Abstract
Background
The optimal neoadjuvant radiation therapy (RT) dose prior to esophagectomy is unknown. We compared patients receiving lower-dose RT (LD-RT) of 41.4–45 Gy versus those receiving higher-dose RT (HD-RT) of 50–54 Gy.
Methods
Patients with non-metastatic esophageal or gastroesophageal cancer diagnosed from 2004 to 2015 who underwent neoadjuvant chemoradiation (CRT) followed by esophagectomy were identified using the National Cancer Database (NCDB) and divided into LD-RT and HD-RT groups. Logistic regression was used to evaluate predictors of HD-RT utilization and propensity score matching. Overall survival (OS) was compared between HD-RT and LD-RT groups using Cox regression. Logistic regression was performed with respect to pathologic complete response (pCR), positive surgical margins, postoperative mortality, and readmission rates.
Results
We identified 7,996 patients meeting inclusion criteria, of which 5,732 (71.7%) received HD-RT. At median follow-up of 3.3 years, 3-year OS was 48.7% for HD-RT versus 48.4% for LD-RT (P=0.734). pCR rates were 20.3% with HD-RT versus 16.3% with LD-RT [odds ratio (OR) 1.24; 95% CI: 1.06–1.44; P=0.006]. There were no statistically significant differences between HD-RT and LD-RT with respect to positive margins, 90-day postoperative mortality, or readmission rates. In a separate analysis of patients treated with CRT alone and no subsequent esophagectomy, HD-RT was associated with improved OS (HR 0.83; 95% CI: 0.78–0.88; P<0.001).
Conclusions
Our analysis suggests that 41.4–45 and 50–54 Gy dose regimens are similar in survival and postoperative outcomes. However, in cases of equivocal resectability, a higher RT dose of 50–54 Gy may be preferred.
Keywords: Esophageal cancer, neoadjuvant therapy, radiation therapy (RT), radiation dose
Introduction
Esophageal cancer is the eighth most common cancer worldwide, affecting an estimated 17,290 patients in the United States and 455,800 patients globally on an annual basis (1,2). Neoadjuvant chemoradiation (CRT) followed by esophagectomy is considered the standard of care for locally advanced disease (3,4).
The publication of the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) trial in 2012 firmly established the role of neoadjuvant CRT (5,6). However, substantial heterogeneity exists among different clinical trial protocols in the dosage and fractionation used for neoadjuvant radiation therapy (RT). The CROSS trial utilized neoadjuvant RT to a dose of 41.4 Gy in 23 fractions. Other published clinical trial protocols use widely heterogeneous dosages, ranging from 30 Gy in the PreOperative therapy in Esophagogastric adenocarcinoma Trial (POET) trial to 50.4 Gy in the Cancer and Leukemia Group B (CALGB) 9781 trial (7-11).
The landmark Integroup-0123 trial investigated the impact of dose escalation to 64.8 Gy compared to 50.4 Gy in esophageal cancer, and found no difference in survival outcomes (12). However, this trial specifically analyzed patients receiving definitive CRT. In the neoadjuvant setting, there are minimal data and no randomized studies to guide selection of RT dose. Guidelines from the National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO) allow for a dose range from 41.4 to 50.4 Gy (3,4).
Therefore, the optimal neoadjuvant RT dose for esophageal cancer remains unclear. Given the scarcity of data to guide RT dose in the preoperative setting, we utilized the National Cancer Database (NCDB) to compare esophageal cancer patients receiving a lower dose of 41.4–45 Gy (LD-RT) to those receiving a higher dose of 50–54 Gy (HD-RT). We analyzed these two cohorts with respect to survival outcomes, pathologic response, and postoperative mortality and readmissions. We additionally identified a substantial population of patients who do not undergo esophagectomy after receiving neoadjuvant RT dosing, and compared survival by RT dose in this population as well.
Methods
The NCDB is a national oncology database sponsored by the College of Surgeons and the American Cancer Society. It includes patient data from over 1,500 accredited facilities and captures >70% of newly diagnosed cancer cases in the United States (13). The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used, or the conclusions drawn from these data by the investigators. Ethical statement: this study uses only de-identified data from the National Cancer Database and is considered exempt from Institutional Board Review.
Patient cohort definition
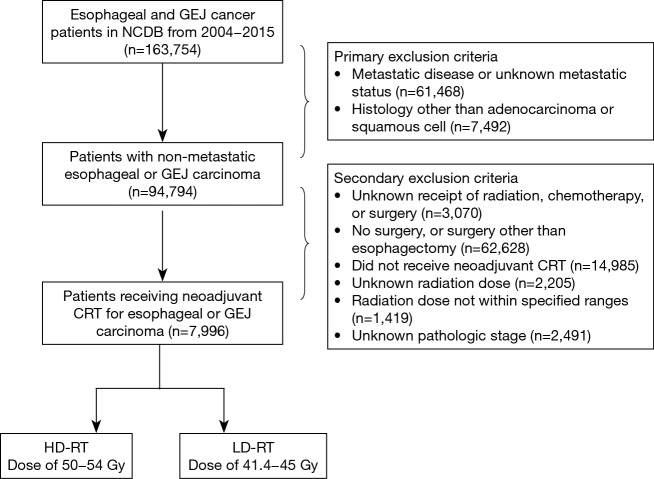
Using the NCDB, a total of 163,754 patients were identified with cancers of the esophagus or the gastroesophageal junction (GEJ) diagnosed from 2004 to 2015. Patients were excluded if they had histology other than adenocarcinoma or squamous cell carcinoma (SCC), had metastatic disease or unknown metastatic status at presentation, did not undergo esophagectomy, did not receive CRT followed by surgery, had unknown RT dose, or had a RT dose outside the ranges of 41.4–45 and 50–54 Gy. The remaining patients were stratified into a lower-dose RT (LD-RT) group receiving 41.4–45 Gy, and a higher-dose RT group (HD-RT) receiving 50–54 Gy. Exclusion criteria for this cohort, are summarized in Figure 1.
