Abstract
Extraneural metastatic disease of glioma is rare and poses unique therapeutic challenges. Increasingly, the ability to sequence genetic alterations in tumors has allowed for the identification of common oncogenic signatures such as the activating BRAFV600E mutation and may be useful in therapeutic decision making. We report two patients with widespread aggressive gliomas whose tumors were found to express the BRAFV600E mutation and then responded robustly albeit transiently when exposed to vemurafenib. Although both patients succumbed to their disease, our results suggest that targeting BRAF might be appropriate for patients with aggressive gliomas that express this mutation.
Keywords: BRAF, metastatic glioma, vemurafenib
Thought to be locally invasive, brain tumors rarely present with extraneural metastases, at an incidence estimated to be 0.2% and typically with pulmonary involvement.1,2 Across all tumor types, activating mutations in the BRAF oncogene (BRAFV600E point mutations) are common genetic alterations, seen in an estimated 7% of human tumors,3 including multiple subtypes of primary CNS neoplasms.4 Specifically, BRAFV600E mutations have been identified in 60% to 70% of pleomorphic xanthoastrocytomas5–7 and approximately 50% of glioblastomas with epithelioid features.8
Vemurafenib is an ATP-competitive inhibitor of the kinase domain for BRAF. Its selective therapeutic effect is seen in BRAFV600E activating mutations by blocking MEK in the BRAF-MEK-ERK pathway.9 Concurrent therapy with trametinib, a selective MEK inhibitor, has also been approved for use in BRAFV600E-mutated malignant melanomas and achieved dramatic and durable clinical responses.10 In a rapidly expanding number of case reports and series, vemurafenib has shown efficacy in BRAFV600E-mutated CNS tumors.7,11–17
We report two cases of CNS tumors with BRAFV600E mutations that responded to treatment with vemurafenib. These patients were exceptional in that each presented with extraneural metastases of their gliomas and responded robustly to treatment with this targeted therapy. In one case, there was a dramatic and complete response to treatment by both the primary and metastatic lesions, while in the other case, only the pulmonary lesions responded. These cases further illustrate the therapeutic potential for BRAF inhibition in these aggressive and lethal cancers.
Case 1
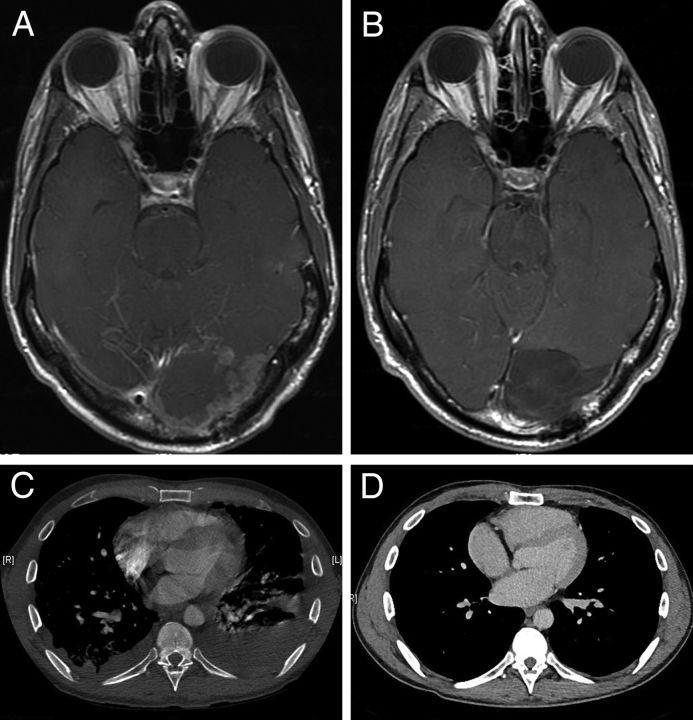
A 39-year-old man initially presented with peripheral vision loss due to an enhancing, left occipital mass. Gross total resection of the brain lesion revealed anaplastic pleomorphic xanthoastrocytoma (PXA), testing positive for the BRAFV600E mutation. Almost immediate regrowth was noted, requiring a second gross total resection. Because of the aggressive nature of his tumor and dural involvement, craniospinal radiation with a boost to the tumor bed was administered. Three weeks after completion of irradiation, he presented with dyspnea due to multiple lung lesions. One of the lung lesions was biopsied and demonstrated pathology consistent with lung metastases from anaplastic PXA. Vemurafenib at a dose of 960 mg orally twice daily was started with a virtually complete response as assessed by MR brain imaging 2 months later (Fig. 1). One month after that time point, a subcutaneous mass was noted in the area of his craniotomy that proved to be PXA on subsequent biopsy. Unfortunately, the complete intracranial response to vemurafenib treatment was not sustained beyond the 2-month time point from the initiation of treatment. Despite initiation of trametinib therapy in addition to ongoing vemurafenib therapy, followed by alkylating chemotherapy with temozolomide and bevacizumab treatment, the disease progressed and the patient died 10 months after initial diagnosis.
