Abstract
Purpose:
To determine if a negative tuberculin skin test (TST) results is associated with increased risk of mortality during tuberculosis (TB) treatment.
Methods:
We conducted a retrospective cohort study among patients aged ≥15 years with culture-positive TB reported to the Georgia State Notifiable Disease Surveillance System from 2009 – 2014. TST positivity was defined by US Centers for Disease Control guidelines. All-cause mortality during TB treatment as well as HIV, diabetes, and end-stage renal disease (ESRD) status were collected from surveillance data. Log-binomial regression was used to estimate adjusted risk ratios (aRR) and 95% confidence intervals (CI).
Results:
Among 1,186 culture-confirmed TB patients, 780 (65.8%) with a valid TST and TB treatment outcomes were eligible. Nearly one-third (242/780) had a negative TST result, and 5.6% died during treatment. The highest risk of death was observed among patients with a negative TST and HIV (12.5%) and a negative TST and diabetes (15.4%). Adjusting for confounders, the risk of death among patients with a negative TST was significantly greater compared to those with a positive TST (aRR 2.33 95% CI 1.23 – 4.43).
Conclusions:
A negative TST was associated with more than twice the risk of mortality during TB treatment after adjusting for immunosuppressive conditions.
Keywords: tuberculosis, tuberculin skin test, mortality risk, diabetes, HIV
INTRODUCTION
An estimated one-quarter of the world’s population is infected by Mycobacterium tuberculosis (MTB) [1] and 10.4 million people are newly diagnosed with tuberculosis (TB) disease annually [2]. TB remains the most common infectious disease cause of death and was estimated to cause > 4,000 deaths per day in 2016 [2]. Although the greatest burden of TB disease and mortality exists in low- and middle-income countries, deaths from TB remain an important clinical problem in high-income countries. In the United States (U.S.), nearly 5% of patients with TB disease died in 2015 [3]. While the global TB mortality rate declined during the past four years, [2, 4] achieving further reductions in the TB morality rates remains a central pillar of the End TB Strategy [2]. Developing simple and inexpensive tools to identify patients at higher risk of death during TB treatment will be vital to substantially reduce TB mortality [5].
Approximately 10-30% of patients with culture-confirmed TB have a negative tuberculin skin test (TST) [6-9]. Previous work from the U.S. suggests that a negative TST is associated with increased risk of TB mortality [6]. However, mechanisms underlying the association between a negative TST and increased risk of mortality during TB treatment remain unclear. It is well established that TB mortality risk is greater for patients with immunosuppressive comorbidities such as diabetes mellitus, HIV infection, end-stage renal disease [ESRD] [10-13]. But whether the association between a negative TST and TB mortality is differentially affected by the presence of immunosuppressive comorbidities is unknown.
Because the TST relies partially on cell-mediated immune responses to MTB [7, 14], it is an unreliable screening tool among persons with immunosuppression comorbidities [15]. For example, persons living with HIV with lower CD4 cell counts may have a smaller TST induration size or may be non-reactive (i.e., anergic) despite the presence of TB infection [16]. Among patients with TB disease and comorbid HIV, diabetes, or ESRD, it is unknown whether a negative TST is a marker of inadequate immune response. If a negative TST result among patients with TB and immunosuppressive comorbidities is associated with increased risk of mortality, a negative TST could be a simple indicator for increased clinical monitoring during TB treatment.
Given existing gaps in knowledge regarding TST results and TB mortality, we aimed to 1) estimate the prevalence of the common immunosuppressive comorbidities of diabetes, HIV and ESRD among TB patients with a negative TST result, and 2) determine whether the relationship between TST result and risk of all-cause mortality during TB treatment differed by the presence of immunosuppressive comorbidities.
METHODS
Setting, Study Design, and Measures
We conducted a retrospective cohort study among adult patients with TB disease (≥15 years old) reported to the Georgia Department of Public Health’s (GDPH) State Electronic Notifiable Disease Surveillance System (SENDSS) from 2009 to 2014. All healthcare providers and laboratories are required by law to report TB cases to Georgia public health authorities using standardized form (i.e., Report of Verified Case of Tuberculosis form) within SENDSS which then transferred to the Tuberculosis Information Management System (TIMS) and US Centers for Disease Control and Prevention (CDC) server [17]. All adults with culture-confirmed pulmonary or extrapulmonary TB at time of TB diagnosis were eligible for this study. Patients received individualized TB treatment regimens prescribed by health department affiliated providers, typically under directly observed therapy. TB treatment end date and outcomes were extracted from SENDSS.
The primary exposure for this study was TST result. The tuberculin testing was administered by healthcare workers in county health departments, hospitals, or community clinics. As recommended by US CDC guidelines, in the state of Georgia the TST and Interferon-Gamma Release Assays (IGRAs) are used as an aid to establish TB diagnosis, but are not required for all cases [18]. TST induration size was measured and defined as positive based on US CDC guidelines: a) >5mm for persons with high risk of TB, b) >10mm for persons with low to medium risk of TB, and c) >15mm for persons with no known TB risk factors [19]. The primary outcome for this study was all-cause mortality during TB treatment which was reported and confirmed by death certificate records in SENDSS.
