Abstract
To investigate the involvement of peripheral adenosine receptors in the effect of electroacupuncture (EA) on visceral pain in mice with inflammatory bowel disease (IBD). 2,4,6-Trinitrobenzene sulfonic acid (TNBS) was used to induce the visceral pain model. EA (1 mA, 2 Hz, 30 min) treatment was applied to bilateral acupoints “Dachangshu” (BL25) 1 day after TNBS injection once daily for 7 consecutive days. Von Frey filaments were used to measure the mechanical pain threshold. Western blot was used to detect the protein expression levels of adenosine 1 receptor (A1R), adenosine 2a receptor (A2aR), adenosine 2b receptor (A2bR), adenosine 3 receptor (A3R), substance P (SP), and interleukin 1 beta (IL-1β) in colon tissue. EA significantly ameliorated the disease-related indices and reduced the expression of SP and IL-1β in the colon tissues of mice with IBD. EA increased the expression of A1R, A2aR, and A3R and decreased the expression of A2bR in the colon tissue. Furthermore, the administration of adenosine receptor antagonists influenced the effect of EA. EA can inhibit the expression of the inflammatory factors SP and IL-1β by regulating peripheral A1, A2a, A2b, and A3 receptors, thus inhibiting visceral pain in IBD mice.
Electronic supplementary material
The online version of this article (10.1007/s11302-019-09655-4) contains supplementary material, which is available to authorized users.
Keywords: Inflammatory bowel disease (IBD), Electroacupuncture (EA), Adenosine receptor, Visceral pain
Introduction
Visceral pain is one of the common symptoms of inflammatory bowel disease [1], which is associated with decreased health-related quality-of-life scores. Patients with visceral pain usually suffer from increasing pressure and concurrently become anxious and depressed [2, 3]. Narcotics are usually used for chronic visceral pain, but they have many side effects [4, 5]. Therefore, it is of vital importance for patients with IBD to seek new treatments to alleviate visceral pain.
EA, a non-drug treatment method, has been used to alleviate visceral pain for a long time in China. Previous studies have shown that EA can significantly relieve inflammation and allodynia in mice or rats with colitis induced by TNBS [6, 7]. EA can also downregulate the disease activity index and histological scores in rats with TNBS-induced colitis [8].
Purine compounds incorporate adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), and adenosine (ADO), all of which are contained in neurotransmitters. As an endogenous purine nucleoside with multiple physiological functions, adenosine was recently deemed to be able to change a variety of inflammatory/immune responses [9]. Adenosine acts on four kinds of receptors: adenosine 1 receptor (A1R), adenosine 2a receptor (A2aR), adenosine 2b receptor (A2bR), and adenosine 3 receptor (A3R). Recent research has reported that adenosine and its receptors participate in the pathogenesis of IBD [10]. However, few studies have focused on the relationships between adenosine and the antinociceptive effect of EA on visceral pain induced by IBD.
In this study, we investigated the efficacy of EA treatment for inflammatory visceral pain and the relationships between adenosine receptors and EA effect on visceral pain.
Material and methods
Experimental animals
All experimental procedures were approved by the Animal Care Committee at Huazhong University of Science and Technology and conformed to the ethical guidelines of the International Association for the Study of Pain (IASP).
Male adult mice (20–25 g) were used for the experiments. They were purchased from the Experimental Animal Center of Tongji Medical College of Huazhong University of Science and Technology. After arrival, the mice were housed in cages at 23 °C ± 1 °C and a 12-h light/dark cycle and had free access to food and water. The mice fasted 24 h before the experiment.
Two series experiments were performed respectively. In experiment 1, 40 C57BL/6 mice were randomly divided into 4 groups (n = 10): CON (vehicle of TNBS), TNBS, TNBS mice treated with EA (TNBS+EA), and TNBS mice treated with sham EA (TNBS+sham EA). In experiment 2, 80 C57BL/6 mice were randomly divided into 8 groups (n = 10): CON, TNBS, TNBS+EA, TNBS+sham EA, TNBS+EA mice pre-treated with the A1R antagonist DPCPX (TNBS+EA+DPCPX), TNBS+EA mice pre-treated with the A2aR antagonist ZM241385 (TNBS+EA+ZM241385), TNBS+EA mice pre-treated with the A2bR antagonist PSB603 (TNBS+EA+PSB603), and TNBS+EA mice pre-treated with the A3R antagonist MRS3777 (TNBS+EA+MRS3777). An experimental design timeline with the day and time of all manipulations is presented in Fig. S1.
Reagents and instruments
A1R antagonist DPCPX (No: C101-25MG, Sigma-Aldrich, China), A2aR antagonist ZM241385 (No: Z0153, Sigma, USA), A2bR antagonist PSB603 (No: 3198/10, Tocris, UK), and A3R antagonist MRS3777 (No: SC-204105, Santa Cruz, CA, USA) were intraperitoneally injected to stimulate the effect of the adenosine receptors. Sheep anti-A1R antibody (No: sc-7500, Santa Cruz), sheep anti-A2aR antibody (No: sc-7504, Santa Cruz), sheep anti-A2bR antibody (No: sc-7507, Santa Cruz), rabbit anti-A3R antibody (No: sc-13,938, Santa Cruz), rabbit anti-IL-1β antibody (No: sc-7884, Santa Cruz), and sheep anti-SP antibody (No: sc-9758, Santa Cruz) were used in Western blotting as the primary antibodies. Mouse anti-GAPDH and β-actin (Santa Cruz) were used in Western blotting as an internal reference. Goat anti-rabbit IgG, rabbit anti-goat IgG, and rabbit anti-mouse IgG (Kerry Company, Wuhan, China) were used in Western blotting as the secondary antibodies.
