Abstract
Epileptic seizures are common in patients with primary or secondary malignant brain tumor. However, current knowledge on the occurrence of seizures during the end of life (EOL) phase of brain tumor patients is limited. Because symptom management with preservation of quality of life is of major importance for patients with a malignant brain tumor, particularly in the EOL, it is necessary to gain a deeper understanding of seizures and their management during this phase. We performed a systematic review of literature related to epilepsy in the EOL phase of brain tumor patients, based on the electronic resources PubMed, Embase, and Cinahl. The search yielded 442 unique records, of which 11 articles were eligible for further analysis after applying predefined inclusion criteria. Seizures occur relatively frequently in the EOL phase, particularly in patients with high-grade glioma. However, seizure management is often hampered by swallowing difficulties and impaired consciousness. Treatment decisions are largely dependent on expert opinion because a standardized approach for treating seizures in the terminal stage of brain tumor patients is still lacking.
Keywords: brain tumor, end of life, epilepsy, glioma, metastasis
Epileptic seizures are a common symptom in patients with a primary or secondary malignant brain tumor, and seizures are the presenting sign of a tumor in up to 50% of patients. Tumor type, the location of the tumor, and its proximity to the cortical gray matter influence the chance of developing a seizure.1–3 In general, the epileptogenicity of the tumor is inversely correlated with its growth rate. Low-grade gliomas are often the most epileptogenic, particularly slow-growing tumors such as gangliogliomas and dysembryoblastic neuroepithelial tumors, whereas fewer than 50% of patients with brain metastases and high-grade gliomas (HGGs) have seizures.1,4 Antiepileptic drugs (AEDs), as well as tumor-directed treatment, may lead to seizure control.5–8 However, anticonvulsant treatment may be hampered by pharmacoresistance, side effects, and interactions with other drugs or anticancer agents.2,9 Eventually, more than 30% of patients will be refractory to AED treatment during the course of their disease despite AED treatment.10
Median survival of patients with a malignant brain tumor varies widely from less than 6 months to more than 10 years, depending on tumor type and grade, age, performance status, and, in cases of metastases, the number of cerebral lesions and systemic disease activity.11,12 Patients will enter the end of life (EOL) phase at some point, which starts (in our definition) once tumor-directed treatment has been deemed no longer meaningful and symptom burden has increased significantly. Although the length of the EOL phase in individual cases may range from days to weeks before death, it has been confined to the last 3 months prior to death in previous studies.13,14 During this phase, preservation of quality of life by reducing clinical symptoms, such as pain, agitation, and seizures, is the mainstay of care.15–17 Seizure occurrence at the EOL often leads to rehospitalization and increased emergency room visits, with an increase in health care economic system costs and worsening of the patient's quality of life.18 Swallowing difficulties and impaired consciousness, in particular, can interfere with the regular oral administration of AEDs.19,20 In addition to further tumor growth, metabolic disturbances due to organ dysfunction or interactions of AEDs with other drugs, such as antibiotics and neuroleptics, may also contribute to an increased risk of seizures in the EOL phase.21 In other words, the context in which a seizure takes place during the EOL may vary considerably among brain tumor patients. A seizure may be the expression of epilepsy, which is defined as the enduring predisposition to generate epileptic seizures and requires the history of at least one seizure.22 Other patients may have a seizure without having epilepsy (eg, in the case of an acute symptomatic or provoked seizure) in which a proximate cause is clearly identifiable.23 Both treatment and prevention of seizures in the EOL, whether in the context of epilepsy or not, are serious challenges. Ongoing seizures may cause additional distress for caregivers as well, who are already experiencing a heavy burden of care.19,24 Because preservation of quality of life is of major importance for patients with a malignant brain tumor as well as their relatives, especially in the EOL, it is important to gain a deeper understanding of seizures and their management during this phase.
Current knowledge about the occurrence and treatment of seizures in the EOL phase is limited. In this report, we present the results of a systematic review of literature regarding seizures in the EOL phase of brain tumor patients. In particular, we aimed to outline the epidemiology, pathogenesis of seizures, and treatment strategies regarding the use of AEDs in the EOL phase.
Materials and Methods
Search Strategy
We performed a systematic review of literature in the electronic resources PubMed, Embase, and Cinahl. There were no limits with regard to publication years. We used a combination of 3 search strings related to (i) brain tumors, (ii) epilepsy, and (iii) end of life. The complete search string was as follows and was limited to terms that appeared in the title or abstract: (glioma OR gbm OR glioblastoma OR astrocytoma OR oligodendroglioma OR brain tumor OR brain tumors OR brain tumour OR brain tumours OR brain neoplasm OR brain cancer OR brain metastasis OR brain metastases) AND (epilepsy OR seizures OR convulsion OR anticonvulsant OR antiepileptic OR symptoms) AND (end of life OR palliative OR supportive OR terminal OR dying OR issues) (see online supplementary file).