Figure 1.
Diagram illustrating exclusion criteria and case selection for patient cohort. NCDB, National Cancer Database; GEJ, gastroesophageal junction; CRT, chemoradiation; RT, radiation therapy; HD, higher-dose; LD, lower-dose.
Variable definitions
All variables were selected a priori. Demographic variables including age, year of diagnosis, race, insurance status, facility type, and facility location were defined according to their respective data fields in the NCDB data dictionary (14). Facility regions were grouped into Northeast, South, Midwest, and West regions. Insurance status was grouped into government (Medicare/Medicaid/other), private, or uninsured. Histologies of adenocarcinoma and SCC were defined using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) codes (15). All TNM staging was done according to the American Joint Committee on Cancer (AJCC) 7th edition staging manual.
For treatment variables, receipt of surgery, type of surgery performed, as well as chemotherapy and radiation were defined by their respective fields in the data dictionary. The total RT dose was defined as the sum of the primary site and boost site radiation dosages. Time from CRT to surgery was calculated using the formula: (days from diagnosis to surgery) − (days from diagnosis to radiation start) − (days elapsed for radiation); patients were stratified by delay of ≥10 weeks (16). Finally, pathologic complete response (pCR) was defined by ypT0N0 staging, similar to the CROSS trial.
Analysis of high-dose versus low-dose neoadjuvant radiation
Multivariate logistic regression was performed to assess factors predictive of receiving HD-RT. This was also used to generate propensity scores, with all factors included in propensity score generation regardless of statistical significance.
We performed survival analysis in the two cohorts as defined above. Survival analyses were performed using the log-rank test for univariate analysis and Cox proportional hazards regression for multivariate analysis. The final parsimonious multivariate Cox model was formed by using hierarchical backwards selection of variables significant at P<0.10. The proportional hazards assumption was assessed for all variables in the final multivariate analysis and was not violated (17).
Propensity score-matched analysis was performed. One-to-one nearest neighbor matching without replacement with caliper width of 0.10. Log-rank test was performed in the final matched cohort.
Analysis of CRT without esophagectomy
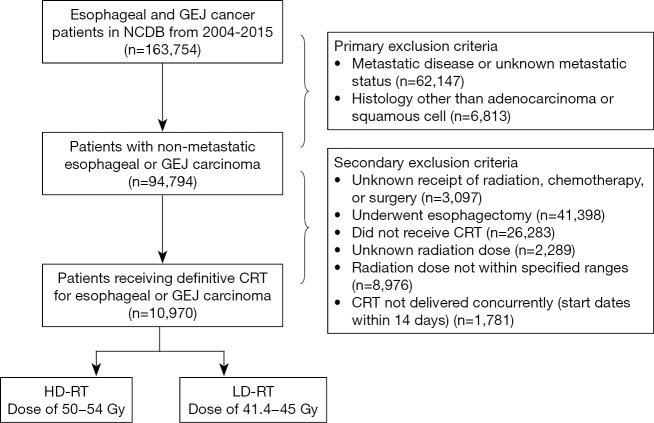
Our analysis identified a population of patients who do not undergo esophagectomy after receiving neoadjuvant RT dosing. We therefore defined a separate cohort of patients receiving CRT without esophagectomy, using identical exclusion criteria other than receipt of esophagectomy (Figure S1). This second cohort was used for survival analysis, and multivariate Cox proportional hazards regression was used to analyze the association between radiation dose and survival using identical methodology as described in the previous section.
Figure S1.
Diagram illustrating exclusion criteria and case selection for patient cohort receiving chemoradiation without esophagectomy. NCDB, National Cancer Database; GEJ, gastroesophageal junction; CRT, chemoradiation; RT, radiation therapy; HD, higher-dose; LD, lower-dose.
Analysis of secondary endpoints
We defined the following four secondary endpoints: pCR, surgery with positive surgical margins, 90-day mortality following surgery, and readmission within 30 days of surgical discharge. For each of these endpoints, we performed multivariate analysis using logistic regression (again using hierarchical backwards selection of variables significant at P<0.10). For the analysis of pCR, we specified a priori a subset analysis by underlying histology (SCC versus adenocarcinoma). All statistical analyses were performed using SPSS statistical software (version 23.0; IBM Corporation, Armonk, NY, USA). A significance level of 0.05 was used for all analyses.
