Abstract
The common γ-chain cytokine interleukin-15 (IL-15) plays a significant role in regulating innate and adaptive lymphocyte homeostasis and can stimulate anti-tumor activity of leukocytes. We have previously shown that the circulating IL-15 in the plasma is the heterodimeric form (hetIL-15), produced upon co-expression of IL-15 and IL-15 Receptor alpha (IL-15Rα) polypeptides in the same cell, heterodimerization of the two chains and secretion. We investigated the pharmacokinetic and pharmacodynamic profile and toxicity of purified human hetIL-15 cytokine upon injection in rhesus macaques. We compared the effects of repeated hetIL-15 administration during a two-week dosing cycle, using different subcutaneous dosing schemata, i.e. fixed doses of 0.5, 5 and 50 μg/kg or a doubling step-dose scheme ranging from 2 to 64 μg/kg. Following a fixed-dose regimen, dose-dependent peak plasma IL-15 levels decreased significantly between the first and last injection. The trough plasma IL-15 levels measured at 48 h after injections were significantly higher after the first dose, compared to subsequent doses. In contrast, following the step-dose regimen, the systemic exposure increased by more than 1 log between the first injection given at 2 μg/kg and the last injection given at 64 μg/kg, and the trough levels were comparable after each injection. Blood lymphocyte cell count, proliferation, and plasma IL-18 levels peaked at day 8 when hetIL-15 was provided at fixed doses, and at the end of the cycle following a step-dose regimen, suggesting that sustained expansion of target cells requires increasing doses of cytokine. Macaques treated with a 50 μg/kg dose showed moderate and transient toxicity, including fever, signs of capillary leak syndrome and renal dysfunction. In contrast, these effects were mild or absent using the step-dose regimen. The results provide a new method of optimal administration of this homeostatic cytokine and may have applications for the delivery of other cytokines.
Keywords: IL-15, IL-15 Receptor Alpha, Lymphocytes, Pharmacokinetics, Homeostatic cytokine
1. Introduction
Interleukin-15 is a gamma-chain cytokine important for the survival, proliferation, mobilization and function of many lymphocyte subsets including NK, CD8, IEL and CD4 [1–6]. IL-15 is produced by stromal cells in several tissues, some blood endothelial cells and antigen presenting cells [7–9]. IL-15 is co-produced in the same cells with a second polypeptide chain, named IL-15 Receptor alpha (IL-15Rα) [10–13], and the two proteins form stable heterodimers in the Endoplasmic Reticulum (ER) of the producing cell [14,15]. The heterodimeric complex is transported to the plasma membrane where the IL-15Rα chain is cleaved at the extracellular domain by membrane associated proteases [14,16,17]. The soluble IL-15:IL-15Rα complex is released in the extracellular space and circulates in the plasma in both mice and humans [18]. This soluble IL-15:IL-15Rα complex is bioactive and has a long plasma half-life [14,16,19–21], thus representing a long-acting gamma-chain cytokine form in vivo.
Recombinant human single-chain IL-15 has been produced in E. coli (sch rhIL-15) as a non-glycosylated monomer of ~12kDa [22] and has been shown to be immunostimulatory in macaques and humans. sch rhIL-15 has been tested in preclinical studies in macaques upon intravenous (IV) [23], subcutaneous (SC) [24] and continuous intravenous infusion (CIV) [24]. The first-in-human clinical trial of sch rhIL-15 delivered IV has been concluded [25], while a phase I clinical study of CIV is currently on-going in patients with advanced cancer ( NCT01572493). Administration of IL-15 resulted in increased frequency and proliferation of peripheral NK cells and effector memory CD8+ T cells [23,26]. Increased proliferation and tissue migration of CD4+ effector memory cells were also reported [6]. Although administration of IL-15 was overall well tolerated, some toxicity was observed. In both non-human primates and human cancer patients, IV IL-15 administration was associated with hypotension, fever, chills and rigors beginning 2 h after IL-15 administrations [25,27]. Transient neutropenia with hepatic granulocyte accumulation was also observed in animals receiving the higher dose. Toxicities associated with IL-15 administration resolved after discontinuation of the treatment [27]. Limitations for the clinical use of sch rhIL-15 are its rapid plasma clearance and potential immunogenicity [24,27]. These problems can be overcome by the use of the heterodimeric complex of IL-15:IL-15Rα (hetIL-15) [16]. This form represents the naturally produced IL-15 in humans and it is currently evaluated in clinical trials in patients with refractory metastatic or unresectable cancer ( NCT02452268). In the present work, we evaluated the pharmacokinetic and pharmacodynamic profile and toxicity of hetIL-15 in rhesus macaques. We also compare different doses and administration schemes to provide a regimen for the sustained optimal expansion of lymphocytes in the body.
2. Material and methods
2.1. hetIL-15 source
hetIL-15 is a heterodimer cytokine consisting of IL-15 and soluble IL-15Rα and was produced and purified from a cloned cell line derived from the human embryonic kidney HEK293 cell line transfected with optimized plasmid DNA encoding IL-15 and IL-15Rα [14,16,28,29], and grown in serum-Free Medium (Lonza). Purified, lyophilized hetIL-15 was reconstituted to the desired concentration using water for injection.
2.2. Treatment of rhesus macaques with hetIL-15
Rhesus macaques (Macaca mulatta) of Indian or Chinese origin were housed and handled in accordance with the Institutional Animal Care and Use Committee. Animals were housed at BIOQUAL, Inc. (Rockville, MD, US; animal welfare assurance no. A3086–01; protocol numbers 14-A478–11 and 17–024 and USDA Certificate number 51-R0036) and at Wuxi AppTec Animal Facility protocol #206–0001-TX (Suzhou, China). Animals were treated with hetIL-15 at the indicated doses either by IV or SC routes. The dose of hetIL-15 was calculated and expressed as the equivalent amount of single-chain IL-15 found within the heterodimer. Both single and repeated injection treatments were performed. A cohort of 48 monkeys was randomly assigned to 4 groups of 6/sex/group to determine the pharmacokinetics, pharmacodynamics and toxicity of hetIL-15 when administered in two dosing cycles over 6 weeks by the subcutaneous injection route. hetIL-15 was provided at 3 different doses, 0.5, 5, or 50 μg/kg, and the same dose was used for all the injections (fixed-dose regimen). The control group was administered vehicle (saline). Animals were randomly assigned to groups using a computer-generated randomization method (Provantis) based on body weight. Dosing cycle 1 was conducted on days 1, 3, 5, 8, 10 and 12 and dosing cycle 2 was conducted on days 29, 31, 33, 36, 38, and 40. The first day of dosing was designated as day 1. Animals were sacrificed at day 41 and 68. In additional studies with animals housed at BIOQUAL, Inc., monkeys were enrolled in a 2-week cycle following a doubling step-dose regimen. Injections were performed on days 1, 3, 5, 8, 10, and 12 at doses of 2, 4, 8, 16, 32 and 64 μg/kg, respectively (step-dose regimen). Animals were sacrificed at day 15. A list of animals enrolled in the protocols is shown as Supplementary Tables 1, 2 and 3.
2.3. Cytokine measurements in rhesus macaques
Rhesus macaques were bled at different time points prior, during and after either single or repeated IL-15 administrations and plasma was collected for cytokine measurements. IL-15 plasma levels were evaluated using a colorimetric immunoassay (Quantikine, D1500; R&D Systems), according to the manufacturer’s instructions. IL-18 plasma levels were evaluated using a colorimetric immunoassay (MBL International), according to the manufacturer’s instructions. These assays detect both human and rhesus macaque cytokines and allow the determination of endogenous plasma IL-15 and IL-18 levels prior to treatment or in control animals.