Relevant covariates in our study, including the immunosuppressive comorbidities of HIV infection, diabetes mellitus, and ESRD, were defined according to SENDSS records. Covariate definitions and categories utilized in this analysis, including patients’ demographic characteristics, TB clinical manifestation, behavioral risk factors, history of LTBI/TB treatment, and drug-resistance profile, were previously described [20].
Statistical Analyses
Chi-square and Fisher’s exact tests were performed to assess the bivariate association between patients’ characteristics and both TST results and all-cause mortality during TB treatment. We performed log-binomial regression to compare the relative risk of all-cause mortality among patients with a negative TST to patients with a positive TST. Covariates included in regression models were based on observed associations in the bivariate analyses, confounding factors established from published literature, and directed acyclic graph theory [21]. Statistical and biological interactions between TST and comorbid conditions (e.g., HIV and diabetes) were assessed to determine if the association between TST result and all-cause mortality varied by immunosuppressive comorbidities status. Statistical interaction was tested using cross-product terms within multivariable models. Biological interaction was assessed using three measures: 1) relative excess risk due to interaction (RERI), 2) attributable proportion (AP) due to biologic interaction, and 3) synergy index [22]. We defined the absence of biological interaction if the 95% confidence interval (CI) of the RERI and AP included 0 and the 95% CI of synergy index included 1.0. Analyses were performed using SAS version 9.4 (Cary, USA) with a two-sided p-value <0.05 considered significant in all analyses.
Sensitivity Analyses
Sensitivity analyses were performed to quantify systematic errors due to: a) misclassification of TST results, b) unmeasured confounders, c) distribution assumptions used in regression analyses, and d) misspecification of covariate selection for the multivariable models. To quantify error from misclassification of TST results, we performed additional regressions including those with a missing TST to determine changes in our estimates. We also compared the risk of mortality across different groups of TST induration. We used Lash et al.’s approach [23] to externally adjust the association between negative TST and all-cause mortality during TB treatment based on the unmeasured confounders of smoking, non-adherence to TB treatment, and HIV severity (CD4 count). We compared our log-binomial regression to Cox proportional hazard models to assess whether a negative TST was associated with a higher hazard rate of death during TB treatment. Last, to assess covariate misspecification, we reported results from several multivariable models to compare changes in the estimated risk ratios with different subsets of covariates.
Institutional Review Board
This study was approved by the Institutional Review Boards at Georgia State University, Emory University, and the Georgia Department of Public Health, Atlanta, USA.
RESULTS
Study population and baseline characteristics
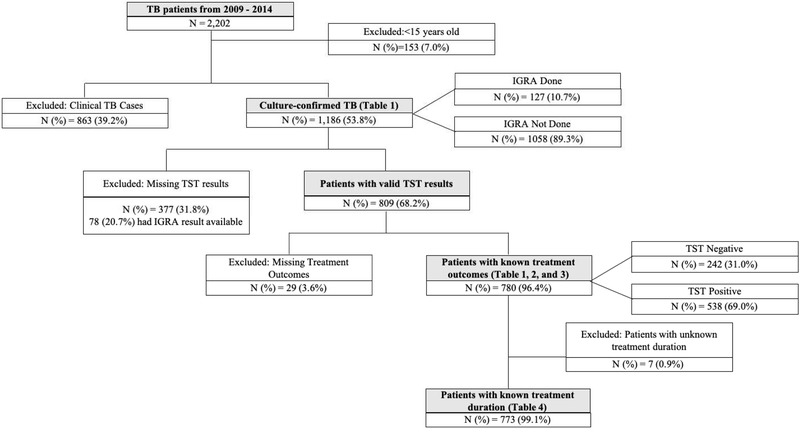
There were 2,202 patients with TB disease reported to SENDSS from 2009 – 2014, of whom 2,049 (93%) were 15 years or older (Figure 1). Among these patients diagnosed with TB, 58% (1,186/2,049) had culture-confirmed disease. Of those with a positive culture, 68.2% (809/1,186) had a valid TST result, of whom the majority (96.4%, 780/890) had a documented TB treatment and, thus, were included in the final analyses. Among those with culture-confirmed TB, those with valid TST results (n=809) were similar to those with a missing TST result (n=377) with regard to gender, country of birth, history of homelessness and imprisonment, diabetes status, presence of cavitary or miliary disease, and TB site of disease (p>0.05, Supplemental Table 1). However, HIV infection and ESRD were more prevalent among those with missing TST (p<0.01). Of the 780 patients included in the final analyses, 242 (31.0%, 95%CI 27.9 – 34.3%) had a negative TST (Table 1). The overall prevalence of HIV infection and diabetes in this group were 11.4% (95%CI 9.3 – 13.8%) and 12.9% (95%CI 10.6 – 15.4%), respectively. ESRD was reported among 0.9% (95%CI 0.4 – 1.8%) of study participants. The overall all-cause mortality rate was 5.6% (95%CI 4.2 – 7.4%) with a median time to death of 69.5 days (IQR 25.0 – 132.0 days) post-TB treatment initiation.
Figure 1:
Study flow diagram of among adult patients with culture-confirmed TB, Georgia, 2009 – 2014
Table 1.