Selection of Articles
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were followed.25 Articles were included when they reported on the epidemiology or treatment of epilepsy in adult brain tumor patients in the EOL phase. We included only peer-reviewed articles that were available full text in English. Case eports and reviews were excluded, as were studies not focusing on the EOL phase. Two authors (J.A.F.K. and L.D.) determined whether the articles were eligible for inclusion after screening title and abstract and served as reviewers of the full texts of all selected and potentially relevant articles. When full-text articles could not be retrieved through electronic resources, we contacted one of the authors to determine if the full text was available. The reference lists of the selected full-text articles were searched manually to identify additional studies. The 2 authors resolved any disagreements by reaching consensus through discussion. From the selected articles, we extracted the following study characteristics: publication year, study design, sample size, brain tumor type (primary or secondary malignant brain tumor including subtype), seizure prevalence, time evaluated before death, type of AED treatment, and other available data with regard to epilepsy in the EOL phase.
Results
Search Results
Through our search strategy, we retrieved 442 unique records, of which 33 were assessed for further eligibility. Of these, 23 were excluded for the following reasons: abstract only (n = 11), no seizures described (n = 5), no EOL phase described (n = 3), reviews (n = 3), and full text unavailable (n = 1). One additional study was found after manually searching the reference lists.26 The complete selection of articles is outlined in Fig. 1.
Fig. 1.
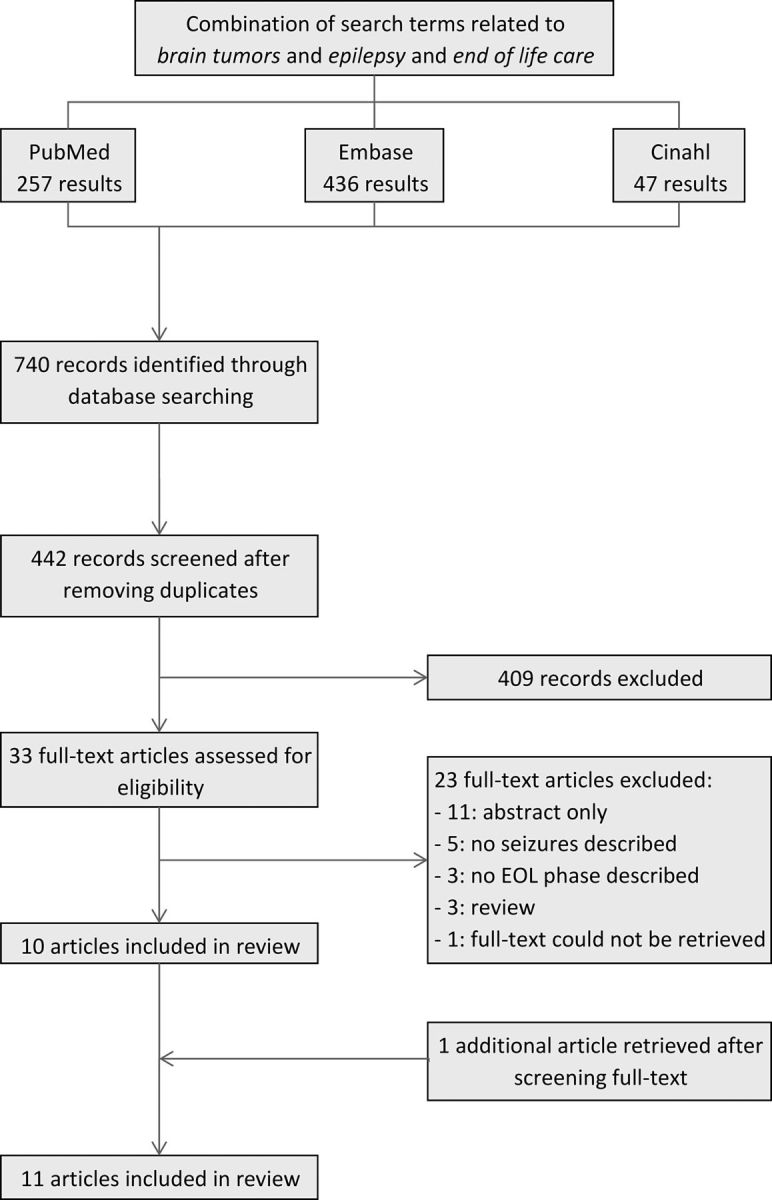
Identification of articles.