Preparation of the IBD mouse model
The mouse model of IBD was established according to the method described by Hollenbach E. et al. [11] with a minor modification. Briefly, the mice were anaesthetized with 80 mg/kg pentobarbital (ethanol 20%), given as an i.p. bolus of 10 ml/kg [12]. One end of a PVC-Fr4 catheter (Φ 2.7 mm, YN Medical Instrument, Yangzhou, China) was inserted into the anus to the colon at a depth of approximately 4 cm and the other end was connected with a 1-ml syringe. Each mouse of the TNBS group was injected with 50 μl TNBS (5% w/v) (Sigma-Aldrich, St. Louis, MO, USA) and 50 μl absolute ethanol through the catheter to induce IBD. Each mouse of the control group was injected with 50 μl absolute ethanol and 50 μl distilled water. The mice were placed on their heads for 1 min after the lysis fluid infusions.
EA treatment
The mice in the TNBS+EA group were treated with EA 1 day after TNBS injection. Bilateral acupoints “Dachangshu” (BL25) were selected. The acupoints BL25 of the mouse are 7-mm lateral to the fourth lumbar spinous process on both side of waist [13]. A pair of acupuncture needles (Φ 0.30 mm × 25 mm, Huatuo, Suzhou, China) was inserted into the bilateral BL25 at a depth of 4 mm and then connected to Han’s acupoint nerve stimulator (Hans-200A, Jisheng Medical Technology Co., Ltd., Nanjing, China) with a frequency of 2 Hz and an intensity of 1 mA for 30 min. EA was applied once daily for 7 consecutive days. The acupuncture needles were inserted at the same depth in the sham EA group but not connected to the apparatus. During the EA treatment, the mouse was placed in homemade clothes but not given any anaesthetics. The homemade clothes were made with a piece of 10×10-cm denim. The limbs of the mouse were pulled out through the holes in the clothes. The edge of the clothes was fastened by clips. The animals remained awake and still during the treatment and showed no evident signs of distress. The control group and TNBS group were only lightly held in homemade clothes without other treatment.
Adenosine receptor antagonist injection
One day after TNBS injection, corresponding adenosine receptor antagonists were intraperitoneally injected into the mice in the TNBS+EA+antagonist groups 30 min before the EA treatment every day. The antagonists were injected with the following concentrations: A1R antagonist DPCPX: 3 mg/kg [14]; A2aR antagonist ZM241385: 1 mg/kg [15]; A2bR antagonist PSB603: 3 mg/kg [16]; and A3R antagonist MRS3777: 5 mg/kg [17].
Nociceptive behaviour tests
The mechanical threshold
The mice were first habituated to the testing environment for 30 min. The mechanical thresholds were tested for 3 days before TNBS injection, and the average value of which was calculated as the baseline threshold. After TNBS injection, the nociceptive thresholds were tested after EA/sham EA treatment once daily for 7 consecutive days. The mechanical threshold of the mice was measured by using the “up and down” method [18]. The mice were placed in a transparent plexiglass box with a metal mesh pad (5 mm × 5 mm mesh area) at the bottom for an adaptation period of 30 min. Then, a series of calibrated Von Frey filaments (Wood Dale, USA) were applied perpendicularly to the plantar surface of the left hind paw to bend the filament for 6 s. The range of Von Frey filament forces was 0.07–1.4 g. Paw withdrawal or the action of licking feet was considered a positive response. The test was repeated twice at 5-min intervals to calculate the average value.
Body weight determination
The body weight of the mice was tested for 3 days before TNBS injection, and the average value of which was calculated as the baseline. After TNBS injection, the weight of each group of mice was measured after EA/sham EA treatment once daily for 7 days. The daily weight/baseline *100% was used as the measurement index.
Diarrhoea score
Diarrhoea scores were observed according to the method described by Do A1 et al. (2017) [19]. For the stool consistency score, 0 points were assigned for well-formed pellets, 1 point was assigned for well-formed but soft and either very dark or light-coloured faeces, 2 points were assigned for pasty and semi-formed stools that did not adhere to the anus, 3 points were assigned for semi-formed stool that contained mucus and adhered to the anus, and 4 points were assigned for liquid stool. The diarrhoea scores were tested for 3 days before TNBS injection, and the average value of which was calculated as the baseline. After TNBS injection, the diarrhoea score of each group was measured after EA/sham EA treatment once daily for 7 days.
Colon length measurement
The mice were deeply anaesthetized with pentobarbital after the last behaviour tests. The abdomens of the mice were quickly opened. The entire intestine from the anus to the end of the caecum was removed and cleaned with 0.1-M phosphate buffer brine. Then, the length (cm) of the entire intestine was measured [20–22].