Tuberculin skin test status among adult patients with culture-confirmed TB, Georgia, 2009 – 2014 (N=780)
| TST Results | Total N=780 N % |
P-Value (Χ2) |
||
|---|---|---|---|---|
| Patient Characteristic | Negative N % = 242 (31.0) N % |
Positive* N % = 538 (69.0) N % |
||
| Age group | ||||
| 15 – 24 | 18 (7.4) | 85 (15.8) | 103 (13.2) | <0.01 |
| 25 – 44 | 84 (34.7) | 214 (39.8) | 298 (38.2) | |
| 45 – 64 | 93 (38.4) | 184 (34.2) | 277 (35.5) | |
| ≥ 65 | 47 (19.4) | 55 (10.2) | 102 (13.1) | |
| Male Gender | 177 (73.1) | 365 (67.8) | 542 (69.5) | 0.14 |
| Race, Black | 104 (43.0) | 271 (50.5) | 375 (48.1) | 0.05 |
| Foreign-born | 84 (34.7) | 255 (47.4) | 339 (43.5) | <0.01 |
| Homeless | 37 (15.3) | 68 (12.6) | 105 (13.5) | 0.32 |
| History of imprisonment | 4 (1.7) | 39 (7.3) | 43 (5.5) | <0.01 |
| History of contact with TB patient | 19 (8.1) | 95 (18.0) | 114 (15.0) | <0.01 |
| History of TB disease | 8 (3.3) | 32 (5.9) | 40 (5.1) | 0.12 |
| Illicit drug use | 28 (11.7) | 67 (12.5) | 95 (12.2) | 0.75 |
| Alcohol abuse | 44 (18.3) | 94 (17.5) | 138 (17.8) | 0.78 |
| HIV status, Positive | 40 (17.5) | 46 (8.7) | 86 (11.4) | <0.01 |
| Diabetes | 39 (16.7) | 59 (11.2) | 98 (12.9) | 0.04 |
| ESRD | 4 (1.7) | 3 (0.6) | 7 (0.9) | 0.21¶ |
| History of LTBI prophylaxis | 2 (0.8) | 31 (5.9) | 33 (4.3) | <0.01 |
| Positive smear at baseline | 168 (69.7) | 361 (67.2) | 529 (68.0) | 0.49 |
| Abnormal CXR reading at baseline | 214 (96.0) | 475 (92.8) | 689 (93.7) | 0.10 |
| Cavitary disease | 70 (31.8) | 196 (38.5) | 266 (36.5) | 0.09 |
| Miliary disease | 15 (6.8) | 9 (1.8) | 24 (3.3) | <0.01 |
| INH-monoresistant TB | 36 (15.6) | 60 (11.6) | 96 (12.8) | 0.13 |
| TB Site | ||||
| Pulmonary | 210 (86.8) | 495 (92.0) | 705 (90.4) | 0.04 |
| Pulmonary + Extrapulmonary | 27 (11.2) | 32 (6.0) | 59 (7.6) | |
| Extrapulmonary only | 5 (2.0) | 11 (2.0) | 16 (2.1) | |
| TB Treatment Outcome | ||||
| Completed | 207 (85.5) | 505 (93.9) | 712 (91.3) | <0.01¶ |
| Died | 26 (10.7) | 18 (3.4) | 44 (5.6) | |
| Lost to follow up | 6 (2.5) | 13 (2.4) | 19 (2.4) | |
| Uncooperative/refused to continue treatment | 0 (0.0) | 1 (0.2) | 1 (0.1) | |
| Stopped due to adverse reaction | 3 (1.24) | 1 (0.2) | 4 (0.5) | |
Classification of positive TST results based on US Centers for Disease Control and Prevention guidelines [19]
Abbreviations: HIV – human immunodeficiency virus; ESRD – end-stage renal disease; TNF – tumor necrosis factor; LTBI – latent TB infection; TST – tuberculin skin test; CXR – chest x-ray; INH – isoniazid
Bold indicates statistical significance (two-sided P-value <0.05)
Factors associated with a negative TST
Compared to TB patients with a positive TST, patients with a negative TST were significantly older (i.e., 44.4% vs. 57.8% ≥45 years old) and less likely to be foreign-born (47.4% vs. 34.7%, p<0.05), but more likely to have miliary TB (1.8% vs 6.8%, p=<0.01) or concurrent pulmonary and extrapulmonary TB (6.0% vs. 11.2%) (p=0.04) (Table 1). Compared to patients with a positive TST, the prevalence of HIV infection and diabetes were significantly higher among those with a negative TST (8.7% vs. 17.5% for HIV, p=<0.01; 11.2% vs. 16.7% for diabetes, p=0.04). The prevalence of ESRD was also slightly higher among patients with a negative TST (0.6% vs. 1.7%, p=0.21), but this difference was not statistically significant.