2.4. Analysis of lymphocyte subsets in blood and tissue
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation and cryopreserved in liquid nitrogen until analysis. Immunophenotypic analysis was performed using the following directly conjugated anti-human antibodies: APC-Cy7 CD3 (Clone SP34–2) and V500 CD4 (clone L200) obtained from BD Biosciences; AF405-CD8 (Caltag; clone 3B5). Cell proliferation was monitored by staining with AF700- or FITC-Ki-67 Ab in cells permeabilized with the Foxp3 Staining Buffer Set (eBioscience). All the samples were acquired in a LSR II Flow Cytometer (BD) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.5. Physical examinations and clinical pathology
2.5.1. Body temperature
Body temperature was recorded pre-dose and at approximately 4–6 and 24 h post-dose.
2.5.2. Body weight
Each animal was weighed once during pretest, on Day −1, and then once weekly throughout the dosing phases.
2.5.3. Hematology and clinical chemistry
All study animals were evaluated for hematology and clinical chemistry at the indicated time points. Hematology blood samples were also collected on days 1, 3, 8 and 15 for lymphocyte counts. Blood samples for hematology, coagulation, and clinical chemistry evaluations were obtained from a cephalic vein. Animals were compared to control animals assayed at the same time in the same facility.
2.6. Data and statistical analysis
Pharmacokinetic analysis of hetIL-15 plasma concentration-time data was performed using WinNonlin v6.3 (Pharsight Corp, Cary, NC). AUC was calculated using the linear up/log down trapezoidal rule by noncompartmental methods from drug-treated animals only. AUC and Cmax ratios were used to evaluate dose-proportionality and drug accumulation. Differences were evaluated by 1-way ANOVA or unpaired student’s t test. The p-values were corrected for multiple comparisons using Holm-Sidak test. Prism 6.0c software package (GraphPad Software, Inc., La Jolla, CA) was used for analysis.
3. Results
3.1. Administration of hetIL-15 via SC route results in increased half-life and reduced Cmax in comparison to IV route
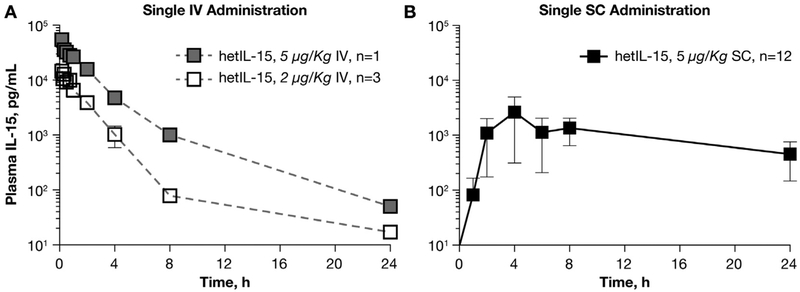
We have previously shown that administration of purified human hetIL-15 in mice resulted in sustained IL-15 plasma levels and in robust expansion of NK and T cells [16], demonstrating in vivo stability and bioactivity superior to E. coli derived single-chain sch rhIL-15. In this study, we evaluated the pharmacokinetic/pharmacodynamic profiles and toxicity of hetIL-15 upon administration in rhesus macaques. To determine the hetIL-15 pharmacokinetics, a total of 16 macaques received a single injection of the cytokine via either IV or SC routes. At the dose of 5 μg/kg delivered IV, the peak IL-15 plasma level was determined as ~55 ng/ml at 10 min after injection. The exponential decay analysis showed that hetIL-15 had a plasma half-life (T1/2) ~1.5 h. IV injection of a lower dose of hetIL-15 (2 μg/kg) resulted in lower Cmax (~11 ng/ml), with similar decay kinetics and T1/2 (Fig. 1A and Table 1). The pharmacokinetic profile of hetIL-15 was also evaluated upon a single SC administration of 5 μg/kg in 12 macaques (Fig. 1B). Peak IL-15 plasma levels of ~2 ng/ml were achieved 4 h after injection and levels of IL-15 were detected in plasma between 8 and 24 h. Administration of hetIL-15 via the SC route resulted in lower Cmax in comparison to the IV route at the same dose (2 ng/ml vs 55 ng/ml, Fig. 1B and Table 1). However, at 24 h after injection, the plasma IL-15 levels in macaques treated via the SC route were ~1 log higher in comparison to the levels found in macaques treated via the IV route (p < .01). SC administration of hetIL-15 resulted in a prolonged T1/2 of ~12 h. Therefore, SC delivery of hetIL-15 achieved lower peak plasma levels (Cmax) and longer plasma T1/2 compared to IV delivery, suggesting that SC delivery may require less frequent injections for effective treatment.
Fig. 1.
Plasma levels of IL-15 after a single administration of hetIL-15 in rhesus macaques. Macaques received a single injection of hetIL-15 at 5 μg/kg IV (A, grey square, n = 1), at 2 μg/kg IV (A, open square, n = 5 ) and at 5 μg/kg SC (B, filled square, n = 12) at time point 0. IL-15 levels were determined in plasma over the course of 24 h. Each point corresponds to the mean ± SD.
Table 1.
Pharmacokinetics upon single administration of hetIL-15 via IV and SC routes in rhesus macaques.
| Dose (μg/kg) | n | Route | Cmax (ng/mL) | AUCInf (h * ng/mL) | T1/2 (h) |
|---|---|---|---|---|---|
| 5 | 1 | IV | 54.84 | 86.91 | 1.5 |
| 2 | 3 | IV | 11.41 ( ± 2.03) | 21.2 ( ± 6.1) | 1.8 (0.9–2.9) |
| 5 | 12 | SC | 1.72 ( ± 1.53) | 55.24 ( ± 10.67) | 12.2 |
| (8.3–28.3) |
3.2. Delivery of hetIL-15 at fixed doses resulted in reduced peak and trough IL-15 levels at the end of the treatment
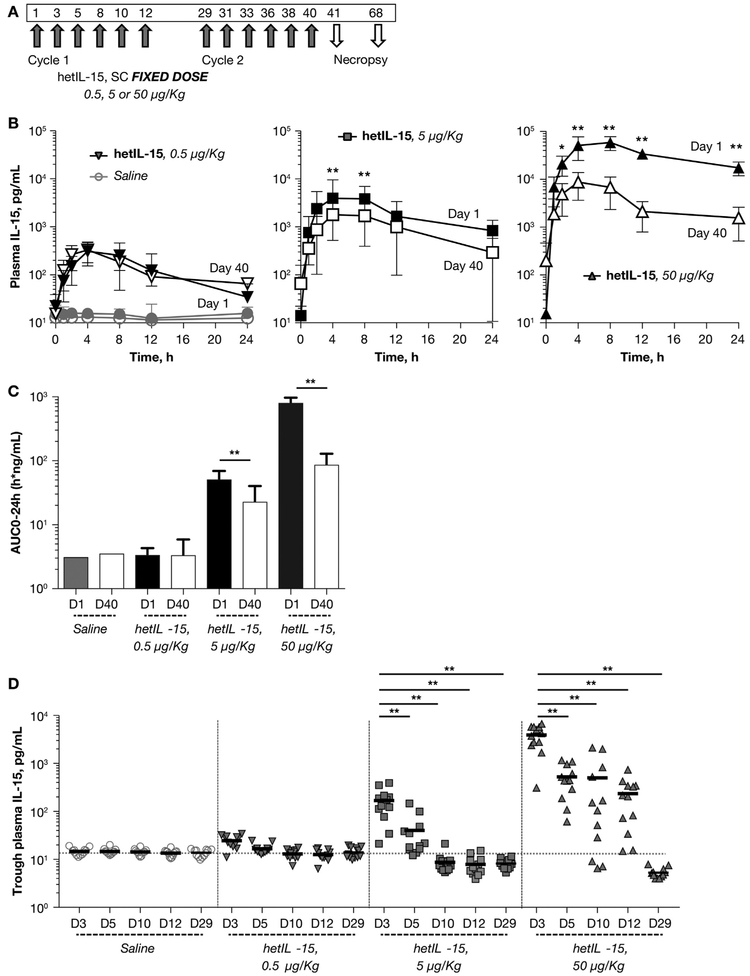
To evaluate the pharmacokinetic profile of hetIL-15 upon repeated SC administration, we analyzed the circulating IL-15 levels over time in 48 macaques treated as illustrated in Fig. 2A. Groups of 12 macaques (6 males and 6 females) received either vehicle (saline control), 0.5, 5 or 50 μg/kg hetIL-15 per injection, for a total of 6 SC injections in a 2-week cycle. Two cycles were performed, separated by a rest period of 14 days. A detailed pharmacokinetic analysis was performed upon the first injection on day 1 and the last injection on day 40. The endogenous level of IL-15 in the control group ranged from 9.1 to 21.4 pg/mL, (mean = 15.14, SD = 2.16) calculated as single chain IL-15. Upon hetIL-15 administration, Tmax values were reached at 4–8 h post-dose and T1/2 values were estimated as 6.7–12.6 h for day 1. At the end of the second cycle (day 40) T1/2 values were lower (5.3–9.1 h, Fig. 2B and Table 2). As the dosage increased from 0.5 to 50 μg/kg/dose, the systemic exposure (measured by plasma AUC0–24h and Cmax) increased more than dose-proportionally on day 1, but increased less than dose-proportionally on day 40 in all the treated animals (Fig. 2B–C, Table 2). No significant sex difference in systemic exposure were detected at any dose levels (Table 2). Importantly, comparison between the circulating plasma IL-15 levels of day 1 and day 40 showed a significant lower Cmax (Fig. 2B) and AUC0–24h at day 40 (Fig. 2C) at hetIL-15 doses of 5 and 50 μg/kg. In macaques treated with a fixed dose of 5 μg/kg, AUC0–24h and Cmax were reduced by 2-fold and 4-fold, respectively, between day 1 and 40, whereas in macaques treated with a fixed dose of 50 μg/kg, AUC0–24h and Cmax were reduced by 9-fold and 8-fold, respectively, between day 1 and 40 (Fig. 2B–C). No significant differences between day 1 and day 40 were observed at the lowest dose of 0.5 μg/kg.
