Abstract
Background
Metastatic pancreatic cancer (MPC) is associated with an extremely high mortality. Current NCCN guidelines recommend systemic therapy, as it is superior to best supportive care. Undertreatment of MPC continues to be an issue. Recent treatment and survival data of MPC in Veterans’ Affairs’ (VA) hospitals have not been published. The relationship between MPC treatment and survival and the American College of Surgeons’ (ACS) Committee on Cancer (CoC) accreditation in VA hospitals has not been studied.
Methods
Nationwide data from the National Veterans Affairs Cancer Cube Registry was analyzed. In total, 6,775 patients were diagnosed with MPC between 2000 and 2014. CoC accreditation of each VA hospital was obtained using the ACS website.
Results
MPC constitutes 52.31% of all pancreatic cancer diagnosed (6,775/12,951 cases). The near totality was men (97.44%). The above 70 years age group and the 60–70 years age group were the most common ages at diagnosis with 39.39% and 38.02% respectively. The proportion of early-onset pancreatic cancer (EOPC) was 2.84%. When compared to all stages of pancreatic cancer, stage IV pancreatic cancer had a lower proportion of cancer originating from the head of the pancreas (39.33% versus 50.63%) and more originating from the tail (17.99% versus 13.39%). Tumors originating from head of the pancreas are more likely to cause biliary symptoms and thus are more likely to be caught at an earlier stage. Overall, treatment rate in the VA at the national level with first-line chemotherapy was 37.61%. The rate of treatment over the years has increased in a linear fashion from 33.01% in 2000 to 41.95% in 2014. This has corresponded with an increase of 1–5 years survival of 9.29% in 2000 to 22.99% in 2014 and 5–10 years survival from 0.96% in 2000 to 6.00% in 2012. Treatment rates in CoC-accredited and non-CoC accredited VA hospitals were similar (38.94% and 38.12%, respectively). Survival rates in CoC-accredited and non-COC accredited VAs were similar with a 1–5 years survival rate of 8.89% and 8.57%, respectively.
Conclusions
Treatment and survival of MPC have risen significantly in the past decade at VA hospitals. CoC accreditation is not associated with a change in treatment or survival rates.
Keywords: Pancreatic neoplasms, pancreatic neoplasms/epidemiology, pancreatic neoplasms/therapy, pancreatic neoplasms/classification
Introduction
Pancreatic cancer is the twelfth most common cancer in incidence in the United States and the fourth most common cause of cancer-related death (1,2). The incidence of pancreatic cancer increased from 1999 to 2008, possibly due to increasing obesity and an aging population (3). Interestingly, mortality due to this disease remained largely unchanged with overall 1-year survival at 18% and 5-year survival at less than 5% (4,5). In 2017, over 53,000 new cases of pancreatic cancer were diagnosed and over 43,000 deaths were attributed to the disease (1).
Clinical staging of pancreatic cancer revolves around resectability, as surgical resection is a key factor in treatment of early pancreatic cancer. However, chemotherapy is indicated in all stages of the disease (2). Clinical staging ranges from resectable, to borderline resectable, locally advanced unresectable, and metastatic.
The prognosis of metastatic pancreatic cancer (MPC) is dismal, with life expectancy of less than one year at diagnosis (6). Chemotherapy remains the mainstay of treatment of metastatic disease. Given the poor prognosis and severe side effects of the chemotherapy agents used, functional status at diagnosis is of particular importance (2). Patients with poor performance status are generally recommended to undergo more palliative-based treatment. Traditionally, gemcitabine was the only available therapy in late stage disease. Recent trials (6-11) have shown multiple gemcitabine combinations as well as FOLFIRINOX to be effective in increasing median survival by several months, while decreasing quality of life impairment (12-16). Current NCCN guidelines (2) have been updated to recommend such regimens.
Despite the benefits of treatment, undertreatment of MPC remains an ongoing area of concern within the GI oncology community. A 1996 review of the National Cancer Database (NCDB) (17) revealed that only 35% of patients with MPC received chemotherapy. Two decades later, estimates rose to only 50% (18). This treatment deficiency is not particular to the United States, with a study in Nova Scotia, Canada (19) showing that only 36% of patients with non-resectable disease got referred to a medical oncologist and that only 33% of those referrals were completed. Similarly, a study in Ireland (20) showed that chemotherapy administration in unresected pancreatic cancer peaked at 20%. The treatment and survival rate of MPC in the VA has not been studied.