All 11 studies included for further analysis had a retrospective design,20,26–35 8 studies included chart or database reviews,20,26–32 and 3 studies were based on questionnaires.33–35 The numbers of cases studied ranged considerably from 29 to 812 patients.28,31 Seven studies focused primarily on patients with an HGG.20,27,28,30,33–35 Three studies focused on primary as well as secondary brain tumors,26,29,31 and one study focused on brain metastases from lung carcinoma only.32 All relevant study characteristics are summarized in Table 1.
Table 1.
Summary of main characteristics of selected articles
| Article | Study Design | Population | Location | Time Before Death | Seizure Prevalence | Additional Data |
|---|---|---|---|---|---|---|
| Bausewein, 200326 | Retrospective chart review | 31 patients: | Palliative care unit, hospital | Last 72 h | 10% | Nonpeaceful death due to seizures |
| 17 GBM | ||||||
| 9 grade II astrocytoma | ||||||
| 5 other | ||||||
| Faithfull, 200527 | Retrospective chart review | 39 primary malignant brain tumor | Hospice | Time between referral to hospice and death, ranging from 1–10 months with a mean of 3.7 months | 56% | |
| Oberndorfer, 200828 | Retrospective chart review | 29 GBM | Hospital | 6–10 weeks | 21% | |
| 2–6 weeks | 28% | |||||
| Last 2 weeks | 50% | |||||
| Pace, 200929 | Retrospective chart review | 169 patients: | At home | Last month | 10%–34% | Seizure type |
| 135 GBM | AED treatment | |||||
| 30 metastases | ||||||
| 4 other | ||||||
| Sizoo, 201030 | Retrospective chart review | 55 high-grade glioma | Not specified | Anytime during EOL phase, ranging from 1–296 days (median, 46 days) | 45% | Cause of death |
| Last week | 28% | |||||
| Ostgathe, 201031 | Retrospective review of database | 812 patients | Palliative care unit, hospice, at home | Upon admission to palliative care unit | 6%–19% | |
| 151 primary brain tumor | ||||||
| 661 secondary brain tumor | ||||||
| Yamanaka, 201132 | Retrospective chart review | 55 brain metastases from lung cancer | Palliative care center | Anytime during stay at palliative care center, ranging from 2–196 days (mean, 42.6 days) | 9% | |
| Flechl, 201333 | Retrospective cohort study, questionnaires | 52 GBM | Hospital, at home, hospice, nursing home | Last 3 months | 52% | |
| Last week | 38% | |||||
| Pace, 201320 | Retrospective review of database | 157 high-grade glioma | At home | Last month before death | 37% | Seizure type |
| AED treatment | ||||||
| Heese, 201334 | Retrospective cohort study, questionnaires | 605 patients: | At home, hospital, hospice, nursing home | Last 4 weeks before death | n/a | Percentage of caregivers satisfied with seizure treatment |
| 74 grade II astrocytoma | ||||||
| 86 grade III astrocytoma | ||||||
| 398 GBM | ||||||
| Sizoo, 201331 | Retrospective cohort study, questionnaires | 92 high-grade glioma | At home, hospital, hospice, nursing home | Last week | 29% | Seizure type |
| AED treatment |
Abbreviation: GBM, glioblastoma multiforme; n/a, not applicable.
Epidemiology of Seizures
Ten studies described the seizure prevalence at some point during the EOL phase (Table 1). The prevalence of seizures differed considerably, ranging from 6% to 56%,20,26–33,35 and the time of evaluation before death varied widely as well. In 6 of 10 studies, a specific period of time before death was analyzed, ranging from 3 days to 3 months.20,26,28,29,33,35 One other study reported the time between referral to a hospice and death, which ranged from 1 to 10 months (mean, 3.7 months).27 Another study analyzed the time between referral to a palliative care center and death, which ranged from 2 to 196 days (mean, 42.6 days).32 Moreover, one study reported on seizure occurrence anytime during the EOL phase (range, 1–294 days; mean, 46 days),30 and one study described seizures upon admission to a palliative care unit without a documented time between admission and death.31
In 2 studies that reported on seizures during the last month in 326 consecutive patients (90% HGG), the prevalence varied from 30% to 37%.20,29 Moreover, seizure prevalence appeared to increase towards death in a study evaluating 29 GBM patients in a hospital setting.28 A seizure prevalence between 28%–38% during the last week was reported in 3 other studies.30,33,35
Pace et al reported that of 58 HGG patients with epilepsy in the last month before death, 79% had focal seizures, 18% had generalized seizures, and 3% had a status epilepticus. The risk of seizures was higher in patients with a previous history of epilepsy, and only 5% of patients with seizures during the last month had been seizure-free before.20 In contrast, Sizoo et al reported that of 27 HGG patients with epilepsy in the last week before death, 22% had no seizures before the EOL phase.35 Moreover, only 9% of patients with previous focal epilepsy experienced focal seizures during the last week before death. Patients with a previous status epilepticus showed a higher prevalence of seizures during the last week before death. No other risk factors were found for the occurrence of seizures in the last week of life.35