Fig. 2.
Pharmacokinetics and consumption of IL-15 upon repeated administration of hetIL-15 at fixed-dose. (A) Fixed-dose regimen of hetIL-15 administration. hetIL-15 was administered in 2 dosing cycles over 6 weeks by the SC route. Each cycle consisted of 6 injections performed over the course of 2 weeks (3 injections/week). A rest period of 2 weeks separated the 2 treatment cycles. hetIL-15 was provided at 3 different doses, 0.5, 5, and 50 μg/kg/dose (12 animal/group). A group of 12 animals received vehicle and was used as control. Necropsies were performed at day 41 and 68. (B) Detailed pharmacokinetics of IL-15 was performed after injection at day 1 (filled symbols) and at day 40 (open symbols). Macaques were bled over the course of 24 h after injections and plasma IL-15 were determined overtime. Left panel depicts IL-15 plasma levels in control animals (circles) and in animals treated at 0.5 μg/kg/dose (inverted triangles). Middle panel depicts IL-15 plasma levels in animals treated at 5 μg/kg/dose (squares). Right panel depicts IL-15 plasma levels in animals treated at 50 μg/kg/dose (triangles). Each data point shows mean ± SD. * indicates time points with statistical significant difference between day 1 and day 40 by t-test analysis; *, p < .05; **, p < .01. (C) AUC0–24h (as measure of systemic exposure IL-15) was determined for injection at day 1 (filled bar) and at day 40 (open bar) for macaques in control, 0.5, 5 μg/kg/dose and 50 μg/kg/dose groups. Bar represents mean ± SD. **, p < .01, unpaired student t-test analysis. (D) Trough plasma IL-15 levels (as measure of IL-15 consumption) were evaluated at 48 h after injections, on day 3, 5, 10, 12 and at day 29 (after 2 weeks rest period). The IL-15 levels for the animal in the control (circles), 0.5 (inverted triangles), 5 μg/kg/dose (squares) and 50 μg/kg/dose (triangles) groups are depicted. Values from individual animals and mean are shown. **, p < .01.
Table 2.
Pharmacokinetics upon repeated SC administration of hetIL-15 at the fixed-dose of 5 and 50 μg/kg/dose in rhesus macaques.
| Dosel (μg/kg) | Study day | Sex | Cmax (ng/mL) | Tmax (h) | AUC0–24h (h * ng/mL) | T1/2 (h) |
|---|---|---|---|---|---|---|
| 0.5 | 1 | Male | 0.327 ± 0.170 | 4.0 (2.0–4.0) | 3.00 ± 1.14 | 12.1 (7.3–33.2) |
| Female | 0.363 ± 0.131 | 4.0 (2.0–4.0) | 3.62 ± 0.780 | 12.6 (8.5–19.0) | ||
| 40 | Male | 0.244 ± 0.0494 | 4.0 (4.0–8.0) | 2.26 ± 0.825 | 5.3 (4.4–8.3) | |
| Female | 0.416 ± 0.221 | 4.0 (2.0–8.0) | 4.31 ± 3.37 | 5.5 (4.4–6.7) | ||
| 5 | 1 | Male | 4.51 ± 0.740 | 6.0 (4.0–8.0) | 50.7 ± 9.82 | 6.7 (5.1–7.1) |
| Female | 4.63 ± 2.74 | 4.0 (4.0–8.0) | 49.9 ± 26.0 | 11.2 (7.3–17.1) | ||
| 40 | Male | 1.70 ± 1.63 | 4.0 (4.0–8.0) | 18.0 ± 17.3 | 5.4 (3.2–6.9) | |
| Female | 2.12 ± 1.26 | 6.0 (4.0–8.0) | 26.9 ± 18.8 | NA | ||
| 50 | 1 | Male | 61.9 ± 24.8 | 8.0 (4.0–8.0) | 795 ± 220 | NA |
| Female | 61.5 ± 21.0 | 8.0 (4.0–8.0) | 791 ± 148 | NA | ||
| 40 | Male | 7.00 ± 4.81 | 4.0 (4.0–8.0) | 63.8 ± 42.2 | 9.1 (3.6–24.2) | |
| Female | 11.7 ± 4.65 | 4.0 (4.0–8.0) | 106 ± 37.7 | 8.1 (7.8–65.5) | ||
Data presented as mean ± SD for Cmax and AUC0–24h values, and median (range) for Tmax and T1/2 values.
To evaluate the kinetics of IL-15 consumption during treatment, we also determined the trough levels of IL-15 measured at 48 h after each injection. At fixed doses of 5 and 50 μg/kg, the trough levels were significantly higher at day 3 in comparison to the following time points (p < .01, One-way ANOVA), suggesting that injected hetIL-15 remained in the plasma for > 48 h early in the treatment cycle (Fig. 2D). In macaques receiving hetIL-15 at the dose of 5 μg/kg, no differences were observed in the IL-15 trough levels at day 5, however, at day 10 and day 12, the plasma IL-15 levels were significantly reduced in comparison to the endogenous IL-15 levels detected in control animals (p < .01, unpaired student’s t test). In macaques receiving hetIL-15 at the dose of 50 μg/kg, the trough levels at day 5, 10 and 12 progressively declined but remained significantly elevated in comparison to endogenous IL-15 levels (Fig. 2D). The decrease in plasma IL-15 continued after the end of the first cycle. At day 29, after 2 weeks of rest, the macaques in the groups receiving hetIL-15 at the dose of 5 and 50 μg/kg had lower IL-15 blood levels in comparison to controls (Fig. 2D). These data suggested that the consumption of the administered hetIL-15 progressively increased during the treatment cycle, reflecting an increase in the pool of cells responding to IL-15 (see below). The amount of IL-15 utilized by the body lymphocytes after the first injection was less than the available IL-15, resulting in a substantial amount of free circulating cytokine two days after injection (trough level). During subsequent injections, expansion creates a pool of lymphocytes able to use hetIL-15, as a result the trough levels are significantly lower at the end of the two-week rest period (day 29).