Western blot
The mice were deeply anaesthetized with pentobarbital after the last behaviour tests. Their descending colon tissue was removed and minced with scissors. The tissues were then lysed by adding 40-mg/ml RIPA lysis buffer (Biosharp, China) and 40 mg/ml phenylmethylsulfonyl fluoride to the samples for 30 min. The lysate was collected and centrifuged at 12,000 rpm at 4 °C for 15 min, and the protein contents were quantified by using the Enhanced BCA Protein Assay Kit (Beyotime Biotechnology, China). The protein (60 μg) was denatured in a loading buffer at 95 °C for 5 min, separated on a 10%/12% glycine-SDS-PAGE gel (10%: A1R, A2aR, A2bR, and A3R; 12%: SP, IL-1β) (Beyotime Biotechnology, China), and then transferred onto a PVDF membrane (Millipore Immobilon-P, USA). The membrane was probed with the following primary antibodies at 4 °C overnight: sheep anti-A1R antibody (1:500), sheep anti-A2aR antibody (1:500), sheep anti-A2bR antibody (1:500), rabbit anti-A3R antibody (1:500), sheep anti-SP antibody (1:500), and rabbit anti-IL-1β antibody (1:1000). Then, the membranes were incubated with horseradish peroxidase-conjugated-labelled IgG (1:20,000). The signals were developed using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, USA). The densitometric analysis of the protein band images was performed using Image J software (NIH, Bethesda, MD, USA). In Western blot experiments, each group included 10 mice (n = 10).
Statistical analysis
The results are presented as the mean ± SEM. Bivariate ANOVA and Bonferroni post hoc tests were used to compare the mechanical pain threshold at different time points in each group. One-way ANOVA and Newman-Keuls post hoc tests were used for the body weight (%baseline), colon length, and biochemical data. SPSS 23.0 was used for the data analysis. A P value of less than 0.05 was considered statistically significant.
Results
Effect of EA on TNBS-induced IBD in mice
EA improved the mechanical pain threshold of the TNBS-induced IBD mice
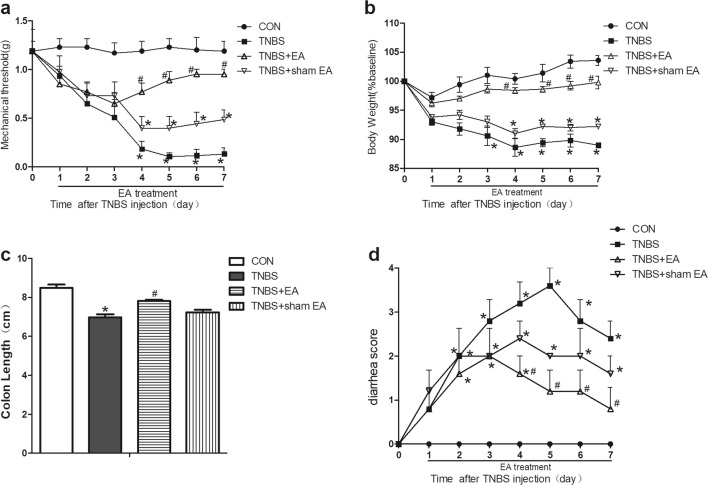
TNBS remarkably decreased the mechanical pain threshold of mice compared with that in the control group (P < 0.05, Fig. 1a), which indicated that the TNBS-induced IBD model had mechanical allodynia. The mechanical pain threshold in the TNBS+EA group but not that in the TNBS+sham EA group was notably upregulated on the fourth day after treatment compared with that of the TNBS group (P < 0.05, Fig. 1a). These results suggested that EA significantly alleviated the mechanical allodynia of TNBS-induced IBD mice.
Fig. 1.
Time course of the effect of EA on the behaviour of TNBS-induced mice. a Effect of EA on the mechanical withdrawal threshold of TNBS-induced mice. b Effect of EA on body weight (body weight divided by the baseline *%). c Effect of EA on colon length. d Effect of EA on diarrhoea score. EA treatment was applied 1 day after TNBS injection once daily for 7 consecutive days. Data were expressed as the means ± SEM (n = 10 mice per group). *P < 0.05, compared with the control group; #P < 0.05, compared with the TNBS group; △P < 0.05, compared with the TNBS+EA group
EA ameliorated the body weight loss of the TNBS-induced IBD mice
The body weight in the TNBS group was obviously reduced compared with that in the control group (P < 0.05, Fig. 1b). Compared with the body weight in the TNBS group, the body weight in the TNBS+EA group but not that in the TNBS+sham EA group was significantly increased (P < 0.05, Fig. 1b). This result illustrated that EA significantly ameliorated the TNBS-induced weight loss in the IBD model.
EA reversed the shortening of the colon in the TNBS-induced IBD mice
The length of the colon is inversely proportional to the severity of colon inflammation and is an indirect indicator of colon inflammation [23]. The length of the colon in the TNBS group was obviously shorter than that in the control group (P < 0.05, Fig. 1c). Compared with the length of the colon in the TNBS group, the length of the colon of mice in the TNBS+EA group but not that in the TNBS+sham EA group distinctly increased (P < 0.05, Fig. 1c). These results indicated that EA reversed the shortening of the colon in the TNBS-induced IBD mice.
