Abstract
Background
Capecitabine (Cap) is an established treatment alternative to 5-fluorouracil (5-FU) for chemoradiation in rectal cancer. Few studies have compared the two agents in anal cancer. We compared outcomes and toxicities using Cap versus 5-FU in non-metastatic anal cancer patients at Stanford.
Methods
All non-metastatic anal cancer patients treated with definitive chemoradiation at Stanford from 1997–2016 were included. Fisher’s exact and Mann-Whitney U tests were used to compare nominal and continuous variables. Gray’s test was used to compare incidence of recurrence and colostomy, and Log-rank test was used to compare survival.
Results
Sixty-eight patients were included. Thirty-six patients received Cap and 32 received 5-FU (12 received standard 5-FU and 20 received low-dose continuous 5-FU). Patient characteristics were similar between the two groups. There was no difference in the 3-year overall and disease-specific survival between Cap and 5-FU (94% vs. 80%, P=0.197; 100% vs. 86%, P=0.051). Overall incidence of recurrence was equivalent between Cap and 5-FU (11% vs. 13%, P=0.703), but incidence of locoregional recurrence was higher in the 5-FU group (0% vs. 13%, P=0.042); patients treated with Cap had longer recurrence-free intervals (18 vs. 6 months, P=0.400), and all recurrences were distant. More colostomies were needed with 5-FU (3% vs. 13%, P=0.133). Toxicities were similar between the two groups. The most common grade ≥2 toxicities were dermatitis (77%), anal pain (78%), and diarrhea (56%).
Conclusions
Overall survival (OS), cancer-specific survival and incidence of recurrence were equivalent between Cap and 5-FU in anal cancer. Patients treated with Cap had statistically significant lower incidence of loco-regional relapses.
Keywords: Anal cancer, capecitabine (Cap), 5-fluorouracil (5-FU), cancer-specific survival, recurrence
Introduction
Anal cancer is a relatively rare malignancy, accounting for approximately 2.5% of all gastrointestinal (GI) cancers in the US (1,2). HIV and HPV infection, history of cervical cancer or cervical intraepithelial neoplasia, anal warts, and smoking are risk factors for the development of anal cancer (3). As this at-risk population grows in absolute numbers, so have the total number of anal cancer cases, fostering a growing interest in this disease (4).
Treatment for locally advanced anal cancer has evolved from abdominoperineal resection (APR) and permanent colostomy for all patients, to treatment with a combination of radiotherapy (RT) and radio-sensitizing chemotherapy using 5-fluorouracil (5-FU) and mitomycin-C (MMC). Most patients today are treated on the Nigro protocol, with 5-FU delivered via a 96 hour infusion on days 1–4 and 29–32, MMC given on days 1 and 29, and a total dose of ~45 Gy radiation delivered over 22 sessions (1,5). Five-year overall survival (OS) is excellent, averaging between 72–89% (1).
Although this regimen is typically well tolerated, toxicities force dose reductions and treatment breaks in 20–55% of patients treated using the Nigro protocol (6,7). There is evidence in other GI malignancies that a protracted infusion of 5-FU at a low dose of 200 mg/m2/day throughout the duration on RT, instead of the 96-hour infusions used in the Nigro protocol, is better tolerated (8). In anal cancer, two studies showed promising results (equivalent survival and lower toxicities) using protracted 5-FU infusion and another study showed increased loco-regional relapse rates with low-dose protracted 5-FU and MMC compared to cisplatin and MMC (9-11). Although low-dose protracted 5-FU infusion is not standard of care in anal cancer, some oncologists have favored this approach both due to its better tolerability, and because of the theoretical benefit of having continuous exposure to radio-sensitizing chemotherapy throughout the duration of RT.
Capecitabine (Cap) is an oral pro-drug preferentially converted to 5-FU at the tumor site (12). Numerous studies, including a large randomized controlled study in rectal cancer, have shown equivalent outcomes between Cap and 5-FU infusion in several GI malignancies (13,14). In anal cancer, a number of studies have been published using Cap in place of 5-FU, but few studies have examined Cap vs. 5-FU (7,12,15,16).
Cap offers obvious benefits over 5-FU infusion as it can be conveniently taken at home, and obviates the need for central venous catheter placement and its associated complications. Additionally dosing Cap daily with RT emulates a protracted low-dose 5-FU infusion, and thus may be associated with lower hematological toxicities and may be overall better tolerated than the 96-hour 5-FU infusion (7).
In this paper, we retrospectively reviewed anal cancer patients treated at Stanford in order to compare outcomes and toxicities between 5-FU and Cap, adding to the growing retrospective data comparing these two regimens in anal cancer. In addition, our experience is unique because several patients were treated with low-dose continuous 5-FU throughout their RT treatment course, instead of 96-hour 5-FU infusions during weeks 1 and 5 of treatment.
