (Shimadzu) on a Polar-RP column (150 × 2 mm; 4 μm; Phenomenex) with mobile-phase solvents A and B comprised of 10% and 60% acetonitrile, respectively (both with 0.1% trifluoroacetic acid) a flow rate of 0.1 mL/min. Solvent gradient was as follows (values for solvent B): 0–5 min, 40%; increase to 100% by 25 min, 25–30 min, 100%; decrease to 40% by 35 min; 35–40 min, 40%. Fluorescence detection was used to identify peaks at known retention times for DHE (Ex 358, Em 440 nm), 2-OH-E+ (Ex 490, Em 567 nm), and E+ (Ex 490, Em 596 nm) were then quantified (Lab Solutions) against a 2-OH-E+ standard curve.
Culture of C. elegans and strains
C. elegans were grown on plates containing NGM and OP50 bacterial food using established laboratory techniques (4). All strains used are shown in Table 1 and were provided by the C. elegans Genetics Center (CGC, University of Minnesota, Minneapolis, MN). All alleles generated in this study were backcrossed to N2 at least four times.
Table 1.
List of Caenorhabditis elegans Strains
| Strain | Genotype | Source |
|---|---|---|
| N2-Bristol | Wild-type | CGC |
| APW54 | sdhb-1(jbmSi12 [sdhb-1::SuperNova]) II | This study |
| APW149 | sdhc-1(jbmSi34 [sdhc-1::SuperNova]) III | This study |
| APW47 | sdhc-1(jbm1 [sdhc-1::mCherry]) III | This study |
| APW49 | sdhb-1(jbm4 [sdhb-1::mCherry]) II | This study |
| APW40 | immt-1(jbmSi7 [immt-1::link::GFP]) X | This study |
| APW75 | sdhb-1(jbmSi4 [sdhb-1::mCherry]) II; immt-1(jbmSi7 [immt-1::link::GFP]) X | This study |
| APW76 | sdhc-1(jbmSi1 [sdhc-1::mCherry]) III; immt-1(jbmSi7 [immt-1::link::GFP]) X | This study |
| APW224 | sdhb-1(jbm12 [sdhb-1::SuperNova]) II; immt-1(jbmSi7 [immt-1::link::GFP]) X | This study |
| APW225 | sdhc-1(jbm34 [sdhc-1::SuperNova]) III; immt-1(jbmSi7 [immt-1::link::GFP]) X | This study |
| CL2166 | dvIs19[pAF15(Pgst-4::GFP::NLS) | CGC |
| APW61 | sdhb-1(jbmSi12 [sdhb-1::SuperNova]) II; dvIs19 [(pAF15) gst-4p::GFP::NLS] | This study |
| APW150 | sdhc-1(jbmSi36 [sdhc-1::SuperNova]) III; dvIs19 [(pAF15) gst-4p::GFP::NLS] | This study |
| TK22 | mev-1(kn1)III | CGC |
All alleles generated in this study were backcrossed to N2 four times. Strains were provided by the CGC.
CGC, C. elegans Genetics Center.
C. elegans transgene construction with CRISPR/Cas9
Transgenic worms were generated via established C. elegans CRISPR/Cas9 gene editing protocols (33). In brief, repair template amplicons were designed with 35 nucleotide homology to either side of the cut site. All primers used for repair template amplicons, and target crRNA sequences are detailed in Tables 2 and 3, respectively. Repair templates were PCR amplified and purified using Qiagen MinElute kit. The injection mix was prepared consisting of (final concentrations) 25 mM KCl, 7.5 mM HEPES, 4 μg/μL tracrRNA, 0.8 μg/μL target crRNA, 0.8 μg/μL dpy-10 crRNA, 50 ng/μL dpy-10 ssODN, 2.5 μg/μL Cas9 enzyme, and nuclease-free water to a final volume of 10 μL. Cas9 enzyme was cloned and purified in-house as described in Materials and Methods section above. Injection mix was then centrifuged for 2 min at 17,000 g, and incubated at 37°C for 10 min before microinjection into the germline of ∼25 young adult worms per construct under a DIC microscope. After injection, worms were transferred to individual plates, then again on day 2. Progeny were screened for the dpy-10 dumpy/roller phenotype (or for fluorescence), then at least three independent homozygous lines were selected for subsequent genotyping to confirm that the coding region of SuperNova or mCherry was correctly inserted before the translational stop codon/3′ UTR of mev-1 (ortholog of mammalian sdhc-1) or sdhb-1 to create a translational fusion protein. The coding sequence for GFP was inserted before the translational stop codon/3′ UTR of immt-1 (aka mitofilin) yielding IMMT-1::GFP expressing worms.