EA improved the diarrhoea scores of the TNBS-induced IBD mice
The diarrhoea score in the TNBS group was obviously higher than that in the control group (P < 0.05, Fig. 1d). Compared with the TNBS group, the diarrhoea score of the mice in the TNBS+EA group distinctly decreased (P < 0.05, Fig. 1d). These results indicated that EA improved the diarrhoea score of the TNBS-induced IBD mice.
EA reduced the expression of SP and IL-1β in the colon tissue of mice with TNBS-induced visceral pain
Using Western blotting, we observed the expression of SP and IL-1β in colon tissues. The protein expression of SP and IL-1β in the colon tissues of the TNBS group was obviously increased compared with that in the control group (P < 0.05, Fig. 2). EA significantly decreased the expression of SP in the colon compared with that in the TNBS group but not that in the TNBS+sham EA group (P < 0.05, Fig. 2). The expression of IL-1β was also significantly decreased in the TNBS+EA group. In the TNBS+sham EA group, the IL-1β expression was slightly reduced but still higher than that in the TNBS+EA group. These results indicated that EA inhibited visceral pain by downregulating the expression of SP and IL-1β in colon tissues.
Fig. 2.
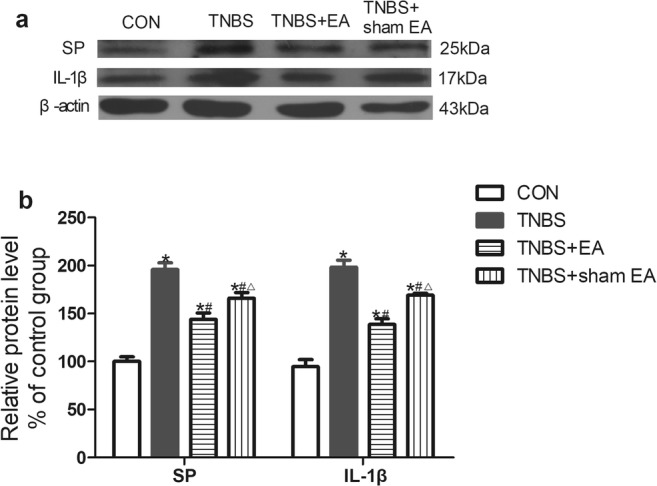
Effect of EA on the protein levels of SP and IL-1β in colon tissue. a Representative gel images of SP and IL-1β in colon tissue. β-Actin was used as a loading control. b Summary data of SP and IL-1β in colon tissue. Data were expressed as the means ± SEM (n = 10 mice per group). *P < 0.05, compared with the control group; #P < 0.05, compared with the TNBS group; △P < 0.05, compared with the TNBS+EA group
The role of the A1 receptor (A1R) in the antinociceptive effect of EA on TNBS-induced IBD mice
EA increased the expression of A1R in colon tissues
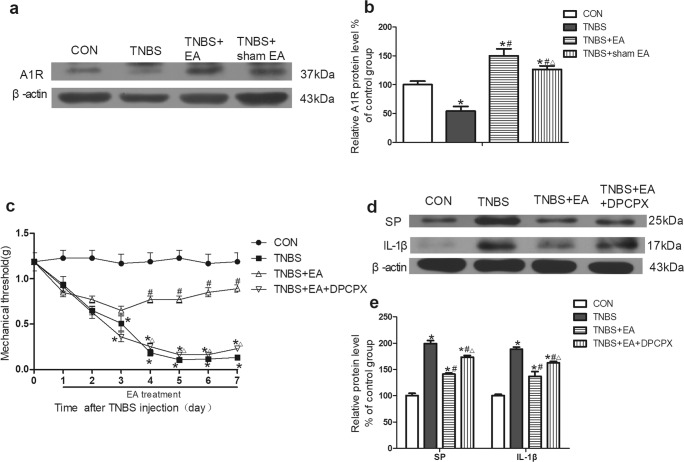
Western blotting was used to detect the expression of A1R in colon tissues. The expression of A1R was decreased in the colon tissues of the TNBS group compared with that of the control group (P < 0.05, Fig. 3a, b). The expression of A1R was significantly upregulated in the TNBS+EA group but not in the TNBS+sham EA group compared with that in the TNBS group (P < 0.05, Fig. 3a, b). These results indicated that A1R was involved in the effect of EA on TNBS-induced IBD mice.
Fig. 3.
Role of A1R in the effect of EA on TNBS-induced mice. a Representative gel images of A1R in colon tissue. β-Actin was used as a loading control. b Summary data of A1R in colon tissue. c Time course of the mechanical withdrawal threshold when used A1R antagonist. d Representative gel images of SP and IL-1β in colon tissue. β-Actin was used as a loading control. e Summary data of SP and IL-1β in colon tissue. Data were expressed as the means ± SEM (n = 10 mice per group). *P < 0.05, compared with the control group; #P < 0.05, compared with the TNBS group; △P < 0.05, compared with the TNBS+EA group
The A1R antagonist inhibited the effect of EA that increased the mechanical threshold of the mice
TNBS remarkably decreased the mechanical pain threshold of the mice compared with that in the control group (P < 0.05, Fig. 3c). The mechanical pain threshold in the TNBS+EA group was significantly elevated on the fourth day after treatment compared with that in the TNBS group (P < 0.05, Fig. 3c). Pretreatment with the A1R antagonist DPCPX obviously counteracted the EA effect in the TNBS+EA+DPCPX group compared with that in the TNBS+EA group (P < 0.05, Fig. 3c).
