Abstract
Background
Mutations in the KRAS gene predict for resistance to anti-EGFR therapies, including cetuximab. Upregulation of VEGF-A has been implicated in resistance to anti-EGFR treatment. Abrogation of the VEGF and RAS/RAF/MEK/ERK pathways has the potential to restore cetuximab sensitivity.
Patients and Methods
Adult patients with histologically documented, measurable, EGFR-expressing, KRAS-mutated metastatic colorectal cancer (mCRC), that had progressed following prior 5-fluorouracil-based regimens were treated with sorafenib 400-mg orally BID and cetuximab IV weekly in 28-day cycles. Primary endpoint was the response rate (CR + PR + SD at 4 cycles); secondary endpoints included plasma biomarker analysis of angiogenic cytokines and correlative imaging studies with DCE-MRI and 89Zr-panitumumab.
Results
Of 30 patients enrolled, 26 were evaluable for response. Four patients had stable disease at 4 cycles, one with stable disease at 8 cycles. Median progression-free survival was 1.84 months. Common toxicities were rash, diarrhea, and liver enzyme elevations. Of the angiogenic cytokines evaluated, only placental growth factor was significantly increased with treatment (p < 0.0001). No pharmacodynamic parameters were associated with response.
Conclusion
We report the results of a trial that combined cetuximab and sorafenib for the treatment of KRAS-mutated mCRC, with correlative imaging studies and pharmacodynamic angiogenic cytokine profiling as downstream markers of EGFR and VEGFR signaling. No objective responses were observed. Further development of biomarkers for patient selection is needed to evaluate combined EGFR and VEGFR blockade as a therapeutic option in KRAS-mutated CRC.
Keywords: KRAS, anti-angiogenic therapy, biomarkers, pharmacodynamics, panitumumab imaging
MicroAbstract
KRAS-mutated colorectal cancers (CRC) respond poorly to cetuximab. Inhibiting resistance pathways with sorafenib may restore cetuximab sensitivity in KRAS-mutated CRC. We conducted a phase II study of this combination in 30 patients with metastatic CRC harboring KRAS mutations. Four patients had durable stable disease past 4 months, with one lasting 8+ months. Further biomarker development for patient selection is needed.
Introduction
Colorectal cancer (CRC) is the third most common cancer in both men and women in the United States, accounting for an estimated 136,830 new cases and 50,310 deaths in 2014.1 Over the past decade, molecularly targeted agents have added to the armamentarium for the treatment of metastatic CRC. Cetuximab is an IgG3 chimeric antibody directed against the extracellular portion of the epidermal growth factor receptor (EGFR), resulting in inhibition of downstream EGFR-directed intracellular signals, including the RAS-RAF-MEK-MAPK and PTEN-PIK3CA-AKT pathways. Cetuximab is approved by the FDA for the treatment of metastatic CRC in combination with folinic acid, fluorouracil, and irinotecan (FOLFIRI) or as a single-agent in the second-progression setting after irinotecan-based or oxaliplatin-based chemotherapy; as a single agent it produces responses in approximately 10% of patients with EGFR-expressing CRC refractory to treatment with irinotecan.2 EGFR upregulation occurs in an estimated 60–80% of CRC, and has been associated with poor survival.3,4 Mutations in the KRAS gene that result in constitutive activation of the RAS-RAF-MEK-MAPK pathway have been implicated in resistance to anti-EGFR therapies.5,6 However, even among KRAS wild-type CRC, cetuximab response is attenuated, implicating other resistance mechanisms to EGFR-directed therapies.7 One of these proposed resistance mechanism involves the upregulation of angiogenic growth factors (VEGF, bFGF, and TGF-α) and stimulation of tumor angiogenesis in response to EGFR inhibition.8 Chronic administration of cetuximab to nude mice bearing tumor xenografts that were initially sensitive to the EGFR inhibitor resulted in upregulation of VEGF-A and acquired resistance to EGFR treatment.9
Sorafenib is a small-molecule inhibitor of multiple targets including C-RAF, B-RAF, VEGFR-1,2,3, PDGFR-b, FLT-3, RET, and c-KIT.10 Simultaneous abrogation of several growth factor receptor pathways, including the VEGF pathway, has the potential to restore tumor sensitivity in EGFR-resistant tumors. In addition, inhibition of downstream RAF kinase may result in restoration of the activity of cetuximab in KRAS-mutated tumors. Synergism of antitumor activity has been demonstrated with the combination of sorafenib and cetuximab in preclinical models, and the abrogation of the EGFR and VEGF pathways has resulted in response rates of 20% in clinical studies.11,12 Here we report the results from a single-arm, optimal two-stage design, phase II study of cetuximab and sorafenib in patients with metastatic CRC harboring KRAS mutations (codon 12/13), with the objectives of determining the safety, tolerability, and the response rate of the regimen. We also evaluated the effect of the combination on levels of various cytokines (epidermal growth factor [EGF], vascular endothelial growth factor [VEGF-A], placental growth factor [PlGF], basic fibroblast growth factor [bFGF], and soluble vascular endothelial growth factor receptor 1 [sVEGFR1]) in plasma and on tumor vascularity and blood flow using dynamic contrast-enhanced (DCE) MRI. As an exploratory correlative study for tumor distribution of EGFR, we also explored PET/CT imaging with the EGFR-targeting antibody panitumumab.13,14