NCCN guidelines (2) currently recommend that clinical decision making regarding diagnostic management and resectability should involve multidisciplinary teams at a high-volume treatment centers. The American College of Surgeons has published multiple studies (17,21) demonstrating increased survival rate in early pancreatic cancer at larger academic medical centers.
The relationship between the American College of Surgeons commission of cancer accreditation and the treatment and survival rates of pancreatic cancer has not been studied in VA hospitals.
Methods
Nationwide data from the National Veterans Affairs Cancer Care Cube Registry (CCCR) (22) was analyzed. No patient charts were accessed. The main data source for the CCCR is the Oncology Domain tables on the Corporate Data Warehouse (CDW) raw server which is updated every 2 weeks. The Oncology Domain tables are created from the VISTA OncoTrax software package. The registry was accessed on 12/8/17, and data input after this date is not included in this study. Unique cases of pancreatic cancer with accession year between 2000 and 2014 were analyzed (as the CCCR first began recording data on 1/1/2000). The registry defines unique cases as those with the same combination of the following data points: patient SSN, diagnosis date, primary tumor site, sequence number, histology ICD 03 code, grade differentiation ID, and laterality. Accession year refers to the year in which the patient was first seen at the reporting institution for diagnosis and/or treatment of the primary cancer recorded. The registrar further classifies cases based on abstract status. “Complete” abstract status indicates that all data points have been entered by the Tumor Registrar for that particular case. Only cases with complete abstract status were considered for this study. The cancer primary site (pancreas) was identified by a computed VISTA field which recorded the primary site/group major body system. After application of the above qualifiers (unique cases, complete abstract, primary site, all stages, and accession year span 2000–2014) 12,951 total cases of pancreatic cancer were identified including 6,775 of MPC which were the cases analyzed in more detail.
Demographic data on the CCCR including age at diagnosis, gender, and survival were generated from the VA Health Eligibility Center (HEC) demographic file. Survival in the CCCR was defined as <1 year, 1–5 years, 5–10 years, 10–15, and >15 years. Race, and ethnicity were derived from the CDW. Anatomic site/distribution was defined by ICD-10 codes CD25.0 through CD25.9. Treatment was analyzed as first course treatment groups where patients received one of the following eight groups chemotherapy, radiation therapy, surgery, immunotherapy, hormone therapy, other, unknown, or no therapy. Chemotherapeutic agents administered as part of the first course of treatment were analyzed. Up to 5 chemotherapy agents can be recorded in VISTA OncoTrax as part of a cancer case’s first course of treatment. The Surveillance, Epidemiology and End Results (SEER) Self-Instructional Manual for Tumor Registrars Book 8-Antineoplastic Drugs, third edition is the data source for the chemotherapy agents Demographic characteristics including race and ethnicity were determined based on information provided by patients at initial contact with the VA hospital. Local IRB approval was obtained for the study.
The American College of Surgeons (ACS) Committee on Cancer (CoC) accreditation status of each VA hospital was obtained from the ACS website (23). The VA oncology directory was employed to verify accreditation data found on ACS website.
Microsoft Excel was used for data tabulation and graph formulation. Discreet data points were described using percentages and compared using Chi-squared test with two-sided P value of <0.05 as statistically significant. Graph slopes and Chi-squared tests were calculated using Vassarstats (24).
Results
Demographic data
Of the 12,951 cases of pancreatic cancer diagnosed among the VA patient population nationwide in the years spanning 2000 to 2014, 6,775 (52.31%) had stage IV pancreatic cancer at diagnosis. The demographic data of the VA cancer cube population is described in Table 1. The quasi-totality of the population is male with 12,619 men diagnosed (97.44%) with pancreatic cancer. Almost two-thirds of the population identified as White (7,878 or 62.43%), with the other major subgroup identifying as Black or African American 2,036 (16.13%). Most patients [9,675] identified as Non-Hispanic (76.67%). Across all demographic subtypes, stage IV pancreatic cancer was the most common stage at diagnosis.