The A1R antagonist inhibited the effect of EA that reduced the expression of SP and IL-1β in the colon tissue
The expression of SP and IL-1β in colon tissues was detected by Western blotting. The expression of SP and IL-1β was significantly increased in the TNBS group compared with that in the control group (P < 0.05, Fig. 3d, e). EA significantly reduced the expression of SP and IL-1β compared with that in the TNBS group (P < 0.05, Fig. 3d, e). The above effect of EA was apparently weakened by pretreatment with the A1R antagonist DPCPX in the TNBS+EA + DPCPX group compared with that in the TNBS+EA group (P < 0.05, Fig. 3d, e).
The role of the A2a receptor (A2aR) in the analgesia action of EA on visceral pain
EA increased the expression of A2aR in colon tissues
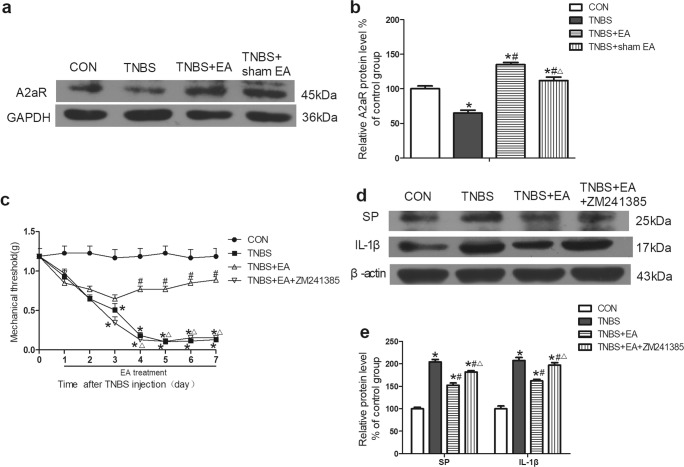
Western blotting was used to detect the expression of A2aR in colon tissues. The expression of A2aR was decreased in the colon tissues of the TNBS group compared with that of the control group (P < 0.05, Fig. 4a, b). The expression of A2aR was significantly upregulated in the TNBS+EA group but not in the TNBS+sham EA group compared with that in the TNBS group (P < 0.05, Fig. 4a, b). This finding indicated that A2aR was involved in the effect of EA on TNBS-induced IBD mice.
Fig. 4.
Role of A2aR in the effect of EA on TNBS-induced mice. a Representative gel images of A2aR in colon tissue. GAPDH was used as a loading control. b Summary data of A2aR in colon tissue. c Time course of the mechanical withdrawal threshold when used A2aR antagonist. d Representative gel images of SP and IL-1β in colon tissue. β-Actin was used as a loading control. e Summary data of SP and IL-1β in colon tissue. Data were expressed as the means ± SEM (n = 10 mice per group). *P < 0.05, compared with the control group; #P < 0.05, compared with the TNBS group; △P < 0.05, compared with the TNBS+EA group
The A2aR antagonist inhibited the effect of EA that increased the mechanical threshold
TNBS remarkably lowered the mechanical pain threshold of mice compared with that in the control group (P < 0.05, Fig. 4c). The mechanical pain threshold in the TNBS+EA group was significantly upregulated on the fourth day after treatment compared with that in the TNBS group (P < 0.05, Fig. 4c). Pretreatment with the A2aR antagonist ZM241385 obviously counteracted the EA effect in the TNBS+EA+ZM241385 group compared with that in the TNBS+EA group (P < 0.05, Fig. 4c).
The A2aR antagonist inhibited the effect of EA that reduced the expression of SP and IL-1β in the colon tissue
The expression of SP and IL-1β in the colon tissue was detected by Western blotting. The expression of SP and IL-1β was significantly increased in the TNBS group compared with that in the control group (P < 0.05, Fig. 4d, e). EA significantly reduced the expression of SP and IL-1β compared with that in the TNBS group (P < 0.05, Fig. 4d, e). The above effect of EA was apparently weakened by pretreatment with the A2aR antagonist ZM241385 in the TNBS+EA+ZM241385 group compared with that in the TNBS+EA group (P < 0.05, Fig. 4d, e).