Results
Patient cohort characteristics
A total of 7,996 patients at a median follow-up of 3.3 years for living patients were identified in the NCDB that met the inclusion criteria (Figure 1). The demographic, pathologic, and treatment characteristics of our patient cohort are summarized in Table 1. The median time between completion of radiation treatment and esophagectomy was 50 days [interquartile range (IQR) 40 to 64 days].
Table 1. Baseline characteristics of patients.
| Baseline characteristics | All patients (n=7,996), n (%) |
|---|---|
| Sociodemographic factors | |
| Year of diagnosis | |
| 2004–2006 | 730 (9.1) |
| 2007–2009 | 1,307 (16.3) |
| 2010–2012 | 2,307 (28.9) |
| 2013–2015 | 2,163 (27.1) |
| Age | |
| <65 years | 4,678 (58.5) |
| ≥65 years | 3,318 (41.5) |
| Charlson-Deyo comorbidity score | |
| 0 | 5,885 (73.6) |
| 1 | 1,698 (21.2) |
| ≥2 | 413 (5.2) |
| Race | |
| Non-Hispanic White | 7,345 (91.9) |
| Black | 285 (3.6) |
| Hispanic | 166 (2.1) |
| Other | 146 (1.8) |
| Unknown | 54 (0.7) |
| Insurance status | |
| Government | 3,771 (47.2) |
| Private | 3,978 (49.7) |
| Uninsured | 165 (2.1) |
| Unknown | 82 (1.0) |
| Facility type | |
| Academic/research | 4,799 (60.0) |
| Community/comprehensive community | 2,979 (37.3) |
| Unknown | 218 (2.7) |
| Facility location | |
| Midwest | 3,418 (42.7) |
| Northeast | 2,608 (32.6) |
| South | 757 (9.5) |
| West | 995 (12.4) |
| Unknown | 218 (2.7) |
| Clinical/pathologic factors | |
| Histology | |
| Adenocarcinoma | 6,930 (86.7) |
| Squamous cell carcinoma | 1,066 (13.3) |
| Primary site | |
| Esophagus | 6,742 (84.3) |
| Gastroesophageal junction | 1,254 (15.7) |
| Clinical T stage | |
| T1 | 397 (5.0) |
| T2 | 1,452 (18.2) |
| T3 | 5,201 (65.0) |
| T4 | 194 (2.4) |
| Unknown | 752 (9.4) |
| Clinical N stage | |
| N0 | 2,828 (35.4) |
| N1 | 3,849 (48.1) |
| N2 | 707 (8.8) |
| N3 | 105 (1.3) |
| Unknown | 507 (6.3) |
| AJCC stage | |
| I | 535 (6.7) |
| II | 2,922 (36.5) |
| III | 3,773 (47.2) |
| Unknown | 766 (9.6) |
| Treatment factors | |
| Time from completion of radiation to surgery | |
| <10 weeks | 6,528 (81.6) |
| ≥10 weeks | 1,468 (18.4) |
| Radiation therapy dose | |
| Lower dose: 41.4 to 45 Gy | 5,732 (71.7) |
| Higher dose: 50 to 54 Gy | 2,264 (28.3) |
Utilization of higher-dose RT
Of the total 7,996 patients, there were 5,732 patients (71.7%) who received HD-RT of 50–54 Gy and 2,264 patients (28.3%) who received LD-RT of 41.4–45 Gy. Within the HD-RT group 5,425 patients (94.6%) received 50–51 Gy, and within the LD-RT group 1,802 patients (79.6%) received 45 Gy. Table 2 illustrates the distribution of factors between the HD-RT and LD-RT cohorts, in addition to odds ratios (OR) based on logistic regression. Predictors of increased HD-RT utilization included: time between CRT and surgery ≥10 weeks (OR 1.30; 95% CI: 1.12–1.52; P<0.001). Decreased HD-RT utilization was associated with age ≥65 (OR 0.83; 95% CI: 0.72–0.95; P=0.009), treatment in the south region (OR 0.62; 95% CI: 0.51–0.76; P<0.001), and GEJ primary (OR 0.78; 95% CI: 0.67–0.91; P=0.002). Notably, clinical T and N stage were not associated with HD-RT utilization.
Table 2. Comparative utilization of radiation therapy dose by baseline characteristics.