Methods
Patient population
IRB approval was obtained from Stanford Cancer Institute. Patients with non-metastatic anal cancer who received curative-intent radiation in combination with either Cap or 5-FU at our institution from January 1997 through January 2016 were retrospectively reviewed. Patients were included if they had biopsy-proven anal cancer, received entirety of RT at Stanford, and received chemotherapy either at Stanford or at the Palo Alto VA. Patients were clinically staged via digital rectal exam/anoscopy, CT abdomen-pelvis, and routine lab work. Patients were excluded if they presented with metastatic disease, or if they received cisplatin-based or induction chemotherapy.
Treatment details and toxicity evaluation
We extracted data on exact doses of chemotherapy prescribed, and number of days chemotherapy was received. Data on any planned or unplanned chemotherapy dose reductions, treatment breaks, and treatment discontinuation were obtained from physician notes. During treatment, acute toxicity was recorded during weekly clinic visits with the radiation oncologist and monthly visits with the medical oncologist. Acute toxicity was assessed retrospectively according to the NCI-CTCAE, v4.0. Toxicities were scored as worst grade occurring from start of treatment until 30 days after the last fraction of RT.
Similarly, detailed radiation therapy data was obtained, including total dose received and total number of therapy days. Radiation treatment interruptions and radiation dose reductions were recorded, and reason for treatment interruptions ascertained from physician notes.
Treatment outcomes and follow up
Patients were followed clinically with digital rectal exam and via CT imaging after completion of CRT. Loco-regional recurrence (LRR) was defined as recurrence or persistence of disease within the anal canal or elsewhere in the pelvic or inguinal nodes. Distant metastasis was defined as development of disease outside the pelvic or inguinal lymph nodes (LN). Rates of colostomy were recorded and used to calculate cumulative incidence of colostomy. For deceased patients, cause of death was determined from the chart and/or from the California Cancer Registry. If the cause of death was unable to be determined, the event was excluded from disease-specific survival calculations.
Statistical methods
Baseline patient characteristics, treatment details and toxicities were summarized for each group using descriptive statistics. Pearson chi square (Fisher’s exact) test was used for comparing nominal variables across the two groups and Mann-Whitney U test was used for continuous variables. Non-hematological toxicities between the two treatment groups were compared as dichotomized outcomes (none or grade 1 toxicity vs. grade 2 or higher toxicity), using Fisher’s exact test. Hematological toxicities between the two treatment groups were compared as dichotomized outcomes (none or grade 1–2 toxicity vs. grade 3 or higher toxicity), also using Fisher’s exact test.
Relevant events were death from any cause, death related to anal cancer, recurrence (local vs. distant) and colostomy. Time to event was calculated from date of first radiation treatment to date of event occurrence. The 3-year OS and 3-year anal cancer specific survival (ACSS) were estimated for the two groups using the Kaplan Meier method. Differences in survival outcomes were assessed using Log-rank test. Gray’s test for equality was used to compare cumulative incidence of overall recurrence, LRR, distant metastasis, and colostomy; we used competing risks method to account for deaths that occurred prior to recurrence event and/or colostomy.
Statistical significance for all analyses was two-sided and used a 5% significance level (P<0.05). Statistical analyses were performed using SPSS and SAS.
Results
Patient and treatment characteristics
A total of 68 patients were included in the study. Patient characteristics are summarized in Table 1; there were no significant differences between the two groups with the exception of median date of diagnosis, which was earlier in the 5-FU group. The median follow up was also longer for the 5-FU group (41.4 vs. 64.4 months, P=0.037). The majority of patients presented with stage 2 disease.
Table 1. Patient characteristics.
| Characteristics | Cap (N=36), n [%] | 5-FU (N=32), n [%] | Total (N=68), n [%] | P value |
|---|---|---|---|---|
| Median age [95% CI] | 64 [58–69] | 58 [55–65] | 60 [56–64] | 0.33 |
| Gender, female | 21 [58] | 17 [53] | 38 [56] | 0.81 |
| Ethnicity/race | 0.52 | |||
| Caucasian | 27 [75] | 24 [75] | 51 [75] | |
| Hispanic | 3 [8] | 2 [6] | 5 [7] | |
| Black | 2 [6] | 0 [0] | 2 [3] | |
| Asian | 1 [3] | 1 [3] | 2 [3] | |
| Other | 2 [6] | 1 [3] | 3 [4] | |
| Missing | 1 [3] | 4 [13] | 5 [7] | |
| Stage (AJCC 7th edition) | 0.38 | |||
| Stage 1 | 8 [22] | 5 [16] | 13 [19] | |
| Stage 2 | 15 [42] | 19 [59] | 34 [50] | |
| Stage 3 | 13 [36] | 8 [25] | 21 [31] | |
| Tumor size | 0.47 | |||
| ≥5 cm | 7 [19] | 8 [25] | 15 [22] | |
| Missing | 0 [0] | 1 [3] | 1 [1] | |
| Node status, positive | 9 [25] | 8 [25] | 17 [25] | 1.00 |
| ECOG status | 0.57 | |||
| 0 | 11 [31] | 11 [34] | 22 [32] | |
| 1 | 21 [58] | 13 [41] | 34 [50] | |
| 2 | 4 [11] | 2 [6] | 6 [9] | |
| 3 | 0 [0] | 1 [3] | 1 [1] | |
| Missing | 0 [0] | 5 [16] | 5 [7] | |
| Pathology, poorly differentiated | 4 [11] | 6 [19] | 10 [15] | 0.53 |
| HIV status | 0.30 | |||
| Positive | 1 [3] | 3 [9] | 4 [6] | |
| Missing | 16 [44] | 16 [50] | 32 [47] | |
| Ever smoker, yes | 17 [47] | 18 [56] | 35 [51] | 0.48 |
| Median date of diagnosis (95% CI) | 1/18/2012 (6/8/2010–3/22/2013) | 2/09/2008 (9/24/2003–3/24/2010) | 05/28/2010 (7/02/2009–05/13/2011) | <0.001 |
Cap, capecitabine; 5-FU, 5 fluorouracil.