Materials and Methods
Eligibility Criteria
Adult (≥ 18 years old) patients with histologically documented, measurable, EGFR-expressing metastatic CRC which had recurred or progressed following at least one prior 5-fluorouracil–based combination chemotherapy regimen administered in the metastatic setting, and documented KRAS mutations (codon 12/13) from archival tissue were eligible. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Adequate organ function, defined as absolute neutrophil count ≥ 1500/μL, platelet count ≥ 100,000/μL, total bilirubin ≤ 1.5 x institutional upper limit of normal (ULN), AST and ALT ≤ 2.5 x ULN (up to 5 x ULN was allowed in the setting of liver metastases), and creatinine ≤ 1.5 x ULN, were required. Systolic blood pressure had to be ≤ 150 mmHg and diastolic blood pressure ≤ 90 mmHg at enrollment. Prior anticancer therapy had to be completed at least 4 weeks prior to enrollment. Exclusion criteria included prior cetuximab or sorafenib treatment, pregnancy, history of a second malignancy within the preceding 5 years, active brain metastases, bleeding diathesis, or inability to swallow whole tablets. Concurrent antiretroviral therapy, therapeutic anticoagulation, and agents known to be strong inhibitors or inducers of the cytochrome P450 enzymes were not permitted due to potential pharmacokinetic interaction. This trial was conducted under a National Cancer Institute-sponsored IND with institutional review board approval. Protocol design and conduct followed all applicable regulations, guidances, and local policies [ClinicalTrials.gov Identifier: ].
Study Design and Treatment
This was an open-label, single-arm phase 2 study. Sorafenib was provided by the Division of Cancer Treatment and Diagnosis, NCI, under a collaborative agreement with Bayer. Cetuximab was obtained from commercial sources. Sorafenib was administered orally twice daily at a dose of 400 mg. Cetuximab was administered intravenously at a loading dose of 400 mg/m2, followed by 250 mg/m2 weekly, in a 28-day cycle; patients were premedicated with diphenhydramine (50 mg intravenously) and acetaminophen (650 mg orally). Because this was a combination of an antibody along with the tyrosine kinase inhibitor with extensive single agent safety data for both agents and a low likelihood of PK interaction, we opted to proceed with a phase 2 study in the absence of an initial dose escalation safety study. All patients were monitored closely and stringent dose modification criteria were defined. Patients were monitored weekly with history, physical examination, and laboratory evaluations (complete blood count and serum chemistries). Radiologic assessments with CT scans were obtained at baseline and every 2 cycles using the Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1).15 Toxicity was assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 4.0). Both drugs were held for grade 2 skin rash or grade 3 dry skin for 1–2 weeks (until improvement to grade 1 for rash and grade 2 for dry skin), and restarted on the same dose with the first occurrence; doses were reduced for both agents for subsequent occurrences. In the absence of improvement or with a fourth occurrence patients were taken off study. For hand-foot skin reaction, sorafenib was held for 1–2 weeks until improvement to grade 1, restarted at the same dose with the first occurrence, and dose reduced for subsequent occurrences. Sorafenib was held for grade 3 hypertension and refractory diarrhea and restarted at a lower dose with improvement to grade 2. Both drugs were held for all grade ≥ 3 hematologic toxicities except lymphopenia and alopecia, and restarted with dose reduction upon resolution to ≤ grade 2. Up to two dose reductions were allowed. Treatment could be delayed up to 4 weeks to allow for recovery from toxicity; patients who did not meet retreatment criteria after a 4 week delay were removed from the study.
Pharmacokinetic Assessments
Blood samples collected at several time points during the 24 hours after the initial dose of sorafenib were analyzed using a validated HPLC/MS/MS method.16 Noncompartmental pharmacokinetic assessment of sorafenib was performed using WinNonlin v5 (Pharsight Corp, Mountainview, CA) on cycle 1 day 1. The maximum plasma concentration (Cmax) and time to Cmax (Tmax) were recorded as observed values and the area under the plasma-concentration time curve was calculated using the Linear Trapezoidal rule. Exposure (AUC) was calculated from 0–12 hours to eliminate complications from BID dosing.