3.3. Doubling step-dose regimen of hetIL-15 sustained constant trough levels of IL-15 throughout the treatment
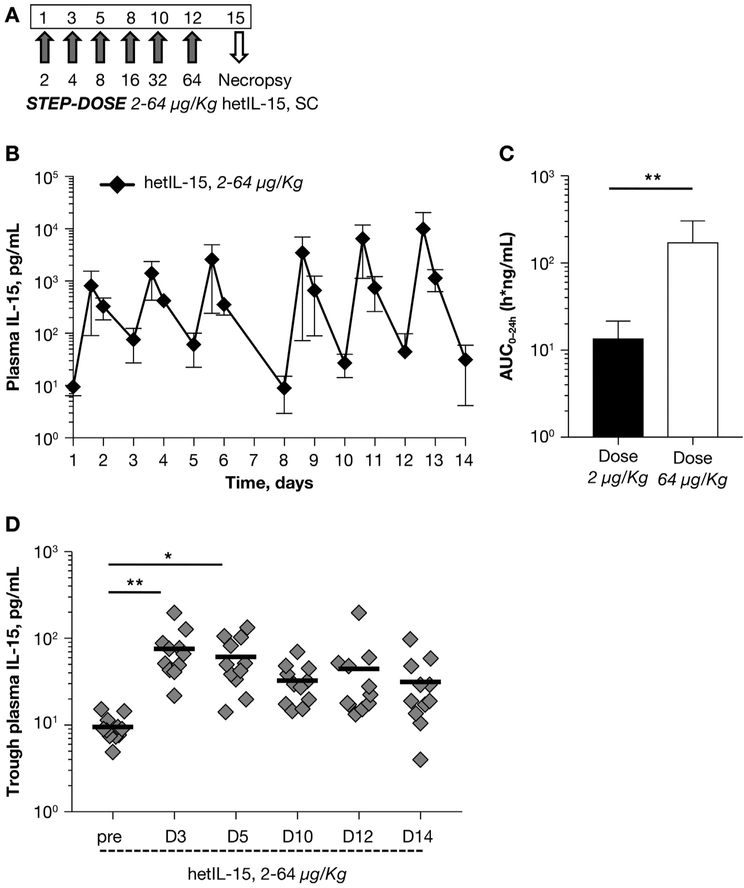
The study performed with hetIL-15 at doses of 0.5, 5, and 50 μg/kg suggested that the fixed-dose regimen provided an excess of IL-15 early in the 2-week cycle but not enough cytokine later in the treatment cycle (Fig. 2D). These results are compatible with the hypothesis that hetIL-15 leads to increased numbers of lymphocytes having IL-2/IL-15 receptors, and therefore more cytokine is binding to the lymphocytes and is removed from the plasma at the later times of the 2-week administration. It was therefore postulated that hetIL-15 is a homeostatic cytokine in continuous equilibrium with the body lymphocytes. This is also in agreement with the experimental observation that lymphodepletion leads to a transient increase in IL-15 plasma levels [18,30]. Based on these considerations, we tested a novel administration regimen in 11 rhesus macaques, consisting of 6 progressively doubling doses of hetIL-15 administration over the course of two weeks, increasing from 2 to 64 μg/kg (called “step-dose regimen”; Fig. 3A). We found that each injection resulted in a dose-proportional increase (1.3–1.8-fold) in the IL-15 peak plasma levels detected at 4 h after cytokine administration (Fig. 3B). A~13-fold increase in the AUC0–24h was found after the injection on day 12 (performed at 64 μg/kg) in comparison to the injection on day 1 (performed at 2 μg/kg). The step-dose regimen was associated with a progressive increase in systemic exposure to the cytokine during the treatment (Fig. 3C). Importantly, the trough levels of plasma IL-15 were comparable 48 h after each injection and slightly elevated in comparison to basal level (Fig. 3D), suggesting a better utilization of hetIL-15 during the entire treatment. Overall, these data indicated that the step-dose regimen better matched the increasing IL-15 need by the expanding pool of target cells during treatment.
Fig. 3.
Pharmacokinetics and consumption of IL-15 upon repeated administration of hetIL-15 following a step-dose regimen. (A) Step-dose regimen of hetIL-15 administration. Six SC hetIL-15 injections were given over the course of 2 weeks (3 injections/week) in 11 rhesus macaques. Starting with 2 μg/kg, each subsequent dose was doubled (from 2 to 64 μg/kg). Necropsies were performed at day 15. (B) Plasma IL-15 levels were determined overtime in macaques treated with the hetIL-15 step-dose regimen. Injections performed on day 1, 3, 5, 8, 10 and 12. Each data point shows mean ± SD. (C) AUC0–24h (as measure of systemic exposure of IL-15) was determined for injection at day 1 (dose: 2 μg/kg; filled bar) and at day 12 (dose: 64 μg/kg; open bar). Bar represents mean ± SD. **, p < .01. (D) Trough plasma IL-15 levels (as measure of IL-15 consumption) were evaluated at 48 h after injections, on day 3, 5, 10, 12 and 14. The IL-15 levels before treatment are also plotted. Individual animal values and mean are showed. *, p < .05; **, p < .01.
3.4. Fixed-dose and step-dose regimens differentially supported the expansion of lymphocytes and plasma IL-18 levels
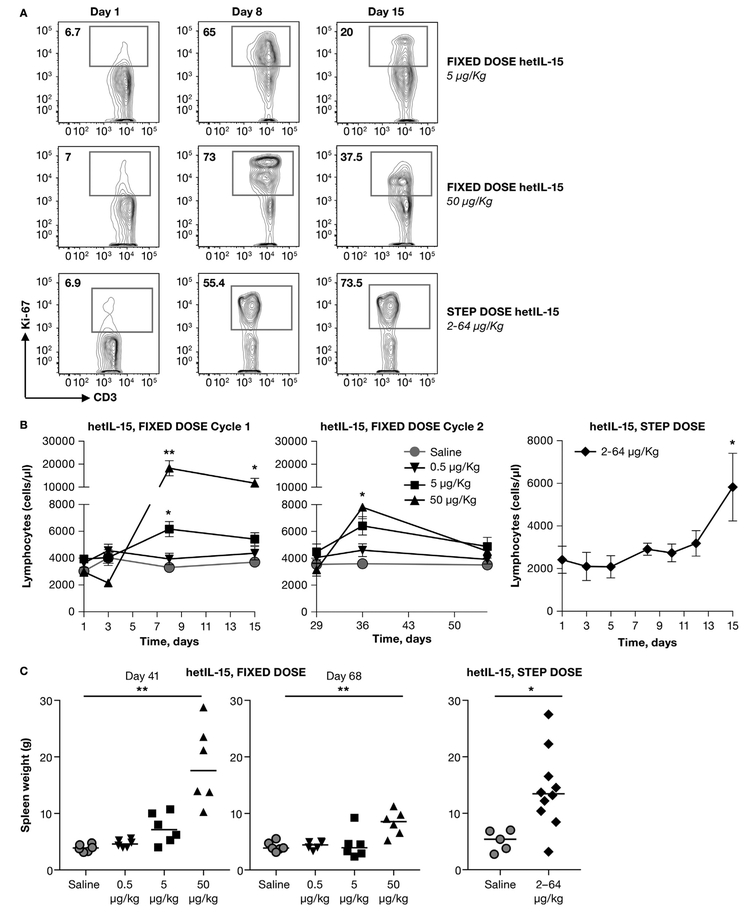
IL-15 has been previously reported to act as a homeostatic cytokine, influencing the survival, growth and mobilization of lymphocytes [3]. Previous studies in mice have demonstrated that hetIL-15 administration results in proliferation of CD8+ T and NK cells, lymphocyte subsets known to express the IL-2/IL-15βγ receptor [14,16,20,31]. We hypothesized that the progressively increased cytokine consumption observed during treatment was a reflection of expansion of the target cell pool, acting as sink for IL-15 [32]. For this reason, we evaluated the extend of proliferation of CD8+ T cells in blood by analyzing expression of the proliferation marker Ki67 by intracellular staining (Fig. 4A). In all macaques, <10% of CD8+ T cells express Ki67 at pre-treatment. By day 8, we observed increased CD8+ T cell proliferation (65–75% of total CD8+ T cell). The percentage of proliferating CD8+ T cells declined at day 15 in macaques treated with fixed-dose regimens, although it remained elevated in comparison to baseline levels (Fig. 4A, top and middle panels). In contrast, macaques treated with the step-dose regimen showed high and comparable CD8+ T cell proliferation rate on day 8 and 15 (Fig. 4A, bottom panels). We also evaluated the absolute blood lymphocyte count. In macaques treated with a fixed-dose regimen, lymphocyte counts decreased in the 50 μg/kg/dose on Day 3, in agreement with previous findings [25]. These data reflect the activity of hetIL-15 inducing lymphocyte movement out of the circulation and into the tissues [25]. Lymphocyte counts significantly increased after day 3 in a dose-dependent manner, reaching peak at day 8 (90% and 450% increase over baseline levels in the 5 and 50 μg/kg/dose, respectively) (Fig. 4B, left panel). On day 15, lymphocyte counts remained significantly elevated only in macaques treated with the higher dose (200% increase over baseline levels) and returned to baseline levels by day 29. During the second cycle of dosing starting at day 29, lymphocyte counts increased peaking on Day 36 (100% over baseline levels in both groups), and returned to levels comparable to pre-treatment by Day 55 (Fig. 4B, middle panel). Interestingly, the peak during the second cycle (Day 36) was significantly lower than the one during the first cycle (Day 8) only in the macaques that were treated with 50 μg/kg/dose. This may reflect altered properties of lymphocytes, tissue redistribution or other processes. No differences in the absolute lymphocyte counts were observed in macaques treated with 0.5 μg/kg/dose in comparison to baseline levels through cycle 1 and 2. In general, treatment with hetIL-15 resulted in a dose-dependent increase in the lymphocyte count in blood. The increase was transient and the lymphocyte count was normalized after the end of hetIL-15 dosing. A different profile in the absolute blood lymphocyte numbers was observed in macaques treated with a step-dose regimen (Fig. 4B, right panel). The lymphocyte counts remained similar to baseline levels during the first week of treatment, and started to progressively increase from day 8, reaching significantly elevated peak at day 15 (225% increase over baseline level).