Table 2.
List of Amplicon Repair Templates for CRISPR-Homology Directed Repair Editing
| Strain | Repair template primers | Template | Product |
|---|---|---|---|
| APW49 | F: GCTTACTGGATTCACATCGAAGCCAGCCGCTGAGCCATCTGCATTCATGGTCTCAAAGGGTGAAG | pCFJ90 (Addgene No. 19327) | mCherry |
| R: CATCGAATCTAGGCAAAACAATGGAATTTTCTTATTTATACAATTCATCCATGCCACCTG | |||
| APW54 | F: GCTTACTGGATTCACATCGAAGCCAGCCGCTGAGCCATCTGCATTCATGGGTTCAGAGGTCGGCC | SuperNova/pRSETB (Addgene No. 53234) | SuperNova |
| R: CATCGAATCTAGGCAAAACAATGGAATTTTCTTATTTAATCCTCGTCGCTACCGATGGCG | |||
| APW47 | F: CTTCAACTCTTGCCAGAACAAGAGCAACAAGACTGCtATGGTCTCAAAGGGTGAAGAAG | pCFJ90 (Addgene No. 19327) | mCherry |
| R: GCGGAGTAAGAAAAAAGAAGGCGGAGCATCTGTGCTTATACAATTCAATCCATGCCACCTG | |||
| APW149 | F: CTTCAACTCTTGCCAGAACAAGAGCAACAAGACTGCCGGAGCATCGGGAGCCTCAGGAGCATCGATGGGTTCAGAGGTCGGCCC | SuperNova/pRSETB (Addgene No. 53234) | SuperNova |
| R: GAGTAAGAAAAAAGAAGGCGGAGCATCTGTGCCTAATCCTCGTCGCTACCGATG | |||
| APW40 | F: CTCGCCCAGCTTCTTGTGGCTCACGCCGCCGTCTCATCGATTCGCTCAACTTATCCTCGTAGCCCTCGGACTCCTCGTAGCATGAGCAAGGGCGAGG | pFCK-Arch_GFP (Addgene No. 22217) | GFP |
| R: TTTTAAAAAACGGTGACGCAAGACAATCAATTGTTTTACTTGTACAGCTCGTCCATGCCG |
GFP, green fluorescent protein.
Table 3.
List of crRNA for CRISPR-Homology Directed Repair editing
| Gene target | crRNA target sequence |
|---|---|
| sdhb-1 | TTTCTTATTTAAAATGCTGA |
| sdhc-1 | CAAGAGCAACAAGACTGCCT |
| immt-1 | CTAATAAGTTGAGCGAATCG |
RNAi
RNAi was performed as previously described (55). Worms were placed onto NGM plates seeded with HT115 Escherichia coli that transcribes double-stranded RNA (dsRNA) homologous to skn-1 (23).
