Abstract
Background
The impact of primary malignant brain tumors on patient quality of life and psychological functioning is poorly understood, limiting the development of an evidence base for supportive interventions. We conducted a thorough systematic review and quality appraisal of the relevant literature to identify correlates of health-related quality of life (HRQoL) and psychological functioning (depression, anxiety and distress) in adults with primary malignant brain tumors.
Method
Twenty-three articles met predefined inclusion criteria from a pool of peer-reviewed literature published between January 1984 and July 2015 (N = 2407). Methodological quality of included studies was assessed using an adapted version of the Newcastle-Ottawa Scale.
Results
The overall methodological quality of the literature was moderate. Factors relating consistently with HRQoL and/or psychological functioning were cognitive impairment, corticosteroid use, current or previous mental health difficulties, fatigue, functional impairment, performance status and motor impairment.
Conclusions
Practitioners should remain alert to the presence of these factors as they may indicate patients at greater risk of poor HRQoL and psychological functioning. Attention should be directed towards improving patients' psychological functioning and maximizing functional independence to promote HRQoL. We outline several areas of future research with emphasis on improved methodological rigor.
Keywords: anxiety, depression, distress, health-related quality of life, primary malignant brain tumors
Health-related quality of life (HRQoL) has become increasingly important throughout the health sciences with the widespread recognition that objective improvements in clinical presentation rarely correlate with patient-reported satisfaction.1 Defined as a measurement of the “radiating impact of pathology on the patient's wider world,”2 HRQoL has been present in medical oncology literature from the 1990s, and is an increasingly important end point in treatment trials.3 Comprehensive assessment of patients with brain tumors has, however, lagged behind other conditions.4,5
Patients' experiences of primary malignant brain tumor (PMBT) can vary depending on the size, location, and specific variant of tumor.6 Seizures, increased fatigue, and headaches are common, as are progressive neurological and cognitive deficits such as hemiplegia, dysphasia, memory loss, confusion, and difficulties regulating affect and behaviors.7–11 Symptoms can be precipitated or exacerbated as a consequence of surgical intervention and adjuvant therapy.9,12 Psychological distress is estimated to affect between 30% and 73% of patients with PMBT.13–15 Approximately one-third of patients experience clinically significant levels of depression and anxiety.16–18 The reported incidence of emotional problems is likely not representative of reality: patients typically under-report psychological concerns and such difficulties can go undetected by clinicians.19,20
Despite advances in detection and treatment over the past 3 decades, survival for PMBT remains low with limited improvement.21–23 It is important, therefore, that protection and maintenance of HRQoL remains central to care, as per national guidelines.24,25 Unfortunately, much still remains unknown about the impact of the illness experience on patients' HRQoL, psychological functioning, and overall adjustment to PMBT, which consequently limits supportive interventions.4 A previous systematic review sought to identify the supportive care needs of patients with PMBT26; we intend to supplement this work by identifying key patient and treatment factors that may identify red flags for the provision of these interventions. Others have attempted to describe and delineate factors related to HRQoL in patients with malignant and nonmalignant brain tumors,4,5,27 however none have focused exclusively on PMBT. As illness experiences, degree of impairment and prognoses differ substantially between patients with malignant and non-malignant disease.5 We aim to improve on existing evaluations with a specific focus on PMBT.
The present report describes a systematic review of evidence for factors associated with HRQoL and psychological functioning in adults with PMBT published over the previous 3 decades. The quality of the evidence is assessed and findings synthesized narratively. We endeavor to make tentative suggestions as to which subgroups of patients may be at greater risk of impaired HRQoL or psychological functioning. The review concludes with recommendations for clinical care and the ongoing research agenda.
Method
Search Strategy
A systematic search of electronic databases (CINAHL, PsycInfo, and PubMed) was conducted to identify relevant articles published between January 1, 1984 and July 25, 2015. The search was restricted to English language, peer-reviewed studies. Conference abstracts, case reports, and grey literature were omitted. The search terms for this review are listed in Box 1. The review was completed in accordance with the PRISMA statement.28
Box 1. Search terms used to identify articles for inclusion in the review.
(“brain tumo?r*” or “brain neoplasm*” or “glioblastoma” or “GBM” or “astrocytoma” or “oligodendroglioma” or “oligoastrocytoma” or “high-grade glioma” or “high grade glioma” or “primary malignant brain tumo?r*”)
(“adjustment” or “adaptation” or “acceptance” or “satisfaction” or “happiness” or “happy” or “adaptation” or “optimism” or “optimistic” or “well?being” or “quality of life” or “QoL” or “anxiety” or “depression” or “stress disorder*” or “stress, psychological” or “mood” or “affect”)
1 and 2
3 not (“child*” or “p?ediatric” or “adolescen*”)
limit 4 to english language
limit 5 yr=“1989-2016”
Studies were considered for review if they: (i) aimed to delineate independent variables that may be related to HRQoL and/or psychological functioning outcomes; (ii) investigated samples where greater than 75% of the participants had a diagnosis of PMBT; (iii) recruited adult patients (≥18 years old) exclusively; (iv) used at least 1 validated outcome measure; and (v) reported statistics in sufficient detail to describe the relationship between the independent variables and the outcomes under consideration. All study designs were considered for inclusion.