Fig. 1.
Pleomorphic xanthoastrocytoma. (A) Contrast-enhanced, T1-weighted MR image shows postsurgical and radiation therapy changes with extensive irregular nodular enhancement in the resection margin. (B) Contrast-enhanced, T1-weighted MR image after vemurafenib initiation shows marked interval regression of enhancing mass without recurrence. (C) Chest CT scan before treatment shows bilateral pleural effusions with nodularity of the pleura and multiple scattered pulmonary nodules. (D) Chest CT scan after vemurafenib shows marked interval response with resolution of pulmonary and pleural metastatic disease with only minimal residual bilateral major fissure nodularity.
Case 2
A 26-year-old man with history of bipolar affective disorder presented with headache, vomiting, and memory problems. A large, enhancing mass centered in the right superior temporal lobe was noted on MR of the brain in addition to numerous bilateral pulmonary nodules. Gross total resection of the brain lesion revealed glioblastoma, epithelioid type, testing positive for the BRAFV600E mutation (Fig. 2). Although the lung nodules were not biopsied, they were presumed to be metastatic disease from the primary brain location.
Fig. 2.
Hematoxylin and eosin stained sections revealed a densely cellular and necrotic malignant neoplasm (A, scale bar = 200 µm) composed of epithelioid cells with eosinophilic cytoplasm, eccentrically placed nuclei, and variably prominent nucleoli (B, scale bar = 50 µm). Immunohistochemical stains demonstrate expression of glial fibrillary acidic protein (C, scale bar = 100 µm) and presence of BRAFV600E mutation (D, scale bar = 100 µm).
The patient's postoperative course was complicated by steroid-induced psychosis requiring inpatient psychiatric care leading to a delay in the start of treatment. Specifically, standard therapy using temozolomide and radiation therapy was not possible due to the patient's inability to comply with treatment. One month after resection, the patient continued to experience headaches, nausea, and vomiting. Follow-up MRI showed significant progression of the brain tumor, including leptomeningeal involvement. Follow-up CT of the thorax at this time also showed significant interval increase in the size and number of bilateral, pleural-based, necrotic nodules and masses. Because of the progression of disease, the finding of the BRAF V600E mutation, and the patient's inability to cooperate with standard therapies, he was treated with Vemurafenib at a dose of 960 mg twice daily.
One week later, chest imaging demonstrated a profound reduction in the size of his paramediastinal and perihilar opacities consistent with a partial response to treatment (Fig. 3). MR of brain, however, revealed increased hemorrhage and edema. Due to his persistently diminished mental status, it was decided to transition to comfort measures. The patient died 3 days later.
Fig. 3.
Glioblastoma epithelioid type. (A) Contrast-enhanced, T1-weighted MR image shows partial debulking of the tumor, with extensive irregular enhancement within the right frontal, parietal, and temporal lobes, areas of hemorrhage, surrounding edema, and adjacent leptomeningeal enhancement. (B) Contrast-enhanced T1-weighted MR image 7 days after initiation of vemurafenib shows increased size of the right frontotemporal mass with increased hemorrhage, edema, and mass effect. (C) Chest X-ray shows a 3.2-cm mass over the left upper lung, a soft tissue prominence in the right hilar region over a 5.5-cm area. (D) Chest X-ray 7 days after initiation of vemurafenib shows decreased perihilar opacities and resolution of previously visualized masses.
Discussion
Vemurafenib was approved by the FDA in 2011 for use in BRAFV600E-mutated melanomas. Soon after introduction, an antitumor effect on brain metastasis was reported,18 which led to an open label pilot study demonstrating safety and efficacy of vemurafenib in treating intracranial disease.19 Based on the experience in melanoma, 10 cases have been reported in the literature assessing responsiveness to vemurafenib in BRAFV600E-mutated primary CNS tumors with mixed results.20,21 Of noteworthy mention, no toxicities resulted from vemurafenib therapy for our aforementioned patients.
This report adds two additional patients with CNS tumors harboring the BRAFV600E mutation who are distinct in that they both presented with extra-neural metastases. Although neither of these patients had a long lasting remission, the prompt and robust response suggests potential utility in cases of brain tumors with BRAFV600E mutation. Importantly, the first case described of the intracranial anaplastic PXA lesion, demonstrated a complete response, a therapeutic finding not often seen in this disease population, which persisted 3 months before disease recurrence.
No large or randomized studies exist to assess the rate of treatment response to vemurafenib among patients with brain tumors, but the response rate of patients with malignant melanoma is approximately 50%.22,23 In the aforementioned pilot study of BRAFV600E-mutated melanoma with symptomatic brain metastases, there was a greater response seen with extracranial disease than with intracranial disease.19 While vemurafenib crosses the blood-brain barrier, perhaps it does not do so with perfect fidelity. Further studies on the pharmacokinetics of this drug would need to be completed to address CNS penetrance.