In 55 patients with brain metastases from lung carcinoma, 9% had seizures during their stay at a palliative care center (mean, 43 days).32 In a study of 661 patients with brain metastases and 151 patients with a primary malignant brain tumor referred to a palliative care unit, 6% and 19%, respectively, were reported as having had seizures.31 Similar differences in terms of seizure prevalence during the EOL phase in 30 patients with brain metastases and 135 patients with HGG were found in another study, with 10% versus 34%, respectively.29
In a study of 55 HGG patients, seizures were thought to be the cause of death in 5% of all patients.30 In 3% of patients with a malignant brain tumor, death was considered to be nonpeaceful due to seizures in the last 72 hours.28
Seizure Treatment
Three studies described the treatment of epilepsy during the EOL phase.20,29,35 All patients with epilepsy during the course of their disease were on AEDs. However, the use of prophylactic AEDs among patients without seizures before the EOL phase varied widely from none to 88% of patients.20,29,35 No correlation was found between treatment with AEDs and the presence of seizures during the EOL phase.20
Regular intake of oral AEDs in the EOL phase appeared to be often hampered by swallowing difficulties and/or impaired consciousness.13,14 In the last month before death, mild to severe dysphagia was observed in 70% of patients, and 63% had impaired consciousness. Eighty-two percent of patients were unable to continue oral AEDs through the last days of life, and the route of AED administration was changed into parenteral use during the EOL phase for 83% of patients.20 Sizoo et al found that AEDs were tapered in the last week before death in 29 of 64 (45%) of patients. In 10 of 29 patients (35%) whose AEDs were tapered, seizures occurred during the last week before death.35 None of the studies addressed the impact of changes or discontinuation of AEDs on seizure prevalence during the EOL phase. A large study (605 glioma patients) reporting on the caregivers' perception found that 64% of caregivers thought epilepsy was treated effectively during the EOL phase.34
Discussion
EOL care in patients with a malignant brain tumor is primarily aimed at reducing clinical symptoms, relieving suffering, and avoiding unnecessary prolongation of dying.36–39 Next to a wide range of neurological deficits and cognitive impairments, seizures occur frequently in the EOL phase.19,24
Overall, seizures occur in up to 56% of patients.26,27 About one-third of HGG patients develop seizures in the last month or last week before death, whereas fewer than ≤10% of patients with brain metastases were reported to have had seizures during this phase.31,32 Possibly, the expansive, rather than the infiltrative, character of brain metastases contributes to a lower seizure burden in this group.40 It is difficult to characterize the type of seizures that occur in the EOL phase. For example, Pace et al showed that 46 of 58 patients (79%) had partial seizures, whereas Sizoo et al showed that 15 of 20 patients (75%) with epilepsy during the last week before death had generalized seizures during the EOL phase.20,35 It would be helpful to know which type of seizures are more prevalent because generalized seizures may require more aggressive treatment compared with seizures of focal origin.41
The EOL phase in brain tumor patients is characterized by a rapid progression of neurological and cognitive deficits, which could interfere with adequate seizure treatment.42 Swallowing difficulties are reported in 10%–85% of brain tumors, particularly in patients with HGG in the EOL phase, and can be caused by paresis, apraxia, and impaired consciousness. In up to 94% of brain tumor patients, progressive confusion or coma are reported in the last days before death.26–31 Thus, regular oral AED treatment is hampered in most patients with brain tumor-related epilepsy, particularly in the last days before death, leading to a higher risk of seizures.20
Besides subtherapeutic drug levels due to inadequate AED intake, several other factors may contribute to the occurrence of seizures during the EOL. Tumor progression itself may lead to an elevated risk of seizures in both patients with and without a history of seizures. The abrupt tissue damage in gliomas, which causes necrosis and edema, is thought to result in epileptogenicity of the tumor.10 In rapidly progressive tumors, infiltration of the peritumoral environment may lead to excitability.43 Metabolic encephalopathy due to electrolyte abnormalities or organ dysfunction may result in an additional seizure risk towards the EOL as well.21 Serum AED levels may also be affected by interactions with other drugs. For example, corticosteroids may impair the activity of phenytoin through shared pathways of hepatic metabolism.44 Concomitant use of antipsychotics such as clonazepine or antidepressants (particularly tricyclic antidepressants) may lead to seizures as well, due to a lower seizure threshold.45 On the other hand, anxiolytics such as benzodiazepines, which are frequently administered in the terminal phase for relief of intractable pain or agitation, may additionally reduce the risk of seizures. The potential role of brain imaging or other diagnostic tools to differentiate between causes of seizures during the EOL, which might subsequently direct treatment, was not examined in any of these studies.