| Baseline characteristics | RT dose: 41.4 to 45 Gy (n=2,264), n (%) | RT dose: 50 to 54 Gy (n=5,732), n (%) | Odds ratio | 95% CI | P |
|---|---|---|---|---|---|
| Sociodemographic factors | |||||
| Year of diagnosis | |||||
| 2004–2006 | 289 (39.6) | 441 (60.4) | 1 | Reference | |
| 2007–2009 | 409 (31.3) | 898 (68.7) | 1.43 | 1.18–1.74 | <0.001* |
| 2010–2012 | 554 (24.0) | 1,753 (76.0) | 2.03 | 1.68–2.45 | <0.001* |
| 2013–2015 | 531 (24.5) | 1,632 (75.5) | 2.00 | 1.65–2.43 | <0.001* |
| Age | |||||
| <65 years | 1,298 (27.7) | 3,380 (72.3) | 1 | Reference | |
| ≥65 years | 966 (29.1) | 2,352 (70.9) | 0.83 | 0.72–0.95 | 0.009* |
| Charlson-Deyo comorbidity score | |||||
| 0 | 1,669 (28.4) | 4,216 (71.6) | 1 | Reference | |
| 1 | 476 (28.0) | 1,222 (72.0) | 0.98 | 0.85–1.12 | 0.733 |
| ≥2 | 119 (28.8) | 294 (71.2) | 1.06 | 0.81–1.37 | 0.674 |
| Race | |||||
| Non-Hispanic White | 2,065 (28.1) | 5,280 (71.9) | 1 | Reference | |
| Black | 80 (28.1) | 205 (71.9) | 0.81 | 0.59–1.11 | 0.195 |
| Hispanic | 57 (34.3) | 109 (65.7) | 0.67 | 0.46–0.99 | 0.045* |
| Other | 47 (32.2) | 99 (67.8) | 0.82 | 0.54–1.24 | 0.336 |
| Insurance status | |||||
| Government | 1,066 (28.3) | 2,705 (71.7) | 1 | Reference | |
| Private | 1,137 (28.6) | 2,841 (71.4) | 0.88 | 0.76–1.02 | 0.084 |
| Uninsured | 40 (24.2) | 125 (75.8) | 1.11 | 0.73–1.67 | 0.627 |
| Facility type | |||||
| Academic/research | 1,329 (27.7) | 3,470 (72.3) | 1 | Reference | |
| Community/comprehensive community | 877 (29.4) | 2,102 (70.6) | 0.95 | 0.85–1.07 | 0.428 |
| Facility location | |||||
| Midwest | 740 (28.4) | 1,868 (71.6) | 1 | Reference | |
| Northeast | 891 (26.1) | 2,527 (73.9) | 1.20 | 1.05–1.36 | 0.007* |
| South | 288 (38.0) | 469 (62.0) | 0.62 | 0.51–0.76 | <0.001* |
| West | 287 (28.8) | 708 (71.2) | 1.04 | 0.86–1.25 | 0.676 |
| Clinical/pathologic factors | |||||
| Histology | |||||
| Adenocarcinoma | 1,971 (28.4) | 4,959 (71.6) | 1 | Reference | |
| Squamous cell carcinoma | 293 (27.5) | 773 (72.5) | 1.03 | 0.86–1.23 | 0.723 |
| Primary site | |||||
| Esophagus | 1,878 (27.9) | 4,864 (72.1) | 1 | Reference | |
| Gastroesophageal junction | 386 (30.8) | 868 (69.2) | 0.78 | 0.67–0.91 | 0.002* |
| Clinical T stage | |||||
| T1 | 119 (30.0) | 278 (70.0) | 1 | Reference | |
| T2 | 434 (29.9) | 1,018 (70.1) | 1.05 | 0.81–1.36 | 0.731 |
| T3 | 1,375 (26.4) | 3,826 (73.6) | 1.16 | 0.91–1.47 | 0.228 |
| T4 | 52 (26.8) | 142 (73.2) | 1.19 | 0.78–1.80 | 0.415 |
| Unknown | 284 (37.8) | 468 (62.2) | 0.98 | 0.71–1.36 | 0.921 |
| Clinical N stage | |||||
| N0 | 814 (28.8) | 2,014 (71.2) | 1 | Reference | |
| N1 | 1,052 (27.3) | 2,797 (72.7) | 1.05 | 0.93–1.19 | 0.457 |
| N2 | 165 (23.3) | 542 (76.7) | 1.21 | 0.96–1.53 | 0.112 |
| N3 | 27 (25.7) | 78 (74.3) | 0.95 | 0.57–1.58 | 0.830 |
| Unknown | 206 (40.6) | 301 (59.4) | 1.06 | 0.78–1.42 | 0.722 |
| AJCC stage | |||||
| I | 165 (30.8) | 370 (69.2) | |||
| II | 834 (28.5) | 2,088 (71.5) | |||
| III | 975 (25.8) | 2,798 (74.2) | |||
| Unknown | 290 (37.9) | 476 (62.1) | |||
| Treatment factors | |||||
| Time from completion of radiation to surgery | 0.001* | ||||
| <10 weeks | 1,923 (29.5) | 4,605 (70.5) | 1 | Reference | |
| ≥10 weeks | 341 (23.2) | 1,127 (76.8) | 1.30 | 1.12–1.52 |
*, statistically significant. RT, radiation therapy.
Survival analysis
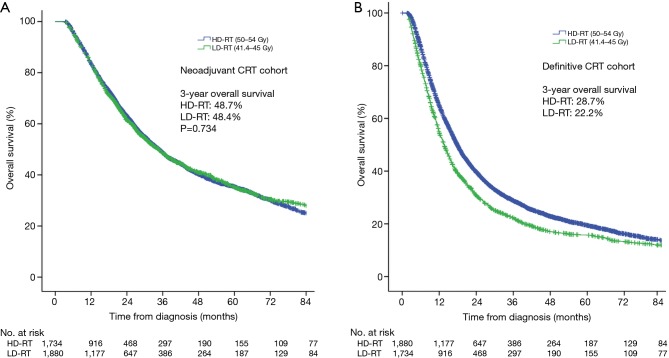
Kaplan-Meier curves comparing HD-RT and LD-RT groups are depicted in Figure 2A. Three-year overall survival (OS) was 48.7% and median survival 34.8 months in patients receiving HD-RT compared to 48.4% and 34.7 months in patients receiving LD-RT (log-rank P=0.734).