Of the 32 patients in the 5-FU group, 20 patients (63%) were treated with a continuous low-dose 5-FU infusion (median 200 mg/m2/day for 31 days), and 12 patients (38%) were treated with standard 96-hour infusion of 5-FU on days 1–4 and 29–32 (median 1,000 mg/m2/day for 8 days total). Patients who received low-dose continuous 5-FU infusion received on average a lower total dose of the drug, but this was not statistically significant (median dose 6,300 vs. 8,000 mg/m2, P=0.407). Three patients were switched from continuous 5-FU to Cap early on in their course (mean 7±4 days of continuous 5-FU received prior to switching to Cap); these patients were analyzed with the 5-FU group using intention-to-treat analysis. The median dose of Cap was 852 mg/m2 twice daily, over 29 days.
Five patients (2 in Cap group, 3 in 5-FU group) did not receive any doses of MMC; reasons for omitting MMC from the start included multiple medical comorbidities, poor performance status, and patient non-compliance. The median dose of MMC given was 8 mg/m2 in the Cap group and 10 mg/m2 in the 5-FU group, which was statistically significant (P=0.021).
Most patients (91%) were treated with intensity-modulated radiation therapy (IMRT); all patients treated with 3D radiotherapy (3DRT) were in the 5-FU group (19% vs. 0%, P=0.008). Table 2 summarizes the RT details. There were no differences between the two groups in the median dose of RT delivered to the primary tumor or LN.
Table 2. Treatment details.
| Variable | Cap (N=36), n [%] | 5-FU (N=32), n [%] | Total (N=68), n [%] | P value |
|---|---|---|---|---|
| IMRT | 36 [100] | 26 [81] | 62 [91] | 0.008 |
| Median radiation dose to primary tumor [range], Gy | 50.4 [45.0–59.4] | 50.4 [50.4–54.0] | 50.4 [50.4–54.0] | 0.597 |
| Median radiation dose to LN [range], Gy | 45.0 [40.0–45.0] | 45.0 [5.4–45.0] | 45.0 [5.4–45.0] | 0.435 |
| Median radiation dose to involved LN [range], Gy | 54.0 [50.4–59.4] | 54.0 [54.0–54.0] | 54.0 [50.4–59.4] | 0.190 |
| Median RT treatment days [range], day | 41 [39–43] | 41 [37–43] | 41 [39–43] | 0.923 |
| RT interruptions | 0.097 | |||
| Yes | 6 [17] | 12 [38] | 18 [26] | |
| Missing | 1 [3] | 0 [0] | 1 [1] | |
| Median duration of RT interruption [range], day | 1 [1–3] | 3 [1–10] | 1 [1–10] | 0.054 |
| Hospitalization | 0.298 | |||
| Yes | 10 [28] | 12 [38] | 22 [32] | |
| Missing | 0 [0] | 3 [9] | 3 [4] | |
| Median days in hospital [range], day | 4 [2–5] | 2.5 [1–14] | 3.5 [1–14] | 0.461 |
| Chemotherapy treatment interruption | 0.058 | |||
| Yes | 8 [22] | 14 [44] | 22 [32] | |
| Missing | 4 [11] | 5 [16] | 9 [13] | |
| Cap or 5-FU dose reduction | 0.310 | |||
| Yes | 4 [11] | 3 [9] | 7 [10] | |
| Discontinued | 1 [3] | 4 [13] | 5 [7] | |
| Missing | 3 [8] | 6 [19] | 9 [13] | |
| Median MMC dose 1 [range], mg/m2 | 8 [0–10] | 10 [0–10] | 9 [0–10] | 0.021 |
| MMC | 0.723 | |||
| Dose reduction or discontinuation | 4 [11] | 4 [13] | 8 [12] | |
| Missing | 3 [8] | 6 [19] | 9 [13] |
Cap, capecitabine; 5-FU, 5 fluorouracil; IMRT, intensity modulated radiotherapy; MMC, mitomycin; RT, radiotherapy; LN, lymph nodes.