Where possible, anticonvulsant therapy needs to be continued during the EOL phase, particularly for patients with a higher risk of seizures such as those with previous seizures or a status epilepticus during the course of their disease.20,35 In patients with a mass effect contributing to the development of seizures, a temporary start or continuation of dexamethasone might be considered. The consequences of discontinuing AEDs in the EOL phase remain largely unknown. Sizoo et al found that seizures took place in 10 of 29 patients (35%) whose AEDs were tapered during the EOL phase.35 However, seizures also occur in patients who are on AEDs, suggesting a relative inefficacy of AEDs.35 Before the EOL phase, the use of prophylaxis is not recommended for brain tumor patients without a history of seizures.15,46 Two meta-analyses found insufficient evidence to support prophylactic use of phenobarbital, phenytoin, and valproic acid.47,48 Prospective data on newer AEDs are unavailable. However, as there are no trials of prophylactic AEDs in the EOL phase of brain tumor patients, it is uncertain whether the benefits of prophylactics outweigh the risks. Therefore, future research should focus on the role of prophylactic anticonvulsant treatment in the EOL phase, particularly in HGG patients. An initial study to assess the efficacy of non-oral AEDs for patients with swallowing difficulties toward the EOL would be helpful.
Several studies advocate alternative routes of administration when patients are unable to continue oral AEDs.20,35 The preferred route of nonoral AED administration depends on the location of care in the EOL phase, but also on the availability of AEDs and the physician's experience with their administration.49 Achieving seizure control at the patient's preferred place of care is warranted because it could prevent unnecessary transitions and hospitalization during the EOL.27,28 Intravenous administration is used in a clinical setting when a rapid response is needed. In the out-of-hospital setting, intranasal, buccal, or rectal administration is more appropriate because as it can easily be administered by the caregiver.50
Results from randomized trials analyzing the use of non-oral AEDs in non-oncological patients with epilepsy can be helpful in examining alternative treatment options for brain tumor patients towards the EOL. In comparison with intravenous or rectal diazepam, intranasal midazolam shows a similar efficacy and safety in the acute treatment of seizures.51,52 Moreover, caregivers are more satisfied with the use of intranasal midazolam compared with rectal diazepam in treating acute seizures.50,53 A meta-analysis by McMullan54 et al, evaluating 3 clinical trials, shows that buccal midazolam is superior to rectal diazepam in achieving seizure control.55–57 Rectal administration of carbamazepine or valproic acid could contribute to the maintenance of serum levels of orally administered AEDs. However, evidence for rectal use of AEDs that are normally taken orally is limited to a few small prospective studies.50,58 Buccal or intranasal clonazepam can be safely administered in the out-of-hospital setting, although evidence for its efficacy is limited as well.59 Other reports show that intramuscular or subcutaneous phenobarbital in the EOL setting can be effective, as is repeated use of subcutaneous levetiracetam.20,60–62 In the case of unbearable suffering in the EOL phase (eg, due to refractory seizures), palliative sedation with benzodiazepines can be initiated. However, it is preferable to restrict the use of palliative sedation for epilepsy to those patients in the terminal phase in whom other AED treatment options have failed.63,64 A list of non-oral AEDs that can easily be applied in the out-of-hospital setting is outlined in Table 2.
Table 2.
List of non-oral antiepileptic drugs that can easily be applied in the out-of-hospital setting
| Route of Administration | Drug | Dose | Indication |
|---|---|---|---|
| Intranasal | Midazolam | 27.8 mg/mL, 1 spray of 2.5 mg in each nostril (total dose 5 mg); if necessary, repeat after 5 min | Emergency treatment |
| Rectal | Diazepam | 10 mg; if necessary, repeat after 5 min | Emergency treatment |
| Buccal | Clonazepam | 2–4 times 0.5 mg/d; maintenance dose 4 times, 0.5–1.0 mg/d; maximum dose 20 mg/d | Prophylactic treatment |
| Subcutaneous | Phenobarbital | 100 mg; if necessary, repeat after 4–6 h; maintenance dose 2–4 mg/kg | Prophylactic treatment |
| Subcutaneous | Midazolam | Start dose 10 mg; maintenance dose 1.5–2.5 mg/h | Palliative sedation in patients with refractory seizures where other non-oral AEDs have failed. |
Abbreviations: d, day; h, hour; min, minute
There are some limitations of this review. First, the level of evidence is low according to international classifications because all included studies had a retrospective design. Second, there is a remarkable heterogeneity of study populations in terms of histological diagnosis and place of care. Moreover, because a standardized definition of the EOL phase is lacking, each study used its own time interval prior to death, and these ranged from days to months. Therefore, comparisons between studies should be interpreted with caution. Third, there is a paucity of high-quality published data on the frequency of epilepsy and management of seizures in the EOL phase. Although the highest level of evidence would be achieved by randomized controlled studies, there are serious ethical objections to randomizing patients with regard to AED treatment in the EOL phase. In general, a retrospective study design is well accepted in EOL research and has the advantage of allowing all patients who enter the EOL phase to be identified.65–67
In conclusion, seizures occur relatively frequently in the EOL of patients with a malignant brain tumor, particularly in patients with HGG. Treatment decisions are largely dependent on expert opinion, as a standardized approach for treating seizures in the terminal stage of the disease is lacking. Currently, evidence is too limited to recommend the use of certain AEDs. Prospective studies, taking into account the complexity of AED administration during the EOL, should contribute to the development of more targeted seizure treatment.