Western blotting
Each sample lysate was prepared from a single 35 mm NGM plate containing ∼200 developmentally staged adult worms. These were collected into microcentrifuge tubes using room temperature M9 media (22 mM KH2PO4, 42 mM Na2HPO4, 86 mM NaCl, 1 mM MgSO4), then shortly centrifuged. Excess M9 was removed before snap freezing in liquid nitrogen. Protein extraction was then performed with the addition of 30–50 μL modified RIPA buffer (50 mM Tris HCl, 150 mM NaCl, 20 mM EDTA, 0.5% sodium deoxycholate, 1% Triton-X, 0.1% SDS, and Halt™ Protease & Phosphatase Inhibitor cocktail [No. 78440; Thermo Fisher], pH 8.0) combined with mechanical homogenization by vortexing for 8 × 30 s on/off ice with 3 × 2 mm zirconin beads (No. 11079124ZX; BioSpec) to disrupt the worm cuticle. After centrifugation at 8000 g for 5 min at 4°C, supernatant was collected, and protein concentration determined by the Lowry assay. After equalizing protein concentration, loading buffer (100 mM Tris HCl, 10% v/v glycerol, 0.2% w/v bromophenol blue, 2% v/v β-mercaptoethanol) was added in equal volume. Samples were then loaded onto 10%–12% gels and separated by SDS-PAGE. Proteins were transferred to nitrocellulose membranes and blocked in 5% nonfat milk/Tris-buffered saline with tween (TBST) for 1 h at room temperature. Membranes were then incubated overnight at 4°C in the appropriate primary antibodies diluted 1:1000 in 5% BSA: anti-KillerRed (No. AB961; Evrogen), anti-GFP (No. 11814460001; Roche), anti-HSP60 (Developmental Studies Hybridoma Bank, University of Iowa, lot No. 20150409), anti-mCherry (No. PA5-34974, lot No. SH2437272B; Thermo Fisher), anti-ATP5a (No. ab14748, lot No. GR160041-12; Abcam), anti-Actin (No. ab14128, lot No. GR319639-1; Abcam), antiactive PMK-1 (No. V1211, lot No. 0000290588; Promega), and anti-2,4-dinitrophenylhydrazine for OxyBlot (No. S7150; Millipore Sigma). After washing in TBST, membranes were incubated in HRP-conjugated secondary antibodies: antirabbit IgG (No. 7074S; Cell Signaling) or antimouse IgG (No. 32430, lot No. RF234708; Thermo Scientific) for 1 h at room temperature. Finally, proteins were visualized using ECL (Clarity Western ECL Substrate; Bio Rad) for image capture (ChemiDoc; Bio Rad).
Mitochondrial respiration and isolated enzyme activity assay
Mitochondria were isolated from ∼200,000 day-1 adult C. elegans in mannitol, sucrose, (3-(N-morpholino) propanesulfonic acid), EDTA buffer (MSM-E) buffer (MSM-E: 220 mM mannitol, 70 mM sucrose, 5 mM 3-morpholinopropane-1-sulfonic acid, 2mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), pH 7.4 at 4°C), then resuspended in MRB (120 mM KCl, 25 mM sucrose, 5 mM MgCl2, 5 mM KH2PO4, 1 mM EGTA, 10 mM HEPES, 1 mg/mL fatty-acid free bovine serum albumin (FF-BSA), pH 7.35 at 25°C) as described previously (55). Mitochondria were added to a clark-type electrode chamber (Hansatech Instruments, United Kingdom) at 1 mg/mL−1 in MRB also containing 1 mg/mL−1 FF-BSA. Substrates and inhibitors were then sequentially added: 5 mM succinate and 2 μM rotenone, followed by 0.4 mM ADP to induce state-3 oxidative phosphorylation, then 2.5 mM malonate to inhibit complex-II activity.
Frozen mitochondria isolates were used to measure enzyme activity spectrophotometrically. Mitochondria were freeze-thawed, then Complex II activity was determined as the succinate-driven, malonate-sensitive rate of 2,6-dichlorophenolindophenol reduction (ɛ = 21,000 M−1 at 600 nm) (51). Citrate synthase activity was determined as the rate of 5,5′-disulfanediylbis(2-nitrobenzoic acid)-coenzyme A formation (ɛ = 13,600 M−1 at 412 nm) (55).
