Abstract
Background
To evaluate the clinical impact of fluorescence-guided surgery (FGS) in glioblastoma, we analyzed the clinical data of 80 consecutive patients operated on by a single surgeon with or without 5-aminolevulinic acid (5-ALA).
Methods
We compared 3-dimensional volumetric extent of resection and clinical outcomes between 40 consecutive patients undergoing resection using a white-light (WL) microscope and 40 subsequent consecutive patients undergoing resection using FGS with 5ALA.
Results
By introducing FGS, there was a significant difference in the mean volumetric extent of the resection rate of T1-enhancing lesions (84.7% in the white-light group and 97.0% in the 5-ALA group, P = .002). The complete resection rate was improved from 43% to 80%, and the proportion of resections that were <80% was reduced from 26% to 4% by FGS. The median progression-free survival was significantly better in the 5-ALA group (18.0 months vs. 6.0 months; P = .001). Although the immediate postoperative functional status was slightly worse in the 5-ALA group, this trend had reversed itself by 3 months postoperatively.
Conclusions
The present study adds practical evidence of the clinical impact of 5-ALA FGS on glioblastomas from the surgeon's standpoint.
Keywords: aminolevulinic acid, extent of resection, fluorescence-guided surgery, glioblastoma, volumetric analysis
In the modern era of glioblastoma management, it is accepted that the extent of resection (EOR) on its own is a critical factor for improving prognosis, aside from the original surgical role of confirming the histological diagnosis and reducing the tumor burden for subsequent chemoradiotherapy.1–4 However, previous studies assessing the EOR based on postoperative magnetic resonance imaging (MRI) have revealed that no more than a 40% gross total resection (GTR) rate can be identified in surgeries for high-grade glioma.5–8 The hurdles for maximal safe resection are largely due to the difficulty in defining tumor margins and functional preservation in cases of eloquent area tumors.9
Since the introduction of fluorescence-guided surgery (FGS) using 5-aminolevulinic acid (5-ALA) for the resection of malignant gliomas, it has become obvious that the EOR and GTR rates could be improved significantly.9,10 A landmark study of FGS using 5-ALA for malignant gliomas by Stummer et al reported that GTR could be obtained in 65% of 139 patients using 5-ALA, a significantly higher rate compared with the 36% of 131 patients who were resected using the conventional white-light (WL) microscope (P < .0001).9 They reported that progression-free survival (PFS) was better in the 5-ALA group, but no statistically significant difference was found in overall survival (OS).9 However, subsequent reanalysis by the same study group showed that the improved OS in those participants with complete resections was found predominantly in the FGS group, after controlling for compounding factors.11 Other studies have also supported the clinical usefulness of FGS using 5-ALA for high-grade glioma patients.10,12 However, the impact of introducing 5-ALA FGS into the clinical field from the standpoint of a single surgeon is difficult to predict, as previous studies have assessed the outcomes for multiple surgeons collectively.
In this study, we analyzed the clinical data of 80 consecutive, newly diagnosed glioblastoma patients who underwent surgery by a single surgeon during the period in which 5-ALA FGS was introduced, in order to evaluate its impact on clinical practice and ability to minimize bias.
Materials and Methods
Between January 2009 and May 2011, we analyzed the clinical data of 80 consecutive adult patients who were newly diagnosed with glioblastoma according to the WHO 2007 classification, after obtaining approval for retrospective analysis by the Institutional Review Board of the Seoul National University Hospital. All participants were intended to achieve the maximal safe resection under general anesthesia using neuronavigation and intraoperative monitoring by a single surgeon (C.-K.P.). We used July 30, 2010, the date 5-ALA FGS was introduced at our institution, as a separation reference point, Forty preintroduction participants and 40 postintroduction participants were assigned to the white-light group and the 5-ALA group, respectively. There were no significant differences between the 2 groups in baseline characteristics such as age, sex ratio, or performance status (Table 1). The distribution of tumor locations was also similar in both groups, with involvement of the eloquent area observed in 52.5% of the white-light group and 47.5% of the 5-ALA group (Table 1). All participants were treated with standard concomitant chemoradiotherapy with temozolomide after surgery. The rechallenging of temozolomide, bevacizumab/CPT-11, CCNU, PCV chemotherapy, and reoperation had been chosen for salvage management in progressive disease when indicated. Salvage management was carried out in 31.4% and 27.8% of the patients who experienced progression in the white-light group and the 5-ALA group, respectively.