Pharmacodynamic Assessments
Immunohistochemical staining for EGFR in archival tumor formalin-fixed slides was performed using a mouse anti-EGFR antibody (clone: 31G7, Zymed Laboratories, Inc., Invitrogen Immunodetection, San Francisco, CA) according to the manufacturer’s instructions; membrane staining > 1% was considered positive for enrollment purposes. Optional PET/CT imaging with 89Zr-labelled panitumumab was performed as an exploratory, non-invasive method for characterization of EGFR expression within tumors and metastatic lesions.14 Since this was the first-in-human application of this imaging agent, data from the first few patients scanned was utilized to study dosimetry. Scans to obtain dosimetry data were performed at baseline, after 2–6 hours, 1–3 days, and 7–8 days following injection of 89Zr-panitumumab. Because the study was closed early, only 3 patients underwent scanning with 89Zr-labelled panitumumab, and due to high radiation exposure estimates, the maximum dose of 89Zr panitumumab was limited to 37 MBq (1 mCi) pending further dosimetry analyses. DCE-MRI was performed to evaluate the effect of the drug combination on tumor vascularity and blood flow. DCE-MRI scans were analyzed with the two-compartment model to derive ktrans, the transfer constant reflecting permeability and flow generated by the model (units = 1/sec), and kep values, the reflux rate constant describing the rate of transfer from the extravascular space to the vascular space.
Blood samples were collected to measure changes in levels of plasma angiogenic factor biomarkers (EGF, VEGF, PlGF, bFGF, and sVEGFR1)17 prior to treatment, on days 8 and 15, and prior to cycle 2. The EGF assay was developed with the EGF antibody pair from R&D Systems (Minneapolis, MN) adapted to the electrochemiluminescence platform of Meso-Scale Discovery (Rockville, MD). Recombinant EGF was used as the standard to determine the plasma EGF concentrations of the patients.
Statistical Methods
This study followed a phase 2 optimal design18 to rule out 5% (p0=0.05) in favor of a 20% clinical benefit rate (CR + PR + SD at 4 cycles; p1=0.20), using an alpha=0.10 (10% probability of accepting a poor therapy) and beta=0.10 (10% probability of rejecting a good therapy) in KRAS-mutant CRC patients, based on historical data demonstrating 20% progression-free survival (PFS) probability of 4 months (4 cycles) for EGFR-directed therapy in patients with metastatic CRC.2,19 If there was at least one patient with clinical benefit among the first 12 evaluable patients, then accrual would continue until a total of 37 evaluable patients were enrolled. If there were only 1–3 patients with clinical benefit, then the treatment would not warrant subsequent investigation, while if at least 4 of these 37 patients had a response or stable disease at 4 cycles, then the combination would be deemed worthy of further investigation. PFS was determined from the on-study date until the date of progression using the Kaplan-Meier method; 7 patients were censored for toxicity, one for removal from study because of patient preference, and one was still on study. The differences in marker values between cycle 2, day 1 of treatment and pre-treatment were determined by a paired Wilcoxon signed rank test. All p-values are two-tailed and presented without adjustment for multiple comparisons.
Results
Patient characteristics and disposition
Between 2007 and 2014, 30 patients with KRAS-mutant colorectal cancer were enrolled, of whom 26 were evaluable for efficacy. A total of 37 patients were planned to be enrolled; however, given the lack of clinical activity the decision was made to close the study to patient accrual. All patients enrolled were evaluable for toxicity. Median age was 54 years (range 20–80), male/female ratio was 19/11, and median number of prior treatments was 3 (range 1–5), including a number of antiangiogenic therapies (Table 1). Of the inevaluable patients, two had an infusion reaction and were taken off study for safety. One patient had persistent grade 3 transaminitis attributed to disease progression after the first week of treatment and was taken off study. One patient had intermittent bowel obstruction symptoms that developed during the second week of treatment and was taken off study for safety considerations at the discretion of the investigator.
Table 1.
Prior antiangiogenic therapies
| Agent | Number of Patients (n=30) |
|---|---|
| Bevacizumab | 29 |
| Ziv-Aflibercept | 1 |
| Regorafenib | 2 |
| Any antiagiogenic agent within 3 months of enrollment | 16 |
Safety Findings
Common toxicities were dermatologic, hematologic, and gastrointestinal (Table 2). The worst grade 3 or higher toxicities were hypophosphatemia (30%) and lymphopenia (13%). As expected, skin toxicities were the most common overall event observed with the combination. Other events occurring with higher frequency included diarrhea, nausea, vomiting, hypertension, and palmar-plantar erythrodysesthesia.
Table 2.