Toxicities
Toxicity data is presented in Table 3. Severe radiation dermatitis was common in our cohort (grade ≥3 43%), without a difference between chemotherapy treatment groups. In the 5-FU group, grade ≥3 radiation dermatitis was more common in patients treated with 3DRT compared to IMRT, but this was not statistically significant (grade ≥3 67% vs. 42% for 3DRT vs. IMRT, P=0.383).
Table 3. Toxicities.
| Variable | Cap (N=36), n [%] | 5-FU (N=32), n [%] | Total (N=68), n [%] | P value |
|---|---|---|---|---|
| Hematological toxicities (≥ grade 3) | ||||
| Neutropenia | 5 [14] | 6 [19] | 11 [16] | 0.744 |
| Anemia | 3 [8] | 2 [6] | 5 [7] | 0.557 |
| Thrombocytopenia | 0 [0] | 1 [3] | 1 [1] | 0.471 |
| Common non-hematological toxicities | ||||
| Radiation dermatitis | 0.740 | |||
| Grade 2 | 13 [36] | 11 [34] | 24 [35] | |
| Grade 3 | 14 [39] | 15 [47] | 29 [43] | |
| Anal pain | 0.159 | |||
| Grade 2 | 28 [78] | 18 [56] | 46 [68] | |
| Grade 3 | 3 [8] | 4 [13] | 7 [10] | |
| Diarrhea | 0.556 | |||
| Grade 2 | 12 [33] | 15 [47] | 27 [40] | |
| Grade 3 | 6 [17] | 5 [16] | 11 [16] | |
| Nausea | 0.236 | |||
| Grade 2 | 9 [25] | 3 [9] | 12 [18] | |
| Grade 3 | 2 [6] | 2 [6] | 4 [6] | |
| Uncommon non-hematological toxicities (≥ grade 2) | ||||
| Stomatitis | 2 [6] | 10 [31] | 12 [18] | 0.009 |
| Skin/soft tissue infection | 3 [8] | 2 [6] | 5 [7] | 1.000 |
| Cystitis | 8 [22] | 4 [13] | 12 [18] | 0.353 |
| Proctitis | 4 [11] | 2 [6] | 6 [9] | 0.676 |
| Deep vein thrombosis | 0 [0] | 4 [13] | 4 [6] | 0.044 |
| Acute coronary syndrome | 2 [6] | 2 [6] | 4 [6] | 1.000 |
| Hand and foot syndrome | 4 [11] | 5 [16] | 9 [13] | 0.725 |
Cap, capecitabine; 5-FU, 5 fluorouracil.
GI toxicity was the next most commonly reported complication of treatment, without statistically significant differences between the two treatment groups except for stomatitis, which was more common in the 5-FU group (grade 2, 6% vs. 31%, P=0.009).
Rates of infections and hand and foot syndrome were also equivalent between the two treatment groups.
There were four cases of catheter-related deep vein thrombosis reported in the 5-FU group (P=0.044); all were clinically significant and required removal of central line.
Severe hematological toxicities were not common in our cohort and no differences were seen between the two treatment groups.
Treatment breaks and dose reductions
Three patients in the 5-FU group did not complete the full-prescribed RT dose. Two patients discontinued RT after 54 Gy due to grade 3 radiation dermatitis. One patient’s radiation field was decreased significantly due to pain and patient request and he received 51.4 Gy to the primary tumor and only 5.4 Gy to regional LNs. All patients in the Cap group completed the full-prescribed RT dose.
RT interruptions were more common, and longer, in the 5-FU group, but this was not statistically significant (17% vs. 38%, P=0.097; median duration of RT interruption 1 vs. 3 days, P=0.054). Reasons for RT interruptions were similar between the two groups, and included radiation dermatitis (n=6), diarrhea (n=3), cardiovascular complications (n=2), infection (n=2), dehydration (n=1), and unrelated to therapy/patient preference (n=2).
Chemotherapy was also more frequently interrupted in the 5-FU group (22% vs. 44%, P=0.058). Reasons for chemotherapy interruption were similar between the two groups and included GI toxicities (n=9), infectious complications (n=8), neutropenia/thrombocytopenia (n=7) and cardiovascular complications/thromboembolic events (n=6).
Thirty four percent of patients required hospitalization, without a difference between the two groups (Table 2, P=0.298). Median duration of hospitalization was 3.5 days (range, 1–14 days) and the most common reasons for hospitalization were infectious complications (n=12, neutropenic fever, pneumonia, urinary tract infection, perianal skin and soft tissue infections or sepsis of unknown etiology) and GI complications (n=12, diarrhea, anal pain, or bowel inflammation), without differences between the two treatment groups. Four patients were hospitalized due to acute coronary syndrome (2 patients in each treatment group), highlighting the rare but serious cardiac side effects of both 5-FU and Cap.