Table 1.
Baseline characteristics of the study cohort
| Characteristics | White-light Group (n = 40) | 5-ALA Group (n = 40) |
|---|---|---|
| Median age, years | 55 (range, 31–81) | 51 (range, 16–77) |
| Sex, male:female | 24:16 | 23:17 |
| KPS, number of patients | ||
| 100% | 12 | 8 |
| 90% | 9 | 16 |
| 80% | 3 | 3 |
| 70 | 11 | 10 |
| 60% | 4 | 3 |
| 50% | 1 | 0 |
| Mean preoperative tumor volume, cm3 | 57.044 ± 40.571 | 39.582 ± 31.997 |
| Involvement of eloquent area, number of patients | 21 | 19 |
In all patients, the preoperative MRI was performed the day before surgery, and the postoperative MRI was performed within 48 hours after surgery to compare the EOR. The analysis of 3-dimensional volumetry was performed to assess the EOR. The MRI data for the contrast-enhanced T1-weighted images (CE-T1WI) and T2-weighted images (T2WI) were digitally transferred from the PACS workstation to a personal computer and processed with ImageJ (Rasband WS. ImageJ, US National Institutes of Health, Bethesda, Maryland, USA, imagej.nih.gov/ij/) and software developed in-house using Microsoft Visual C++. Regions of interest that contained the entire tumor were drawn in each section of the MR images. Tumor boundaries were defined with reference to the high signal intensity areas thought to represent tumor tissue on the CE-T1WI (or T2WI). The data acquired from each slice were summed to derive the volume for the entire tumor, using the software developed in-house. The volume was measured and double-checked in a blinded fashion by a neurosurgeon and neuroradiologist who were not involved in the surgery and had no information about the patients' management. There were no significant differences in the mean preoperative volume between the 2 groups (57.044 ± 40.571 cm3 in the white-light group and 39.582 ± 31.997 cm3 in the 5-ALA group; P = .053, Table 1).
OS was measured from the date of operation to the date of the participant's death. The surviving participants were submitted to censored observations at the last follow-up. PFS was calculated as the period of progression-free months after the surgery. Disease progression was evaluated using the Response Assessment in Neuro-Oncology (RANO) criteria.13
Functional outcome was evaluated using the Karnofsky performance score (KPS), estimated before and immediately after surgery and again at 3 months, 6 months, and 12 months after surgery. Statistical analysis was performed using t tests and a repeated-measures ANOVA for parametric comparisons. The Kaplan-Meyer method and Breslow test were used for the PFS and OS analysis. P values <.05 were regarded as statistically significant. These analyses were performed using IBM SPSS Statistics software version 21.0 (SPSS IBM).
Results
Extent of Resection
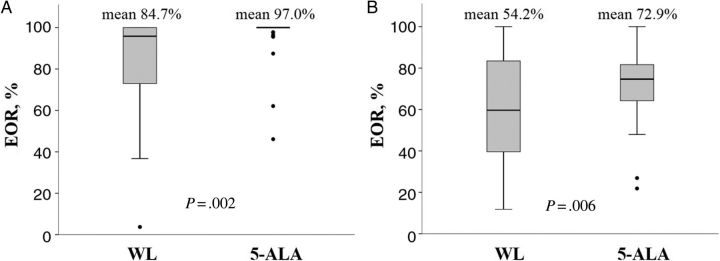
The extent of resection, based on volumetric analysis of resection of the T1-enhancing area in both groups, is shown in Table 2. The GTR rate improved from 43% to 80% using 5-ALA, without any other alterations of the surgical condition. Patients who obtained EOR at <80% were reduced to 4% from 26% using 5-ALA. This superior outcome of EOR in the 5-ALA group over the white-light group was confirmed to be statistically significant with regard to both the T1-enhancing area (P = .002) and the T2 high signal intensity area (P = .006) (Figure 1).
Table 2.