Table 1. VA Cancer Cube pancreatic cancer patients [2000–2014].
| Variable | Stage | N (total) | |||||
|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | Unknown | ||
| n | 55 | 1,218 | 2,333 | 1,390 | 6,775 | 1,208 | 12,951 |
| Incidence (%) | 0.42 | 9.40 | 18.01 | 10.73 | 52.31 | 9.33 | 12,951 |
| Gender (%) | |||||||
| Female | 0.90 | 9.64 | 14.76 | 10.24 | 50.60 | 14.16 | 332 |
| Male | 0.41 | 9.40 | 18.10 | 10.75 | 52.36 | 9.20 | 12,619 |
| Race (%) | |||||||
| American Indian/Alaskan native | 0.00 | 14.75 | 16.39 | 8.20 | 50.82 | 9.84 | 61 |
| Asian | 4.00 | 8.00 | 20.00 | 12.00 | 44.00 | 12.00 | 25 |
| Black or African American | 0.34 | 8.20 | 18.71 | 11.25 | 53.00 | 8.64 | 2,036 |
| Declined to answer | 0.58 | 11.34 | 21.22 | 13.08 | 45.64 | 8.43 | 344 |
| Multiple | 1.96 | 9.80 | 25.49 | 9.80 | 41.18 | 12.75 | 102 |
| Native Hawaiian or Pacific Islander | 1.56 | 7.81 | 20.31 | 20.31 | 46.88 | 3.13 | 64 |
| Unknown | 0.08 | 7.41 | 10.49 | 8.68 | 59.69 | 13.72 | 2,441 |
| White | 0.51 | 10.22 | 19.92 | 11.08 | 50.37 | 8.17 | 7,878 |
| Ethnicity (%) | |||||||
| Declined to answer | 0.67 | 9.73 | 19.13 | 12.08 | 51.01 | 7.72 | 298 |
| Hispanic or Latino | 0.64 | 10.94 | 18.03 | 11.59 | 50.00 | 9.01 | 466 |
| Non-Hispanic or Latino | 0.50 | 9.98 | 20.00 | 11.21 | 50.45 | 8.09 | 9,675 |
| Unknown | 0.08 | 6.85 | 10.23 | 8.56 | 60.07 | 14.33 | 2,512 |
The most common age at diagnosis of MPC is greater than 70 years old with 2,669 (39.39%) patients diagnosed beyond this age. The sixth decade (years 60–69) comes in second with 2,576 (38.02%) of the population diagnosed in this age range, while 1,335 (19.7%) patients were diagnosed between the ages of 50 and 59 (Figure 1).
Figure 1.
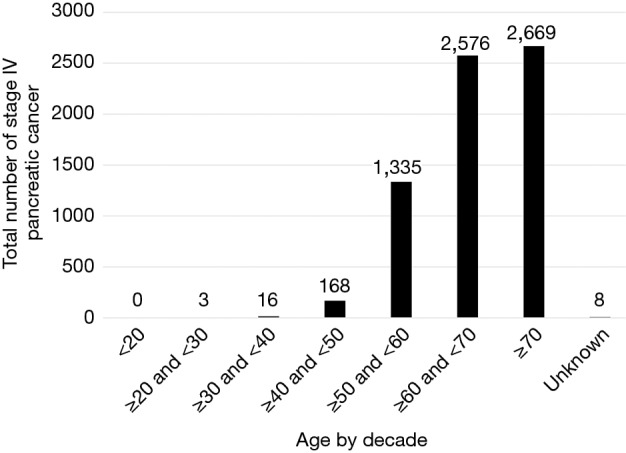
Age distribution of stage IV pancreatic cancer. Patients affected by MPC are older with 39.39% over the age of 70 and 38.02% between 60 and 69 years .
Anatomic site, functional performance status
The anatomic distribution of all stages of pancreatic cancer and MPC are detailed in Figure 2. Of note, while the head of the pancreas constitutes 6,506 (50.63%) cases of all cancers identified, only 2,656 (39.33%) cases originated at this site among patients with MPC. The tail of the pancreas constituted 4.6% more cases of MPC compared to pancreatic cancer at all stages.
Figure 2.
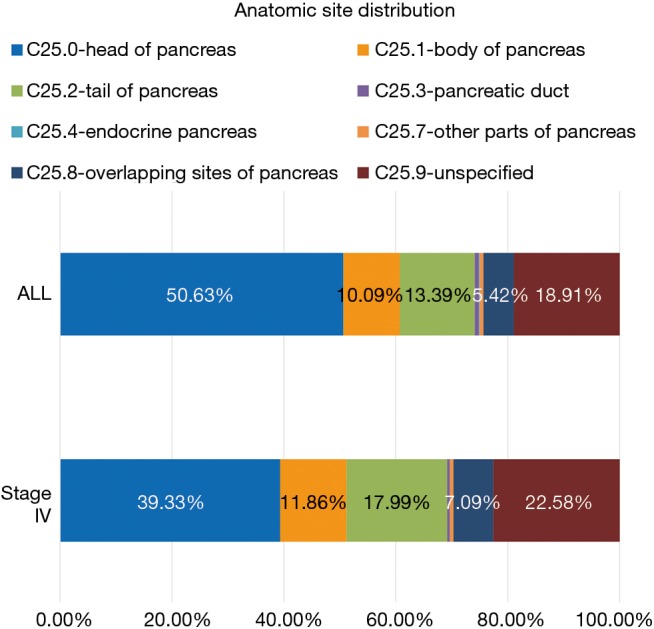
Anatomic distribution of metastatic pancreatic cancer (MPC) and pancreatic cancer. Tail of pancreas constitutes a higher fraction of MPC at presentation.