Outcomes and survival
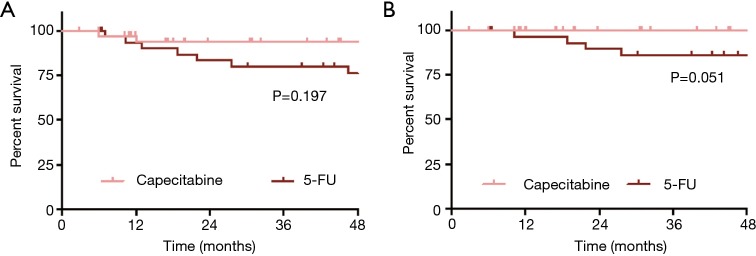
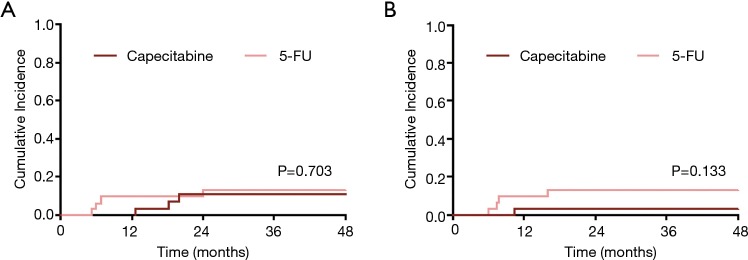
The cohort had favorable outcomes, with a 3-year OS of 87% (95% CI: 78–95%) and 3-year ACSS of 93% (95% CI: 85–100%). There was no difference in 3-year OS between the two treatment groups [Figure 1A, 94% (95% CI: 85–100%) vs. 80% (95% CI: 66–94%) for Cap vs. 5-FU; P=0.197]. There were 4 anal-cancer related deaths, all of which occurred in the 5-FU group (3 in the continuous group, and 1 in the standard group); the 3-year ACSS was 100% vs. 86% (95% CI: 73–99%) in the Cap and 5-FU groups respectively (Figure 1B, P=0.051). Recurrence was a rare event (N=7), and 3-year cumulative incidence for overall recurrence was equivalent between the two treatment groups as shown in Figure 2A [11% (95% CI: 3–26%) vs. 13% (95% CI: 4–27%) for Cap vs. 5-FU, P=0.703].
Figure 1.
Kaplan-Meier curves depicting (A) overall survival and (B) anal cancer specific survival in capecitabine versus 5-FU treated patients.
Figure 2.
Cumulative incidence curves depicting (A) recurrence and (B) treatment related colostomy in capecitabine versus 5-FU treated patients, with death as a competing event.
However, recurrence patterns differed markedly between the two groups, with only early, LRRs in the 5-FU group and only late, distant recurrences in the Cap group [cumulative incidence of LRR 0% vs. 13% (95% CI: 4–27%) for Cap vs. 5-FU, P=0.042; cumulative incidence of distant metastasis 11% (95% CI: 3–26%) vs. 0% for Cap vs. 5-Fu, P=0.079]. The median recurrence free interval was 18 months (95% CI: 13–20 months) vs. 6 months (95% CI: 5–24 months) for Cap vs. 5-FU (P=0.400).
Very few patients required colostomies (Figure 2B, N=5), but more colostomies occurred in the 5-FU group [3-year cumulative incidence 3% (95% CI: 0–14%) vs. 13% (95% CI: 4–28%) for Cap vs. 5-FU, P=0.133], consistent with more loco-regional failures in this group.
Discussion
Our retrospective analysis adds to the existing body of literature supporting the use of Cap in the definitive treatment of anal cancer. In 2016, Souza et al. published a systematic review of the use of Cap to treat locally advanced anal cancer patients and concluded that Cap is likely equivalent to 5-FU in this setting based on a comparable pooled response rate (6). Subsequently, two groups published detailed retrospective analyses comparing anal cancer patients treated with Cap vs. 5-FU at their institutions, also favoring Cap in this setting; Table 4 compares our main results with these two most recent studies (7,17).