Figure 2.
Survival analysis by dosage of radiation therapy. (A) Kaplan-Meier curves of survival in patients treated with neoadjuvant chemoradiation then surgery; (B) Kaplan-Meier curves of survival in patients treated with chemoradiation alone. HD-RT, higher-dose radiation therapy (50–54 Gy); LD-RT, lower-dose radiation therapy (41.4–45 Gy).
On multivariate Cox regression, significant predictors of OS included: year of diagnosis, Charleson-Deyo comorbidity score, insurance, facility type, T stage, N stage, and time between RT and surgery ≥10 weeks (Table 3). Notably, receipt of HD-RT versus LD-RT was not associated with survival (HR 1.02; 95% CI: 0.95−1.10; P=0.580). Similarly, in a propensity matched cohort of 4,022 patients, there was no survival benefit (log-rank P=0.298).
Table 3. Multivariable Cox proportional hazards model for overall survival, stratified by receipt of esophagectomy.
| Significant factors | Neoadjuvant CRT + esophagectomy | Definitive CRT without esophagectomy | |||
|---|---|---|---|---|---|
| Hazard of death (95% confidence) | P value | Hazard of death (95% confidence) | P value | ||
| Year of diagnosis | |||||
| 2004–2006 | Reference | Reference | |||
| 2007–2009 | 1.03 (0.93–1.15) | 0.570 | 0.98 (0.91–1.06) | 0.628 | |
| 2010–2012 | 0.79 (0.71–0.88) | <0.001* | 0.92 (0.85–0.99) | 0.031* | |
| 2013–2015 | 0.75 (0.67–0.84) | <0.001* | 0.84 (0.77–0.91) | <0.001* | |
| Charlson-Deyo comorbidity score | |||||
| 0 | Reference | Reference | |||
| 1 | 1.11 (1.03–1.20) | 0.008* | 1.11 (1.05–1.18) | 0.001* | |
| ≥2 | 1.34 (1.17–1.54) | <0.001* | 1.25 (1.14–1.36) | <0.001* | |
| Insurance status | |||||
| Government | Reference | Reference | |||
| Private | 0.82 (0.75–0.89) | <0.001* | 0.90 (0.85–0.96) | 0.001* | |
| Uninsured | 1.17 (0.94–1.46) | 0.149 | 1.11 (0.96–1.29) | 0.166 | |
| Unknown | 1.00 (0.74–1.37) | 0.986 | 1.09 (0.89–1.34) | 0.399 | |
| Facility type | |||||
| Academic/research | Reference | Reference | |||
| Community | 1.21 (1.13–1.29) | <0.001* | 1.02 (0.98–1.07) | 0.348 | |
| Unknown | 0.87 (0.70–1.07) | 0.189 | 1.02 (0.77–1.35) | 0.868 | |
| Clinical T stage | |||||
| T1 | Reference | Reference | |||
| T2 | 0.90 (0.77–1.05) | 0.192 | 1.07 (0.97–1.18) | 0.184 | |
| T3 | 1.13 (0.98–1.30) | 0.098 | 1.35 (1.24–1.48) | <0.001* | |
| T4 | 1.33 (1.06–1.68) | 0.014* | 1.67 (1.49–1.88) | <0.001* | |
| Unknown | 1.31 (1.09–1.58) | 0.004* | 1.35 (1.21–1.50) | <0.001* | |
| Clinical N stage | |||||
| N0 | Reference | Reference | |||
| N1 | 1.22 (1.13–1.31) | <0.001* | 1.15 (1.09–1.21) | <0.001* | |
| N2 | 1.35 (1.18–1.54) | <0.001* | 1.40 (1.27–1.54) | <0.001* | |
| N3 | 2.16 (1.62–2.86) | <0.001* | 1.44 (1.19–1.75) | <0.001* | |
| Unknown | 1.06 (0.90–1.25) | 0.508 | 1.14 (1.03–1.27) | 0.009* | |
| Time from completion of radiation to surgery | |||||
| <10 weeks | Reference | ||||
| ≥10 weeks | 1.09 (1.01–1.19) | 0.037* | |||
| Radiation therapy dose | |||||
| Lower dose: 41.4 to 45 Gy | Reference | Reference | |||
| Higher dose: 50 to 54 Gy | 1.02 (0.95–1.10) | 0.580 | 0.83 (0.78–0.88) | <0.001* | |
*, statistically significant. CRT, chemoradiation.
Patients receiving CRT without subsequent esophagectomy
Patients receiving CRT without esophagectomy were identified in the NCDB (Figure S1).