Summary of treatment-related adverse events
| Adverse Event‡ | Grade 1 | Grade 2 | Grade 3 | Grade 4 | All Grades N (%) |
|---|---|---|---|---|---|
| Increase in AST | 8 | 7 | 1 | - | 16 (53%) |
| Increase in ALT | 13 | 2 | 2 | - | 17 (57%) |
| Hyperbilirubinemia | 3 | 3 | 1 | - | 7 (23%) |
| Increase in alkaline phosphatase | 5 | 3 | 1 | - | 9 (30%) |
| Hypokalemia | 2 | - | - | - | 2 (7%) |
| Hypomagnesemia | 8 | - | - | - | 8 (27%) |
| Hypophosphatemia | - | 6 | 9 | - | 15 (50%) |
| Hypoalbuminemia | 2 | 2 | - | - | 4 (13%) |
| Leucopenia | 8 | 3 | - | - | 11 (37%) |
| Neutropenia | 1 | 1 | - | - | 2 (7%) |
| Lymphopenia | 6 | 9 | 4 | 1 | 20 (67%) |
| Anemia | 7 | 6 | 1 | - | 14 (47%) |
| Thrombocytopenia | 12 | - | - | - | 12 (40%) |
| Rash acneiform | 17 | 7 | 1 | - | 25 (83%) |
| Rash maculo-papular | 5 | 2 | 1 | - | 8 (27%) |
| Dry skin | 13 | - | - | - | 13 (43%) |
| Pruritis | 11 | - | - | - | 11 (37%) |
| Palmar-plantar erythrodysesthesia syndrome | 5 | 5 | - | - | 10 (33%) |
| Oral mucositis | 8 | - | - | - | 8 (27%) |
| Cheilitis | 2 | 1 | - | - | 3 (10%) |
| Diarrhea | 10 | 3 | 2 | - | 15 (50%) |
| Nausea | 8 | 1 | - | - | 9 (30%) |
| Vomiting | 5 | - | 1 | - | 6 (20%) |
| Anorexia | 6 | 2 | - | - | 8 (27%) |
| Fatigue | 10 | 2 | - | - | 12 (40%) |
| Hypertension | 2 | 5 | 1 | - | 8 (27%) |
| Headache | 2 | 2 | 1 | - | 5 (17%) |
| Dysgeusia | 5 | 1 | - | - | 6 (20%) |
| Hoarseness | 2 | 1 | - | - | 3 (10%) |
| Voice alteration | 6 | 1 | - | - | 7 (23%) |
| Proteinuria | 2 | - | - | - | 2 (7%) |
Number of patients experiencing study-related adverse event by grade (worst grade for each patient and experienced by > 1 patient)
Pharmacokinetic Analyses
Of the pharmacokinetic samples collected on this trial, two were insufficient for pharmacokinetic analysis. The Cmax (1.98 μg/mL) and AUC0–12hr (13.87 hr*μg/mL) are within reported ranges (Cmax 0.92–7.55 μg/mL; AUC0–12hr 13.9–46.4 hr*μg/mL) from comparable studies using 400 mg BID dosing.20–23 Both the Cmax and AUC0–12hr values demonstrated approximately 50–60% interpatient variability, which is consistent with what has been reported in the literature.20–24 The median Tmax (9.3 hr) and level of interpatient variability (approximately 40%) are also consistent with comparable literature.20
Pharmacodynamic Analysis
Of the angiogenic cytokines evaluated (Table 3), only PlGF levels significantly increased following treatment (p < 0.0001). An induction of plasma EGF levels (Table 3) was also observed (p = 0.022). No significant changes in tumor vascularity were measured in either of the two patients who had pre- and post-dose scans by DCE-MRI [Figure 1], so further DCE-MRI scans were not pursued in subsequent patients. With the availability of 89Zr-panitumumab scans as an exploratory modality to evaluate EGFR distribution within tumor, we pursued 89Zr-panitumumab PET/CT scan imaging in the last 3 patients enrolled on study. Radiotracer activity in tumors was not significantly increased at any timepoint (Figure 3).
Table 3.