The functional performance status as calculated by the ECOG-PS score is shown in Figure 3 for all stages. The other established system of ranking performance status, the Karnofsky Performance Status was used in a negligible number of charts. The functional status appears to trend down as the stage at diagnosis advances. For example, 36.41% [150] of stage I patients have a functional ECOG-PS score of 0 while only 20% [373] of stage IV patients have an ECOG-PS of 0. For stage I patients, 7.04% [29] of patients had an ECOG-PS score of 3, and 14.39% [266] of patients diagnosed at stage IV had this ECOG-PS score.
Figure 3.
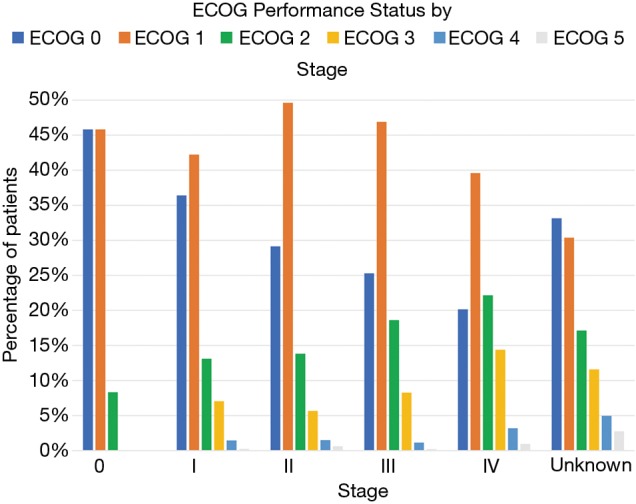
ECOG-PS distribution of pancreatic cancer. Stage IV cancer presents with a worse performance status with increasing numbers of ECOG 2, 3 and 4 (22.17%, 14.39% and 3.19% respectively).
Treatment & survival
Table 2 illustrates the treatment rates of stage IV pancreatic cancer. Out of a total of 6,775 cases, 2,548 (37.61%) received chemotherapy as part of their first course of treatment. The first course of treatment also included 359 patients (5.30%) who received radiation and 198 (2.92%) who underwent surgery. Thirty-four patients (0.50%) underwent hormonal therapy, underscoring the fact that our population contained a few tumors with neuroendocrine features (19 histological cases of adenocarcinoma with neuroendocrine differentiation our of 2,296 histology case were reported). Overall, 41.33% of patients received a defined therapy, while 20.97% of cases were classified as unknown. Table 3 lists the individual chemotherapy agents used in the anti-neoplastic chemotherapy regimens. Of the 2,548 patients who received chemotherapy, 1,992 (78.18%) included agents that were approved by the NCCN guidelines. As expected, Gemcitabine was by far the most used anti-neoplastic agents used in 1,080 cases (42.39%). A list of rarely used non-standard chemotherapy is also provided.
Table 2. Treatment rate of metastatic pancreatic cancer (N=6,775).
| Therapy | N | % |
|---|---|---|
| Chemotherapy | 2,548 | 37.61 |
| Radiation therapy | 359 | 5.30 |
| Surgery | 198 | 2.92 |
| Immunotherapy | 26 | 0.38 |
| Hormone therapy | 34 | 0.50 |
| Other therapy | 51 | 0.75 |
| Unknown | 1,421 | 20.97 |
| No therapy | 2,554 | 37.70 |
Table 3. Chemotherapy agents used in treatment of metastatic pancreatic cancer (N=2,548).
| Chemotherapy | N | % |
|---|---|---|
| NCCN-approved | ||
| Capecitabine | 102 | 4.00 |
| Erlotinib | 131 | 5.14 |
| Fluorouracil | 102 | 4.00 |
| Gemcitabine | 1,080 | 42.39 |
| Oxaliplatin | 278 | 10.91 |
| Nab-Paclitaxel | 105 | 4.12 |
| Total NCCN-approved | 1,992 | 78.18 |
| Other (>10 patients treated) | ||
| Carboplatin | 21 | 0.82 |
| Cisplatin | 30 | 1.18 |
| Dexamethasone | 22 | 0.86 |
| Doxorubicin | 16 | 0.63 |
| Docetaxel | 15 | 0.59 |
| Etoposide | 25 | 0.98 |
| Sunitinib | 19 | 0.75 |
| Unknown | 30 | 1.18 |
Not listed are agents used on less than 10 patients: Bendamustine, Camptothecin, Cyclocytidine, Cyclophosphamide, Epirubicin, Everolimus, Floxuridine, Leucovorin, Lomustine, Mitomycin, Octreotide, Pemetrexed, Streptozocin, RAD001, Temozolomide, Vincristine.