We compared HD-RT and LD-RT in patients receiving definitive CRT without esophagectomy. In this comparison, 3-year OS was 28.7% in the HD-RT group and 22.2% in the LD-RT group (log-rank P<0.001; Figure 2B). After multivariate adjustment (Table 3), HD-RT was associated with improved survival in the definitive setting (HR 0.83; 95% CI: 0.78–0.88; P<0.001). After propensity score matching in a matched cohort of 3,639 patients, 3-year OS was 27.3% with HD-RT versus 22.2% with LD-RT (log-rank P<0.001).
Analysis of secondary outcomes
We compared the HD-RT and LD-RT groups in the primary cohort (neoadjuvant CRT + esophagectomy) on the basis of pCR rate. The overall pCR rate was 20.3% in the HD-RT group and 16.3% in the LD-RT group. On multivariate regression shown in Table S1, HD-RT of 50–54 Gy was associated with higher pCR rate (OR 1.24; 95% CI: 1.06–1.44; P=0.006). In 6,930 patients with adenocarcinoma, the overall pCR rate was 17.1%, with a rate of 18.3% in the HD-RT group and 14.0% in the LD-RT group (OR 1.33; 95% CI: 1.12–1.57; P=0.001). In 1,067 patients with SCC, the overall pCR rate was 32.8%, rate of 33.1% in the HD-RT group and 32.1% in the LD-RT group (OR 0.94; 95% CI: 0.67–1.32; P=0.733; pinteraction=0.115).
Table S1. Multivariable logistic regression for pathologic complete response.
| Significant factors | Odds ratio (95% confidence) | P value |
|---|---|---|
| Year of diagnosis | ||
| 2004–2006 | Reference | |
| 2007–2009 | 1.05 (0.80–1.37) | 0.745 |
| 2010–2012 | 1.46 (1.13–1.88) | 0.003* |
| 2013–2015 | 1.62 (1.25–2.08) | <0.001* |
| Facility type | ||
| Academic/research | Reference | |
| Community | 0.90 (0.78–1.02) | 0.095 |
| Unknown | 0.65 (0.40–1.03) | 0.070 |
| Histology | ||
| Adenocarcinoma | Reference | |
| Squamous cell carcinoma | 2.28 (1.93–2.69) | <0.001* |
| Primary site | ||
| Esophagus | Reference | |
| Gastroesophageal junction | 0.75 (0.61–0.91) | 0.004* |
| Clinical T stage | ||
| T1 | Reference | |
| T2 | 1.12 (0.84–1.51) | 0.434 |
| T3 | 0.84 (0.64–1.11) | 0.220 |
| T4 | 1.50 (0.97–2.31) | 0.069 |
| Unknown | 0.55 (0.37–0.84) | 0.005* |
| Clinical N stage | ||
| N0 | Reference | |
| N1 | 0.89 (0.78–1.03) | 0.120 |
| N2 | 0.73 (0.56–0.94) | 0.053* |
| N3 | 0.94 (0.53–1.66) | 0.808 |
| Unknown | 0.50 (0.31–0.79) | 0.004* |
| Chemotherapy radiation sequence | ||
| Induction chemotherapy | Reference | |
| Concurrent | 0.90 (0.71–1.15) | 0.402 |
| Time from completion of radiation to surgery | ||
| <10 weeks | Reference | |
| ≥10 weeks | 1.29 (1.10–1.51) | 0.001* |
| Radiation therapy dose | ||
| Lower dose: 41.4 to 45 Gy | Reference | |
| Higher dose: 50 to 54 Gy | 1.24 (1.06–1.44) | 0.006* |
*, statistically significant; #, n (%) in group no pCR and pCR: lower dose, 1,907 (83.7)/373 (16.3); higher dose, 4,570 (79.7)/1,167 (20.3). pCR, pathologic complete response.
Separate multivariate logistic regressions were performed for each of the secondary endpoints, which did not show any significant differences between HD-RT and LD-RT groups (data not shown). The rate of positive surgical margins was 6.1% with HD-RT and 6.3% with LD-RT; there was no statistically significant difference (OR 0.99; 95% CI: 0.78–1.24; P=0.908). In terms of 90-day postoperative mortality, the rate was 6.3% with HD-RT and 5.3% with LD-RT; again there was no statistically significant difference (OR 1.17; 95% CI: 0.94–1.45; P=0.173). Finally, in terms of readmission within 30 days of discharge, rate was 6.6% in HD-RT group and 6.5% in the LD-RT group with no statistically significant difference (OR 1.03; 95% CI: 0.84–1.25; P=0.784).
Discussion
To our knowledge, this is the first study to comprehensively compare RT dose of 41.4–45 Gy to dose of 50–54 Gy in esophageal cancer. Our analysis reveals several notable findings. In the neoadjuvant setting, there was no association between RT dose and survival, readmission rates, or early postoperative mortality. However, the higher dose of 50–54 Gy was associated with an improved pCR rate, particularly in cases with adenocarcinoma histology.