Protease-protection assay for determination of inner mitochondrial membrane protein topology
Mitochondria were isolated as described above, and an aliquot of resuspended isolated mitochondria in MSM-E at 5 mg/mL was divided in half, pelleted at 7000 g for 5 min, then the supernatant was removed from each. To form mitoplasts, one of the two aliquots was resuspended in hypotonic swelling buffer (20 mM HEPES, 1 mM EGTA, pH 7.2 at 4°C), and the other in MSM-E to maintain intact mitochondria and incubated on ice for 10 min. Both were pelleted again at 7000 g for 5 min, then resuspended in MRB. Samples were then treated with proteinase-K (No. P81075; Biolabs) at 0.1 U·mg protein−1 for 10 min without or with Triton-X (2.5% v/v), then digestion was inhibited with the serine protease inhibitor, phenylmethyl sulfonyl fluoride (PMSF, 40 mM). Sample loading buffer was added in equal volume, and the SDS-denatured protein lysates were resolved on a 15% gel via SDS-PAGE as described above.
Development rate assay
Day-1 adult worms were moved to an NGM plate and allowed to lay eggs for 30 min, then a single egg was transferred to each new NGM plate and placed in an incubator maintained at 20°C. At regular intervals, images were taken of each worm and body length along the midline determined using ImageJ.
Confocal microscopy
Day 1 adult worms were placed on a 2% agarose pad under anesthetic (0.1% tetramisole), and 1 μm image stacks were acquired using an Olympus FV1000 confocal microscope with a 100 × oil immersion objective (University of Rochester Light Microscopy Core Facility). Single Z-stack images were used to assess cross-section profile plots of large spherical hypodermal mitochondria coexpressing GFP and mCherry or SuperNova using ImageJ. Profile plot data were smoothed by three-point moving average of pixel intensity, then normalized to peak intensity of each profile. To confirm mitochondrial-specific expression of IMMT-1::GFP, these worms were imaged after 2 h incubation in 5 μM Mitotracker Red CMXRos dye (Invitrogen) in M9 media overnight, which preferentially accumulates in the mitochondrial matrix.
Epifluorescence microscopy for GFP transcriptional reporter experiments
Worms were placed on a 2% agarose pad under anesthetic (tetramisole, 0.1% w/v), then images were obtained through GFP and TexasRed filter sets on a fluorescence microscope (Nikon MVX10) equipped with a camera (Lumenera) and acquisition software (Infinity Analyze; Lumenera). GFP reporter expression was then quantified using ImageJ by tracing around individual worms to determine their mean fluorescence intensity.
Glutathione assay
Approximately 20,000 staged day-1 adult worms per plate were exposed to light (1 h, 1 mW/mm2) or kept in dark conditions, then collected in MES buffer [0.2 M 2-(N-morpholino)ethanesulfonic acid, 0.05 M phosphate, 1 mM EDTA, pH 6] and homogenized as described in the Western blotting method using zircon beads. Total and oxidized glutathione were then determined using a commercially available kit (703002, Cayman Glutathione Assay Kit) according to the manufacturer's directions.
AR sensitivity assay
All pretreatment was performed on developmentally synchronized day-1 adult worms. For optogenetic experiments, worms were subjected to 60-min light pretreatment or dark-control, then 1 h later placed at 26°C for 4 h in normoxic conditions in the dark. For anoxia preconditioning, worms were placed in a chamber maintained at <2 ppm O2 (5%/95% H2/N2, palladium catalyst; Coy Lab Products) for the same 4 h period at 26°C. For PQ (N,N′-dimethyl-4,4′-bipyridinium dichloride) pretreatment, worms grown on regular NGM plates were moved to NGM plates containing 4 mM PQ for the 4 h pretreatment period at 26°C, then returned to fresh plates. In all groups, after pretreatment, worms were returned to dark/normoxia conditions in a 20°C incubator for 24 h. Then, day-2 adult worms (≥15 per plate in triplicate per condition, strain and time point) were subjected to anoxia at 26°C for a duration of 18 h unless otherwise indicated. Worms were then returned to normoxia at 20°C to recover for 22–24 h, after which they were scored for the number of surviving worms on each plate. Survival was defined as at least having pharyngeal pumping and side-to-side head movement in response to gentle touch.