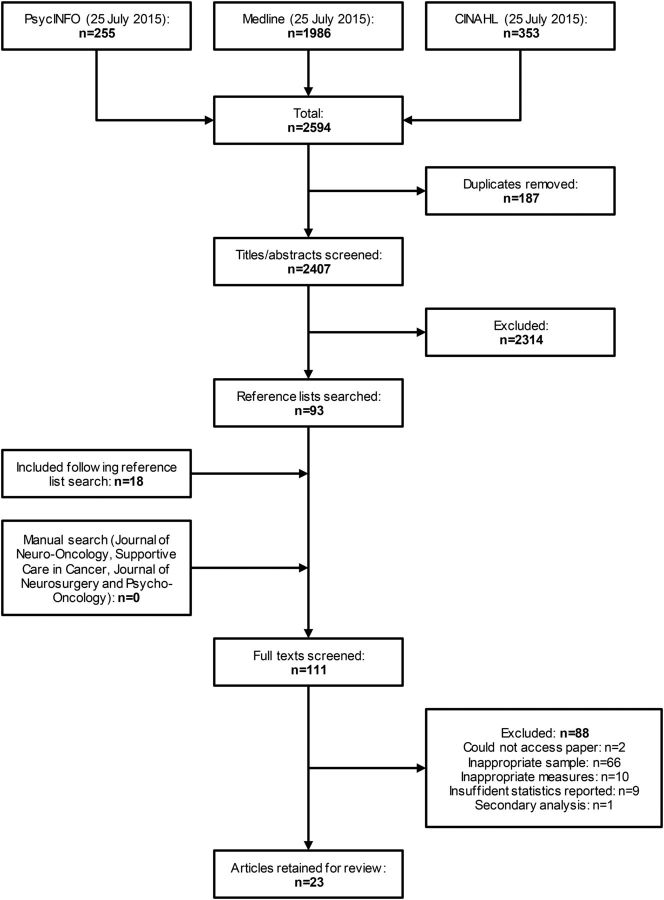
The electronic database search returned 2407 articles, excluding duplicates. The titles and/or abstracts of these articles were screened and 2314 were rejected as they were not considered relevant to the review question. Full versions of the remaining 93 articles were obtained and reviewed against the inclusion criteria above. The bibliographies of these articles were examined to identify further potential studies for inclusion, yielding 18 additional papers. Manual searches of the 4 journals in which the most identified articles had been published returned no additional studies. Of these 111 studies, 23 fulfilled all inclusion criteria and 88 were excluded (Fig. 1).
Fig. 1.
Flowchart of the selection process for the review.
Quality Assessment
All studies selected for inclusion were assessed by the lead author and a randomly selected subset of 10 studies was assessed independently by 2 postgraduate level researchers with experience conducting quality assessments but who were unfamiliar with the field. Studies were assessed against an 8-item quality assessment tool based on the Newcastle-Ottawa Scale29 and tailored to this review. Assessment criteria were consistent with published recommendations.30 The overall methodological quality of a study was defined in terms of the following: clarity of the research aim; validity of outcome measures; clarity of sampling methodology; exclusivity of diagnoses; specificity of diagnoses; representativeness of the sample; justification for sample size; and clarity of statistical tests (see Supplementary material, Table S1). Each criterion was scored on a 3-point scale (2 points = fully met; 1 point = partially met; 0 = not met), yielding scores of 0 to 16. All studies were retained for review.
Synthesis
Due to considerable heterogeneity among the methodologies and outcomes of the studies included, meta-analysis was considered neither viable nor appropriate. In answering the research question, the authors conducted a narrative synthesis, as per guidance developed by Popay and colleagues,31 in which a theory of effect was established, a preliminary synthesis developed, relationships explored, and robustness of the synthesis assessed.
Results
Description of Studies
Studies included in the review (Table 1; Supplementary material, Table S2) used cross-sectional (n = 14) or cohort designs (n = 9). Sample sizes varied substantially; 18 studies featured a sample between 50 and 186 participants, 3 reported results for more than 50 participants, and 2 recruited 363 and 598 participants, respectively. The majority of studies recruited participants opportunistically from routine treatment clinics (n = 21), 1 recruited patients as part of a companion protocol of a clinical trial, and 1 recruited solely using advertisements on a website for patients with brain tumors.
Table 1.
Description of studies included in the review of factors associated with quality of life and psychological functioning in patients with PMBT
| Authors, Year | Outcomes of Interest (measures) | Independent Variables with Statistical Significance (P < .05)a | Quality Score |
|---|---|---|---|
| Arnold et al, 200833 | Depression (mPHQ); generalised anxiety (mPHQ) | Sex; tumor grade; history of mental health difficulties; education | 9/16 |
| Brown et al, 200550 | QoL (LASA, FACT-Br); depression (POMS-SF) | Fatigue; sleep quality; depression; extent of resection; AED use | 10/16 |
| Daigle et al, 201348 | QoL (SNAS) | Extent of resection; tumor volume; pain | 10/16 |
| Fox et al, 200710 | Depression (HADS); QoL (FSQoLS) | Fatigue; sleep quality; cognitive function; depression; QoL | 12/16 |
| Giovagnoli et al, 199640 | QoL (FLIC); anxiety (STAI); depression (SRDS) | KPS; functional impairment; anxiety; depression; education; tumor site | 10/16 |
| Giovagnoli, 199939 | QoL (FLIC); anxiety (STAI, STAI-2); depression (SRDS) | Anxiety; depression; KPS; cognitive function | 11/16 |
| Giovagnoli et al, 200534 | QoL (FLIC); anxiety (STAI, STAI-2); depression (SRDS) | Tumor grade | 9/16 |
| Hahn et al, 200346 | Depression (BDI); QoL (LASA, MHS, HUS) | Tumor laterality | 10/16 |
| Halkett et al, 201541 | Distress (DT); QoL (FACT-Br, FACT-G) | Education; functional impairment | 13/16 |
| Kaplan and Miner, 200035 | Depression (BDI, MAS); anxiety (BAI, STAI) | Anxiety | 9/16 |
| Keir et al, 200813 | Distress (DT) | Sex | 13/16 |
| Kilbride et al, 200743 | Anxiety (HADS); depression (HADS) | No findings of statistical significance | 9/16 |
| Klein et al, 200147 | QoL (SF-36, QLQ-BN20) | Extent of resection; corticosteroid use | 11/16 |
| Kvale et al, 200914 | Distress (DT) | QoL | 12/16 |
| Lin et al, 201351 | Depression (POMS-SF); anxiety (POMS-SF). | Uncertainty; KPS | 11/16 |
| Litofsky et al, 200420 | Depression (SF-36, 3-item binary measure, physician report) | KPS; tumor site; tumor volume; corticosteroid use; consciousness problems; headache; memory loss; personality change; motor deficits; cognitive changes; papilledema | 11/16 |
| Porter et al, 201442 | QoL (FACT-Br, FP-QLI) | TFD; marital statusb | 10/16 |
| Osoba et al, 199749 | QoL (QLQ-C30) | Functional impairment; recurrence; motor deficits | 11/16 |
| Raysi Dehcordi et al, 201236 | Depression (BDI) | Tumor site; KPS | 12/16 |
| Rooney et al, 201118 | Depression (SCID) | History of mental health difficulties; KPS; corticosteroid use; cognitive impairment | 14/16 |
| Rooney et al, 201345 | Distress (DT) | Depression; functional impairment; AED use; extent of resection; age | 10/16 |