The dismal prognosis of MPC is illustrated in Figure 4. Overall, 5,771 out of 6,775 (85.18%) survived less than a year with only 699 (10.32%) making it between one and five years. The percentage of MPC patients receiving chemotherapy from 2000 to 2014 is charted in Figure 5A. Chemotherapy was chosen as the treatment modality of choice as it is the first-line treatment, per NCCN guidelines. A linear trend (r2=0.73; slope =0.85) illustrates the uptick in treatment rates in systemic therapy. The lowest treatment rate recorded was in 2002 with 108/388 malignancies (27.84%) being treated while the highest was in 2014 with 219/522 cases (41.95%). As the large majority of MPC survive less than a year, the increase in percentages of patients surviving 1–10 years was selected as a surrogate for increase in survival. The concomitant increase in both 1–5 years and 5–10 years survival rates is demonstrated in Figure 5B. Significantly, the trendlines for both were polynomial with an order of 2, showing a larger increase in survival than in treatment. These trends correlate with the increased use of novel chemotherapy agents in recent years as they gained approval from both the FDA and the NCCN.
Figure 4.
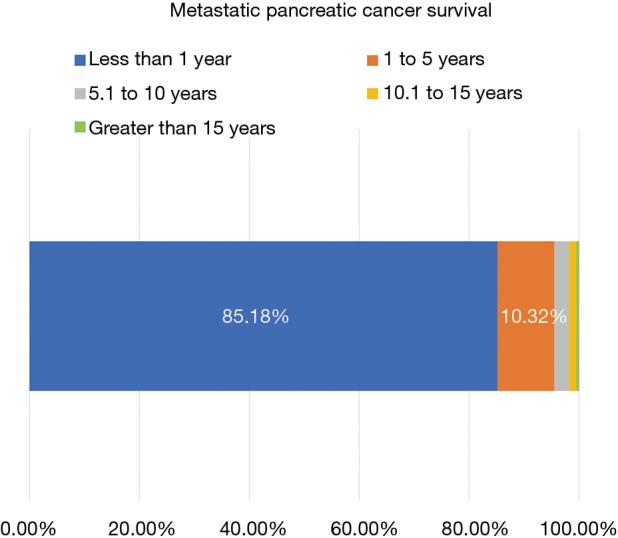
Survival of metastatic pancreatic cancer (MPC). Stage IV cancer has a dismal prognosis with 85.18% surviving less than 1 year.
Figure 5.
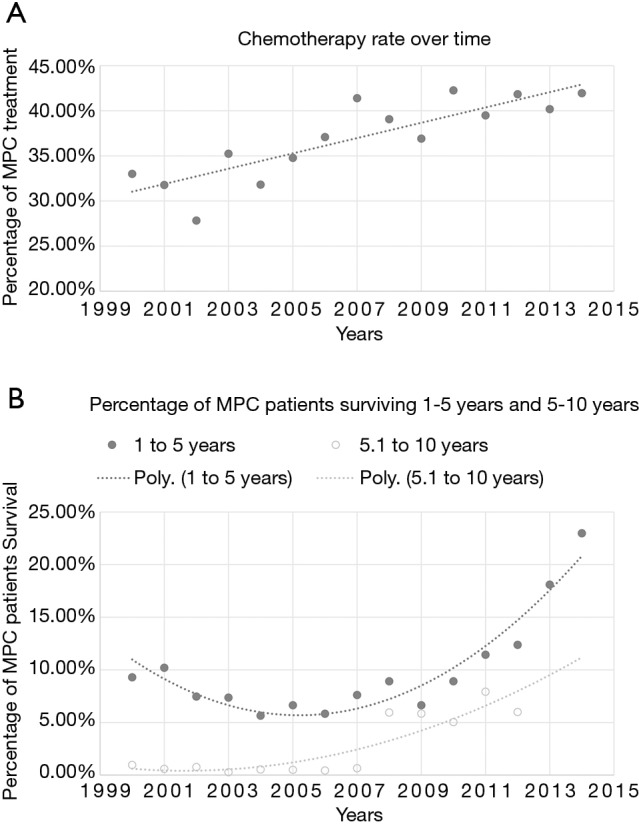
Timeline showing increase in treatment (A) and survival (B) of metastatic pancreatic cancer (MPC). (A) Linear trend (r2=0.73 and slope =0.85) shows increase in treatment of stage IV pancreatic cancer over time; (B) the increase in survival is more significant than the increase treatment rate, as shown by the trendline.