Plasma analysis of cytokines
| Pre-treatment | Post-treatment (cycle 2 day 1) | ||||
|---|---|---|---|---|---|
| Biomarker | Median (pg/mL) (No. patients) | IQR (pg/mL) | Median (pg/mL) (No. patients) | IQR (pg/mL) | P-value* |
| EGF | 10 (20) | 2.3–44.8 | 18.7 (16) | 9.0–56.1 | 0.022 |
| VEGF | 242.3 (27) | 95.6–627.5 | 288.2 (17) | 188.0–675.8 | 0.86 |
| P1GF | 25.2 (27) | 19.7–34.8 | 49.0 (17) | 41.9–63.6 | ˂0.0001 |
| bFGF | 12.1 (27) | 5.1–55.8 | 19.9 (17) | 7.7–79.1 | 0.43 |
| sVEGFRl | 176.1 (27) | 104.7–447.0 | 277.6 (17) | 189.5–840.6 | 0.67 |
Two-tailed p-values from Wilcoxon signed rank test
Abbreviations: IQR, interquartile range
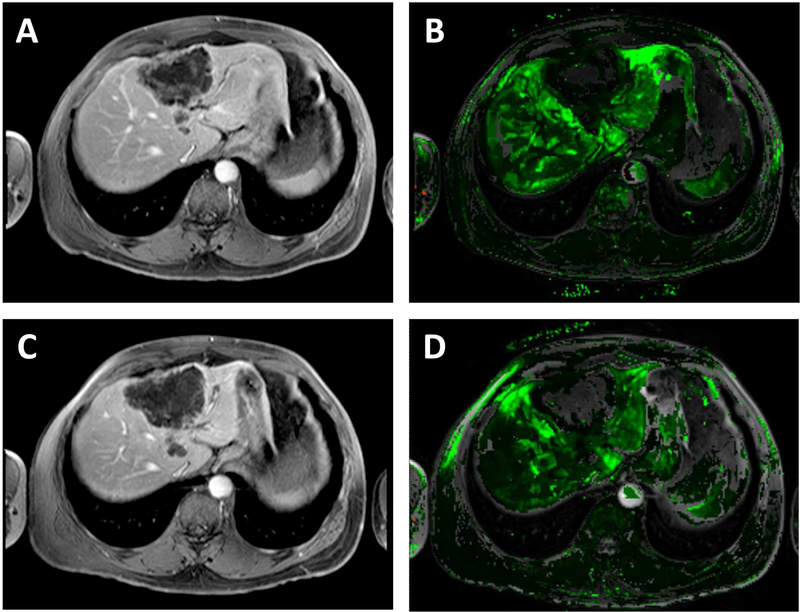
Figure 1. DCE-MRI image of a target metastatic liver lesion consistent with stable disease.
Sample DCE-MRI images of a 58 year-old man with mCRC to the liver having had progressive disease through prior combination therapies containing oxaliplatin, irinotecan, or capecitabine with bevacizumab. The patient had progression of his disease on study after 2 cycles. Target lesion indicated by arrow. Baseline axial (A) raw DCE-MRI and (B) kep (wash out) map derived from DCE-MRI showing a large lesion in the right lobe (mean and median kep values 0.228 and 0.202 min−1, respectively). Post-dose (completion of cycle 2) axial (C) raw DCE-MRI and (D) kep derived from DCE-MRI again localizes the right lobe lesion (mean and median kep values of 0.322 and 0.208 min−1, respectively).
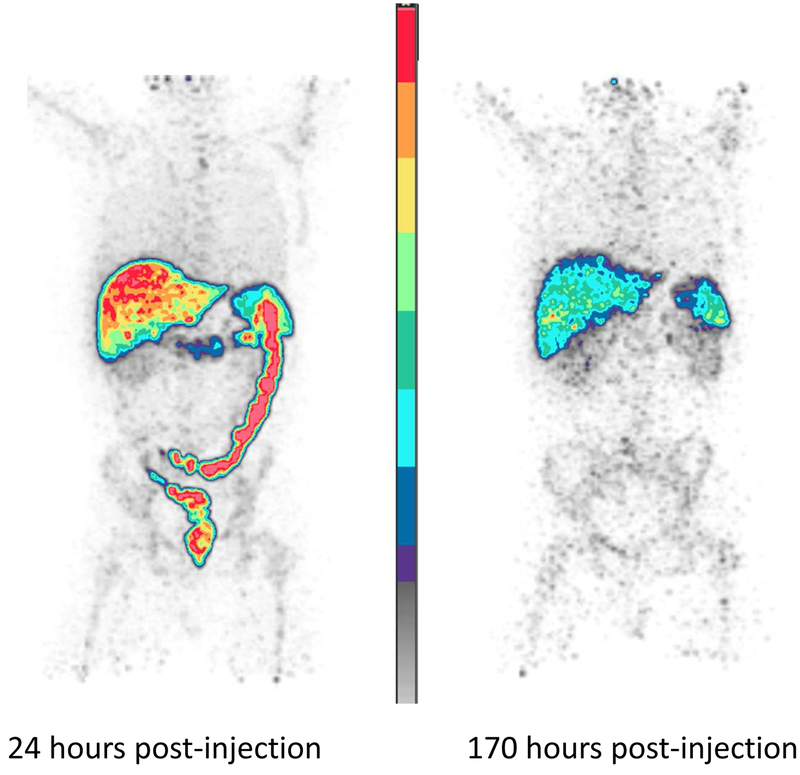
Figure 3. 89Zr-panitumumab biodistribution images for a patient.
Sample 89Zr-panitumumab PET scans from a patient on study with metastatic lesions in the liver performed to study the dosimetry of this agent in humans. Preliminary biodistribution findings demonstrate increased physiologic activity in the liver, spleen, and large bowel detectable 24 and 170 hours after radiotracer injection. (A) Whole-body image of a patient taken 24 hours after injection of 89Zr-panitumumab. (B) Whole-body image of the same patient taken 170 hours after injection.