Table 4. Comparison with prior published studies.
| Variable | Meulendijkis et al., 20141 | Goodman et al., 20172 | Pumpalova et al.1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cap (N=58) | 5-FU (N=47) | P value | Cap (N=44) | 5-FU (N=63) | P value | Cap (N=36) | 5-FU (N=32) | P value | |||
| Outcomes | |||||||||||
| 2-year OS | 98% | 87% | 0.12 | 94% | 83% | 0.197 | |||||
| 3-year OS | 86% | 78% | 0.364 | 94% | 80% | 0.197 | |||||
| 3-year ACSS | 100% | 86% | 0.051 | ||||||||
| 2-year cumulative incidence of LRR3 | 8% | 7% | 0.78 | 0% | 13% | 0.042 | |||||
| 3-year LRC4 | 79% | 76% | 0.690 | ||||||||
| 2-year cumulative incidence of DM3 | 8% | 15% | 0.26 | 11% | 0% | 0.079 | |||||
| Treatment-related colostomies | 9% | 2% | – | 9% | 5% | 0.65 | |||||
| 3-year cumulative incidence of treatment-related colostomies3 | 3% | 13% | 0.133 | ||||||||
| Median duration of follow up (months) | 23 | 49 | – | 22 | 49 | – | 41 | 64 | – | ||
| Toxicity (≥ grade 3) | |||||||||||
| Radiation dermatitis | 31% | 13% | 0.035 | 2% | 13% | 0.08 | 39% | 47% | 0.740 | ||
| Gastrointestinal toxicity | 3% | 2% | 1.000 | ||||||||
| Diarrhea | 2% | 0% | 0.41 | 17% | 16% | 0.556 | |||||
| Hematological toxicity | 6% | 6% | 1.000 | ||||||||
| Neutropenia | 20% | 52% | 0.001 | 14% | 19% | 0.744 | |||||
| Thrombocytopenia | 9% | 16% | 0.39 | 0% | 3% | 0.471 | |||||
| Anemia | 7% | 8% | 0.4 | 8% | 6% | 0.557 | |||||
1, time to event was defined as the interval between the first day of radiation and the day of event of interest; 2, time to event was defined as the interval between the last day of radiation and the day of event of interest; 3, cumulative incidence of LRR, DM and colostomy were evaluated using competing risks methods. The risk of each event was estimated using a cumulative incidence function that accounted for death without the event; 4, the Kaplan-Meier method was used to determine LRC. CaP, capecitabine; 5-FU, 5 fluorouracil; OS, overall survival; ACSS, anal cancer specific survival; DM, distant metastasis; LRR, locoregional recurrence; LRC, locoregional control.
Similar to previously published data, we show equivalent OS between the two groups. All anal-cancer related deaths in our cohort occurred in the 5-FU group (3 in the continuous group, and 1 in the standard group), resulting in a pronounced difference in ACSS that has not been previously reported, and supports the use of Cap in this setting (6,7,17).
The patterns of recurrence in our cohort were notably different from those previously published, with a statistically significant higher cumulative incidence of LRR in patients treated with 5-FU, and a trend towards more metastatic disease in the Cap group (Table 5) (7,12). Given the small number of overall recurrences in our cohort, we must be cautious in the interpretation of this unexpected result.
Table 5. Events of Interest.
| Recurrence site | Time to recurrence (months) | Site of distant recurrence | Surgery | Status | Cause of death | Time to death or last follow up (months) |
|---|---|---|---|---|---|---|
| Cap | ||||||
| Distant | 20.0 | Pulmonary | VATs | Alive | – | 84.0 |
| Distant | 18.2 | Pulmonary | No palliative chemotherapy | Alive | – | 44.9 |
| Distant | 12.7 | Pulmonary and hepatic | VATs and hepatectomy | Alive | – | 89.5 |
| No known recurrence | – | – | Colostomy for anal stenosis | Alive | – | 10.9 |
| 5-FU | ||||||
| Loco-regional | 6.8 | – | APR | Died | Metastatic anal cancer | 18.7 |
| Presumed loco-regional (based on exam only) | 6.0 | – | Unknown | Died | Unknown | 13.0 |
| Loco-regional | 24.0 | – | Right inguinal node dissection | Died | Metastatic anal cancer | 27.5 |
| Loco-regional | 5.3 | – | APR | Alive | – | 105.3 |
| Unknown | Missing | – | Unknown | Died | Anal cancer (per death registry) | 21.9 |
| No known recurrence | – | – | Colostomy for fecal incontinence | Died | Chronic aspiration | 46.4 |
| No known recurrence | – | – | Colostomy for anal perforation | Died | Surgical complications | 10.3 |
Cap, capecitabine; 5-FU, 5 fluorouracil; VATS, video-assisted thoracic surgery; APR, abdominoperineal resection.
As detailed in the Results section, the 5-FU group experienced more RT and chemotherapy interruptions compared to the Cap group, and although these differences were not statistically significant, the combined effect may explain the higher rate of loco-regional failure seen in our 5-FU group. Furthermore, all Cap-treated patients received IMRT radiation while 19% of 5-FU treated patients received 3DRT, which is considered inferior to the newer modality and may have further contributed to higher LRR. Additionally more than half the patients in our 5-FU group were treated with low-dose continuous infusion over the duration of their RT. While there is support for the use of a continuous infusion regimen in the literature, this regimen is not in the NCCN guidelines (10,11,18). Similarly to Cap, a continuous low-dose 5-FU infusion is expected to deliver a more stable and continuous radio-sensitizing dose during radiation therapy. One study in anal cancer patients showed that compared to MMC + cisplatin, MMC + low-dose continuous 5-FU had higher rates of LRR, which may further explain the relapse patterns seen in our data (11). We did not observe a difference in OS, ACSS, or incidence of recurrence between the continuous and standard 5-FU groups, but our dataset is underpowered for this analysis (data not shown).