The aggregated accreditation status of all VA hospitals by the ACS CoC is shown in Table 4. The specific accreditation status of each individual VA hospital is detailed in Figure S1. The interested reader can find them in Supplementary file online. Thirty-nine hospitals treating 3,669 patients were ACS-certified while 93 hospitals taking care of 4,116 patients were not certified. The larger patient load of the CoC-accredited hospitals represents their presumed status as a referral center. The chemotherapy treatment rates of CoC-accredited and non-accredited VA hospitals is similar with 38.94% and 38.12%, respectively (P value =0.466). Survival rates in CoC-accredited and non-CoC accredited VAs were similar with a 1–5 years survival rate of 12.35% and 11.83% (P value =0.5071) and a 5–10 years survival rates of 2.61% and 2.12% (P value =0.175).
Table 4. Metastatic pancreatic cancer in VA hospitals accredited and non-accredited by the Commission on Cancer.
| Variable | VA hospitals | Total | P value | |
|---|---|---|---|---|
| CoC-accredited | CoC non-accredited | |||
| Number of hospitals | 39 | 93 | 132 | – |
| Number of MPC patients | 3,669 | 4,116 | 7,785 | – |
| Treatment rate (%) | 38.94 | 38.12 | – | 0.466 |
| 1–5 years survival rate (%) | 12.35 | 11.83 | – | 0.5071 |
| 5–10 years survival rate (%) | 2.61 | 2.12 | – | 0.175 |
CoC, Committee on Cancer; MPC, metastatic pancreatic cancer.
Discussion
Slightly more than half of all VA patients diagnosed with pancreatic cancer between 2000 and 2014 had metastatic disease at the time of diagnosis (Table 1). This is consistent with previously published reports in the literature (25). This finding holds true even though most of the VA database patients identified as male and white. The distribution of pancreatic cancer by stage did not vary by race, as there was no clinical significance in the percentage of patients diagnosed at each stage in whites, blacks and other races.
Our data shows that MPC remains a disease of the elderly with 77.38% of cases in our database diagnosed after the age of 60 (Figure 1). We also demonstrate that 2.76% of MPCs and 2.84% of pancreatic cancers are diagnosed before the age of 50. This is slightly lower than the national rates of early-onset pancreatic cancer (EOPC) incidence which range 5% and 10% (26,27). The VA patient population being over-represented by the elderly (28) could explain the lower proportion of EOPC.
About 50% of all pancreatic cancer presented at the head of the pancreas (Figure 2). With the bulk of pancreatic tissue concentrated in the head, this finding is not surprising. Other epidemiological studies (29-31) have shown the head of the pancreas to be the site of 56–78% of pancreatic cancers, depending on the study. Our lower incidence of pancreatic cancer originating in the head of the pancreas could be due to the fact that almost 19% of pancreatic cancers in our database were coded as “unspecified” in regard to anatomic location within the pancreas. When examining MPC, the percentage originating in the pancreatic head diminishes from 50% to 39%, and the percentage originating in the pancreatic tail increases from 13% to 18%. This is likely explained by the vicinity of the pancreatic head to the biliary system often resulting in biliary obstruction and associated symptoms prior to spread of pancreatic malignancy. Pancreatic cancer in the tail has been linked to new-onset diabetes (32,33), a vague and wide-spread diagnosis that can easily be overlooked and attributed to old age, obesity, and the metabolic syndrome. The finding of sudden new-onset diabetes paired with systemic symptoms such as fatigue and weight loss should prompt consideration of underlying pancreatic malignancy. The widespread nature of type 2 diabetes in the general population renders using hyperglycemia as a tool to screen for pancreatic cancer problematic but coupling it with a serological marker of pancreatic cancer might offer a reasonable solution (32). Cost and feasibility data regarding screening based on such findings is not yet available.