Funding
This work was supported by the St. Jacobusstichting, The Hague (J.A.F.K. and M.J.B.T); Foundation ZOLEON (J.A.F.K. and M.J.B.T), and Foundation Chanrone (J.A.F.K. and M.J.B.T).
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Smits A, Duffau H. Seizures and the natural history of World Health Organization Grade II gliomas: a review. Neurosurgery. 2011;68(5):1326–1333. doi: 10.1227/NEU.0b013e31820c3419. [DOI] [PubMed] [Google Scholar]
- 2.Ruda R, Bello L, Duffau H, et al. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;(14(Suppl 4)):iv55–iv64. doi: 10.1093/neuonc/nos199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol. 2012;13(9):e375–e382. doi: 10.1016/S1470-2045(12)70266-8. [DOI] [PubMed] [Google Scholar]
- 4.Rosati A, Tomassini A, Pollo B, et al. Epilepsy in cerebral glioma: timing of appearance and histological correlations. J Neurooncol. 2009;93(3):395–400. doi: 10.1007/s11060-009-9796-5. [DOI] [PubMed] [Google Scholar]
- 5.Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 6.Sherman JH, Moldovan K, Yeoh HK, et al. Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg. 2011;114(6):1617–1621. doi: 10.3171/2010.12.JNS101602. [DOI] [PubMed] [Google Scholar]
- 7.Englot DJ, Han SJ, Berger MS, et al. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70(4):921–928. doi: 10.1227/NEU.0b013e31823c3a30. [DOI] [PubMed] [Google Scholar]
- 8.Kerrigan S, Grant R. Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database Syst Rev. 2011;8:CD008586. doi: 10.1002/14651858.CD008586.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Breemen MS, Wilms EB, Vecht CJ. Seizure control in brain tumors. Handb Clin Neurol. 2012;104:381–389. doi: 10.1016/B978-0-444-52138-5.00026-8. [DOI] [PubMed] [Google Scholar]
- 10.De Groot M, Reijneveld JC, Aronica E, et al. Epilepsy in patients with a brain tumour: focal epilepsy requires focused treatment. Brain. 2012;135(Pt 4):1002–1016. doi: 10.1093/brain/awr310. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JH, Carter JH, Jr., Friedman AH, et al. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 12.Crocetti E, Trama A, Stiller C, et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur J Cancer. 2012;48(10):1532–1542. doi: 10.1016/j.ejca.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Walbert T, Khan M. End-of-life symptoms and care in patients with primary malignant brain tumors: a systematic literature review. J Neurooncol. 2014;117(2):217–224. doi: 10.1007/s11060-014-1393-6. [DOI] [PubMed] [Google Scholar]
- 14.Sizoo EM, Pasman HR, Dirven L, et al. The end-of-life phase of high-grade glioma patients: a systematic review. Support Care Cancer. 2014;22(3):847–857. doi: 10.1007/s00520-013-2088-9. [DOI] [PubMed] [Google Scholar]
- 15.Preusser M, de RS, Wohrer A, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70(1):9–21. doi: 10.1002/ana.22425. [DOI] [PubMed] [Google Scholar]
- 16.Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54(4):514–520. doi: 10.1002/ana.10712. [DOI] [PubMed] [Google Scholar]
- 17.Aaronson NK, Taphoorn MJ, Heimans JJ, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. doi: 10.1200/JCO.2011.35.5750. [DOI] [PubMed] [Google Scholar]
- 18.Pace A, Di LC, Capon A, et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227. doi: 10.1089/jpm.2011.0306. [DOI] [PubMed] [Google Scholar]
- 19.Krouwer HG, Pallagi JL, Graves NM. Management of seizures in brain tumor patients at the end of life. J Palliat Med. 2000;3(4):465–475. doi: 10.1089/jpm.2000.3.4.465. [DOI] [PubMed] [Google Scholar]
- 20.Pace A, Villani V, Di Lorenzo C, et al. Epilepsy in the end-of-life phase in patients with high-grade gliomas. J Neurooncol. 2013;111(1):83–86. doi: 10.1007/s11060-012-0993-2. [DOI] [PubMed] [Google Scholar]
- 21.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 22.Fisher RS, van Emde BW, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 23.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671–675. doi: 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 24.Wideheim AK, Edvardsson T, Pahlson A, et al. A family's perspective on living with a highly malignant brain tumor. Cancer Nurs. 2002;25(3):236–244. doi: 10.1097/00002820-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Bausewein C, Hau P, Borasio GD, et al. How do patients with primary brain tumours die? Palliat Med. 2003;17(6):558–559. doi: 10.1177/026921630301700615. [DOI] [PubMed] [Google Scholar]