At 2 years, we report an 11% cumulative incidence of distant metastasis in the Cap group, which is comparable to previously published results (7). Despite the occurrence of distant metastases in the Cap group, all patients are still alive at this time with two patients having undergone lung surgery to resect lung oligometastases and one with stable disease on palliative chemotherapy (Table 5). Although there were no cases of distant metastasis in our 5-FU group, given the small number of patients and lower OS in this group, this may be due to chance alone.
The rates of hematological toxicities in our Cap group were lower compared to those reported by Goodman et al., despite similar doses of Cap used (7). However, patients in our Cap cohort received a median MMC dose of 8 mg/m2, compared to 10 mg/m2 in the Goodman et al. cohort, which may partly explain this difference. The lower median dose of MMC in our Cap cohort is a result of capping the maximum dose at 15 mg total, regardless of BSA. While Goodman et al. showed significantly lower rates of hematological toxicities in patients treated with Cap vs. 5FU, we show equivalent rates, most likely due to the difference in dosing and delivery schedule of 5-FU, leading to overall lower rates of hematological toxicities in that group.
The review article by Souza et al. all highlights the frequency of severe radiation dermatitis in anal cancer patients treated with CRT (23–63.6% grade ≥3 dermatitis in patients treated with Cap) (6). Meulendijks et al. reported higher rates of severe radiation dermatitis with Cap vs. 5-FU (grade ≥3, 31% vs. 13%, P=0.035), while Goodman et al. report higher grade 2 but not grade 3 dermatitis with Cap, and overall observed very low rates of severe radiation dermatitis across both groups (Cap vs. 5-FU; grade 2, 86% vs. 52%, P<0.001; grade 3, 2% vs. 13%, P=0.08) (7,19). The rates of grade ≥3 radiation dermatitis in our Cap cohort are much closer to those reported by Meulendijks et al. and prior studies as summarized by Souza et al. (Table 4). In our Cap cohort, 38.9% of patients experienced severe radiation dermatitis, compared to 46.9% in the 5-FU group (grade ≥3, P=0.338). Similarly to the Goodman et al. cohort, all the patients in our Cap group were treated with IMRT, thus this is unlikely to explain the observed difference in severe radiation dermatitis. The rates of dermatological toxicity observed in our 5-FU group are higher than previously reported, possibly due to the fact that more than half our patients received 5-FU continuously, which more closely emulates Cap dosing (7,19).
Our study has several limitations. Our sample size is small, and may limit the statistical comparison between the two groups. However, given the low incidence of anal cancer, and the relatively small number of institutions using Cap in anal cancer, a large prospective study is not feasible, and studies like ours provide important information about treatment options. Given the retrospective nature of our analysis, there is a risk of selection bias, since patients were not randomly assigned to either treatment group. However, baseline characteristics were not statistically different between the two groups, and treatment decision regarding 5-FU versus Cap was largely driven by when the patient was diagnosed, with most patients in the Cap group being diagnosed after 2010. Patients in the 5-FU group were on average diagnosed and treated at an earlier date compared to Cap patients, and we cannot exclude the possibility that supportive oncological care improved in the interval period, contributing to some of the differences we see between the two groups. More than 50% of the patients in our 5-FU group were treated with low-dose continuous 5-FU infusion throughout the duration of their RT. This dosing regimen may confound our pooled analysis, and makes it harder to compare our results to previously published data. A 5-FU sub-group analysis is limited by the small size of the dataset, and thus a statistically significant difference in baseline characteristics, OS, ACSS, incidence of recurrence, incidence of colostomy, or toxicities may be missed between the continuous low-dose and conventional 5-FU groups. One of the strengths of our study is the long follow-up time for both groups, especially compared to other published data for Cap in anal cancer patients.
Conclusions
In this study, we show that Cap is a reasonable alternative to 5-FU in the treatment of locally advanced anal cancer patients. We show a lower cumulative incidence of LRR and a trend towards improved ACSS with Cap. A Cap-based regimen is as well tolerated as 5-FU based chemotherapy, with less stomatitis and fewer thromboembolic events. Despite the limitations of our study, we believe that our data supports the use of Cap in anal cancer patients.
Acknowledgments
None.