The percentage of MPC that received standard of care chemotherapy as first-course treatment was 37.61% while 37.7% did not receive any form of therapy (Table 2). About 5% received radiation therapy while about 3% received surgery; likely in a palliative setting. About 0.50% received hormonal therapy, reflecting the miniscule share of pancreatic neuro-endocrine tumors (PNET) amongst all pancreatic cancer. About 20.97% of patients received treatment labelled as “Unknown”. These include cancer patients diagnosed at the VA who decided to seek care at outside facilities. This large share, about a fifth of the database, makes the treatment rate harder to accurately pin down. When compared to national averages (~50%) (18), the VA treatment rate is lower. Estimating the number of MPC eligible for chemotherapy based on performance status is especially hard as the reported documentation of ECOG-PS in the VA Cancer Care Cube is only 28.59% (3,692/12,951) and ECOG itself is a subjective measure. Overall, the estimate of MPC eligible for treatment was about 60% (ECOG 0: 20% and ECOG 1: 40%).
Of the chemotherapy regimens used, Gemcitabine-based chemotherapy was by far the most popular with 42.39% of patients receiving it (Table 3). Oxaliplatin, as part of FOLFORINOX, was the second most common regimen. These numbers reflect adherence to NCCN guidelines.
Our survival data reflects the dismal prognosis MPC carries. Our 1-year survival is slightly less than 15% and our 5-year survival is less than 5%. Unfortunately, the data input method used in the current version of the VA Cancer Cube does not allow us to create a survival curve.
Treatment rate over the past 15 years has increased in a linear fashion (Figure 5); with the rate of chemotherapy administered in the early 2000s between 25% and 35% and the rate of chemotherapy after 2010 between 39% and 45%. During the same period, overall survival has increased exponentially (Figure 5B). The percentage of MPC patients living 1 to 5 years slowly decreased from 10% to 5% from 2000 to 2007 and later picks up to above 10% in 2011. The change in the percentage of patients living 5 to 10 years is even starker with the number increasing from 1% to over 5% after 2008. The steeper increase in survival than treatment rate likely reflects the increase in number of available chemotherapy regimens.
Data from our own local QI at the Stratton Veterans Affairs Medical Center (SVAMC) showed a 54.28% treatment rate for MPC. SVAMC is accredited by the American College of Surgeons Commission on Cancer. Multiple studies have shown increased survival in pancreatic cancer patients in high-volume centers and centers accredited by the CoC. In fact, the NCCN guidelines recommend determining resectability at a high-volume center. We examined the relationship between CoC accreditation and treatment and survival rates of MPC within the VA network of hospitals. As expected CoC accredited VA hospitals treated a higher volume of MPC patients. However, there was no statistically significant difference in either treatment or survival rates between CoC accredited and non-accredited facilities. This may represent the homogenous uniformity seen in the VA, as opposed to the private sector. Given the scarcity of high volume VA centers nationwide many patients have to travel long distances to reach such centers, losing valuable time in the setting of an aggressive disease. The decision to travel such distances may be worth reconsidering given the lack of difference in treatment and survival rates.
Conclusions
Incidence of MPC in the VA is similar to the general population. EOPC is slightly under-represented, likely due to the older VA population. While treatment and survival of MPC have risen significantly in the past decade at VA hospitals, undertreatment of MPC remains a concern. The availability of novel anti-neoplastic agents has resulted in increased survival of MPC patients. The ACoS CoC accreditation of VA hospitals is not associated with a change in treatment or survival rates of MPC. This likely reflects the high degree of uniformity and conformity of standards of care amongst VA hospitals.
Figure S1.
Italicized hospitals are CoC-accredited, while underlined are not. CoC-accredited VA hospitals are italicized and CoC-non-accredited hospitals are underlined. Accreditation status obtained in September 2017.
Acknowledgments
We would like to thank Michael Kelley MD, Laurence Kaminsky PhD and Tariq Khreis MD for their support of our work and their reading of our manuscript and Patty Coke for her help with VA Cancer Cube database.
Ethical Statement: The research was approved by the ethical committee-U.S/Department of Health and Human Services (HHS) Registration of an Institutional Review Board (IRB) (No. IORG0000615). Informed consent is not required as this research was retrospective study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.NCCN Guidelines Pancreatic adenocarcinoma. Version 3. 2017.