- 27.Faithfull S, Cook K, Lucas C. Palliative care of patients with a primary malignant brain tumour: Case review of service use and support provided. Palliative Med. 2005;19(7):545–550. doi: 10.1191/0269216305pm1068oa. [DOI] [PubMed] [Google Scholar]
- 28.Oberndorfer S, Lindeck-Pozza E, Lahrmann H, et al. The end-of-life hospital setting in patients with glioblastoma. J Palliative Med. 2008;11(1):26–30. doi: 10.1089/jpm.2007.0137. [DOI] [PubMed] [Google Scholar]
- 29.Pace A, Lorenzo CD, Guariglia L, et al. End of life issues in brain tumor patients. J Neurooncol. 2009;91(1):39–43. doi: 10.1007/s11060-008-9670-x. [DOI] [PubMed] [Google Scholar]
- 30.Sizoo EM, Braam L, Postma TJ, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol. 2010;12(11):1162–1166. doi: 10.1093/neuonc/nop045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostgathe C, Gaertner J, Kotterba M, et al. Differential palliative care issues in patients with primary and secondary brain tumours. Support Care Cancer. 2010;18(9):1157–1163. doi: 10.1007/s00520-009-0735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka R, Koga H, Yamamoto Y, et al. Characteristics of patients with brain metastases from lung cancer in a palliative care center. Support Care Cancer. 2011;19(4):467–473. doi: 10.1007/s00520-010-0838-5. [DOI] [PubMed] [Google Scholar]
- 33.Flechl B, Ackerl M, Sax C, et al. The caregivers’ perspective on the end-of-life phase of glioblastoma patients. J Neurooncol. 2013;112(3):403–411. doi: 10.1007/s11060-013-1069-7. [DOI] [PubMed] [Google Scholar]
- 34.Heese O, Vogeler E, Martens T, et al. End-of-life caregivers’ perception of medical and psychological support during the final weeks of glioma patients: a questionnaire-based survey. Neuro Oncol. 2013;15(9):1251–1256. doi: 10.1093/neuonc/not089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sizoo EM, Koekkoek JA, Postma TJ, et al. Seizures in high-grade glioma patients: a serious challenge in the end of life phase. BMJ Support Palliat Care. 2014;4(1):77–80. doi: 10.1136/bmjspcare-2013-000456. [DOI] [PubMed] [Google Scholar]
- 36.Sizoo E, Taphoom MJB, Uitdehaag B, et al. The end-of-life phase of high-grade glioma patients: Dying with dignity? Oncologist. 2013;18(2):198–203. doi: 10.1634/theoncologist.2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foley KM, Carver AC. Palliative care in neurology: An overview. Neurol Clin. 2001;19(4):789–799. doi: 10.1016/s0733-8619(05)70047-5. [DOI] [PubMed] [Google Scholar]
- 38.Pace A, Metro G, Fabi A. Supportive care in neurooncology. Curr Opin Oncol. 2010;22(6):621–626. doi: 10.1097/CCO.0b013e32833e078c. [DOI] [PubMed] [Google Scholar]
- 39.Bennett MI, Davies EA, Higginson IJ. Delivering research in end-of-life care: problems, pitfalls and future priorities. Palliat Med. 2010;24(5):456–461. doi: 10.1177/0269216310366064. [DOI] [PubMed] [Google Scholar]
- 40.Cha S. Update on brain tumor imaging: from anatomy to physiology. Am J Neuroradiol. 2006;27(3):475–487. [PMC free article] [PubMed] [Google Scholar]
- 41.Devinsky O. Sudden, unexpected death in epilepsy. N Engl J Med. 2011;365(19):1801–1811. doi: 10.1056/NEJMra1010481. [DOI] [PubMed] [Google Scholar]
- 42.Catt S, Chalmers A, Fallowfield L. Psychosocial and supportive-care needs in high-grade glioma. Lancet Oncol. 2008;9(9):884–891. doi: 10.1016/S1470-2045(08)70230-4. [DOI] [PubMed] [Google Scholar]
- 43.Kohling R, Senner V, Paulus W, et al. Epileptiform activity preferentially arises outside tumor invasion zone in glioma xenotransplants. Neurobiol Dis. 2006;22(1):64–75. doi: 10.1016/j.nbd.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Vecht CJ, Wagner GL, Wilms EB. Interactions between antiepileptic and chemotherapeutic drugs. Lancet Neurol. 2003;2(7):404–409. doi: 10.1016/s1474-4422(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 45.Haddad PM, Dursun SM. Neurological complications of psychiatric drugs: clinical features and management. Hum Psychopharmacol. 2008;23(Suppl 1):15–26. doi: 10.1002/hup.918. [DOI] [PubMed] [Google Scholar]