To our knowledge, there are no randomized studies comparing these two dose regimens for esophageal cancer. We found no difference in OS between these two dose ranges, supporting the recommended dose ranges in national guidelines (3,4). This is consistent with low rates of in-field recurrence using 41.4 Gy in the CROSS trial (18). A prior NCDB analysis by Haque et al. investigated a smaller cohort and narrower dose ranges, similarly finding no difference in OS (19). Notably, we did observe improved pCR range with higher dose in patients with adenocarcinoma, consistent with several contemporary trials indicating a dose-response relationship in esophageal cancer (20-22).
We note that the rates of pCR in our study were lower than those in the CROSS trial. By histology, 28 of 131 patients with adenocarcinoma (23%) and 18 of 37 patients with SCC (49%) achieved pCR in CROSS compared to 17% and 33% in our study (5). However, we note several differences in patient cohort and methodology that make direct comparison difficult. Our study cohort contains a higher proportion of patients with adenocarcinoma, T4 tumors, and esophageal rather than GEJ tumors. Additionally, the CROSS trial protocol specified five cycles of carboplatin/paclitaxel as chemotherapy and preference for surgery within 4–6 weeks of neoadjuvant therapy, whereas patients in this national sample had significant variation in terms of chemotherapy regimens and resection timing.
Several authors have raised concerns regarding postoperative complications associated with neoadjuvant CRT (23-26). Markar et al. found substantially increased in-hospital mortality and complication rates in salvage esophagectomy patients receiving ≥55 Gy (27); however, at the RT dose ranges used in the CALGB 9781 and CROSS trials, the relationship between dose and postoperative morbidity/mortality is unclear. Although detailed data regarding postoperative complications was not available, our findings indicate no differences in postoperative mortality and readmissions between 41.4–45 and 50–54 Gy.
Notably, we identified a substantial portion of patients receiving 41.4–45 Gy who do not undergo esophagectomy. Although prior studies in the definitive setting have not demonstrated a survival benefit for dose escalation, these studies all analyzed dose escalation above 50 Gy (12,28-30). In our analysis, patients receiving LD-RT of 41.4–45 Gy without esophagectomy had significantly worse survival. Radiation doses ≤45 Gy may be insufficient for macroscopic disease control (31). Although we are unable to precisely delineate treatment intent, further research is needed to elucidate the underlying reasons for the absence of surgical resection in this patient population.
Our study is subject to the standard limitations of retrospective analysis and database studies, with limited capacity to control for factors such as chemotherapy regimen, medical comorbidities, clinical tumor extent. Detailed information regarding chemotherapy regimens, including use of specific agents and number of cycles, is not available within the NCDB. Additionally, we note that utilization of 41.4 Gy per CROSS trial was low (16.4% of the LD-RT group). This is likely attributable to the publication of CROSS in the year 2012, near the end of our study period, and subsequent slow adoption (5). Treatment intent is not included in the NCDB, and therefore we cannot distinguish between patients who were intended for neoadjuvant CRT but could not undergo esophagectomy versus those who failed to complete a prescribed course of definitive CRT. Despite these limitations, we feel that our analysis offers significant value in the absence of randomized data. We look forward to the results of clinical trials investigating this question (32).
Our results suggest that both HD-RT and LD-RT regimens are viable and offer similar rates of OS, 90 day postoperative mortality, and 30 day readmission rate. However, if esophagectomy is not performed, HD-RT is associated with superior survival outcomes, although this finding must be interpreted with caution as it does not account for treatment intent. Therefore, in cases where the eventual surgical resectability is uncertain, a higher RT dose of 50.4 Gy may be advantageous. Future prospective trials are needed to further validate our findings.
Acknowledgments
None.
Ethical Statement: This study uses only de-identified data from the National Cancer Database and is considered exempt from Institutional Board Review.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA. Cancer J Clin 2018;68:7-30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2016;27:v50-7. 10.1093/annonc/mdw329 [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Guidelines: Esophageal and esophagogastric junction cancers. Version 2.2018. Available online: https://www.nccn.org. Accessed 10 July 2018.
- 5.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 6.Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 7.Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer 2017;81:183-90. 10.1016/j.ejca.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 8.Mariette C, Dahan L, Mornex F, et al. Surgery Alone Versus Chemoradiotherapy Followed by Surgery for Stage I and II Esophageal Cancer: Final Analysis of Randomized Controlled Phase III Trial FFCD 9901. J Clin Oncol 2014;32:2416-22. 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 9.Walsh TN, Noonan N, Hollywood D, et al. A Comparison of Multimodal Therapy and Surgery for Esophageal Adenocarcinoma. N Engl J Med 1996;335:462-7. 10.1056/NEJM199608153350702 [DOI] [PubMed] [Google Scholar]
- 10.Urba SG, Orringer MB, Turrisi A, et al. Randomized Trial of Preoperative Chemoradiation Versus Surgery Alone in Patients With Locoregional Esophageal Carcinoma. J Clin Oncol 2001;19:305-13. 10.1200/JCO.2001.19.2.305 [DOI] [PubMed] [Google Scholar]
- 11.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III Trial of Trimodality Therapy With Cisplatin, Fluorouracil, Radiotherapy, and Surgery Compared With Surgery Alone for Esophageal Cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. 10.1200/JCO.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J Clin Oncol 2002;20:1167-74. 10.1200/JCO.2002.20.5.1167 [DOI] [PubMed] [Google Scholar]
- 13.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: A Powerful Initiative to Improve Cancer Care in the United States. Ann Surg Oncol 2008;15:683-90. 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Surgeons. National Cancer Data Base: Participant user file data dictionary. Available online: http://ncdbpuf.facs.org. Accessed 13 November 2017.