- 3.Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-28. 10.3322/caac.20141 [DOI] [PubMed] [Google Scholar]
- 4.StatBite U.S. pancreatic cancer rates. J Natl Cancer Inst 2010;102:1822. 10.1093/jnci/djq517 [DOI] [PubMed] [Google Scholar]
- 5.Worni M, Guller U, White RR, et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas 2013;42:1157-63. 10.1097/MPA.0b013e318291fbc5 [DOI] [PubMed] [Google Scholar]
- 6.Golan T, Sella T, Margalit O, et al. Short- and Long-Term Survival in Metastatic Pancreatic Adenocarcinoma, 1993-2013. J Natl Compr Canc Netw 2017;15:1022-7. 10.6004/jnccn.2017.0138 [DOI] [PubMed] [Google Scholar]
- 7.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 8.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 9.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 10.Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640-8. 10.1200/JCO.2012.43.3680 [DOI] [PubMed] [Google Scholar]
- 11.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristensen A, Vagnildhaug OM, Gronberg BH, et al. Does chemotherapy improve health-related quality of life in advanced pancreatic cancer? A systematic review. Crit Rev Oncol Hematol 2016;99:286-98. 10.1016/j.critrevonc.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 13.Zabernigg A, Giesinger JM, Pall G, et al. Quality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tract. BMC Cancer 2012;12:390. 10.1186/1471-2407-12-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. 10.1093/oxfordjournals.annonc.a010676 [DOI] [PubMed] [Google Scholar]
- 15.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23-9. 10.1200/JCO.2012.44.4869 [DOI] [PubMed] [Google Scholar]
- 16.Hagiwara Y, Ohashi Y, Uesaka K, et al. Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: Results from a randomised phase III trial (JASPAC 01). Eur J Cancer 2018;93:79-88. 10.1016/j.ejca.2018.01.081 [DOI] [PubMed] [Google Scholar]
- 17.Janes RH, Niederhuber JE, Chmiel JS, et al. National patterns of care for pancreatic cancer. Results of a survey by the Commission on Cancer. Ann Surg 1996;223:261-72. 10.1097/00000658-199603000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enewold L, Harlan LC, Tucker T, et al. Pancreatic cancer in the USA: persistence of undertreatment and poor outcome. J Gastrointest Cancer 2015;46:9-20. 10.1007/s12029-014-9668-x [DOI] [PubMed] [Google Scholar]
- 19.Hurton S, Urquhart R, Kendell C, et al. Variations in Medical Oncology Utilization for Pancreatic Cancer Patients in Nova Scotia. JOP 2016;18:62-8. [Google Scholar]
- 20.Sharp L, Carsin AE, Cronin-Fenton DP, et al. Is there under-treatment of pancreatic cancer? Evidence from a population-based study in Ireland. Eur J Cancer 2009;45:1450-9. 10.1016/j.ejca.2009.01.033 [DOI] [PubMed] [Google Scholar]
- 21.White MG, Applewhite MK, Kaplan EL, et al. A Tale of Two Cancers: Traveling to Treat Pancreatic and Thyroid Cancer. J Am Coll Surg 2017;225:125-36.e6. 10.1016/j.jamcollsurg.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 22.Coke P, Gill T. National Cancer Care Cube. 2014 AVAHO Meeting; Portland, Oregon: Federal Practitioner, 2014. [Google Scholar]
- 23.Surgeons ACoS Available online: https://www.facs.org/search/cancer-programs, 2017.
- 24.Lowry R. VassarStats. Available online: http://vassarstats.net/, 2018.
- 25.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 26.Raimondi S, Maisonneuve P, Lohr JM, et al. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev 2007;16:1894-7. 10.1158/1055-9965.EPI-07-0341 [DOI] [PubMed] [Google Scholar]
- 27.Piciucchi M, Capurso G, Valente R, et al. Early onset pancreatic cancer: risk factors, presentation and outcome. Pancreatology 2015;15:151-5. 10.1016/j.pan.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 28.Horgan C, Taylor A, Wilensky G. Aging veterans: will they overwhelm the VA medical care system? Health Aff (Millwood) 1983;2:77-86. 10.1377/hlthaff.2.3.77 [DOI] [PubMed] [Google Scholar]
- 29.Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371-6. 10.1080/13651820802291233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg 1999;189:1-7. 10.1016/S1072-7515(99)00075-7 [DOI] [PubMed] [Google Scholar]
- 31.Modolell I, Guarner L, Malagelada JR. Vagaries of clinical presentation of pancreatic and biliary tract cancer. Ann Oncol 1999;10 Suppl 4:82-4. 10.1093/annonc/10.suppl_4.S82 [DOI] [PubMed] [Google Scholar]
- 32.Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10:88-95. 10.1016/S1470-2045(08)70337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Herrington M, Larsson J, et al. The relationship between diabetes and pancreatic cancer. Molecular Cancer 2003;2:4. 10.1186/1476-4598-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]