Response
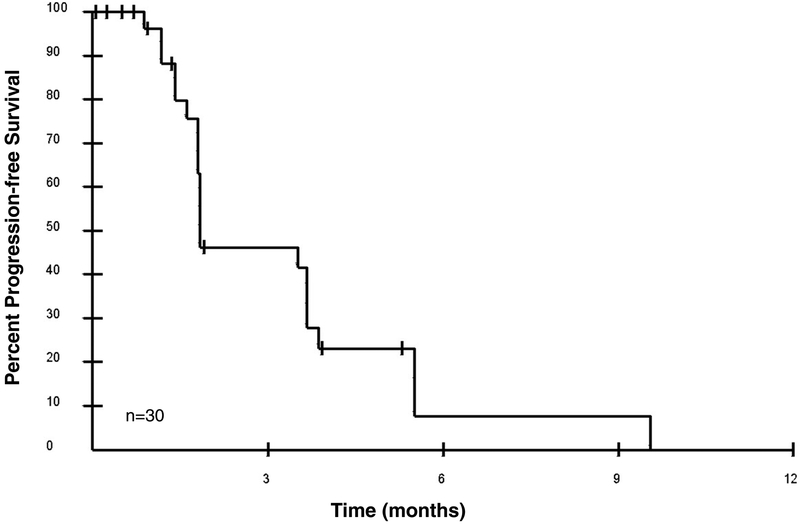
Analysis of the initial 12 patients indicated that two met criteria for stable disease at 4 cycles, justifying continuation of accrual to a goal of 37 patients. Of 30 patients enrolled at the time of this analysis, median PFS was 1.84 months (Figure 2); a total of 4 patients met the primary objective of clinical benefit, defined as CR + PR + SD at 4 cycles (~4 months). One patient with rectal adenocarcinoma who had progressed through oxaliplatin and irinotecan-based therapy with bevacizumab remained on study with stable disease at 8 cycles, with subsequent progression at restaging after 10 cycles. Two patients had stable disease at 4 cycles, with progression of disease at restaging after 6 cycles. One patient had stable disease at 4 cycles, but was hospitalized during cycle 6 for small bowel obstruction related to adhesions from prior abdominal surgeries, and was taken off study for safety although scans did not show evidence of disease progression.
Figure 2. Progression-free survival probability as a function of time.
Median progression-free survival was 1.84 months (n=30).
Discussion
This study evaluated the efficacy of sorafenib in combination with cetuximab for patients with metastatic CRC after failure of fluorouracil-based regimens in the metastatic setting. The defined target of 20% rate of clinical benefit was based on historical data of single-agent cetuximab in irinotecan-refractory metastatic CRC (mCRC).2 In these studies, patients receiving single-agent cetuximab achieved response rates of 10.8% [95% CI, 5.7 to 18.1], with a median time to progression of 1.5 months [p < 0.001]. Subset analyses of response based on KRAS mutation status were not available at the time of design of this trial, however. Subsequent panitumumab studies with analysis of KRAS mutation status showed a 0% response rate in the KRAS-mutant group with median PFS of 1.7 months, comparable to that of best supportive care [HR 0.99; 95% CI, 0.73 to 1.36].19 In the current study, 4 of 26 evaluable patients had stable disease at restaging after 4 cycles, with one remaining on study for 10 cycles with stable disease at 8 cycles. There were no objectives clinical responses in 26 patients, a lower fraction than that observed in other clinical trials with combination targeted therapies directed at EGFR and VEGF pathways. This may be partly due to differences in patient selection.12,25–27 In the BOND-2 study which evaluated the safety and efficacy of cetuximab and bevacizumab with or without irinotecan in patients with irinotecan-refractory mCRC, patients receiving cetuximab and bevacizumab without the addition of irinotecan had a time to tumor progression of 4.9 months and a response rate of 20%.12 Patients in the BOND-2 study were naïve to both cetuximab and bevacizumab, whereas in our study all but one patient had previously received bevacizumab, reflecting a patient population likely to be more resistant to anti-angiogenic therapy. Activation of multiple parallel angiogenic pathways has been linked to resistance to anti-angiogenic therapies and EGFR inhibitors.8,28 HIF-1α, a mediator of VEGF activation, has been implicated in resistance to cetuximab and is upregulated in response to tumor hypoxia and treatment with anti-angiogenic agents.29,30 Levels of PlGF, a ligand for VEGFR1, have also been shown to be increased in the circulation prior to radiographic evidence of disease progression in response to bevacizumab treatment.31 Of the plasma cytokines evaluated in this study, only PlGF showed a statistically significant increase with treatment; that the 4 patients who had stable disease after 4 cycles also had increased levels of this cytokine suggests this marker reflects a downstream target effect of VEGFR inhibition rather than an early marker of disease progression. Several factors limited our interpretation of these results, including the effects of prior angiogenic therapies, most notably bevacizumab with its long (20 day) half-life and potential continued effect on VEGF levels. Additionally, circulating cytokine levels may not be reflective of intratumoral concentrations of these same factors. Finally, the degree to which the tumor microenvironment may play a role in the induction of compensatory pathways to overcome environmental stresses remains incompletely characterized.