Statistical analysis
Data were analyzed by one- or two-way ANOVA where appropriate, with Tukey's correction for multiple comparisons using GraphPad Prism (v7). All data are expressed as mean ± SEM unless otherwise indicated. Exact n for each experiment is reported in the respective figure legend. Significance was accepted at p < 0.05.
Supplementary Material
Acknowledgments
This work was funded by a grant from the National Institutes of Health to A.P.W. (R01 NS092558), American Heart Association, Founders Affiliate Undergraduate Student Summer Fellowship Award (A.Y.W.), an Institutional Ruth L. Kirschstein National Research Service Award (GM068411), and an American Heart Association Predoctoral Fellowship to B.J.B. (18PRE33990054). Some C. elegans strains were provided by the C. elegans Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Dr. Keith Nehrke, Dr. Paul S. Brooks, and members of the mitochondrial research group at the University of Rochester Medical Center for their valuable discussions. The anti-HSP60 monoclonal antibody developed by Nonet M.L., Hadwiger G. and Dour S. of the Washington University Medical School was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Abbreviations Used
- 2-OH-E+
2-hydroxyethidium
- ADP
adenosine diphosphate
- AR
anoxia reoxygenation
- ASK1
apoptosis signal-regulating kinase 1
- Cu/Zn-SOD
copper/zinc superoxide dismutase
- DHE
dihydroethidium
- E+
ethidium
- EGTA
3-morpholinopropane-1-sulfonic acid, 2mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- ETC
electron transport chain
- FF-BSA
fatty-acid free bovine serum albumin
- GFP
green fluorescent protein
- gst-4p
glutathione S-transferase 4 promoter region
- H2O2
hydrogen peroxide
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- HPLC
high-performance liquid chromatography
- HSP60
heat shock protein 60 kDa
- IMMT
inner mitochondrial membrane protein 1 (C. elegans ortholog of mitofilin)
- IMS
intermembrane space
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- LED
light-emitting diode
- MAPK
mitogen activated protein kinase
- mev-1
succinate dehydrogenase cytochrome b560 subunit (C. elegans—methyl viologen sensitive mutant)
- MRB
mitochondrial respiration buffer
- MSM-E
mannitol, sucrose, (3-(N-morpholino)propanesulfonic acid), EDTA buffer
- N2
C. elegans wild-type strain
- NGM
nematode growth medium
- Ni-NTA
nickel-charged affinity resin
- NSY-1
mitogen-activated protein kinase, C. elegans ortholog of ASK1
- O2˙−
superoxide
- OD
optical density
- PBS
phosphate-buffered saline
- PMK-1
mitogen-activated protein kinase (C. elegans ortholog of p38 MAPK)
- PMSF
phenylmethylsulfonyl fluoride
- PQ
paraquat (methyl viologen)
- pro-K
proteinase-K
- Q
ubiquinone
- RET
reverse electron transfer
- RNAi
RNA interference
- ROS
reactive oxygen species
- SDHB
succinate dehydrogenase subunit B
- SDHC
succinate dehydrogenase subunit C
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SKN-1
skinhead-1 (C. elegans ortholog of Nrf2)
- SOD
superoxide dismutase
- TBST
Tris-buffered saline with tween
- TCEP
Tris(2-carboxyethyl)phosphine
- TRX
thioredoxin
- WDR-23
WD40 repeat protein, DDB1- and CUL4-associated factor 11 homolog
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. An JH, and Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev 17: 1882–1893, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, and Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol 139: 479–491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, and Isik M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med 88: 290–301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner S. The genetics of Caenorhabditis elegans. Genetics 77: 71–94, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brigelius-Flohe R. and Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal 15: 2335–2381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choe KP, Przybysz AJ, and Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol 29: 2704–2715, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, and Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, and Murphy MP. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab 23: 254–263, 2016 [DOI] [PubMed] [Google Scholar]
- 9. Cocheme HM. and Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 283: 1786–1798, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Cuenda A. and Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773: 1358–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Dasgupta N, Patel AM, Scott BA, and Crowder CM. Hypoxic preconditioning requires the apoptosis protein CED-4 in C. elegans. Curr Biol 17: 1954–1959, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fedorova M, Bollineni RC, and Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 33: 79–97, 2014 [DOI] [PubMed] [Google Scholar]