| Weitzner et al, 199644 | QoL (FP-QLI, PAIS-SR) | Marital status; tumor laterality | 9/16 |
| Yavas et al, 201238 | QoL (QLQ-C30, QLQ-BN20); anxiety (HADS); depression (HADS) | Sex; tumor grade | 11/16 |
Abbreviations: AED, Anti-epileptic drug; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; DT, Distress Thermometer; FACT-Br, Functional Assessment of Cancer Therapy - Brain; FACT-G, Functional Assessment of Cancer Therapy – General; FLIC, Functional Living Index - Cancer; FP-QLI, Ferrans and Powers Quality of Life Index for Cancer; FSQoLS, Fox Simple Quality of Life Scale; HADS, Hospital Anxiety and Depression Scale; HUS, Hassles and Uplifts Scale; KPS, Karnofsky Performance Scale; LASA, Linear analogue scale assessment; MAS, Mood Assessment Scale; MHS, Miller Hope Scale; mPHQ, Modified Patient Health Questionnaire; PAIS-SR, Psychosocial Adjustment to Illness Scale - Self Report; POMS-SF, Profile of Mood States - Short Form; QLQ-C30, Quality of Life Questionnaire for Cancer-30; QLQ-BN20, Quality of Life Questionnaire for Cancer – Brain Cancer Module; SCID, Structured Clinical Interview for DSM-IV Disorders; SF-36, Short Form (36) Health Survey; SNAS, Sherbrooke Neuro-oncology Assessment Scale; SRDS, Zung Self-Rating Depression Scale; STAI-1, State Trait Anxiety Inventory; STAI-2, State Trait Anxiety Inventory-2; TFD, Time from diagnosis.
aFull details of all relevant findings from each study included in the review are listed in Supplementary material, Table S2.
bThe authors report significant findings at the level of P < .1. To maintain consistency with the other studies reviewed, we will only consider findings of this study statistically significant at P < .05.
Sixteen studies recruited patients with PMBT exclusively and 7 included participants with other diagnoses. The mean ± SD participant age across the 14 studies providing sufficient information was 47.87 ± 13.46 years. Of the 21 studies with adequate demographic information, males accounted for at least 50% of the sample in 20 cases.
Study Evaluation and Assessment
The overall quality ratings of included studies varied (mean = 10.74; SD= 1.39; range, 9-14 [out of 16]). The intraclass correlation between raters for a randomly selected subset of 10 studies was 0.72 (95% CI, 0.40-0.91), suggesting satisfactory inter-rater reliability for the assessment tool.32 Strengths of the included papers were that all but one used appropriate statistical tests and reported them sufficiently (1 study used descriptive statistics only), and that 20 studies used validated measures exclusively, with a further study using a combination of validated and nonvalidated measures. Weaknesses included the lack of demonstrable representativeness of the sample to a wider population of patients with PMBT and lack of a priori or post hoc justification for sample sizes. As the aim of the present report was to review factors pertinent to patients with PMBT, the findings of 7 papers were weakened in specificity by their inclusion of small numbers of patients diagnosed with low-grade brain tumors.
Independent Variables and Outcomes
The principal study outcomes considered in this review were HRQoL (14 studies), depression (14 studies), anxiety (8 studies), and distress (4 studies). Multiple outcomes were reported in 14 studies. In reporting HRQoL outcomes, we acknowledge that HRQoL and QoL (quality of life) are often used interchangeably (and erroneously) by a number of authors; as such, we have considered all findings reported for QoL as HRQoL outcomes. A wide range of validated measures (n = 24) were used to quantify these outcomes (Table 2).
Table 2.
Validated measures used to quantify outcomes of interest in studies selected for inclusion
| Measure | Times Used (n)a | Outcomes Measured |
|---|---|---|
| BAI | 1 | Anxiety |
| BDI | 3 | Depression |
| DT | 4 | Distress |
| FACT-Br | 4 | HRQoL |
| FACT-G | 1 | HRQoL |
| FLIC | 3 | HRQoL |
| FP-QLI | 2 | HRQoL |
| FSQoLS | 1 | HRQoL |
| HADS | 5 | Depression; anxiety |
| HUS | 1 | HRQoL |
| LASA | 2 | HRQoL |
| MAS | 1 | Depression |
| MHS | 1 | HRQoL |
| mPHQ | 2 | Depression; anxiety |
| PAIS-SR | 1 | HRQoL |
| POMS-SF | 3 | Depression; anxiety |
| QLQ-BN20 | 2 | HRQoL |
| QLQ-C30 | 2 | HRQoL |
| SCID | 1 | Depression |
| SF-36 | 2 | Depression; HRQoL |
| SNAS | 1 | HRQoL |
| SRDS | 3 | Depression |
| STAI | 4 | Anxiety |
| STAI-2 | 2 | Anxiety |
Abbreviations: BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; DT, Distress Thermometer; FACT-Br, Functional Assessment of Cancer Therapy - Brain; FLIC, Functional Living Index - Cancer; FP-QLI, Ferrans and Powers Quality of Life Index for Cancer; FSQoLS, Fox Simple Quality of Life Scale; HADS, Hospital Anxiety and Depression Scale; HUS, Hassles and Uplifts Scale; LASA, Linear analogue scale assessment; MAS, Mood Assessment Scale; MHS, Miller Hope Scale; mPHQ, Modified Patient Health Questionnaire; PAIS-SR, Psychosocial Adjustment to Illness Scale - Self Report; POMS-SF, Profile of Mood States - Short Form; QLQ-C30, Quality of Life Questionnaire for Cancer-30; QLQ-BN20, Quality of Life Questionnaire for Cancer – Brain Cancer Module; SCID, Structured Clinical Interview for DSM-IV Disorders; SF-36, Short Form (36) Health Survey; SNAS, Sherbrooke Neuro-oncology Assessment Scale; SRDS, Zung Self-Rating Depression Scale; STAI-1, State Trait Anxiety Inventory; STAI-2, State Trait Anxiety Inventory-2.
aNumber of unique uses. If a study used one validated measure to quantify multiple outcomes, the measure is counted for each use.