Fig. 4.
Comparison of lymphocyte expansion by hetIL-15 fixed-dose and step-dose regimens. hetIL-15 was administered SC at day 1, 3, 5, 8, 10 and 12. Twelve macaques received hetIL-15 at 0.5 μg/kg/dose (inverted triangles), 12 macaques received hetIL-15 at 5 μg/kg/dose (squares), 12 macaques received hetIL-15 at 50 μg/kg/dose (triangles), 11 monkeys received hetIL-15 at increasing doses ranging from 2 to 64 μg/kg (diamonds). Animals treated with vehicle (circles) were included as controls. (A) CD8+ T cells were analyzed for the expression of the proliferation marker Ki-67. The frequency of proliferating CD8+ T cells (Ki-67+) in blood prior to treatment (left panels), at day 8 (middle panels) and day 15 (right panels) was determined by intracellular staining followed by flow cytometry. A representative animal for each treatment group is shown. (B) Absolute counts of lymphocytes in blood (reported as cells/μl) were determined at the indicated time points for the fixed-dose regimen during cycle 1 (left panel) and cycle 2 (middle panel), and the step-dose regimen (right panel). Each data point shows mean ± SEM. * indicates time points with statistical significant difference in comparison to day 1; *, p < .05;**, p < .01. (C) Spleen weight measured at necropsy. Animals treated with the fixed dose regimen were euthanized at completion of hetIL-15 treatment (day 41, left panel) or after a 4-week rest period (day 68, middle panel). Animals treated with the step-dose regimen were euthanized after completion of hetIL-15 treatment (right panel). Individual animals and mean are shown. *, p < .05; **, p < .01.
These data suggested that all treatments resulted in lymphocyte proliferation and increase during treatment. Since the majority of the lymphocytes leave the blood and are redistributed in the tissues, measurements of the total lymphocytes during different courses are not easily obtained. As a measure of the extent of lymphocyte expansion and accumulation, as previously reported [14], macaque spleen weights were recorded at necropsy (Fig. 2A and 3A). Macaques treated with the fixed-dose regimen at 50 μg/kg showed significant spleen enlargement at completion of hetIL-15 treatment (day 41, Fig. 4C, left panel). The dose of 5 μg/kg also resulted in a marginal spleen weight increase, although it did not reach statistical significance (one-way ANOVA). After a 4-week rest period (day 68, Fig. 4C, middle panel), spleen weight decreased in the animals receiving 50 μg/kg dose, although remained significantly higher than control animals. This indicates the high dose of 50 μg/kg hetIL-15 treatment had prolonged effects on the body lymphocytes detectable one month after administration. Interestingly, macaques treated by the step-dose regimen showed an increase in spleen weight at completion of hetIL-15 treatment (day 15) that was comparable to the animals receiving 50 μg/kg at fixed dose (Fig. 4C, right panel). Therefore, the step-dose regimen achieved proliferation and tissue distribution of lymphocytes comparable to the high fixed-dose regimen, using less than half of the total dose of hetIL-15 during the two-week treatment. Additional observations showed that both the 50 μg/kg treated and the step-dose treated animals had enlarged lymph nodes, indicating significant lymphocyte expansion. Taken together, these data suggested that the increasing doses of hetIL-15 during the step-dose regimen match the needs of the growing number of target cells and support their continuous expansion. Therefore, delivery of hetIL-15 following a doubling step-dose regimen provides enough cytokine to sustain the continuous expansion of lymphocytes within the two-week cycle.
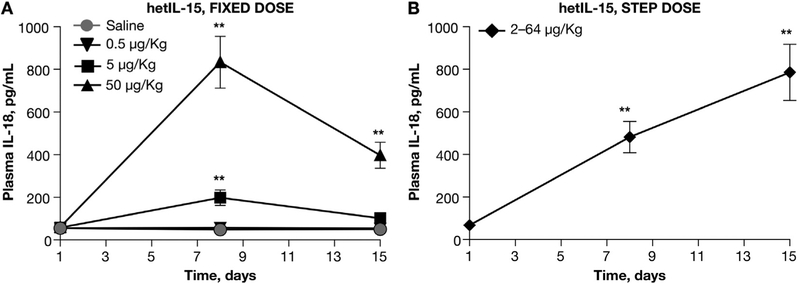
Increased plasma IL-18 concentrations have been previously identified as biomarker of IL-15 activity [27,33,34]. As an additional measure of hetIL-15 effects, we determined the circulating IL-18 levels during hetIL-15 administration following both regimens. In macaques treated with the fixed-dose regimen, the increase in plasma IL-18 levels was dose-dependent (3.5 and 13.5-fold for the 5 and 50 μg/kg/dose, respectively) and peaked at day 8. The plasma IL-18 levels significantly declined during the second week of treatment and, at day 15, only macaques treated with the higher dose showed IL-18 plasma levels significantly higher than controls (6.5-fold; Fig. 5A). In contrast, in animals treated with the hetIL-15 step-dose regimen, we observed a progressive increase in plasma IL-18 levels throughout the treatment with peak levels at day 15 (Fig. 5B). Interestingly, the peak IL-18 levels achieved upon step-dose regimen were as high as the levels obtained after fixed-dose regimen at 50 μg/kg/dose (Fig. 5), supporting the conclusion that progressively increasing doses of hetIL-15 is an effective way to induce the cytokine effects. No increased plasma level of IL-18 was observed throughout the treatment at the fixed dose of 0.5 μg/kg.
Fig. 5.
Plasma IL-18 measurements during hetIL-15 administration. Plasma IL-18 levels were determined at the indicated time points for the fixed-dose regimen (A) and the step-dose regimen (B). Each data point shows mean ± SEM. * indicates time points with statistical significant difference in comparison to day 1; Statistical analysis was done by 1-way ANOVA *, p < .05; **, p < .01.