Comparison of the extent of resection between the white-light and 5-ALA groups. The resection rates are based on the volumetric analysis of the T1-enhancing area in preoperative and postoperative MRIs.
| Extent of Resection | White-light Group | 5-ALA Group |
|---|---|---|
| 100% | 43% | 80% |
| 95%–99% | 10% | 13% |
| 90%–94% | 8% | 0% |
| 85%–89% | 8% | 3% |
| 80%–84% | 5% | 0% |
| <80% | 26% | 4% |
Figure 1.
Comparison of extent of resection between the white-light (WL) group and the 5-ALA group, as measured by 3-dimensional volumetric analysis. (A) 5-ALA group achieved superior extent of resection in both the T1-enhancing area and (B) the T2 high signal intensity area.
Survival Outcome
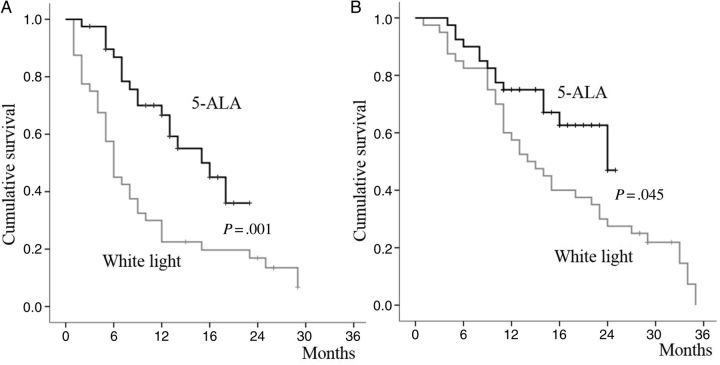
Of the 80 participants, complete resection was achieved in 49 patients, and their median OS was 24.0 months (95% CI, 19.2–29.0 months), which was significantly better than that of the incomplete resection group whose median OS was 13.0 months (95% CI, 5.2–20.8 months; P = .013). There was a significant difference in the survival outcome in the white-light and 5-ALA groups. The PFS was 6.0 months (95% CI, 4.8–7.2 months) in the white-light group and 18.0 months (95% CI, 12.0–24.0 months) in the 5-ALA group (P = .001, Figure 2A). The OS was also statistically superior in the 5-ALA group (median OS, 24.0 months; confidence interval not available) compared with the white-light group (median OS, 14.0 months; 95% CI, 14.1–25.9 months; P = .045, Figure 2B).
Figure 2.
Kaplan–Meier survival plots for (A) progression-free survival and (B) overall survival. There were significant differences in survival between the white-light group and the 5-ALA group.
Functional Outcome
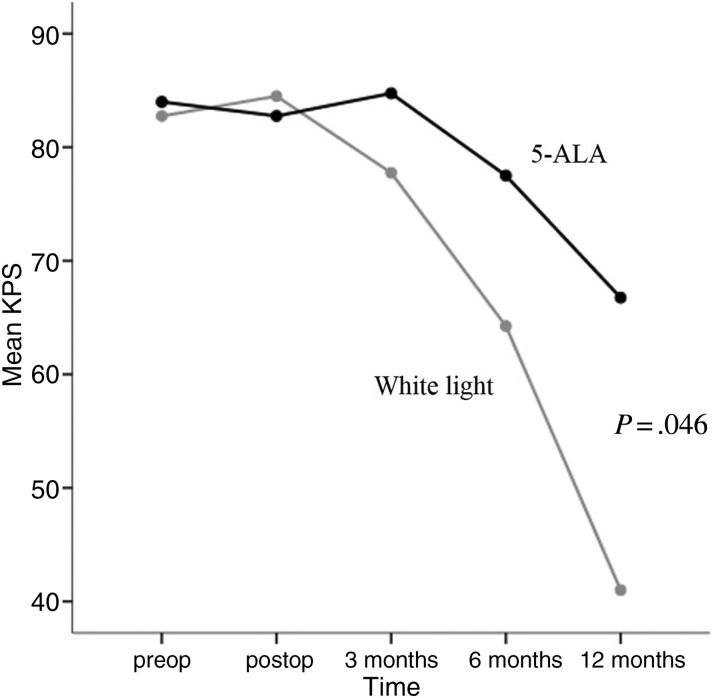
Although the preoperative KPS was similar between the white-light and 5-ALA groups, the immediate postoperative functional outcome was worse in the 5-ALA group than in the white-light group (mean KPS 82.7% vs. 84.5%). However, a reversion of KPS was observed at 3 months after surgery (mean KPS, 84.7% vs. 77.7%), and this gap later widened to a significant level until 12 months after surgery (P = .046; Figure 3). Worsening of neurological functions immediately after surgery was observed in twice as many participants in the 5-ALA group as in the white-light group (motor dysfunction 25.0% vs. 12.5%, language dysfunction 12.5% vs, 5.0%). However, there was significant improvement in the 5-ALA group by 3 months after surgery (motor dysfunction 7.5%, language dysfunction 2.5%), which was similar to those in white-light group (motor dysfunction 7.5%, language dysfunction 0%). No surgical deaths occurred in either group.