Due to the nature of the review question, a large number of independent variables were considered relevant for inclusion. These variables were grouped into 3 thematic categories: demographic factors, clinical factors, and mental health factors.
Demographic Factors
Sex
Patient's sex was included as a variable in 13 studies. Female patients were at a significantly higher risk of developing depression than male patients in 1 study,33 and 5 studies concluded that sex was not significantly related to anxiety or depression.20,34–37 HRQoL was found to be significantly greater in men than women in one study, however only for patients with grade 3 tumors,38 and 5 further studies found no evidence of a significant effect of patient sex on HRQoL.34,39–42 Keir et al found evidence for a significantly greater level of distress in female patients,13 however this finding was not replicated in 2 further studies.14,41
Age
Twelve studies included age as an independent variable, with 11 finding no evidence of a significant effect on levels of anxiety,33,43 depression,20,33,36,37,43 HRQoL,38–40,42,44 or distress.14 One study reported evidence for a significant weak correlation between age and distress at baseline assessment, indicating greater distress for younger patients; this relationship was not significant at reassessment at 3 and 6 months,45 however there was a substantial degree of attrition between the three assessment periods.
Marital Status
Two studies demonstrated that patients who were married or in a relationship reported significantly greater HRQoL than those single42 or divorced.44 A further study demonstrated that married patients experienced greater depression and lower anxiety than nonmarried patients,35 however findings were not evidenced statistically. A further 8 studies found no relationship between marital status and anxiety,33,34,43 depression,33,34,37,43 HRQoL,34,39,40 or distress.41,45
Education
Patients with greater levels of formal education were found to report significantly greater HRQoL in two studies than patients with less education,40,41 yet no significant difference was present in a further 3 studies.39,42,44 Greater previous education was also found to relate significantly to reduced distress,33,41 but not anxiety.33
Ethnicity
No effect of ethnicity was found for anxiety,33 distress,14 depression,33 or HRQoL.42 Litofsky et al demonstrated a significant role of ethnicity for levels of physician-reported depression,20 although findings were highly skewed by small numbers of nonwhite participants.
Clinical Factors
Site and Laterality
The location of the tumor was included as an independent variable in 13 studies. Significant findings included greater HRQoL among patients with unilateral vs bilateral tumors,44 and tumors located in the diencephalon or anterior right hemisphere compared with other areas.40 Significantly greater depressive symptoms were reported for patients with left hemisphere tumors than for those with right hemisphere involvement,46 and patients with frontal tumors compared with other sites.36 Six further studies found no evidence for an effect of laterality on depression,36,37 HRQoL,38,42,47 or distress.45 Seven found no evidence for a significant role of tumor site in predicting levels of anxiety,33 depression,20,33,37 HRQoL,34,39,42 or distress.45
Grade and Variant of Tumor
The role of tumor grade or type of PMBT was examined in 7 studies. Arnold et al reported that rates of depression and anxiety were lower among patients with high-grade tumors than among patients with low-grade tumors, however there was no significant difference between grades 3 and 4.33 Rooney et al identified no difference between tumor grade and rates of depression37; a subsequent study found no evidence for differences in levels of patient-reported distress and tumor grade.45 Patients with grade 3 tumors in a study by Giovagnoli et al reported significantly higher HRQoL than those with grade 4 tumors.34 Five studies considered differences between patients with different variants of PMBT, all finding no evidence for variation in levels of depression,37 HRQoL,39,44 or distress.41,45
Tumor Volume
Four studies considered tumor volume as an independent variable. Litofsky et al observed significantly higher rates of physician-reported depression in high-grade glioma patients with “larger” (not quantified) tumor volume and multifocal tumors.20 Three further studies found no evidence for a significant relationship between volume and HRQoL42,46,48 or depression.46
Recurrence
No significant difference in rates of anxiety or depression was identified by Arnold et al,33 whereas Osoba et al found that HRQoL was greater among patients recently diagnosed with high-grade glioma than among patients with recurrent tumors.49
Time From Diagnosis
Five studies found no significant relationship between time from diagnosis and HRQoL,44 anxiety,33,43 depression33,43 or distress.13,14 Porter et al evidenced that patients 3 to 7 months postdiagnosis reported greater HRQoL than those over 7 months postdiagnosis,42 but this was not significant on all HRQoL measures administered.