3.5. Toxicity associated with hetIL-15 delivery
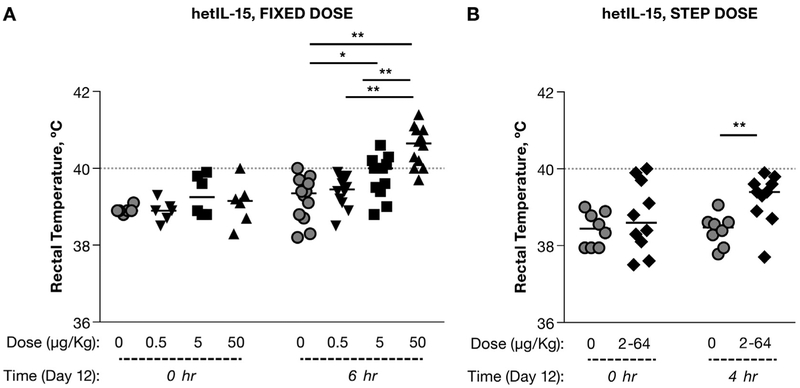
One of the objectives of this study was to determine the potential toxicity of hetIL-15 administration following different treatment regimens. It has been suggested that the fever following the IL-15 treatment is likely associated with the cytokine spike that occurs within the first hours of administration [27]. Body temperature increased at 6 h after each cytokine administration in all macaques treated with the 50 μg/kg, and in few animals treated with the 5 or 0.5 μg/kg fixed-dose regimens (Fig. 6A). In the step-dose regimen, the rectal temperature of hetIL-15 treated animals was also significantly higher than in controls at 4 h after injections performed on day 12 (64 μg/kg dose), (Fig. 6B), although the frequency of animals with high fever (≥40 °C) was significantly lower in the step-dose regimen (Fisher test, p < .001).
Fig. 6.
Comparison of rectal temperature during fixed-dose or step-dose regimen. Rectal temperature was measured before and 4–6 h after the cytokine administration (6th injection) in animals enrolled in the study with fixed-dose regimen (A) and in animals treated with the step-dose regimen (B). Individual animals and mean are shown. Statistical analysis was done by 2-way ANOVA. *, p < .05:**, p < .01. Dotted line corresponds to 40 °C.
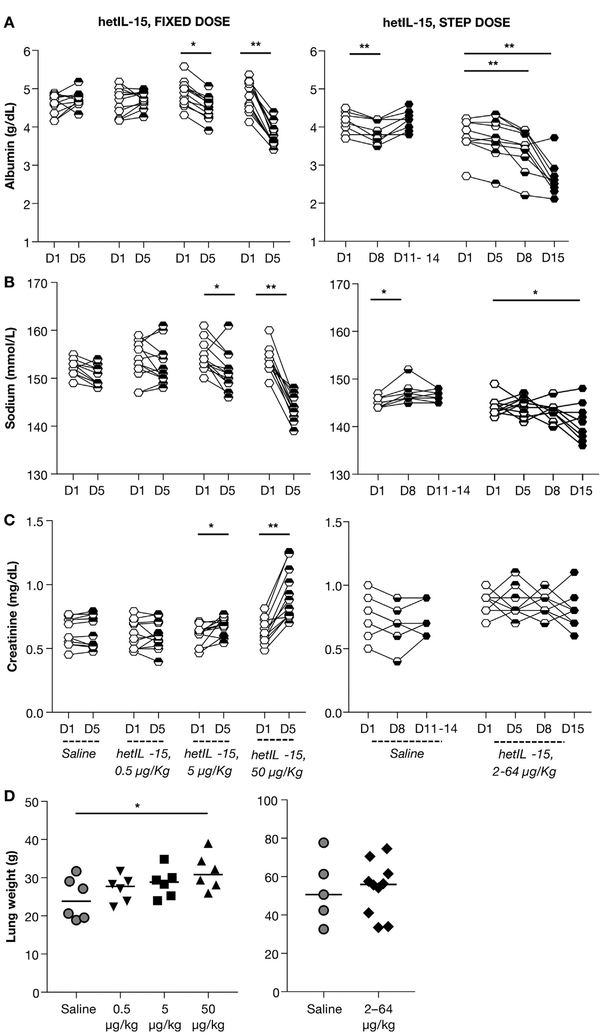
At selected time points, blood samples were also monitored through complete blood counts and blood chemistry. Comparisons of treated animals were done to concurrent controls in the same facility. A decline in albumin (Fig. 7A, left panel) and sodium levels (Fig. 7B, left panel), together with an increase in Creatinine levels (Fig. 7C, left panel) were found in macaques treated with 50 μg/kg, as early as day 5. All levels returned to normal values upon interruption of hetIL-15 treatment. In addition, dependent edema in the lower abdomen, extremities and genital tissues was detected in 9 of 12 (75%) animals treated with 50 μg/kg/dose and in 1 of 12 (8.3%) animals treated with 5 μg/kg/dose. None of the animals treated at 0.5 μg/kg or the controls showed any sign of edema. A Fisher test of the frequency of clinically evident edema showed a strong association with the dose level of hetIL-15 (p < .0001). Taken together, these findings were compatible with decreased oncotic pressure due to the decline in serum albumin, as result of increased vascular permeability (capillary leak). The phenomenon was also associated with renal dysfunction, measured by increased creatinine levels. Given the apparent capillary leak syndrome observed in animals treated with the fixed high-dose regimen of hetIL-15, we also monitored the same clinical pathology parameters in macaques treated with the step-dose regimen. Unlike the animals receiving 50 μg/kg/dose, the step-dose regimen led only to mild effects in the case of albumin (day 8), which became more significant after the end of the treatment cycle (day 15) (Fig. 7A, right panel). A mild decrease in sodium was also evident on day 15 (Fig. 7B, right panel). Importantly, we observed neither signs of edema nor of renal dysfunction (measured by serum creatinine levels), in contrast to the findings of animals in the 50 μg/kg/dose treatment group (Fig. 7C). In agreement with the blood chemistry data, increased lung weight at necropsy was detected only in animals treated at 50 μg/kg/dose (Fig. 7D), suggesting movement of fluids from the intravascular to the interstitial compartment and increased vascular permeability (capillary leak syndrome). Therefore, the drop-in albumin at the end of step-dose regimen was transient and not associated with edema or other sequelae of capillary leak such as fever, decreased blood pressure, and lung edema. In contrast, the animals treated with 50 μg/kg had elevated creatinine, an indication of acute kidney injury. None of these changes were observed in the animals treated at 5 μg/kg/dose, suggesting that the no observed adverse effect level (NOAEL) for the fixed-dose regimen study was 5 μg/kg/dose.
Fig. 7.
Comparison of predictors of capillary leak syndrome and kidney function in animals receiving hetIL-15. Chemistry lab analysis was performed in all macaques prior, during and at the end of the treatment. Albumin levels measured as g/dL (A), sodium levels measured as mmol/L (B), creatinine levels measured as mg/dL (C) were determined at the indicated time points for macaques in the fixed-dose regimen (left panels) and in the step-dose regimen (right panels). (D). Animals were sacrificed at the end of the treatment and lung weight was determined at necropsy. Individual animals and mean are showed. Statistical analyses were performed by unpaired t-test and 1-way ANOVA. *, p < .05; **, p < .01 respectively.
Overall, our data showed that hetIL-15-related toxicity, including altered renal function and clinically apparent capillary leak syndrome, was associated with the provision of higher doses of cytokine earlier in the treatment cycle, and that the step-dose regimen was a preferred administration regimen aimed to improve safety early in the two-week cycle and to better match the administered levels of hetIL-15 to the dynamic changes of the target cell pool.