- 15.International Classification of Diseases for Oncology. ICD-O-3 online. Available online: http://codes.iarc.fr/. Accessed 13 November 2017.
- 16.Giugliano DN, Berger AC, Keith SW, et al. Timing of Esophagectomy after Neoadjuvant Chemoradiation Therapy (nCRT) for Esophageal Cancer (EC) is Associated with Worse Outcomes, without Improvement of Complete Pathologic Response (pCR). J Am Coll Surg 2016;223:e76-7. 10.1016/j.jamcollsurg.2016.08.199 [DOI] [Google Scholar]
- 17.Bellera CA, MacGrogan G, Debled M, et al. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol 2010;10:20. 10.1186/1471-2288-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oppedijk V, van der Gaast A, van Lanschot JJB, et al. Patterns of Recurrence After Surgery Alone Versus Preoperative Chemoradiotherapy and Surgery in the CROSS Trials. J Clin Oncol 2014;32:385-91. 10.1200/JCO.2013.51.2186 [DOI] [PubMed] [Google Scholar]
- 19.Haque W, Verma V, Butler EB, et al. Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol 2018;9:80-9. 10.21037/jgo.2017.09.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Liao Z, Jin J, et al. Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:656-64. 10.1016/j.ijrobp.2004.06.022 [DOI] [PubMed] [Google Scholar]
- 21.Chang CL, Tsai HC, Lin WC, et al. Dose escalation intensity-modulated radiotherapy-based concurrent chemoradiotherapy is effective for advanced-stage thoracic esophageal squamous cell carcinoma. Radiother Oncol 2017;125:73-9. 10.1016/j.radonc.2017.08.025 [DOI] [PubMed] [Google Scholar]
- 22.Venkat PS, Shridhar R, Naghavi AO, et al. Dose escalated neoadjuvant chemoradiotherapy with dose-painting intensity-modulated radiation therapy and improved pathologic complete response in locally advanced esophageal cancer. Dis Esophagus 2017;30:1-9. 10.1093/dote/dox036 [DOI] [PubMed] [Google Scholar]
- 23.Bagheri R, RajabiMashhadi MT, Ghazvini K, et al. The effect of neoadjuvant chemoradiotherapy on airway colonization and postoperative respiratory complications in patients undergoing oesophagectomy for oesophageal cancer. Interact Cardiovasc Thorac Surg 2012;14:725-8. 10.1093/icvts/ivs009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Zhang H. Impact of neoadjuvant chemotherapy and chemoradiotherapy on postoperative cardiopulmonary complications in patients with esophageal cancer. Dis Esophagus 2017;30:1-7. [DOI] [PubMed] [Google Scholar]
- 25.Tapias LF, Mathisen DJ, Wright CD, et al. Outcomes With Open and Minimally Invasive Ivor Lewis Esophagectomy After Neoadjuvant Therapy. Ann Thorac Surg 2016;101:1097-103. 10.1016/j.athoracsur.2015.09.062 [DOI] [PubMed] [Google Scholar]
- 26.Klevebro F, Johnsen G, Johnson E, et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro-oesophageal junction: A randomized clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiation. Eur J Surg Oncol 2015;41:920-6. 10.1016/j.ejso.2015.03.226 [DOI] [PubMed] [Google Scholar]
- 27.Markar S, Gronnier C, Duhamel A, et al. Salvage Surgery After Chemoradiotherapy in the Management of Esophageal Cancer: Is It a Viable Therapeutic Option? J Clin Oncol 2015;33:3866-73. 10.1200/JCO.2014.59.9092 [DOI] [PubMed] [Google Scholar]
- 28.He L, Allen PK, Potter A, et al. Re-evaluating the Optimal Radiation Dose for Definitive Chemoradiotherapy for Esophageal Squamous Cell Carcinoma. J Thorac Oncol 2014;9:1398-405. 10.1097/JTO.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 29.Brower JV, Chen S, Bassetti MF, et al. Radiation Dose Escalation in Esophageal Cancer Revisited: A Contemporary Analysis of the National Cancer Data Base, 2004 to 2012. Int J Radiat Oncol Biol Phys 2016;96:985-93. 10.1016/j.ijrobp.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 30.Shao MS, Wong AT, Schwartz D, et al. Definitive or Preoperative Chemoradiation Therapy for Esophageal Cancer: Patterns of Care and Survival Outcomes. Ann Thorac Surg 2016;101:2148-54. 10.1016/j.athoracsur.2015.12.056 [DOI] [PubMed] [Google Scholar]
- 31.Okunieff P, Morgan D, Niemierko A, et al. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys 1995;32:1227-37. 10.1016/0360-3016(94)00475-Z [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov. Different Radiation Dose of Neoadjuvant Chemoradiation for Resectable Thoracic Esophageal Squamous Carcinoma. NLM Identifier: NCT03381651.