Negative TST and all-cause mortality during TB treatment
The risk of all-cause mortality among patients with a positive TST was significantly lower compared to those with a negative TST (3.4% vs. 10.7%, p=<0.01) (Table 2). The all-cause mortality risk was highest among patients with a negative TST with HIV co-infection (5/40, 12.5%) and those with a negative TST with concomitant diabetes (6/39, 15.4%) (Table 3) compared to those with a positive TST and no comorbidity factor (14/431, 3.3%) (p=<0.01). After adjusting for age, gender, HIV status, diabetes status, ESRD, cavitary and miliary disease, alcohol abuse, and foreign-born status, we found that the risk of all-cause mortality among those with a negative TST was more than twice the risk of those with a positive TST (adjusted risk ratio [aRR] 2.33 95% CI 1.23 – 4.43) (Table 3).
Table 2.
Bivariate analyses of all-cause mortality during treatment among adult patients with culture-confirmed TB, Georgia, 2009 – 2014 (N=780)
| Total N=780* N % |
Treatment Outcome | |||
|---|---|---|---|---|
| Patient Characteristic | Not died† N % = 736 (94.4) N % |
Died N % = 44 (5.6) N % |
cRR (95% CI) | |
| TST Results | ||||
| Negative | 242 (31.0) | 216 (89.3) | 26 (10.7) | 3.21 (1.80 – 5.74) |
| Positive | 538 (69.0) | 520 (96.6) | 18 (3.4) | Reference |
| Age group | ||||
| 15 – 24 | 103 (13.2) | 102 (99.0) | 1 (1.0) | 0.48 (0.06 – 3.96) |
| 25 – 44 | 298 (38.2) | 292 (98.0) | 6 (2.0) | Reference |
| 45 - 64 | 277 (35.5) | 261 (94.2) | 16 (5.8) | 2.87 (1.14 – 7.22) |
| ≥ 65 | 102 (13.1) | 81 (79.4) | 21 (20.6) | 10.23 (4.25 – 24.63) |
| Gender | ||||
| Female | 238 (30.5) | 231 (97.1) | 7 (2.9) | Reference |
| Male | 542 (69.5) | 505 (93.2) | 37 (6.8) | 2.32 (1.05 – 5.13) |
| Race | ||||
| Non-black | 404 (51.9) | 388 (96.0) | 16 (4.0) | Reference |
| Black | 375 (48.1) | 347 (92.5) | 28 (7.5) | 1.89 (1.04 – 3.42) |
| Foreign-born | ||||
| No | 441 (56.5) | 405 (91.8) | 36 (8.2) | Reference |
| Yes | 339 (43.5) | 331 (97.6) | 8 (2.4) | 0.29 (0.14 – 0.61) |
| Homeless | ||||
| No | 675 (86.5) | 636 (94.2) | 39 (5.8) | Reference |
| Yes | 105 (13.5) | 100 (95.2) | 5 (4.8) | 0.82 (0.33 – 2.04) |
| History of imprisonment | ||||
| No | 736 (94.5) | 692 (94.0) | 44 (6.0) | Reference |
| Yes | 43 (5.5) | 43 (100.0) | 0 (0.0) | N/A* |
| History of contact with TB patient | ||||
| No | 648 (85.0) | 609 (94.0) | 39 (6.0) | Reference |
| Yes | 114 (15.0) | 110 (96.5) | 4 (3.5) | 0.58 (0.21 – 1.60) |
| History of TB Disease | ||||
| No | 740 (94.9) | 698 (94.3) | 42 (5.7) | Reference |
| Yes | 40 (5.1) | 38 (95.0) | 2 (5.0) | 0.88 (0.22 – 3.51) |
| Illicit drug use | ||||
| No | 682 (87.8) | 644 (94.4) | 38 (5.6) | Reference |
| Yes | 95 (12.2) | 91 (95.8) | 4 (4.2) | 0.76 (0.28 – 2.07) |
| Alcohol abuse | ||||
| No | 639 (82.2) | 612 (95.8) | 27 (4.2) | Reference |
| Yes | 138 (17.8) | 123 (89.1) | 15 (10.9) | 2.57 (1.41 – 4.71) |
| HIV status | ||||
| Negative | 671 (88.6) | 639 (95.2) | 32 (4.8) | Reference |
| Positive | 86 (11.4) | 78 (90.7) | 8 (9.3) | 1.95 (0.93 – 4.10) |
| Diabetes | ||||
| No | 664 (87.1) | 628 (94.6) | 36 (5.4) | Reference |
| Yes | 98 (12.9) | 91 (92.9) | 7 (7.1) | 1.32 (0.60 – 2.88) |
| ESRD | ||||
| No | 755 (99.1) | 713 (94.4) | 42 (5.6) | Reference |
| Yes | 7 (0.9) | 6 (85.7) | 1 (14.3) | 2.57 (0.41 – 16.14) |
| History of LTBI Prophylaxis | ||||
| No | 729 (95.7) | 686 (94.1) | 43 (5.9) | Reference |
| Yes | 33 (4.3) | 33 (100.0) | 0 (0.0) | N/A* |
| Baseline Smear | ||||
| Negative | 249 (32.0) | 235 (94.4) | 14 (5.6) | Reference |
| Positive | 529 (68.0) | 500 (94.5) | 29 (5.5) | 0.98 (0.53 – 1.81) |
| Baseline CXR Reading | ||||
| Normal | 46 (6.3) | 46 (100.0) | 0 (0.0) | Reference |
| Abnormal | 689 (93.7) | 647 (93.9) | 42 (6.1) | N/A* |
| Cavitary Disease | ||||
| No | 463 (63.5) | 436 (94.2) | 27 (5.8) | Reference |
| Yes | 266 (36.5) | 251 (94.4) | 15 (5.6) | 0.97 (0.52 – 1.79) |
| Miliary Disease | ||||
| No | 699 (96.7) | 659 (94.3) | 40 (5.7) | Reference |
| Yes | 24 (3.3) | 23 (3.4) | 1 (4.2) | 0.73 (0.10 – 5.08) |