In the initial studies of cetuximab in the mCRC setting, grade of skin rash was associated with response with a median PFS of 11.3 months and 9.4 months in those patients with KRAS–wild-type CRC who experienced a grade 3 and 2 rash, respectively, compared to 5.4 months in those patients who had grade 0–1 skin reactions, and 19.7% of patients having grade 3 or higher skin reaction.2,32 Comparable skin toxicities were also found in the BOND-2 study with the combination of bevacizumab and cetuximab.12 In the current study, grade 3 skin toxicity was observed in only one patient, although this may reflect our strict dose modification criteria where both drugs were held for grade 2 or higher skin rash.
An additional toxicity worth noting involves the degree and frequency of hypophosphatemia seen in this study, which occurred in 15 patients (50%), slightly higher compared with prior studies of sorafenib, where hypophosphatemia (all grades) have been reported in up to 45% of patients.33 Physiologic phosphate homeostasis is maintained by the small intestine, bone, parathyroid glands, and kidneys through a complex interaction between calcitriol, PTH, and fibroblast growth factor-23 (FGF-23). Calcitriol serves to increase serum phosphate levels while PTH and FGF-23 serve to decrease circulating serum phosphate levels.34 Recent studies suggest that in addition to proteinuria and renal loss, sorafenib may induce pancreatic exocrine dysfunction and vitamin D malabsorption, with secondary hyperparathyroidism resulting in hypophosphatemia.35 We did not consistently check vitamin D levels in our study, though screening for vitamin D deficiency may be warranted in future studies of this agent.
While mutations in KRAS exon 2 have been shown to predict for overall resistance to EGFR-directed therapies, several retrospective studies have further characterized the clinical response based on the presence of specific mutations within KRAS. Clinical outcome differences have been observed between KRAS exon 2 mutations in codon 12 versus 13.36–38 These analyses have revealed that cetuximab may have activity in the subset of mCRC patients carrying mutations in KRAS exon 2, codon 13, specifically G13D mutations. In a pooled analyses of 579 patients across various clinical trials of cetuximab with or without chemotherapy, patients with the G13D KRAS mutation demonstrated improvement in PFS [4.0 vs. 1.9 months, HR=0.51, p = 0.004] and improvement in overall survival [7.6 vs. 5.7 months, HR=0.50, p = 0.005] compared with patients with other KRAS-mutation subtypes.39 These findings were validated in an independent retrospective analysis of two large randomized phase 3 trials where patients with KRAS 13D mutations had an improved clinical outcome with the addition of cetuximab to chemotherapy.38 However, a recent small prospective study of single-agent cetuximab in patients with KRAS 13D mutations refute these previous findings, demonstrating disease stabilization of 4 months in only 3 of 12 patients, with a median PFS of 1.9 months and overall survival of 7.2 months. No RECIST responses were seen in this study, and investigators concluded there was no clinically relevant benefit to warrant further study.40 In our study, 3 of the 4 patients who met criteria for stable disease at restaging after 4 cycles had mutations in codon 12. We were unable to determine the specific mutation for the one patient who had SD for >8 cycles on study due to limited archival tissue. It is clear from these data that mCRC is a heterogeneous disease, and further characterization of tumor biology above that of KRAS mutation status is needed to identify that subset of patients who could derive benefit from this treatment combination.
Conclusions
The results of this study highlight some of the major challenges facing the development of targeted therapies not only in CRC but also in all solid tumors: optimizing the therapeutic index, understanding the modulation of compensatory pathways, patient selection, and the development and incorporation of appropriate biomarkers for monitoring response.
Clinical Practice Points
Combined blockade of growth pathways presents a promising strategy for treating refractory cancers. However, its application faces a number of challenges that may account for the lack of observed clinical activity, including determining the optimal dose and schedule to optimize the therapeutic index of the combination, up-regulation of compensatory pathways, and identifying the patients most likely to benefit.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors have no conflict of interest to disclose.