The role of the A2b receptor (A2bR) in the antinociceptive effect of EA on visceral pain
EA decreased the expression of A2bR in colon tissues
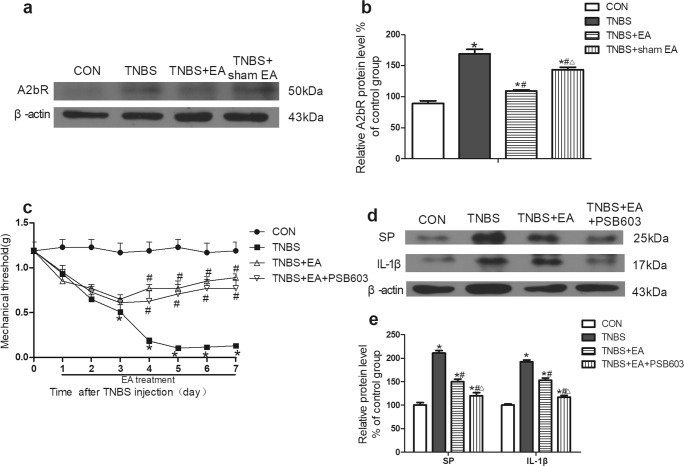
The expression of A2bR in colon tissues was detected by Western blotting. The expression of A2bR was notably increased in the colon tissues of the TNBS group compared with that of the control group (P < 0.05, Fig. 5a, b). EA significantly downregulated the expression of A2bR compared with that in the TNBS group (P < 0.05, Fig. 5a, b). A2bR expression in the TNBS+sham EA group was also slightly decreased compared to that in the TNBS group but not to the same degree as that in the TNBS+EA group (P < 0.05, Fig. 5a, b).
Fig. 5.
Role of A2bR in the effect of EA on TNBS-induced mice. a Representative gel images of A2bR in colon tissue. β-Actin was used as a loading control. b Summary data of A2bR in colon tissue. c Time course of the mechanical withdrawal threshold when used A2bR antagonist. d Representative gel images of SP and IL-1β in colon tissue. β-Actin was used as a loading control. e Summary data of SP and IL-1β in colon tissue. Data were expressed as the means ± SEM (n = 10 mice per group). *P < 0.05, compared with the control group; #P < 0.05, compared with the TNBS group; △P < 0.05, compared with the TNBS+EA group
The A2bR antagonist was of little importance in the inhibitory effect of EA on mechanical hyperalgesia
The mechanical pain threshold was significantly decreased by TNBS compared with that in the control group (P < 0.05, Fig. 5c). The mechanical pain threshold in the TNBS+EA group was significantly elevated on the fourth day after treatment compared with that in the TNBS group (P < 0.05, Fig. 5c). The mechanical pain threshold in the TNBS+EA+PSB603 group was not different from that in the TNBS+EA group (P < 0.05, Fig. 5c).
The A2bR antagonist PSB603 enhanced the inhibitory effect of EA on the expression of SP and IL-1β in colon tissue
The expression of SP and IL-1β in colon tissues was detected by Western blotting. The expression of SP and IL-1β was significantly increased in the TNBS group compared with that in the control group (P < 0.05, Fig. 5d, e). EA significantly reduced the expression of SP and IL-1β compared with that in the TNBS group (P < 0.05, Fig. 5d, e). Pretreatment with the A2bR antagonist PSB603 promoted the effect of EA compared with that in the TNBS+EA group (P < 0.05, Fig. 5d, e).
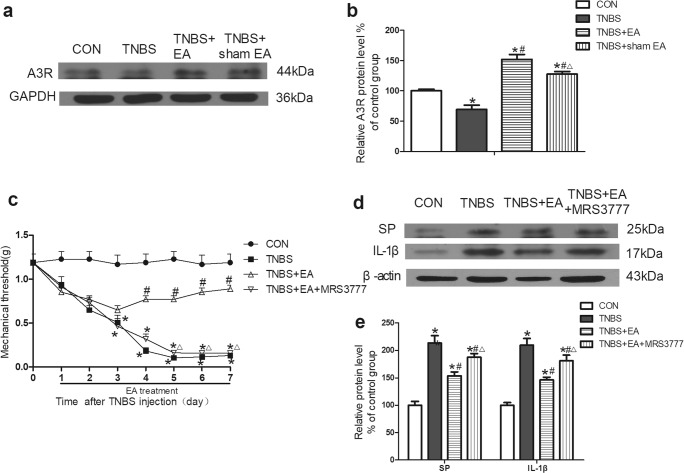
The role of the A3 receptor (A3R) in the analgesia action of EA on visceral pain
EA increased the expression of A3R in colon tissues
Western blotting was used to detect the expression of A3R in colon tissues. The expression of A3R was decreased in the colon tissues of mice in the TNBS group compared with that in the control group (P < 0.05, Fig. 6a, b). The expression of A3R was significantly increased in the TNBS+EA group but not in the sham EA group compared with that in the TNBS group (P < 0.05, Fig. 6a, b).
Fig. 6.
Role of A3R in the effect of EA on TNBS-induced mice. a Representative gel images of A3R in colon tissue. GAPDH was used as a loading control. b Summary data of A3R in colon tissue. c Time course of the mechanical withdrawal threshold when used A3R antagonist. d Representative gel images of SP and IL-1β in colon tissue. β-Actin was used as a loading control. e Summary data of SP and IL-1β in colon tissue. Data were expressed as the means ± SEM (n = 10 mice per group). *P < 0.05, compared with the control group; #P < 0.05, compared with the TNBS group; △P < 0.05, compared with the TNBS+EA group
The A3R antagonist inhibited the effect of EA that increased the mechanical threshold
TNBS significantly decreased the mechanical pain threshold of mice compared with that in the control group (P < 0.05, Fig. 6c).The mechanical pain threshold in the TNBS+EA group was significantly increased on the fourth day after treatment compared with that in the TNBS group (P < 0.05, Fig. 6c). Pretreatment with the A3R antagonist MRS3777 noticeably counteracted the EA effect in the TNBS+EA+MRS3777 group compared with that in the TNBS+EA group (P < 0.05, Fig. 6c).