| Mono-INH resistant | ||||
| No | 652 (87.2) | 617 (94.6) | 35 (5.4) | Reference |
| Yes | 96 (12.8) | 89 (92.7) | 7 (7.3) | 1.36 (0.62 – 2.97) |
| TB Site | ||||
| Pulmonary | 705 (90.4) | 667 (94.6) | 38 (5.4) | Reference |
| Pulmonary + Extrapulmonary | 59 (7.6) | 55 (93.2) | 4 (6.8) | 1.26 (0.47 – 3.40) |
| Extrapulmonary only | 16 (2.1) | 14 (87.5) | 2 (12.5) | 2.32 (0.62 – 8.79) |
Abbreviations: HIV – human immunodeficiency virus; ESRD – end-stage renal disease; TNF – tumor necrosis factor; LTBI – latent TB infection; TST – tuberculin skin test; CXR – chest x-ray; MDR – multidrug resistant; cRR – crude risk ratio; CI – Confidence Interval
Patients completed TB treatment based on Georgia Department of Public Health’s guidelines
Patients with adverse TB treatment outcomes including lost to follow-up, stopped due to adverse events, and refused to continue treatment.
crude risk ratios were not calculated due to zero “0” value in one of the cells
All-cause mortality was categorized dichotomously and patients with adverse outcomes (i.e., loss to follow-up, refusal to continue TB treatment, or stopped TB treatment due to adverse reaction) were classified as survivors (i.e., not died).
Bold indicates statistical significance (two-sided P-value <0.05)
Table 3.
Statistical interaction between TST status with immunocompromised comorbidities among adult patients with culture-confirmed TB, Georgia, 2009 – 2014 (N=780)
| Comorbidities | TST results | Mortality (%) | cRR (95% CI) | aRR (95% CI) * | |
|---|---|---|---|---|---|
| Total cohort | Negative Positive |
26/242 (10.7) 18/538 (3.4) |
3.21 (1.80 –
5.74) Reference |
2.33 (1.23 –
4.43)† Reference |
|
| Statistical Interaction | |||||
| HIV Infection | No | Negative Positive |
19/189 (10.1) 13/482 (2.7) |
3.73 (1.88 –
7.40) Reference |
2.30 (1.12 –
4.73) Reference |
| Yes | Negative Positive |
5/40 (12.5) 3/46 (6.5) |
1.92 (0.49 – 7.52) Reference |
1.91 (0.44 – 8.29) Reference |
|
| Diabetes | No | Negative Positive |
20/195 (10.3) 16/469 (3.4) |
3.01 (1.59 –
5.68) Reference |
2.10 (1.08 –
4.09) Reference |
| Yes | Negative Positive |
6/39 (15.4) 1/59 (1.7) |
9.08 (1.14 –
72.51) Reference |
7.13 (0.78 – 65.01) Reference |
|
| ESRD | No | Negative Positive |
25/230 (10.9) 17/525 (3.2) |
3.36 (1.85 –
6.09) Reference |
2.18 (1.16 –
4.09) Reference |
| Yes | Negative Positive |
1/4 (25.0) 0/3 (0.0) |
N/A‡ Reference |
N/A‡ Reference |
|
Adjusted for age, gender, and foreign-born status
Adjusted age, gender, HIV status, diabetes status, ESRD, cavitary and miliary disease, alcohol abuse, and foreign-born status
Risk ratios were not calculated due to zero “0” value in one of the cells
Bold indicates statistical significance (two-sided P-value <0.05)
Statistical and biological interaction between TST results and comorbidity factors
We found that the multiplicative effect of TST status on mortality risk was non-significantly different across HIV and diabetes status (statistical interaction p>0.05). For example, the risk ratio of all-cause mortality during TB treatment comparing those with negative TST to those with positive TST was 3.73 (95%CI 1.88 – 7.40) among those without HIV infection and 1.92 (95%CI 0.49 – 7.52) among those with HIV infection (Table 3). We also observed that the risk ratio of mortality comparing those with a negative TST to those with a positive TST was 9.08 (95%CI 1.14 – 72.51) among those with diabetes and 3.01 (95%CI 1.59 – 5.68) among patients without diabetes. In additional analyses to assess biologic interaction between a negative TST and HIV infection, we did not find evidence of biological interaction (adjusted RERI=4.31, 95%CI −4.80 – 13.42; adjusted AP=0.50, 95%CI −0.11 – 1.11; and adjusted synergy index=2.30, 95%CI 0.52 – 10.15). Similarly, no biological interaction was observed between a negative TST and diabetes (adjusted RERI=1.36, 95%CI −1.61 – 4.32; adjusted AP=0.44, 95%CI −0.23 – 1.11; and adjusted synergy index=2.85, 95%CI 0.23 – 35.17) (Supplemental Table 2).