References
- 1. American Cancer Society. Cancer Facts and Figures. 2014 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/. Accessed October 20, 2014. [Google Scholar]
- 2.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004; 351:337–45. [DOI] [PubMed] [Google Scholar]
- 3.Messa C, Russo F, Caruso MG, et al. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998; 37:285–9. [DOI] [PubMed] [Google Scholar]
- 4.Mayer A, Takimoto M, Fritz E, et al. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993; 71:2454–60. [DOI] [PubMed] [Google Scholar]
- 5.Normanno N, Tejpar S, Morgillo F, et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009; 6:519–27. [DOI] [PubMed] [Google Scholar]
- 6.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010; 28:1254–61. [DOI] [PubMed] [Google Scholar]
- 7.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009; 27:663–71. [DOI] [PubMed] [Google Scholar]
- 8.Kedar D, Baker CH, Killion JJ, et al. Blockade of the epidermal growth factor receptor signaling inhibits angiogenesis leading to regression of human renal cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 2002; 8:3592–600. [PubMed] [Google Scholar]
- 9.Viloria-Petit A, Crombet T, Jothy S, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001; 61:5090–101. [PubMed] [Google Scholar]
- 10.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008; 7:3129–40. [DOI] [PubMed] [Google Scholar]
- 11.Martinelli E, Troiani T, Morgillo F, et al. Synergistic antitumor activity of sorafenib in combination with epidermal growth factor receptor inhibitors in colorectal and lung cancer cells. Clin Cancer Res. 2010; 16:4990–5001. [DOI] [PubMed] [Google Scholar]
- 12.Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007; 25:4557–61. [DOI] [PubMed] [Google Scholar]
- 13.Argiles G, Dienstmann R, Elez E, et al. Panitumumab: a summary of clinical development in colorectal cancer and future directions. Future Oncol. 2012; 8:373–89. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya S, Kurdziel K, Wei L, et al. Zirconium-89 labeled panitumumab: a potential immuno-PET probe for HER1-expressing carcinomas. Nucl Med Biol. 2013; 40:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Zhao M, Navid F, et al. Quantitation of sorafenib and its active metabolite sorafenib N-oxide in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:3033–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Druzgal CH, Chen Z, Yeh NT, et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2005; 27:771–84. [DOI] [PubMed] [Google Scholar]
- 18.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989; 10:1–10. [DOI] [PubMed] [Google Scholar]
- 19.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008; 26:1626–34. [DOI] [PubMed] [Google Scholar]
- 20.Minami H, Kawada K, Ebi H, et al. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci. 2008; 99:1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009; 27:1800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecuchet N, Lebbe C, Mir O, et al. Sorafenib in advanced melanoma: a critical role for pharmacokinetics? Br J Cancer. 2012; 107:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain L, Woo S, Gardner ER, et al. Population pharmacokinetic analysis of sorafenib in patients with solid tumours. Br J Clin Pharmacol. 2011; 72:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awada A, Hendlisz A, Gil T, et al. Phase I safety and pharmacokinetics of BAY 43–9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005; 92:1855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009; 27:672–80. [DOI] [PubMed] [Google Scholar]
- 26.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009; 360:563–72. [DOI] [PubMed] [Google Scholar]
- 27.Segal NH, Reidy-Lagunes D, Capanu M, et al. Phase II study of bevacizumab in combination with cetuximab plus irinotecan in irinotecan-refractory colorectal cancer (CRC) patients who have progressed on a bevacizumab-containing regimen (The BOND 2.5 Study). J Clin Oncol. 2009; 27(15 suppl), abstract 4087. [Google Scholar]
- 28.Giuliano S, Pages G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie. 2013; 95:1110–9. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Lu Y, Liang K, et al. Requirement of hypoxia-inducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol Cancer Ther. 2008; 7:1207–17. [DOI] [PubMed] [Google Scholar]
- 30.van den Beucken T, Koritzinsky M, Wouters BG. Translational control of gene expression during hypoxia. Cancer Biol Ther. 2006; 5:749–55. [DOI] [PubMed] [Google Scholar]
- 31.Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010; 28:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009; 360:1408–17. [DOI] [PubMed] [Google Scholar]
- 33.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007; 356:125–34. [DOI] [PubMed] [Google Scholar]
- 34.Bellini E, Pia A, Brizzi MP, et al. Sorafenib may induce hypophosphatemia through a fibroblast growth factor-23 (FGF23)-independent mechanism. Ann Oncol. 2011; 22:988–90. [DOI] [PubMed] [Google Scholar]
- 35.Mir O, Coriat R, Boudou-Rouquette P, et al. Sorafenib-induced diarrhea and hypophosphatemia: mechanisms and therapeutic implications. Ann Oncol. 2012; 23:280–1. [DOI] [PubMed] [Google Scholar]
- 36.Modest DP, Stintzing S, Laubender RP, et al. Clinical characterization of patients with metastatic colorectal cancer depending on the KRAS status. Anticancer Drugs. 2011; 22:913–8. [DOI] [PubMed] [Google Scholar]
- 37.De Roock W, Jonker DJ, Di Nicolantonio F, et al. ASsociation of kras p.g13d mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010; 304:1812–20. [DOI] [PubMed] [Google Scholar]
- 38.Tejpar S, Celik I, Schlichting M, et al. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012; 30:3570–7. [DOI] [PubMed] [Google Scholar]
- 39.De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010; 304:1812–20. [DOI] [PubMed] [Google Scholar]
- 40.Schirripa M, Lonardi S, Cremolini C, et al. Phase II study of single agent cetuximab in KRAS G13D mutant metastatic colorectal cancer (MCRC). Annals Oncol. 2014; 25 (suppl 2):ii6–ii7. [DOI] [PubMed] [Google Scholar]