4. Discussion
Cytokine administration is a promising immunotherapy strategy to fight cancer. Recombinant human interleukin-2 (rhIL-2) is a prototypic immunotherapy treatment approved for subjects with metastatic malignancy [35–40]. High dose interleukin-2 (HDIL-2) stimulates the proliferation of effector cells capable of killing cancer cells but also suppresses immune responses through the increase of inhibitory CD25+Foxp3+T regulatory cells (Tregs) [41,42], causing activation-induced cell death (AICD) and serious toxicities such as hypotension, renal failure, and capillary leak syndrome that can lead to respiratory failure [43,44]. These detrimental factors have prompted a search for immunotherapies possessing the benefits of HDIL-2 but fewer negative features. Extensive preclinical investigations have demonstrated that IL-15 has favorable characteristics that could lead to more effective and less toxic immunotherapy for the treatment of metastatic cancers. IL-15 is pivotal in the generation and maintenance of natural killer (NK) cells and CD8+ T-cells [45], leading to tumor growth control in different murine models [31,46–50]. Recently, high serum levels of IL-15 were found to be associated with CAR T cell treatment effectiveness in patients with lymphoma [51].
A first-in-human trial using E. coli-derived sch rhIL-15 was recently concluded in patients with advanced stage metastatic disease [25]. IL-15 was shown to affect lymphocyte homeostasis through both redistribution and proliferation of target cells. However, the administration of sch rhIL-15 as IV bolus resulted in clinical toxicities occurring within 2 h after treatment. Cytokine on- and off-target effects were similar in macaques administered IL-15, suggesting that the rhesus macaque is a useful animal model to evaluate effects of cytokine administration. In this study, we took advantage of the favorable PK properties of hetIL-15 [14,16] to develop delivery methods that avoid cytokine spikes. This was accomplished by SC injections of hetIL-15, that resulted in persistent bioactive levels of plasma IL-15 with lower Cmax and much longer T1/2 (~12 h) compared to the IV route (~1.5 h). The study was also designed to monitor the toxicity of hetIL-15 administered in two 2-week cycles over a 6-week period at dose levels of 0.5, 5, or 50 μg/kg. A phase I clinical trial using the same schedule is currently underway ( NCT02452268). Edema in the lower abdomen, extremities and genital tissues and swollen axillary and inguinal lymph nodes were observed mainly in animals treated at 50 μg/kg/dose. Body temperature generally increased following dose administration. Other clinical pathology findings included signs of capillary leak syndrome and renal dysfunction. Based on the absence of adverse findings related to the hetIL-15, the NOAEL for this study was 5 μg/kg/dose.
While a fixed-dose regimen of cytokine administration is simple, it may not be optimal for achieving maximal immune cell expansion and activation with minimal toxicity. IL-15 is a homeostatic cytokine known to induce the expansion of lymphocyte subsets, including NK and CD8+ T cells, that express high levels of the IL-2/IL-15Rβγ receptor complex [52]. These cells constitute the IL-15 cytokine sink [32]. Following a fixed-dose regimen, systemic exposure to the cytokine decreased during the treatment, while the IL-15 consumption by the cytokine sink increased. The decreasing trough levels throughout the treatment were attributed to the increased consumption of circulating IL-15 after repeated injections, which in turn reflect the dynamic of expansion of lymphocytes targeted by IL-15. In agreement with these data, peak in IL-15-related effects was observed at day 8, suggesting that the provided cytokine was not sufficient to further increase the rate of lymphocyte expansion during the second week of treatment. The peak at day 8 has also been observed in clinical trials. These results support our hypothesis that the capacity of the treated animals to utilize IL-15 increases as the responding cells expand. This explains the high trough levels after the first administration, when few lymphocytes are available, and the low trough levels at the end of the cycles (Fig. 2D). An additional hypothesis fitting the observations is that toxicity is associated with high free plasma levels of IL-15.
Only 7 of the 36 treated animals showed low/moderate and transient antibodies against human hetIL-15 (data not shown). Anti-hetIL-15 antibodies (ADA) developed in 2 macaques treated with the 0.5 μg/kg/dose and in 5 macaques treated with the 5 μg/kg/dose. None of the animals that received the higher dose of 50 μg/kg developed antibodies. These data indicate that antibodies against hetIL-15 have no effects in the different PK profiles between day 1 and day 40 illustrated in Fig. 2, where the greatest effects were seen in the 50 μg/kg/dose group, i.e., in the group of animals with no ADA. In addition, plasma IL-15 measurements at day 40 were comparable between macaques that developed ADA and macaques that did not, within the same dose treatment group.
In an effort to improve safety as well as to better match the circulating levels of IL-15 to the dynamic changes of the target cell pool, we designed a step-dose regimen of hetIL-15 administration, consisting of increasing (doubling) doses of hetIL-15 ranging from 2 to 64 μg/kg. The administered cytokine was consumed to a similar extent throughout the treatment cycle and toxicity was minimal, while the expansion of circulating lymphocytes was sustained throughout the treatment. This administration scheme resulted in high levels of lymphocytes with minimal adverse effects.
The comparison of PK and PD data from the two regimens supports a model in which, early in treatment, a low dose of hetIL-15 is sufficient to activate target cells, while provision of high doses resulted in an excess of circulating unbound cytokine linked to toxicity. During the treatment, lymphocytes expanded under the influence of IL-15 forming a larger cytokine sink. After expansion of lymphocytes is initiated, sequentially increasing doses of cytokines can be safely administered, to match the needs of the expanding lymphocytes.
In conclusion, our study highlights the benefits of a step-dose regimen for the in vivo administration of homeostatic cytokines. This is a new way to provide hetIL-15 and can be useful in the delivery of other cytokines.
Supplementary Material
Acknowledgement
We thank D. Weiss, J. Treece and team (BIOQUAL,Inc.), G. Ferrari (Advanced BioScience Laboratory), and R. Early (WuXI) for help with macaque studies, and T. Jones for administrative support. Research was supported by the Intramural Research Program, National Cancer Institute (G.N.P; B.K.F). Research supported in part by Novartis through a collaborative agreement with the National Cancer Institute/NIH, USA (G.N.P).
Footnotes
Conflict of interest
G.N.P., C.B., A.V. and B.K.F. are inventors on US Government-owned patents related to hetIL-15.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cyto.2018.01.011.