Sensitivity and subgroup analyses
After including patients with missing TST results (n=377) in our analyses, the adjusted risk of all-cause mortality during TB treatment among patients with a missing TST was 2.64 (95%CI 1.51 – 4.63) times the risk of those with a positive TST (Table 4). In the model where we classifed all patients with a missing TST as TST negative, the adjusted risk ratio of all-cause mortality during TB treatment comparing patients with a negative TST to a positive TST was 2.47 (95%CI 1.45 – 4.20). When classifying all patients with a missing TST as TST positive, the risk of all-cause mortality during TB treatment among patients with a negative TST was 28% higher compared to those with a positive TST (aRR 1.28, 95%CI 0.81 – 2.03). The adjusted risk ratio of all-cause mortality during TB treatment among those with 0mm induration was 1.95 (95%CI 1.01 – 3.79) and 0.88 (95% CI 0.20 – 3.91) among those with 1-10mm induration when compared to those with induration >10mm (Table 4).
Table 4:
Sensitivity and subgroup analyses to assess the role of negative TST on all-cause mortality during TB treatment among adult patients with culture-confirmed TB, Georgia, 2009 – 2014
| No | Various Sensitivity Analyses | Measure of Association | TST Results | Crude Estimates | Adjusted Estimates* |
|---|---|---|---|---|---|
| 1 | TST Misclassification (N=1186) | ||||
| Including patients with invalid TST results | Risk ratio (95% CI) | TST Not Done TST Negative TST Positive |
3.94 (2.32 –
6.68) 3.21 (1.80 – 5.74) Reference |
2.64 (1.51 –
4.63) 2.21 (1.19 – 4.09) Reference |
|
| Patients with TST missing/not done classified as TST negative | Risk ratio (95% CI) | TST Negative TST Positive |
3.64 (2.20 –
6.02) Reference |
2.47 (1.45 –
4.20) Reference |
|
| Patients with TST missing/not done classified as TST positive | Risk ratio (95% CI) | TST Negative TST Positive |
1.49 (0.97 –
2.30) Reference |
1.28 (0.81 –
2.03) Reference |
|
| TST induration classification | Risk Ratio (95% CI) | 0 mm 1 – 10 mm > 10 mm |
2.73 (1.49 – 5.01) 0.85 (0.20 – 3.57) Reference |
1.95 (1.01 –
3.79) 0.88 (0.20 – 3.91) Reference |
|
| 2 | Unmeasured confounding† (N=780) | ||||
| Externally adjusted for smoking | Risk ratio range | TST Negative TST Positive |
3.27 – 5.38 Reference |
||
| Externally adjusted for treatment adherence | Risk ratio range | TST Negative TST Positive |
2.84 – 7.05 Reference |
||
| Externally adjusted for CD4 count suppression (among n=86 patients with HIV) | Risk Ratio Range | TST Negative TST Positive |
0.49 – 1.50 Reference |
||
| 3 | Model Specification (N=773) | ||||
| Cox proportional hazard model | Hazard rate ratio (95% CI) | TST Negative TST Positive |
2.65 (1.39 –
5.04) Reference |
2.08 (1.05 –
4.12) Reference |
|
| 4 | Covariate misspecification | ||||
| Multiple log-binomial logistic regression models1 (N=780) | Risk ratio range | TST Negative TST Positive |
2.10 – 2.37 Reference |
||
| Multiple Cox proportional hazard regression models2 (N=773) | Hazard rate ratio range | TST Negative TST Positive |
1.72 – 2.15 Reference |
Adjusted for age, gender, HIV, diabetes, ESRD, cavitary disease, miliary disease, alcohol abuse, and foreign-born status
Range of risk ratio was calculated using 2×2 table according to Lash et al. [23]
Results were available in Supplemental Table 3
Results were available in Supplemental Table 4
In sensitivity analyses to quantify bias for unmeasured confounders, we found that the risk ratio for all-cause mortality during TB treatment ranged from 3.27 – 5.38 when externally adjusting for smoking, and 2.84 – 7.05 when externally adjusting for treatment adherence (Table 4). Among TB patients with HIV, the range of adjusted risk ratios for all-cause mortality comparing those with negative TST to those with positive TST after externally accounting for CD4 count was 0.49 – 1.50. Using Cox proportional models to estimate the hazard rate of mortality, we found that the adjusted hazard of all-cause mortality among patients with a negative TST was 2.08 (95%CI 1.05– 4.12) times the hazard rate among those with a positive TST after adjusting for age, gender, HIV status, diabetes status, ESRD, cavitary disease, miliary TB, alcohol abuse, and foreign-born status (Table 4). In models to assess covariate misspecification, the risk ratio of all-cause mortality comparing patients with negative TST to positive TST ranged from 2.10 (95%CI 1.13 – 3.93) to 2.37 (95%CI 1.27 – 4.42) when using log-binomial logistic regression (Supplemental Table 3), and 1.72 (95%CI 0.88 – 3.37) to 2.15 (95%CI 1.11 – 4.17) when using Cox proportional hazard model (Supplemental Table 4).