The A3R antagonist inhibited the effect of EA that reduced the expression of SP and IL-1β in the colon tissue
The expression of SP and IL-1β in colon tissues was detected by Western blotting. The expression of SP and IL-1β was significantly increased in the TNBS group compared with that in the control group (P < 0.05, Fig. 6d, e). EA significantly reduced the expression of SP and IL-1β compared with that in the TNBS group (P < 0.05, Fig. 6d, e). The pretreatment with the A3R antagonist MRS3777 clearly weakened the EA effect in the TNBS+EA+MRS3777 group compared with that in the TNBS+EA group (P < 0.05, Fig. 6d, e).
Discussion
EA, as a traditional therapeutic method, has been used to treat visceral pain caused by IBD for a long time in China. Previous studies have shown that EA can decrease the disease activity index and reduce histological scores in rats with TNBS-induced colitis [8]. EA can also improve body weight reduction and colonic lesions, including swelling and haemorrhages, by decreasing the expression of TNF-α, IL-1 β, and IL-6 in colon tissues of colitis rats [24]. In this study, we found that EA significantly improved mechanical allodynia and the disease-related index (colon length, body weight, diarrhoea score). In addition, previous study found that SP and IL-1β were involved in the IBD pathologic mechanism and visceral sensitivity process [25, 26]. SP is an important part of the immune response in IBD cases [27], which can activate mast cells to promote inflammation in patients with diarrhoea-predominate irritable bowel syndrome (IBS-D) [28], whose level of IL-1β is also significantly elevated [29]. Our findings showed that EA downregulated the expression of SP and IL-1β in the colon tissues of the TNBS-induced mouse models, which demonstrated that EA inhibited visceral pain in IBD by reducing inflammatory factors associated with visceral pain.
Purinergic neurotransmission includes ATP, ADP, AMP, and adenosine. Under stress or damaged, cells release the pro-inflammatory substance ATP to the extracellular space. ATP can be swiftly hydrolysed into AMP by extracellular triphosphoric acid and diphosphate acid hydrolases (ENTPD1/CD39) and then be hydrolysed into adenosine by extracellular 5′-nucleotidase (NT5E/CD73) [30]. Nucleotides act on two receptor types called P2X (P2X1–7 subtypes) and P2Y (P2Y1, 2, 4, 6, 11–14 subtypes) [31, 32]. Adenosine acts on its own receptor types (A1R, A2aR, A2bR, A3R) [33]. Current data have shown the distribution of adenosine A1, A2a, A2b, and A3 receptors in the colon [34, 35].
The most salient finding of our present study is the potentiating effects of EA on the adenosine receptors in the process of visceral pain. In colonic distension-induced abdominal withdrawal reflex rats, subcutaneous or intracisternal injections of the A1R agonist increased the threshold volume, which was inhibited by the A1R antagonist [36]. Another study showed that the A1R agonist R-phenyl-isopropyl-adenosine (R-PIA) reduced SP-like immunoreactivity by 50%, which was blocked by the adenosine receptor antagonist (theophylline) [37]. Our study showed that EA significantly reversed the decrease of A1 receptors in IBD. Pretreatment with A1 receptor antagonists DPCPX suppressed the effect of EA in analgesia and inhibiting SP and IL-1β. This finding suggested that A1R participated in the effect of EA on visceral pain in IBD.
A2aR has anti-inflammatory and analgesic effects. A2aR contributes to the inhibition of motor actions in the normal colon at the neuronal level, and the recruitment of A2aR by CD73-dependent endogenous adenosine enhances the suppression of colonic motility during bowel inflammation [38]. A2aR agonist ATL-146e or ATL-313 can significantly improve the inflammation of the colon mucosa by suppressing the release of pro-inflammatory cytokines from neutrophils and macrophages whilst sparing anti-inflammatory activity [39]. The A2aR agonist can act as a pharmacological tool to manage IBD, and the antagonist can be used to treat functional dyspepsia [40]. Furthermore, A2a receptor antagonists can reverse the function of adenosine (0.3–100 mg/kg, i.p.) on reducing IL-1β in pleural effusion of the pleurisy model [41]. However, it is not clear whether A2aR activation could inhibit SP and IL-1β release and relieve visceral pain. In our study, results showed that EA significantly reversed the decrease of A2a receptors in IBD. Pretreatment with A2a receptor antagonist ZM241385 suppressed the effect of EA in analgesia and inhibiting SP and IL-1β. Our study provided new information that A2aR participated in the effect of EA on visceral pain in IBD.