Treatment Factors
Three studies evidenced that patients receiving gross total resection reported greater HRQoL47,48,50 and reduced depression50 following surgery compared with patients receiving biopsy only. Gross total resection was not found to be significantly associated with postsurgery improvement in depression or HRQoL in 5 further studies.20,37,39,40,42 Rooney et al reported partial evidence for an effect of extent of resection on patient-reported distress, as patients who received resections (extent not specified) reported less distress than those receiving biopsy only at 6 months postsurgery, but not at 8 weeks or 3 months postsurgery.45 Neither radiotherapy nor chemotherapy schedules were found to predict differences in psychological or HRQoL outcomes in 2 studies.37,45 Reasonable evidence for a relationship between increased depression and corticosteroid use was demonstrated in 2 studies of patients with newly diagnosed brain tumors,20,37 and with HRQoL in 1 study,47 A nonsignificant difference between corticosteroid use and patient-reported distress was demonstrated in one study.45
Performance and Functional Status
Seven studies reported significant relationships between low Karnofsky Performance Status (KPS) scores and greater depression,20,36,37 greater anxiety,51 and reduced HRQoL.39,40,47 Nonsignificant relationships were reported between KPS and depression in 2 studies46,51 and HRQoL in 1 additional study.46 Functional impairment was included as a variable in 5 studies and was found to relate significantly to decreased HRQoL10,40,41,49 and increased depression.10 Rooney et al reported that functional impairment was not significantly related to greater distress 8 weeks postsurgery, but was a significant correlate at reassessment 3 and 6 months later,37 suggesting an impact of persistent impairment. No significant relationship between functional impairment and distress was found in 1 study.41
Symptoms
Evidence for antiepileptic drug (AED) use was mixed. No significant relationship was apparent between AED use and depression in 1 study.37 Rooney et al found evidence for a significant relationship between AED use and greater distress shortly following surgery,45 but not at reassessment 3 and 6 month postsurgery. Brown et al reported that patients prescribed AEDs reported greater HRQoL than those not,50 whereas Klein et al found no significant difference between these groups.47 Seizures were not found to relate to depression or distress.20,37,45
Impairments in self-reported cognitive functioning were found to be related to increased depression10,20,35 and reduced HRQoL.10,47 Cognitive impairment related significantly to decreased HRQoL39 and more depressive symptoms,37 but not distress.45 Confusion was not related to patient-reported global HRQoL.49 Fatigue was significantly related to increased depression10 and poor HRQoL.10,50 Reduced sleep quality, daytime somnolence, and decreased physical activity were similarly associated with significant impairments in HRQoL10,50 and greater depression.10,35
Litofsky et al reported that physician-reported depression was significantly greater in high-grade glioma patients affected by problems with consciousness, headache, personality changes, papilledema, and progressive motor deficits, but the relationship of depression to patient reports of dysphasia or sensory problems were not significant.20 Changes in appearance and sexual dysfunction were also related to depression.35 Osoba et al observed that a key predictor of reduced HRQoL was motor impairment, whereas language deficits were not significantly related.49 Fox et al found that pain was neither a significant correlate of depression nor HRQoL in patients with high-grade glioma.10 Distress did not appear to be related to patients' self-reports of neurological symptoms in 3 studies.13,14,47
Mental Health Factors
History of Mental Health Difficulties
Three considered the impact of previous mental health problems on the likelihood of developing difficulties postdiagnosis. Arnold et al demonstrated that prior mental health problems significantly predicted postdiagnostic depression and trended towards significance for postdiagnostic anxiety,33 although in both cases numbers of participants disclosing previous difficulties were small. Rooney et al also identified a greater level of current depression in participants reporting previous depression.37 Inferential support could not be provided by Kilbride et al due to their small sample size, but descriptive analyses indicate greater levels of postoperative depression among patients with a history of mental health problems.43 In these 3 studies it was unclear as to whether any participants reporting current depression or anxiety were experiencing significant mental health problems immediately before diagnosis, which would have persisted postoperatively, or whether prior experience of mental health problems predisposed participants to mood disturbances precipitated by diagnosis.
Depression
The relationship of current depression to HRQoL was examined in 4 studies, all providing evidence for a significant relationship between increased depression and decreased HRQoL.10,39,40,50 Rooney et al reported that a diagnosis of major depressive disorder was significantly related to patient-report distress at 8 weeks and 3 months postsurgery, but not at 6 months.45
Anxiety
Relationships between current anxiety and HRQoL were investigated in 2 studies, both reporting evidence of a significant correlation between heightened anxiety and reduced HRQoL.39,40 Kaplan et al reported that specific worries about finances, physical deterioration and marital difficulties were significantly related to greater patient-reported depression.35
Illness-Related Uncertainty
Using structural equation modeling to explore mediating factors in a mixed sample of patients with varying grades of brain tumor, Lin et al identified that increased illness-related uncertainty was significantly related to increased anxiety and greater depression.51 Uncertainty was not investigated as an independent variable in any other study.
Discussion
Our primary aim was to systematically review evidence for factors associated with HRQoL and psychological outcomes in adult patients with PMBT. A secondary, related aim was to assess the quality of the evidence in this field and provide recommendations to guide the ongoing research agenda.
Summary of Findings
In conducting this synthesis, we have tentatively identified important factors that relate to patients' HRQoL and psychological functioning (Table 3). Where greater than two-thirds of studies provided significant evidence for a factor, we have identified this factor as potentially significant to patient functioning. Factors for which less than one-third of studies provided significant evidence were considered not significant. Where factors fall between these categories, or where factors have been investigated in only 1 study, we have considered these inconclusive.
Table 3.
Summary of factors relating to HRQoL/psychological outcomes by degree of evidence
| Factor | Total Studies (n) | Studies Reporting Significant Relationship (n) | Studies Reporting Mixed Evidence (n) |
|---|---|---|---|
| Evidence of significant negative relationships to outcomes | |||
| Current level of anxiety and worry | 3 | 3 | 0 |
| Current level of depression | 5 | 4 | 1 |
| Current level of functional impairment | 5 | 4 | 1 |
| Current level of motor impairment | 2 | 2 | 0 |
| Current use of corticosteroids | 4 | 3 | 1 |
| History of mental health problems | 3 | 2a | 0 |
| Symptom: cognitive impairment | 7 | 6 | 0 |
| Symptom: fatigue | 2 | 2 | 0 |
| Evidence of significant positive relationships to outcomes | |||
| KPS | 8 | 7 | 1 |
| Evidence of no significant relationships to outcomes | |||
| Age | 12 | 0 | 1 |
| Current level of neurological function | 3 | 0 | 0 |
| Ethnicity | 4 | 1 | 0 |
| Marital status | 11 | 1 | 1 |
| Radiotherapy/chemotherapy schedules | 2 | 0 | 0 |
| Sex | 13 | 2 | 1 |
| Symptom: language problems | 2 | 0 | 0 |
| Symptom: seizures | 3 | 0 | 0 |
| Time from diagnosis | 6 | 0 | 1 |
| Tumor grade | 7 | 1 | 0 |
| Tumor site/laterality | 13 | 3 | 1 |
| Tumor volume | 4 | 1 | 0 |
| Inconclusive evidence of relationships to outcomes | |||
| Current use of AEDs | 4 | 1 | 1 |
| Extent of resection | 9 | 3 | 1 |
| Level of education | 6 | 3 | 0 |
| Recurrence | 2 | 1 | 0 |
| Evidence only available from one study | |||
| Changes in appearance | 1 | 1 | 0 |
| Current level of physical inactivity | 1 | 1 | 0 |
| Current level of sleep quality | 1 | 1 | 0 |
| Current level of illness-related uncertainty | 1 | 1 | 0 |
| Symptom: confusion | 1 | 0 | 0 |
| Symptom: daytime sleepiness | 1 | 1 | 0 |
| Symptom: headache | 1 | 1 | 0 |
| Symptom: loss of consciousness | 1 | 1 | 0 |
| Symptom: pain | 1 | 0 | 0 |
| Symptom: papilledema | 1 | 1 | 0 |
| Symptom: personality changes | 1 | 1 | 0 |
| Symptom: sensory problems | 1 | 0 | 0 |
| Symptom: sexual dysfunction | 1 | 1 | 0 |
aOne additional study reported descriptive statistics only, indicating greater postdiagnostic mood difficulties in patients with previous mental health difficulties.