- 46.Tremont-Lukats IW, Ratilal BO, Armstrong T, et al. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev. 2008;2:CD004424. doi: 10.1002/14651858.CD004424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirven JI, Wingerchuk DM, Drazkowski JF, et al. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc. 2004;79(12):1489–1494. doi: 10.4065/79.12.1489. [DOI] [PubMed] [Google Scholar]
- 48.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 49.Raijmakers NJ, van ZL, Furst CJ, et al. Variation in medication use in cancer patients at the end of life: a cross-sectional analysis. Support Care Cancer. 2013;21(4):1003–1011. doi: 10.1007/s00520-012-1619-0. [DOI] [PubMed] [Google Scholar]
- 50.Anderson GD, Saneto RP. Current oral and non-oral routes of antiepileptic drug delivery. Adv Drug Deliv Rev. 2012;64(10):911–918. doi: 10.1016/j.addr.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Lahat E, Goldman M, Barr J, et al. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321(7253):83–86. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhattacharyya M, Kalra V, Gulati S. Intranasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34(5):355–359. doi: 10.1016/j.pediatrneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 53.de Haan GJ, van der GP, Doelman G, et al. A comparison of midazolam nasal spray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia. 2010;51(3):478–482. doi: 10.1111/j.1528-1167.2009.02333.x. [DOI] [PubMed] [Google Scholar]
- 54.McMullan J, Sasson C, Pancioli A, et al. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med. 2010;17(6):575–582. doi: 10.1111/j.1553-2712.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McIntyre J, Robertson S, Norris E, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(9481):205–210. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- 56.Mpimbaza A, Ndeezi G, Staedke S, et al. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Ugandan children: a randomized clinical trial. Pediatrics. 2008;121(1):e58–e64. doi: 10.1542/peds.2007-0930. [DOI] [PubMed] [Google Scholar]
- 57.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353(9153):623–626. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 58.Warren DE. Practical use of rectal medications in palliative care. J Pain Symptom Manage. 1996;11(6):378–387. doi: 10.1016/0885-3924(96)00012-7. [DOI] [PubMed] [Google Scholar]
- 59.Schols-Hendriks MW, Lohman JJ, Janknegt R, et al. Absorption of clonazepam after intranasal and buccal administration. Br J Clin Pharmacol. 1995;39(4):449–451. doi: 10.1111/j.1365-2125.1995.tb04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Junck L. Supportive management in neuro-oncology: opportunities for patient care, teaching, and research. Curr Opin Neurol. 2004;17(6):649–653. doi: 10.1097/00019052-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Keen JC. Re: The use of phenobarbitone in the management of agitation and seizures at the end of life. J Pain Symptom Manage. 2000;19(2):80–81. doi: 10.1016/s0885-3924(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Saca JM, Vaquero J, Larumbe A, et al. Repeated use of subcutaneous levetiracetam in a palliative care patient. J Pain Symptom Manage. 2013;45(5):e7–e8. doi: 10.1016/j.jpainsymman.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30(12):1378–1383. doi: 10.1200/JCO.2011.37.3795. [DOI] [PubMed] [Google Scholar]
- 64.Alonso-Babarro A, Bruera E, Varela-Cerdeira M, et al. Can this patient be discharged home? Factors associated with at-home death among patients with cancer. J Clin Oncol. 2011;29(9):1159–1167. doi: 10.1200/JCO.2010.31.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320(7233):469–472. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Setoguchi S, Earle CC, Glynn R, et al. Comparison of prospective and retrospective indicators of the quality of end-of-life cancer care. J Clin Oncol. 2008;26(35):5671–5678. doi: 10.1200/JCO.2008.16.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Earle CC, Ayanian JZ. Looking back from death: the value of retrospective studies of end-of-life care. J Clin Oncol. 2006;24(6):838–840. doi: 10.1200/JCO.2005.03.9388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