- 13. Hackenbrock CR, Chazotte B, and Gupte SS. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr 18: 331–368, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Hayakawa T, Kato K, Hayakawa R, Hisamoto N, Matsumoto K, Takeda K, and Ichijo H. Regulation of anoxic death in Caenorhabditis elegans by mammalian apoptosis signal-regulating kinase (ASK) family proteins. Genetics 187: 785–792, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsieh CC. and Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J 20: 259–268, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hung CH, Cheng SS, Cheung YT, Wuwongse S, Zhang NQ, Ho YS, Lee SM, and Chang RC. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol 14: 7–19, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inarrea P, Moini H, Rettori D, Han D, Martinez J, Garcia I, Fernandez-Vizarra E, Iturralde M, and Cadenas E. Redox activation of mitochondrial intermembrane space Cu,Zn-superoxide dismutase. Biochem J 387: 203–209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, and Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19: 2278–2283, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, and Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature 394: 694–697, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Ishii N, Takahashi K, Tomita S, Keino T, Honda S, Yoshino K, and Suzuki K. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutat Res 237: 165–171, 1990 [DOI] [PubMed] [Google Scholar]
- 21. John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JM, Rangell L, Bennett MJ, and Zha J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol Biol Cell 16: 1543–1554, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones DP. and Sies H. The redox code. Antioxid Redox Signal 23: 734–746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, and Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Klug D, Rabani J, and Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem 247: 4839–4842, 1972 [PubMed] [Google Scholar]
- 25. Lolli G, Raboni S, Pasqualetto E, Benoni R, Campanini B, Ronda L, Mozzarelli A, Bettati S, and Battistutta R. Insight into GFPmut2 pH dependence by single crystal microspectrophotometry and X-ray crystallography. J Phys Chem B 122: 11326–11337, 2018 [DOI] [PubMed] [Google Scholar]
- 26. Madungwe NB, Zilberstein NF, Feng Y, and Bopassa JC. Critical role of mitochondrial ROS is dependent on their site of production on the electron transport chain in ischemic heart. Am J Cardiovasc Dis 6: 93–108, 2016 [PMC free article] [PubMed] [Google Scholar]
- 27. Marinho HS, Real C, Cyrne L, Soares H, and Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2: 535–562, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muller FL, Liu Y, and Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279: 49064–49073, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Munro D, Banh S, Sotiri E, Tamanna N, and Treberg JR. The thioredoxin and glutathione-dependent H2O2 consumption pathways in muscle mitochondria: involvement in H2O2 metabolism and consequence to H2O2 efflux assays. Free Radic Biol Med 96: 334–346, 2016 [DOI] [PubMed] [Google Scholar]
- 30. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okado-Matsumoto A. and Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276: 38388–38393, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Paek J, Lo JY, Narasimhan SD, Nguyen TN, Glover-Cutter K, Robida-Stubbs S, Suzuki T, Yamamoto M, Blackwell TK, and Curran SP. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab 16: 526–537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paix A, Folkmann A, Rasoloson D, and Seydoux G. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics 201: 47–54, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park J, Lee J, and Choi C. Mitochondrial network determines intracellular ROS dynamics and sensitivity to oxidative stress through switching inter-mitochondrial messengers. PLoS One 6: e23211, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pereverzev MO, Vygodina TV, Konstantinov AA, and Skulachev VP. Cytochrome c, an ideal antioxidant. Biochem Soc Trans 31: 1312–1315, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Poburko D, Santo-Domingo J, and Demaurex N. Dynamic regulation of the mitochondrial proton gradient during cytosolic calcium elevations. J Biol Chem 286: 11672–11684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porcelli AM, Ghelli A, Zanna C, Pinton P, Rizzuto R, and Rugolo M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem Biophys Res Commun 326: 799–804, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Powell-Coffman JA. Hypoxia signaling and resistance in C. elegans. Trends Endocrinol Metab 21: 435–440, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, and Brand MD. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J Biol Chem 287: 27255–27264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ranjan M, Gruber J, Ng LF, and Halliwell B. Repression of the mitochondrial peroxiredoxin antioxidant system does not shorten life span but causes reduced fitness in Caenorhabditis elegans. Free Radic Biol Med 63: 381–389, 2013 [DOI] [PubMed] [Google Scholar]