Abbreviations: AED, antiepileptic drug; HRQoL, health-related quality of life; KPS, Karnofsky Performance Status.
Evidence for an effect of demographic differences was mixed. Although survival declines with age,22 variability in patient-reported outcomes remained comparable between age groups. One reason for this may be that patients' resiliency increases with age,52 possibly offsetting the toll of increased deterioration. Where significant demographic differences were found, these tended towards lower impairment in men,13,33,38 which may reflect greater resiliency or reduced tendency to report difficulties.33 Some evidence indicated a general trend towards greater functioning in patients who were married, white, or from higher educational backgrounds14,20,33,35,40–42,44; reliability of these associations is limited by substantial homogeneity within samples.
Findings concerning mental health variables were generally consistent. A history of mental health problems was related to the incidence of depression and poor HRQoL postdiagnosis; however, this was only considered by 3 studies, of which one study could only provide descriptive support due to a small sample size.33,37,43 Given the apparent importance of previous mental health difficulties for patients with PMBT, it is necessary for further research to consider this factor. The experience of current depression or anxiety was related to impaired patient-reported HRQoL.10,39,40,45,50 Further research is necessary to determine the extent to which postdiagnosis depression is a consequence of illness-related metabolic or structural changes, or negative psychological reactions to the disease.
The nature of the relationship between clinical factors and HRQoL/psychological outcomes varied. Significant correlations between tumor characteristics and HRQoL/psychological outcomes appeared to be due to the uneven recruitment of participants across grades in study samples.33,34 Corticosteroid use was found to be related to increased depression and lower HRQoL.20,37,47 Recurrence appeared to be related to lower HRQoL,49 but not anxiety or depression.13,14,33,43 Low performance status and increased functional impairment were typically related to increased mental health problems and reduced HRQoL,10,20,36,37,39,40,47,49 particularly as patients progress further in the disease trajectory. Relationships between AED use and outcomes were inconclusive and were not sufficiently differentiated from the impact of seizures; when reported separately, seizure activity showed no significant relationship with HRQoL or psychological functioning. With regards to specific symptoms, conclusions are limited by lack of evidence, with many only investigated in 1 study. Where more than 1 study provided evidence, HRQoL/ psychological outcomes were significantly related to the experience of cognitive difficulties (both as formally assessed and self-reported),10,20,35,37,39,47 fatigue,10,50,51 and motor impairment.20,49
Quality of the Literature
The overall quality of the literature was generally moderate, with substantial variability between studies. In general, the majority of studies used validated psychometric tools to assess outcomes of interest. However, as described in Table 2, a broad range of outcome measures were used to assess similar constructs; for example, we identified 12 separate measures of QoL/HRQoL. The quality of the literature would be vastly improved, and allow for comprehensive meta-analysis, if there were greater consistency in the choice of outcome measures.
Our review aimed to focus exclusively on findings for patients with histologically confirmed diagnoses of PMBT. The number of relevant studies in which patients with PMBT were recruited exclusively was low (n = 14). By including studies in which at least 75% of participants recruited were diagnosed with PMBT (n = 7), we broadened the scope of the review at the expense of some specificity. Studies combining PMBT and other brain tumor diagnoses compromise the methodological quality and validity of these findings for patients with PMBT. As such, the 7 papers describing studies in which small numbers of patients with non-PMBT diagnoses were recruited received lower quality scores. In all cases there was no clear rationale to justify why these patients were recruited.
The majority of authors did not demonstrate how their sample represented the wider population of patients with brain tumors, either by explicit statement or by similarity to published prevalence data.21 Most did not provide adequate justification for sample sizes and only 4 referenced a priori power calculations. Furthermore, the majority of literature included in our review reported correlations for cross-sectional data, reducing the strength of this evidence. These limitations may be evident of the practicalities of conducting such research with critically ill patients, where investigators cannot afford to be highly selective during recruitment.53
Limitations of the Review
The wide range of methodologies and outcome measures included in this review limits the robustness of conclusions and meta-analysis was neither possible nor appropriate. We elected pragmatically to not exclude studies on the basis of their methodological quality; although none were of such low quality as to invalidate findings, we acknowledge that the variance in quality can place undue value on conclusions drawn. We have tried to minimize bias and reporting error in our conclusions by adhering to published guidance on narrative synthesis.31
Although we used a quality assessment tool based on a previously validated checklist,29 our appraisal is not unimpeachable. Our modifications to the Newcastle-Ottawa Scale, although compliant with published recommendations,30 have not been validated independently. Although an independent assessment of a subset of studies yielded adequate inter-rater reliability there was significant variance between raters on some cases, diminishing the validity of the individual quality scores ascribed. However, we identified a number of methodological issues throughout the literature overall that we consider valid, in spite of the limitations to our appraisal, which we aim to address below.
Considerations for Further Research
Our review has identified a number of methodological weaknesses in the current evidence base that limit our understanding of patients' HRQoL and psychological functioning following diagnosis of PMBT.
Exclusivity of Sample
Many studies identified during electronic database searches reported findings for a unified group of “brain tumor patients,” consisting of both patients with PMBT and those diagnosed with low-grade brain tumors. The findings of studies which combined diagnostic categories are limited, as prognoses, treatment options, and disability vary widely between low and high grades of tumor.5 Although recruitment of both groups of patients has value, further research should endeavor to clearly delineate these groups in analysis.