Ethical Statement: The study was approved by institutional review board of Stanford University. The study was granted exempt status by the IRB, thus informed consent was not required to use patient data.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ghosn M, Kourie HR, Abdayem P, et al. Anal cancer treatment: Current status and future perspectives. World J Gastroenterol 2015;21:2294-302. 10.3748/wjg.v21.i8.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shridhar R, Shibata D, Chan E, et al. Anal cancer: current standards in care and recent changes in practice. CA Cancer J Clin 2015;65:139-62. 10.3322/caac.21259 [DOI] [PubMed] [Google Scholar]
- 3.Nelson VM, Benson AB. Epidemiology of Anal Canal Cancer. Surg Oncol Clin N Am 2017;26:9-15. 10.1016/j.soc.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 4.van der Zee RP, Richel O, de Vries HJ, et al. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med 2013;71:401-11. [PubMed] [Google Scholar]
- 5.Glynne-Jones R, Saleem W, Harrison M, et al. Background and Current Treatment of Squamous Cell Carcinoma of the Anus. Oncol Ther 2016;4:135-72. 10.1007/s40487-016-0024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza KT, Pereira AAL, Araujo RL, et al. Replacing 5-fluorouracil by capecitabine in localised squamous cell carcinoma of the anal canal: Systematic review and meta-analysis. Ecancermedicalscience 2016;10(699). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman KA, Julie D, Cercek A, et al. Capecitabine With Mitomycin Reduces Acute Hematologic Toxicity and Treatment Delays in Patients Undergoing Definitive Chemoradiation Using Intensity Modulated Radiation Therapy for Anal Cancer. Int J Radiat Oncol Biol Phys 2017;98:1087-95. 10.1016/j.ijrobp.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Ohta A, Abe E, et al. Definitive chemoradiotherapy with low-dose continuous 5-fluorouracil reduces hematological toxicity without compromising survival in esophageal squamous cell carcinoma patients. Clin Transl Radiat Oncol 2017;9:12-7. 10.1016/j.ctro.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosset JF, Roelofsen F, Morgan DAL, et al. Shortened irradiation scheme, continuous infusion of 5-fluorouracil and fractionation of mitomycin C in locally advanced anal carcinomas. Results of a phase II study of the European Organization for Research and Treatment of Cancer. Radiotherapy and Gastr. Eur J Cancer 2003;39:45-51. 10.1016/S0959-8049(02)00377-5 [DOI] [PubMed] [Google Scholar]
- 10.Rich TA, Ajani JA, Morrison WH, et al. Chemoradiation therapy for anal cancer: radiation plus continuous infusion of 5-fluorouracil with or without cisplatin. Radiother Oncol 1993;27:209-15. 10.1016/0167-8140(93)90076-K [DOI] [PubMed] [Google Scholar]
- 11.Matzinger O, Roelofsen F, Mineur L, et al. Mitomycin C with continuous fluorouracil or with cisplatin in combination with radiotherapy for locally advanced anal cancer (European Organisation for Research and Treatment of Cancer phase II study 22011-40014). Eur J Cancer 2009;45:2782-91. 10.1016/j.ejca.2009.06.020 [DOI] [PubMed] [Google Scholar]
- 12.Glynne-Jones R, Meadows HM, Wan S, et al. EXTRA - A Multicenter Phase II Study of Chemoradiation using a 5 day per week Oral Regimen of Capecitabine and Intravenous Mitomycin C in Anal Cancer. Int J Radiat Oncol Biol Phys 2008;72:119-26. 10.1016/j.ijrobp.2007.12.012 [DOI] [PubMed] [Google Scholar]
- 13.Cassidy J, Clarke S, Daz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006-12. 10.1200/JCO.2007.14.9898 [DOI] [PubMed] [Google Scholar]
- 14.Hofheinz R, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. 10.1016/S1470-2045(12)70116-X [DOI] [PubMed] [Google Scholar]
- 15.Oliveira SCR, Moniz CMV, Riechelmann R, et al. Phase II Study of Capecitabine in Substitution of 5-FU in the Chemoradiotherapy Regimen for Patients with Localized Squamous Cell Carcinoma of the Anal Canal. J Gastrointest Cancer 2016;47:75-81. 10.1007/s12029-015-9790-4 [DOI] [PubMed] [Google Scholar]
- 16.Thind G, Johal B, Follwell M, et al. Chemoradiation with capecitabine and mitomycin-C for stage I-III anal squamous cell carcinoma. Radiat Oncol 2014;9(124). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peixoto RDA, Wan DD, Schellenberg D, et al. A comparison between 5-fluorouracil/mitomycin and capecitabine/mitomycin in combination with radiation for anal cancer. J Gastrointest Oncol 2016;7:665-72. 10.21037/jgo.2016.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anal Carcinoma (Version 2.2018). Available online: https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf
- 19.Meulendijks D, Dewit L, Tomasoa NB, et al. Chemoradiotherapy with capecitabine for locally advanced anal carcinoma: an alternative treatment option. Br J Cancer 2014;111:1726-33. 10.1038/bjc.2014.467 [DOI] [PMC free article] [PubMed] [Google Scholar]