DISCUSSION
In this large observational cohort study, we found that one-third of patients with culture-confirmed TB had a negative TST result and that the risk of death in these patients was nearly 11%, a risk almost 2.5 times greater than that of patients with a positive TST. Further, the relative effect of a negative TST was three times greater among patients with both TB and diabetes, as compared to patients without diabetes. Although further risk prediction and validation studies are needed, our results confirm existing empirical evidence suggesting that a negative TST result may be a simple, widely available, and inexpensive clinical marker of increased mortality risk during TB treatment.
Our study results suggesting an association between a negative TST result and an increased risk of death are consistent with prior studies [6, 24-26]. For example, a retrospective cohort study among children with TB disease from Peru found that TST induration <5mm among children was predictive of all-cause mortality during TB treatment (aHR 3.01, 95%CI 2.15 – 4.21) [26]. Additionally, a prospective cohort study conducted in Uganda among TB patients living with HIV infection found that a negative TST was significantly associated with an increased hazard rate of death compared to those with positive TST (cHR 1.9, 95%CI 1.42 – 2.77) [25]. Third, a previous analyses of US National Tuberculosis Surveillance System data found that TST positivity was associated with significantly lower odds of death during TB treatment (aOR 0.33, 95%CI 0.30 – 0.36) [6]. However, unlike our study, previous studies findings did not adjust for common comorbidities among patients with TB such as diabetes and ESRD. Thus, our study adds new information on the synergistic effect of a negative TST and common comorbidities on mortality risk during TB disease treatment.
Plausible mechanisms regarding how a negative TST result may increase the risk of mortality during TB treatment are likely linked to an insufficient immune response. Anergy, defined as the absence of normal immune response to a particular antigen (e.g., purified protein derivative), is common among patients with malnutrition, HIV infection, and chronic inflammatory diseases such as diabetes, and ESRD [27]. These patients may have lower expression of cytokines such as interleukin-2 and interferon-γ [24, 28], which may preclude a positive TST result, even in the face of confirmed TB disease. Previous studies among HIV-negative individuals also reported that CD4 cell counts in TB patients with a negative TST were significantly lower than those with a positive TST [29, 30]. Thus, a negative TST could be a signal of impaired cytokine expression and serve as a proxy for immunosuppression, either relative or absolute, in the setting of active TB disease.
We found that almost one-third of patients diagnosed with culture-confirmed TB did not have a documented TST result, indicating that either a TST was not performed or patients did not return for reading. Notably, approximately one-third of these culture-confirmed TB patients with a missing TST had a negative or missing smear. Among these, a limited proportion (29%) had IGRA results recorded in SENDSS system. Such patients typically had lower socioeconomic status and other conditions indicating social instability (e.g., homelessness and alcohol abuse), and a TST may have been less likely to be placed among these patients. Because patients in our study with negative TST results were at higher risk of mortality during TB treatment, increased efforts to document TST results in settings where TST is still commonly used may help identify TB patients with highest risk of death.
This study was subject to limitations. First, our primary analyses only included patients with culture-confirmed TB, an available TST result, and a documented TB treatment outcome. Consequently, our findings may not be generalizable to patients with culture-negative TB, those who did not have a TST performed, or those who were lost to follow-up during treatment. However, results from our sensitivity analyses indicated that the demographic and clinical charactersitics of patients included in the final analyses were similar to those excluded. The generalizability of our findings may also be limited by the potential cohort effects due to changes in TST and IGRA use during the study period. Second, we did not have information on the date TST was performed. Thus, we were unable to confirm whether the TST results in our data were part of a diagnostic evaluation for TB disease or whether they were performed as part of routine TB screening among high-risk groups, such as homeless individuals. Furthermore, as we used TB surveillance data, we did not have access to patients’ detailed clinical records including blood glucose level, CD4 counts, smoking status, hematologic malignancies, TB treatment adherence, the use of hypoglycemic or antiretroviral therapy, or specific cause of death. Our study did not estimate the direct and indirect effects (via negative TST) of immunosuppressed conditions on the risk of mortality during TB treatment. Additionally, as patients with TB are not systematically checked for diabetes in the state of Georgia, missclassification on diabetes status is possible. Finally, the lack of statistical significance in our models assessing the multiplicative and additive effects of a negative TST and various comorbidities may suggest that our study was underpowered. However, our various sensitivity analyses indicated robust and consistent findings regarding the increased risk of mortality among patients with a negative TST even after accounting for multiple types of biases due to systematic errors.
CONCLUSIONS
We found that a negative TST result among patients with culture-confirmed TB indicated an increased risk of mortality during TB treatment. To determine the clinical prognostic role of the widely available TST among presumptive TB or patients diagnosed with TB disease, further experimental or quasi-experimental research is warranted, especially among patients with immunosuppressive conditions. Our results also highlight the need for clinicians to screen for immunosuppressive comorbidities, particularly diabetes, among patients with a negative TST. Importantly, our findings indicate that patients with TB and a negative TST should receive regular clinical monitoring in an effort to reduce risk of death during TB treatment.
Supplementary Material
Our findings provide evidence that a negative TST is common among TB patients with immunosuppressive comorbidities. More importantly, a negative TST result can be used as a marker to identify TB patients that are at higher risk of mortality during treatment.