Greater Consensus in Choice of Outcome Measures
The use of measures to assess identical or similar outcomes significantly limited comparisons between studies. Although different measures of depression or HRQoL may present with reasonable face validity, there can be subtle differences between validated and widely used outcome measures in their assessment of hypothesized contributing factors.54 As such, it is possible that HRQoL or distress constructs vary significantly between instruments. Greater consensus in the choice of assessment instruments assessing HRQoL/psychological outcomes, guided by increased understanding of theoretical models underlying constructs of HRQoL, would lead to greater consistency in the literature and allow for more-rigorous comparison. The proliferation of studies within oncology using the Distress Thermometer55 may herald a movement towards such standardization; however, such rapid screening measures are criticized for their questionable validity, limited specificity, and oversimplification of a multifaceted patient experience.56
Attention to Mediating Factors
Although our review has demonstrated the complexity of factors relating to psychological functioning and HRQoL, the reliance of many studies on correlation limits our capacity to identify causal and directional relationships between factors and outcomes. Four studies included in the review used regression analyses to identify discrete predictors of HRQoL/psychological outcomes35,37,39,45; although increasing the predictive validity of the results, this does not account sufficiently for causality or simultaneous contributions of the myriad of patient and illness factors. Only 1 study used structural equation modeling to observe the concurrent interaction of variables mediating relationships between illness factors, psychological processes, and patient functioning.51 In order to further our understanding of the patient experience of PMBT, we recommend that further research designs should aim towards mediation analysis, repeated sampling, or through treatment investigations targeting processes of change, rather than causal interpretation of findings from cross-sectional designs and correlations.
Potential Implications for Clinical Practice
Promoting and maintaining patients' HRQoL is central to clinical guidance.24,25 Based on the findings of this review, we advocate the following practice points:
Clinicians should ask patients directly about whether they have experienced mental health difficulties in the past and whether they currently feel depressed or anxious, referring patients for specialist support as appropriate.
Patients reporting difficulties with cognition, functional independence, motor function, or fatigue should be monitored closely, as they may more be more likely to experience greater impairments to their HRQoL and psychological functioning.
Patients prescribed corticosteroids should be advised of possible side effects relating to depression. Their mood should be monitored for the duration of this medication.
Conclusions
Our review has identified tentative evidence for a range of clinical and mental health factors that relate to HRQoL and psychological functioning in patients with PMBT, which could be used to identify individuals at risk and to enhance frameworks of supportive interventions. These findings are, however, limited by a number of methodological flaws present throughout the literature. In order to advance this area of knowledge, the field would benefit from greater consensus on the choice of outcome measures. Investigators must also consider their research designs carefully, as further cross-sectional, correlational evidence in this field is unlikely advance our current understanding.
Funding
None declared.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1. Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14(1):69–82. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong D, Lilford R, Ogden J, Wessely S. Health-related quality of life and the transformation of symptoms. Sociol Health Illn. 2007;29(4):570–583. [DOI] [PubMed] [Google Scholar]
- 3. Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol. 2011;104(3):639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: Current knowledge and future directions. Neuro Oncol. 2009;11(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taphoorn MJB, Sizoo EM, Bottomley A. Review on quality of life issues in patients with primary brain tumors. Oncologist. 2010;15(6):618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louis DN, Ohgaki H, Wiestler OT et al. . The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;144(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Breemen MSM, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 8. Behin A, Hoang-Xuan K, Carpentier AF, Delattre J-Y. Primary brain tumours in adults. Lancet. 2003;361(9354):323–331. [DOI] [PubMed] [Google Scholar]
- 9. Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 10. Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh. 2007;39(1):61–67. [DOI] [PubMed] [Google Scholar]
- 11. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 12. Soussain C, Ricard D, Fike JR, Mazeron J-J, Psimaras D, Delattre J-Y. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374(9701):1639–1651. [DOI] [PubMed] [Google Scholar]
- 13. Keir ST, Farland MM, Lipp ES, Friedman HS. Distress persists in long-term brain tumor survivors with glioblastoma multiforme. J Cancer Surviv. 2008;2(4):269–274. [DOI] [PubMed] [Google Scholar]
- 14. Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Support Care Cancer. 2009;17(7):793–799. [DOI] [PubMed] [Google Scholar]
- 15. Renovanz M, Gutenberg A, Haug M et al. . Postsurgical screening for psychosocial disorders in neurooncological patients. Acta Neurochir (Wien). 2013;155(12):2255–2261. [DOI] [PubMed] [Google Scholar]
- 16. D'Angelo C, Mirijello A, Leggio L et al. . State and trait anxiety and depression in patients with primary brain tumors before and after surgery: 1-year longitudinal study. J Neurosurg. 2008;108(2):281–286. [DOI] [PubMed] [Google Scholar]
- 17. Ownsworth T, Little T, Turner B, Hawkes A, Shum D. Assessing emotional status following acquired brain injury: the clinical potential of the depression, anxiety and stress scales. Brain Inj. 2008;22(11):858–869. [DOI] [PubMed] [Google Scholar]
- 18. Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: A systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76. [DOI] [PubMed] [Google Scholar]
- 19. Mystakidou K, Parpa E, Tsilika E et al. . Self-efficacy, depression, and physical distress in males and females with cancer. Am J Hosp Palliat Me. 2010;27(8):518–525. [DOI] [PubMed] [Google Scholar]
- 20. Litofsky NS, Farace E, Anderson F Jr., Meyers CA, Huang W, Laws ER Jr. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54(2):358–366. [DOI] [PubMed] [Google Scholar]
- 21. Ostrom QT, Gittleman H, Liao P et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McKinney PA. Brain tumours: incidence, survival, and aetiology. J Neurol Neurosurg Psychiatry. 2004;75(suppl 2):ii12–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20(4):E1. [DOI] [PubMed] [Google Scholar]
- 24. Lovely MP. Care of the Adult Patient with a Brain Tumor. Chicago, IL: American Association of Neuroscience Nurses; 2014. [DOI] [PubMed] [Google Scholar]
- 25. National Institute for Health and Care Excellence. Service guidance for improving outcomes for people with brain and other central nervous system tumours. NICE guidelines [CSDBRAINCNS]. London, England: National Institute for Health and Care Excellence; 2006. [Google Scholar]
- 26. Ford E, Catt S, Chalmers A, Fallowfield L. Systematic review of supportive care needs in patients with primary malignant brain tumors. Neuro Oncol. 2012;14(4):392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ownsworth TL, Hawkes A, Steginga S, Walker D, Shum D. A biopsychosocial perspective on adjustment and quality of life following brain tumour: A systematic evaluation of the literature. Disabil Rehabil. 2009;31(13):1038–1055. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 29. Wells G, Shea B, O'Connell D et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed 26 June, 2015.