References
- [1].Berard M, Brandt K, Bulfone-Paus S, Tough DF, IL-15 promotes the survival of naive and memory phenotype CD8+ T cells, J. Immunol 170 (2003) 5018–5026. [DOI] [PubMed] [Google Scholar]
- [2].Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA, Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor, J. Exp. Med 180 (1994) 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ma A, Koka R, Burkett P, Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis, Annu. Rev. Immunol 24 (2006) 657–679. [DOI] [PubMed] [Google Scholar]
- [4].Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD, Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells, J. Exp. Med 195 (2002) 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang X, Sun S, Hwang I, Tough DF, Sprent J, Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15, Immunity 8 (1998) 591–599. [DOI] [PubMed] [Google Scholar]
- [6].Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M Jr., J.D. Lifson, V.C. Maino, M.K. Axthelm, F. Villinger, IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates, J. Clin. Invest 116 (2006) 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Colpitts SL, Stoklasek TA, Plumlee CR, Obar JJ, Guo C, Lefrancois L, Cutting edge: the role of IFN-alpha receptor and MyD88 signaling in induction of IL-15 expression in vivo, J. Immunol 188 (2012) 2483–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Colpitts SL, Stonier SW, Stoklasek TA, Root SH, Aguila HL, Schluns KS, Lefrancois L, Transcriptional regulation of IL-15 expression during hematopoiesis, J. Immunol 191 (2013) 3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, Kitano S, Ishii M, Tani-ichi S, Ikuta K, Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo, Proc. Natl. Acad. Sci. USA 111 (2014) 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A, Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis, J. Exp. Med 200 (2004) 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A, Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice, J. Exp. Med 197 (2003) 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schluns KS, Klonowski KD, Lefrancois L, Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells, Blood 103 (2004) 988–994. [DOI] [PubMed] [Google Scholar]
- [13].Dubois S, Mariner J, Waldmann TA, Tagaya Y, IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells, Immunity 17 (2002) 537–547. [DOI] [PubMed] [Google Scholar]
- [14].Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, Zhang GM, Patel V, Felber BK, Pavlakis GN, Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity, J. Biol. Chem 283 (2008) 4189–4199. [DOI] [PubMed] [Google Scholar]
- [15].Mortier E, Woo T, Advincula R, Gozalo S, Ma A, IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation, J. Exp. Med 205 (2008) 1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chertova E, Bergamaschi C, Chertov O, Sowder R, Bear J, Roser JD, Beach RK, Lifson JD, Felber BK, Pavlakis GN, Characterization and favorable in vivo properties of heterodimeric soluble IL-15.IL-15Ralpha cytokine compared to IL-15 monomer, J. Biol. Chem 288 (2013) 18093–18103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anthony SM, Howard ME, Hailemichael Y, Overwijk WW, Schluns KS, Soluble interleukin-15 complexes are generated in vivo by type I interferon dependent and independent pathways, PloS One 10 (2015) e0120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bergamaschi C, Bear J, Rosati M, Beach RK, Alicea C, Sowder R, Chertova E, Rosenberg SA, Felber BK, Pavlakis GN, Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum, Blood 120 (2012) e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bergamaschi C, Jalah R, Kulkarni V, Rosati M, Zhang GM, Alicea C, Zolotukhin AS, Felber BK, Pavlakis GN, Secretion and biological activity of short signal peptide IL-15 is chaperoned by IL-15 receptor alpha in vivo, J. Immunol 183 (2009) 3064–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J, Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}, Proc. Natl. Acad. Sci. USA 103 (2006) 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stoklasek TA, Schluns KS, Lefrancois L, Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo, J. Immunol 177 (2006) 6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vyas VV, Esposito D, Sumpter TL, Broadt TL, Hartley J, G.C.t. Knapp, W. Cheng, M.S. Jiang, J.M. Roach, X. Yang, S.L. Giardina, G. Mitra, J.L. Yovandich, S.P. Creekmore, T.A. Waldmann, J. Zhu, Clinical manufacturing of recombinant human interleukin 15. I. Production cell line development and protein expression in E. coli with stop codon optimization, Biotechnol. Prog 28 (2011) 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lugli E, Goldman CK, Perera LP, Smedley J, Pung R, Yovandich JL, Creekmore SP, Waldmann TA, Roederer M, Transient and persistent effects ofIL-15 on lymphocyte homeostasis in nonhuman primates, Blood 116 (2010) 3238–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sneller MC, Kopp WC, Engelke KJ, Yovandich JL, Creekmore SP, Waldmann TA, Lane HC, IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood, Blood 118 (2012) 6845–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, Goldman CK, Bryant BR, Decker JM, Chen J, Worthy TA, Figg WD Sr., Peer CJ, Sneller MC, Lane HC, Yovandich JL, Creekmore SP, Roederer M, Waldmann TA, Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer, J. Clin. Oncol 33 (2015) 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, Riddell SR, Safety and immunologic effects of IL-15 administration in nonhuman primates, Blood 114 (2009) 2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, Goldman CK, Bryant BR, Decker JM, Fleisher TA, Lane HC, Sneller MC, Kurlander RJ, Kleiner DE, Pletcher JM, Figg WD, Yovandich JL, Creekmore SP, Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques, Blood 117 (2011) 4787–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bergamaschi C, Kulkarni V, Rosati M, Alicea C, Jalah R, Chen S, Bear J, Sardesai NY, Valentin A, Felber BK, Pavlakis GN, Intramuscular delivery of heterodimeric IL-15 DNA in macaques produces systemic levels of bioactive cytokine inducing proliferation of NK and T cells, Gene Ther. 22 (2015) 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jalah R, Rosati M, Kulkarni V, Patel V, Bergamaschi C, Valentin A, Zhang GM, Sidhu MK, Eldridge JH, Weiner DB, Pavlakis GN, Felber BK, Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids, DNA Cell Biol. 26 (2007) 827–840. [DOI] [PubMed] [Google Scholar]
- [30].Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA, Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens, J. Clin. Oncol 26 (2008) 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR, Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action, J. Immunol 180 (2008) 2099–2106. [DOI] [PubMed] [Google Scholar]
- [32].Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP, Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells, J. Exp. Med 202 (2005) 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sattler A, Dang-Heine C, Reinke P, Babel N, IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions, Eur. J. Immunol 45 (2015) 2286–2298. [DOI] [PubMed] [Google Scholar]
- [34].Verri WA Jr., T.M. Cunha, S.H. Ferreira, X. Wei, B.P. Leung, A. Fraser, I.B. McInnes, F.Y. Liew, F.Q. Cunha, IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production, Eur. J. Immunol 37 (2007) 3373–3380. [DOI] [PubMed] [Google Scholar]
- [35].Heemskerk B, Liu K, Dudley ME, Johnson LA, Kaiser A, Downey S, Zheng Z, Shelton TE, Matsuda K, Robbins PF, Morgan RA, Rosenberg SA, Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2, Hum. Gene Ther 19 (2008) 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rosenberg SA, The development of new immunotherapies for the treatment of cancer using interleukin-2. A review, Ann. Surg 208 (1988) 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT, et al. , Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer, N. Engl. J. Med 313 (1985) 1485–1492. [DOI] [PubMed] [Google Scholar]
- [38].Rosenberg SA, Lotze MT, Yang JC, Aebersold PM, Linehan WM, Seipp CA, White DE, Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients, Ann. Surg 210 (1989) 474–484; discussion 484–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosenberg SA, Yang JC, White DE, Steinberg SM, Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response, Ann. Surg 228 (1998) 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, White DE, Steinberg SM, Rosenberg SA, Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines, Clin. Cancer Res 14 (2008) 5610–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ahmadzadeh M, Rosenberg SA, IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients, Blood 107 (2006) 2409–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, Long LM, Bernstein D, Hill BJ, Douek DC, Berzofsky JA, Carter CS, Read EJ, Helman LJ, Mackall CL, Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+ CD25+ regulatory T cells, Nat. Med 11 (2005) 1238–1243. [DOI] [PubMed] [Google Scholar]
- [43].Maraninchi D, Vey N, Viens P, Stoppa AM, Archimbaud E, Attal M, Baume D, Bouabdallah R, Demeoq F, Fleury J, Michallet M, Olive D, Reiffers J, Sainty D, Tabilio A, Tiberghien P, Brandely M, Hercend T, Blaise D, A phase II study of interleukin-2 in 49 patients with relapsed or refractory acute leukemia, Leuk. Lymphoma 31 (1998) 343–349. [DOI] [PubMed] [Google Scholar]
- [44].Rosenstein M, Ettinghausen SE, Rosenberg SA, Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2, J. Immunol 137 (1986) 1735–1742. [PubMed] [Google Scholar]
- [45].Waldmann TA, The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature reviews, Immunology 6 (2006) 595–601. [DOI] [PubMed] [Google Scholar]
- [46].Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, Tagaya Y, Rosenberg SA, Waldmann TA, Restifo NP, IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells, Proc. Natl. Acad. Sci. USA 101 (2004) 1969–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, Tagaya Y, Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance, Blood 105 (2005) 721–727. [DOI] [PubMed] [Google Scholar]
- [48].Zhang M, Ju W, Yao Z, Yu P, Wei BR, Simpson RM, Waitz R, Fasso M, Allison JP, Waldmann TA, Augmented IL-15Ralpha expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice, J. Immunol 188 (2012) 6156–6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ng SSM, Nagy BA, Jensen SM, Hu X, Alicea C, Fox BA, Felber BK, Bergamaschi C, Pavlakis GN, Heterodimeric IL-15 treatment enhances tumor infiltration, persistence, and effector functions of adoptively transferred tumor-specific T cells in the absence of lymphodepletion, Clin. Cancer Res 23 (2017) 2817–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Basher F, Jeng EK, Wong H, Wu J, Cooperative therapeutic anti-tumor effect of IL-15 agonist ALT-803 and co-targeting soluble NKG2D ligand sMIC, Oncotarget 7 (2016) 814–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA, Kammula US, Sherman M, Perez A, Yuan CM, Feldman T, Friedberg JW, Roschewski MJ, Feldman SA, McIntyre L, Toomey MA, Rosenberg SA, Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels, J. Clin. Oncol 35 (2017) 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA, IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels, Immunity 4 (1996) 329–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.