Typically, therapeutic decisions in brain cancer rely heavily on histopathological characterization. These two cases of aggressive brain tumors with distinct pathologies in whom robust albeit transient responses were observed when treatment was targeted at a shared genomic mutation illustrates that common molecular signatures could be as important as pathology in treatment decision making, thus adding to the growing body of literature for targeted therapy directed against primary CNS tumors.14,23–25 Similar to the experience in other cancer subtypes with targetable mutations, early screening for BRAF mutations in high-grade gliomas may allow for the prompt initiation of directed therapies, thereby opening the potential for treatment options and more durable responses with combination therapy. Further clinical studies including the use of targeted therapies in the upfront setting and in combination with standard therapy are needed to address the dire need for more effective treatment options.
Funding
There was no funding used in support of this study.
Conflicts of interest statement. The authors decline the presence of any conflicts of interest pertaining to this study.
References
- 1. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 2. Piccirilli M, Brunetto GM, Rocchi G, Giangaspero F, Salvati M. Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori. 2008;94(1):40–51. [DOI] [PubMed] [Google Scholar]
- 3. Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45(4):346–356. [DOI] [PubMed] [Google Scholar]
- 4. Berghoff AS, Preusser M. BRAF alterations in brain tumours: molecular pathology and therapeutic opportunities. Curr Opin Neurol. 2014;27(6):689–696. [DOI] [PubMed] [Google Scholar]
- 5. Dias-Santagata D, Lam Q, Vernovsky K et al. . BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6(3):e17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schindler G, Capper D, Meyer J et al. . Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 7. Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neurooncol. 2013;114(2):237–240. [DOI] [PubMed] [Google Scholar]
- 8. Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol. 2013;37(5):685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson GL, Stuhlmiller TJ, Angus SP, Zawistowski JS, Graves LM. Molecular pathways: adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancer. Clin Cancer Res. 2014;20(10):2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chapman PB, Hauschild A, Robert C et al. . Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bautista F, Paci A, Minard-Colin V et al. . Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr Blood Cancer. 2014;61(6):1101–1103. [DOI] [PubMed] [Google Scholar]
- 12. Nicolaides TP, Li H, Solomon DA et al. . Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res. 2011;17(24):7595–7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rush S, Foreman N, Liu A. Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol. 2013;31(10):e159–e160. [DOI] [PubMed] [Google Scholar]
- 14. Thomas R, Ajlan A, Ziskin J et al. . Complete response to vemurafinib in a patient with metastatic anaplastic xanthroastrocytoma [SNO abstract NT-34]. Neuro Oncol. 2014;16(suppl 5):v166–v166. [Google Scholar]
- 15. Arvanitis L, Marchioli C, Flickinger J et al. . Dramatic response induced by vemurafenib in a BRAF V600E-mutated bevacizumab refractory glioblastoma [SNO abstract NT-03]. Neuro Oncol. 2014;16(suppl 5):v159–v159. [Google Scholar]
- 16. Lee EQ, Ruland S, LeBoeuf NR, Wen PY, Santagata S. Successful treatment of a progressive BRAF V600E-mutated anaplastic pleomorphic xanthoastrocytoma with vemurafenib monotherapy [published online August 4, 2014]. J Clin Oncol. 2014. doi: 10.1200/JCO.2013.51.1766. [DOI] [PubMed] [Google Scholar]
- 17. Kleinschmidt-DeMasters BK, Aisner DL, Foreman NK. BRAF VE1 immunoreactivity patterns in epithelioid glioblastomas positive for BRAF V600E mutation. Am J Surg Pathol. 2015;39(4):528-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rochet NM, Kottschade LA, Markovic SN. Vemurafenib for melanoma metastases to the brain. N Engl J Med. 2011;365(25):2439–2441. [DOI] [PubMed] [Google Scholar]
- 19. Dummer R, Goldinger SM, Turtschi CP et al. . Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: final results of an open-label pilot study. Eur J Cancer. 2014;50(3):611–621. [DOI] [PubMed] [Google Scholar]
- 20. Skrypek M, Foreman N, Guillaume D, Moertel C. Pilomyxoid astrocytoma treated successfully with vemurafenib. Pediatr Blood Cancer. 2014;61(11):2099–2100. [DOI] [PubMed] [Google Scholar]
- 21. Capper D, Berghoff AS, Magerle M et al. . Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2012;123(2):223–233. [DOI] [PubMed] [Google Scholar]
- 22. Ravnan MC, Matalka MS. Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma. Clin Ther. 2012;34(7):1474–1486. [DOI] [PubMed] [Google Scholar]
- 23. Sun C, Wang L, Huang S et al. . Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508(7494):118–122. [DOI] [PubMed] [Google Scholar]
- 24. Montero-Conde C, Ruiz-Llorente S, Dominguez JM et al. . Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3(5):520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoadley KA, Yau C, Wolf DM et al. . Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158(4):929–944. [DOI] [PMC free article] [PubMed] [Google Scholar]