- 30. Centre for Reviews and Dissemination. Systematic reviews: CRD's guidance for undertaking reviews in healthcare. http://www.york.ac.uk/inst/crd/index_guidance.htm Published January 2009. Accessed 26 June, 2015.
- 31. Popay J, Roberts H, Sowden A et al. . Guidance on the conduct of narrative synthesis in systematic reviews. Lancaster, England: ESRC Methods Programme, University of Lancaster; 2006. [Google Scholar]
- 32. Rosner B. Fundamentals of Biostatistics. 7th ed.Boston, MA: Brooks/Cole; 2011. [Google Scholar]
- 33. Arnold SD, Forman LM, Brigidi BD et al. . Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol. 2008;10(2):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giovagnoli AR, Silvani A, Colombo E, Boiardi A. Facets and determinants of quality of life in patients with recurrent high grade glioma. J Neurol Neurosurg Psychiatry. 2005;76(4):562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaplan CP, Miner ME. Relationships: importance for patients with cerebral tumours. Brain Inj. 2000;14(3):251–259. [DOI] [PubMed] [Google Scholar]
- 36. Raysi Dehcordi S, De Paulis D, Marzi S et al. . Survival prognostic factors in patients with glioblastoma: our experience. J Neurosurg Sci. 2012;56(3):239–245. [PubMed] [Google Scholar]
- 37. Rooney AG, McNamara S, Mackinnon M et al. . Frequency, clinical associations, and longitudinal course of major depressive disorder in adults with cerebral glioma. J Clin Oncol. 2011;29(32):4307–4312. [DOI] [PubMed] [Google Scholar]
- 38. Yavas C, Zorlu F, Ozyigit G et al. . Health-related quality of life in high-grade glioma patients: a prospective single-center study. Support Care Cancer. 2012;20(10):2315–2325. [DOI] [PubMed] [Google Scholar]
- 39. Giovagnoli AR. Quality of life in patients with stable disease after surgery, radiotherapy, and chemotherapy for malignant brain tumour. J Neurol Neurosurg Psychiatry. 1999;67(3):358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giovagnoli AR, Tamburini M, Boiardi A. Quality of life in brain tumor patients. J Neurooncol. 1996;30(1):71–80. [DOI] [PubMed] [Google Scholar]
- 41. Halkett GK, Lobb EA, Rogers MM et al. . Predictors of distress and poorer quality of life in high grade glioma patients. Patient Educ Couns. 2015;98(4):525–532. [DOI] [PubMed] [Google Scholar]
- 42. Porter KR, Menon U, Vick NA, Villano JL, Berbaum ML, Davis FG. Assessment of clinical and nonclinical characteristics associated with health-related quality of life in patients with high-grade gliomas: a feasibility study. Support Care Cancer. 2014;22(5):1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kilbride L, Smith G, Grant R. The frequency and cause of anxiety and depression amongst patients with malignant brain tumours between surgery and radiotherapy. J Neurooncol. 2007;84(3):297–304. [DOI] [PubMed] [Google Scholar]
- 44. Weitzner MA, Meyers CA, Byrne K. Psychosocial functioning and quality of life in patients with primary brain tumors. J Neurosurg. 1996;84(1):29–34. [DOI] [PubMed] [Google Scholar]
- 45. Rooney AG, McNamara S, Mackinnon M et al. . The frequency, longitudinal course, clinical associations, and causes of emotional distress during primary treatment of cerebral glioma. Neuro Oncol. 2013;15(5):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55(4):992–999. [DOI] [PubMed] [Google Scholar]
- 47. Klein M, Taphoorn MJ, Heimans JJ et al. . Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol. 2001;19(20):4037–4047. [DOI] [PubMed] [Google Scholar]
- 48. Daigle K, Fortin D, Mathieu D et al. . Effects of surgical resection on the evolution of quality of life in newly diagnosed patients with glioblastoma: a report on 19 patients surviving to follow-up. Curr Med Res Opin. 2013;29(10):1307–1313. [DOI] [PubMed] [Google Scholar]
- 49. Osoba D, Aaronson NK, Muller M et al. . Effect of neurological dysfunction on health-related quality of life in patients with high-grade glioma. J Neurooncol. 1997;34(3):263–278. [DOI] [PubMed] [Google Scholar]
- 50. Brown PD, Maurer MJ, Rummans TA et al. . A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57(3):495–504. [DOI] [PubMed] [Google Scholar]
- 51. Lin L, Chiang H-H, Acquaye AA, Vera-Bolanos E, Gilbert MR, Armstrong TS. Uncertainty, mood states, and symptom distress in patients with primary brain tumors: Analysis of a conceptual model using structural equation modeling. Cancer. 2013;119(15):2796–2806. [DOI] [PubMed] [Google Scholar]
- 52. Costanzo ES, Ryff CD, Singer BH. Psychosocial adjustment among cancer survivors: findings from a national survey of health and well-being. Health Psychol. 2009;28(2):147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bell ML, Olivier J, King MT. Scientific rigour in psycho-oncology trials: why and how to avoid common statistical errors. Psychooncology. 2013;22(3):499–505. [DOI] [PubMed] [Google Scholar]
- 54. Kuenstner S, Langelotz C, Budach V, Possinger K, Krause B, Sezer O. The comparability of quality of life scores: a multitrait multimethod analysis of the EORTC QLQ-C30, SF-36 and FLIC questionnaires. Eur J Cancer. 2002;38(3):339–348. [DOI] [PubMed] [Google Scholar]
- 55. Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma. Cancer. 1998;82(10):1904–1908. [DOI] [PubMed] [Google Scholar]
- 56. Mitchell AJ. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorder. J Clin Oncol. 2007;25(29):4670–4681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.