Figure 3.
Changes in performance status analyzed by mean KPS after the surgery. Repeated-measures ANOVA confirmed the significant differences between the white-light group and the 5-ALA group over the course of time.
Discussion
Since the introduction of 5-ALA for surgery of high-grade gliomas, it is obvious that FGS can improve surgical outcome, as has been reported in multiple studies.9,10,12,14–17 However, as the most studies evaluated the surgical results of multiple surgeons, the efficacy of 5-ALA FGS might have been biased by uneven surgical conditions or surgeons’ levels of experience. From the surgeon's practical point of view, there are still doubts about how surgical outcome would be improved by the introduction of FGS to high-grade glioma surgery. In the present study, all participants were operated on by a single surgeon, and 40 from each group were recruited consecutively before and after the introduction of 5-ALA FGS; no other changes, except introducing 5-ALA for surgery, were made during the enrollment period. This study setting might minimize the effect of confounding factors, and the sole expected effect of 5-ALA FGS introduction to current clinical practice could be evaluated.
There have been controversies about whether the EOR influenced patient survival beneficially in the management of glioblastoma. This confusion originated from the lack of objective and appropriate measurement strategies for EOR. The studies analyzing EOR of high-grade glioma based on postoperative MRI showed that GTR could be achieved <40% at the cost of modern surgical techniques.5–8 Recent studies indicate that volumetric analysis of EOR based on postoperative MRI provides the most reliable quantified standard of surgical benefit for high-grade gliomas.3,18,19 Among them, Sanai et al reported that patient survival was increased in proportion to EOR in glioblastoma, and the significant minimal threshold for impacting patient outcome was 78% resection of tumor volume based on T1-enhancing area.3 In the present study of volumetric analysis, the GTR rate could be almost doubled from 43% to 80% by introducing 5-ALA FGS. A more encouraging result is that patients whose EOR was <80% (the cutoff value for the benefit of surgery) were reduced from 26% to 4%. These improvements in EOR were significantly associated with patients' clinical outcome.
Nevertheless, there are still 20% of patients for whom GTR could not be achieved despite FGS introduction. There are various situations limiting the usefulness of 5-ALA FGS. They are (i) extensive or multifocal tumor beyond the surgical field; (ii) risk of definite functional deterioration due to the eloquent location of the tumor; (iii) poor visualization of fluorescence due to deep-seated tumor with narrow surgical window, or copious hemorrhage at the surgical field, or tumor hidden by necrotic tissue; (iv) technical problems such as inappropriate administration of 5-ALA and time window violation; and (v) drug interference such as phenytoin.20 By combining other technologies such as improved functional monitoring or intraoperative MRI, there is still room for improvement in GTR rate.12,21 On the other hand, GTR could be achieved in ∼40% of patients without using the FGS technique. This raises the issue of optimal indication for 5-ALA FGS and the appropriate selection of patients for this treatment, which should be evaluated in further studies.