- 41. Reczek CR, Birsoy K, Kong H, Martinez-Reyes I, Wang T, Gao P, Sabatini DM, and Chandel NS. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat Chem Biol 13: 1274–1279, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rhee SG. and Kil IS. Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm. Free Radic Biol Med 100: 73–80, 2016 [DOI] [PubMed] [Google Scholar]
- 43. Scott BA, Avidan MS, and Crowder CM. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science 296: 2388–2391, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol 11: 613–619, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suthammarak W, Somerlot BH, Opheim E, Sedensky M, and Morgan PG. Novel interactions between mitochondrial superoxide dismutases and the electron transport chain. Aging Cell 12: 1132–1140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takemoto K, Matsuda T, Sakai N, Fu D, Noda M, Uchiyama S, Kotera I, Arai Y, Horiuchi M, Fukui K, Ayabe T, Inagaki F, Suzuki H, and Nagai T. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci Rep 3: 2629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tkatch T, Greotti E, Baranauskas G, Pendin D, Roy S, Nita LI, Wettmarshausen J, Prigge M, Yizhar O, Shirihai OS, Fishman D, Hershfinkel M, Fleidervish IA, Perocchi F, Pozzan T, and Sekler I. Optogenetic control of mitochondrial metabolism and Ca(2+) signaling by mitochondria-targeted opsins. Proc Natl Acad Sci U S A 114: E5167–E5176, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, and Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2: 222–228, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trewin AJ, Berry BJ, Wei AY, Bahr LL, Foster TH, and Wojtovich AP. Light-induced oxidant production by fluorescent proteins. Free Radic Biol Med 128: 157–164, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams DC, Bejjani RE, Ramirez PM, Coakley S, Kim SA, Lee H, Wen Q, Samuel A, Lu H, Hilliard MA, and Hammarlund M. Rapid and permanent neuronal inactivation in vivo via subcellular generation of reactive oxygen with the use of KillerRed. Cell Rep 5: 553–563, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wojtovich AP. and Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochim Biophys Acta 1777: 882–889, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wojtovich AP. and Foster TH. Optogenetic control of ROS production. Redox Biol 2: 368–376, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wojtovich AP, Nadtochiy SM, Brookes PS, and Nehrke K. Ischemic preconditioning: the role of mitochondria and aging. Exp Gerontol 47: 1–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wojtovich AP, Smith CO, Haynes CM, Nehrke KW, and Brookes PS. Physiological consequences of complex II inhibition for aging, disease, and the mKATP channel. Biochim Biophys Acta 1827: 598–611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wojtovich AP, Wei AY, Sherman TA, Foster TH, and Nehrke K. Chromophore-assisted light inactivation of mitochondrial electron transport chain complex II in Caenorhabditis elegans. Sci Rep 6: 29695, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wong HS, Dighe PA, Mezera V, Monternier PA, and Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem 292: 16804–16809, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woo HA, Yim SH, Shin DH, Kang D, Yu DY, and Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 140: 517–528, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Wu CW, Deonarine A, Przybysz A, Strange K, and Choe KP. The Skp1 Homologs SKR-1/2 are required for the Caenorhabditis elegans SKN-1 antioxidant/detoxification response independently of p38 MAPK. PLoS Genet 12: e1006361, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, and Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34: 1359–1368, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Zielonka J, Vasquez-Vivar J, and Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc 3: 8–21, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.