ACKNOWLEDGEMENTS
We would like to thank Dr. Rose-Marie Sales (Georgia Department of Public Health) for her assistance in acquiring the dataset and feedback for this project.
STUDY FUNDING
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [R03AI133172 to M.J.M, K24AI114444 to N.R.G, and K23AI134182 to S.C.A]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
We have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.World Health Organization. Tuberculosis Fact Sheet. Available at: http://www.who.int/mediacentre/factsheets/fs104/en/.
- 2.World Health Organization. Global Tuberculosis Report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2016. Atlanta, GA: Centers for Disease Control and Prevention, 2016. [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Report 2014. Geneva: World Health Organization, 2014. [Google Scholar]
- 5.World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization, 2016. [Google Scholar]
- 6.Auld SC, Click ES, Heilig CM, et al. Tuberculin skin test result and risk of death among persons with active TB. PLoS One 2013; 8(11): e78779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huebner RE, Schein MF, Bass JB Jr., The tuberculin skin test. Clin Infect Dis 1993; 17(6): 968–75. [DOI] [PubMed] [Google Scholar]
- 8.Holden M, Dubin MR, Diamond PH. Frequency of negative intermediate-strength tuberculin sensitivity in patients with active tuberculosis. N Engl J Med 1971; 285(27): 1506–9. [DOI] [PubMed] [Google Scholar]
- 9.Nash DR, Douglass JE. Anergy in active pulmonary tuberculosis. A comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest 1980; 77(1): 32–7. [DOI] [PubMed] [Google Scholar]
- 10.Magee MJ, Foote M, Ray SM, Gandhi NR, Kempker RR. Diabetes mellitus and extrapulmonary tuberculosis: site distribution and risk of mortality. Epidemiol Infect 2016; 144(10): 2209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faurholt-Jepsen D, Range N, PrayGod G, et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health 2013; 18(7): 822–9. [DOI] [PubMed] [Google Scholar]
- 12.Reed GW, Choi H, Lee SY, et al. Impact of diabetes and smoking on mortality in tuberculosis. PloS One 2013; 8(2): e58044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CH, Lin CJ, Kuo YW, et al. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis 2014; 14: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tufariello JM, Chan J, Flynn JL. Latent tuberculosis: mechanisms of host and bacillus that contribute to persistent infection. Lancet Infect Dis 2003; 3(9): 578–90. [DOI] [PubMed] [Google Scholar]
- 15.Lee E, Holzman RS. Evolution and current use of the tuberculin test. Clin Infect Dis 2002; 34(3): 365–70. [DOI] [PubMed] [Google Scholar]
- 16.Cobelens Fg, Egwaga SM, van Ginkel T, Muwinge H, Matee MI, Borgdorff MW. Tuberculin skin testing in patients with HIV infection: limited benefit of reduced cutoff values. Clin Infect Dis 2006; 43(5): 634–9. [DOI] [PubMed] [Google Scholar]
- 17.Georgia Department of Public Health. 2014 Georgia Tuberculosis Report. Atlanta, GA: Georgia Department of Public Health, 2014. [Google Scholar]
- 18.Georgia Department of Public Health. Tuberculosis Policy and Procedure Manual 2016. Atlanta, Georgia: Georgia Department of Public Health, 2016. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Tuberculin Skin Testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.htm. Accessed 11/30/2017.
- 20.Salindri AD, Sales RF, DiMiceli L, Schechter MC, Kempker RR, Magee MJ. Isoniazid-Monoresistance and Rate of Culture Conversion among Confirmed Tuberculosis Patients in the State of Georgia, 2009 – 2014. Ann Am Thorac Soc 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10(1): 37–48. [PubMed] [Google Scholar]
- 22.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005; 20(7): 575–9. [DOI] [PubMed] [Google Scholar]
- 23.Lash TL, Fox MP, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data: Statistics for Biology and Health: Springer Science and Business Media, 2011. [Google Scholar]
- 24.Delgado JC, Tsai EY, Thim S, et al. Antigen-specific and persistent tuberculin anergy in a cohort of pulmonary tuberculosis patients from rural Cambodia. Proc Natl Acad Sci U S A 2002; 99(11): 7576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 2000; 14(9): 1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drobac PC, Shin SS, Huamani P, et al. Risk factors for in-hospital mortality among children with tuberculosis: the 25-year experience in Peru. Pediatrics 2012; 130(2): e373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelly TF, Santillan CF, Gilman RH, et al. Tuberculosis skin testing, anergy and protein malnutrition in Peru. Int J Tuberc Lung Dis 2005; 9(9): 977–84. [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalcanti YV, Brelaz MC, Neves JK, Ferraz JC, Pereira VR. Role of TNF-Alpha, IFN-Gamma, and IL-10 in the Development of Pulmonary Tuberculosis. Pulm Med 2012; 2012: 745483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boussiotis VA, Tsai EY, Yunis EJ, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest 2000; 105(9): 1317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zembrzuski VM, Basta PC, Callegari-Jacques SM, et al. Cytokine genes are associated with tuberculin skin test response in a native Brazilian population. Tuberculosis (Edinb) 2010; 90(1): 44–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.