The differences in functional outcome compared with conventional microsurgery are also important issues. Our data showed favorable performance outcome in the 5-ALA group after surgery except for the immediate postoperative period. Immediate deterioration in postsurgical functioning might be related to EOR, which was an added risk for the 5-ALA group. However, the outcome of functional status was reversed 3 months after surgery, and the more rapid functional deterioration observed in the white-light group was related to the differences in PFS. Stummer et al reported results similar to ours, in which the higher risk of temporary impairment was shown in the extended resection group using 5-ALA and the more rapid neurological deterioration was shown with incomplete resection.16
Conclusions
There was significant objective improvement in the EOR rate by employing 5-ALA FGS for glioblastoma patients, and it was related to better clinical outcome. The present study can be a surgeon's touchstone for estimating the clinical impact of introducing FGS using 5-ALA for management of glioblastoma.
Funding
This study was supported by a grant of the Inviting & Supporting Project of Russia Science Seoul, Seoul R&BD Program, Seoul Business Agency (Study No: 0720121550).
Conflict of interest statement. None declared.
References
- 1.Eyupoglu IY, Buchfelder M, Savaskan NE. Surgical resection of malignant gliomas-role in optimizing patient outcome. Nat Rev Neurol. 2013;9(3):141–151. doi: 10.1038/nrneurol.2012.279. [DOI] [PubMed] [Google Scholar]
- 2.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 3.Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 4.Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26(2):239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Maurer MJ, Rummans TA, et al. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57(3):495–504. doi: 10.1227/01.neu.0000170562.25335.c7. [DOI] [PubMed] [Google Scholar]
- 6.Kowalczuk A, Macdonald RL, Amidei C, et al. Quantitative imaging study of extent of surgical resection and prognosis of malignant astrocytomas. Neurosurgery. 1997;41(5):1028–1036. doi: 10.1097/00006123-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Pope WB, Sayre J, Perlina A, et al. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26(10):2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 8.Tortosa A, Vinolas N, Villa S, et al. Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer. 2003;97(4):1063–1071. doi: 10.1002/cncr.11120. [DOI] [PubMed] [Google Scholar]
- 9.Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 10.Diez Valle R, Tejada Solis S, Idoate Gastearena MA, et al. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol. 2011;102(1):105–113. doi: 10.1007/s11060-010-0296-4. [DOI] [PubMed] [Google Scholar]
- 11.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564–576. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 12.Feigl GC, Ritz R, Moraes M, et al. Resection of malignant brain tumors in eloquent cortical areas: a new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg. 2010;113(2):352–357. doi: 10.3171/2009.10.JNS09447. [DOI] [PubMed] [Google Scholar]
- 13.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1072. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 14.Cortnum S, Laursen RJ. Fluorescence-guided resection of gliomas. Dan Med J. 2012;59(8):A4460. [PubMed] [Google Scholar]
- 15.Schucht P, Beck J, Abu-Isa J, et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71(5):927–935. doi: 10.1227/NEU.0b013e31826d1e6b. [DOI] [PubMed] [Google Scholar]
- 16.Stummer W, Tonn JC, Mehdorn HM, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg. 2011;114(3):613–623. doi: 10.3171/2010.3.JNS097. [DOI] [PubMed] [Google Scholar]
- 17.Tykocki T, Michalik R, Bonicki W, et al. Fluorescence-guided resection of primary and recurrent malignant gliomas with 5-aminolevulinic acid. Preliminary results. Neurol Neurochir Pol. 2012;46(1):47–51. doi: 10.5114/ninp.2012.27212. [DOI] [PubMed] [Google Scholar]
- 18.Kuhnt D, Becker A, Ganslandt O, et al. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol. 2011;13(12):1339–1348. doi: 10.1093/neuonc/nor133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider JP, Trantakis C, Rubach M, et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme-a quantitative radiological analysis. Neuroradiology. 2005;47(7):489–500. doi: 10.1007/s00234-005-1397-1. [DOI] [PubMed] [Google Scholar]
- 20.Hefti M, Albert I, Luginbuehl V. Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells. J Neurooncol. 2012;108(3):443–450. doi: 10.1007/s11060-012-0857-9. [DOI] [PubMed] [Google Scholar]
- 21.Eyupoglu IY, Hore N, Savaskan NE, et al. Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS One. 2012;7(9):e44885. doi: 10.1371/journal.pone.0044885. [DOI] [PMC free article] [PubMed] [Google Scholar]