Another potential adenosine receptor that could be involved in treating colon inflammation is A3R [42, 43]. The application of an A3 agonist can effectively protect the inflamed digestive tract mucosal layer and inhibit several cytokine/chemokine/inflammatory genes, thus facilitating an noticeable decrease in several pro-inflammatory mediators (MIP-1a and MIP-2, IL-1, IL-6, IL-12) and an increase in reactive species of oxygen, contributing to an amelioration of intestinal mucosa damage [44, 45]. A3R agonist can alleviate IBD, and its antagonist is beneficial to treat constipation [40]. In addition, A3 receptor agonists can decrease the release of IL-1β in patients with arthritis, which can be blocked by selective A3 receptor antagonists [46]. Nevertheless, few investigations have reported on the action of A3R in relieving visceral pain by inhibiting SP and IL-1β. The results of this study showed that EA significantly reversed the decrease of A3 receptors in IBD. Pretreatment with A3 receptor antagonist MRS3777 suppressed the effect of EA in analgesia and inhibiting SP and IL-1β. Our findings highlighted the important role of interactions between A3R and the effect of EA on visceral pain in IBD.
A2bR is a particular subtype of adenosine receptors that plays a different role from that of the other three subtypes. Studies have shown an overexpression of A2bR in TNBS-induced or DSS-induced colitis [47]. Similar studies also show that A2bR has a crucial role on pro-inflammatory activity in intestinal epithelial cells [48]. The application of A2b receptor antagonists can improve the inflammatory parameters and thus treat the colitis mouse model [49, 50]. Our study showed that EA significantly reversed the increase of A2b receptors in IBD. However, pretreatment with A2b receptor antagonist PSB603 had little effect on analgesia of EA, which need further research.
In summary, our study provides novel evidence that EA can inhibit the expression of inflammatory factors SP and IL-1β by regulating peripheral A1, A2a, A2b, and A3 receptors, thus inhibiting visceral pain and improving symptoms in IBD mice. However, the different roles of adenosine receptors participating in the effect of EA require further research. This new information improves our understanding in the underlying mechanisms of acupuncture analgesia.
Electronic supplementary material
Experimental design timeline. Abbreviations: EA, electroacupuncture; CLM, colon length measurement; WB, Western blotting. a Design timeline of experiment 1. The mechanical thresholds, body weight and diarrhoea scores were tested for 3 days before TNBS/vehicle injection and the average of which was calculated as the baseline, behaviour tests (mechanical thresholds, body weight and diarrhoea scores) were observed once daily for 7 consecutive days 1 day after vehicle/TNBS injection. In the TNBS+EA/ sham EA group, EA/sham EA was applied once daily for 7 consecutive days 1 day after TNBS injection. Behaviour tests (mechanical thresholds, body weight and diarrhoea scores) were observed after EA/sham EA treatment every day. After the last time of behaviour tests, mice were deeply anaesthetised and their descending colon tissues were removed for colon length measurement and Western blotting. b Design timeline of experiment 2. The mechanical thresholds, body weight and diarrhoea scores were tested for 3 days before TNBS/vehicle injection and the average of which was calculated as the baseline, behaviour tests (mechanical thresholds, body weight and diarrhoea scores) were observed once daily for 7 consecutive days 1 day after vehicle/TNBS injection.In the TNBS+EA/ sham EA group, EA/sham EA was applied once daily for 7 consecutive days 1 day after TNBS injection. In the TNBS+EA + DPCPX/ZM241385/PSB603/MRS3777 group, 4 kinds of adenosine receptor antagonists were injected 30 min before EA treatment every day, respectively. Behaviour tests were observed after EA treatment every day. After the last time of behaviour tests, mice were deeply anaesthetised and their descending colon tissues were removed for colon length measurement and Western blotting. ○, EA treatment; ●, sham EA treatment; △, EA treatment + A1R antagonist; ▲, EA treatment + A2aR antagonist; ◊, EA treatment + A2bR antagonist; ♦, EA treatment + A3R antagonist. (PNG 502 kb)
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the National Natural Science Foundation of China (81674057) and the major project of National Natural Science Foundation of Hubei province (No. 2015CFA094).
Compliance with ethical standards
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All experimental procedures were approved by the Animal Care Committee at Huazhong University of Science and Technology and conformed to the ethical guidelines of the International Association for the Study of Pain (IASP). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hong Zhang, Email: 234154585@qq.com.
Man Li, Email: liman73@mails.tjmu.edu.cn.
References
- 1.Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Crohn’s disease in the elderly: a comparison with young adults. J Clin Gastroenterol. 1998;27(2):129–133. doi: 10.1097/00004836-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Farrell KE, Callister RJ, Keely S. Understanding and targeting centrally mediated visceral pain in inflammatory bowel disease. Front Pharmacol. 2014;5:27. doi: 10.3389/fphar.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis. 2006;12(1):38–46. doi: 10.1097/01.MIB.0000195391.49762.89. [DOI] [PubMed] [Google Scholar]
- 4.Crocker JA, Yu H, Conaway M, Tuskey AG, Behm BW. Narcotic use and misuse in Crohn’s disease. Inflamm Bowel Dis. 2014;20(12):2234–2238. doi: 10.1097/MIB.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 5.Edwards JT, Radford-Smith GL, Florin TH. Chronic narcotic use in inflammatory bowel disease patients: prevalence and clinical characteristics. J Gastroenterol Hepatol. 2001;16(11):1235–1238. doi: 10.1046/j.1440-1746.2001.02468.x. [DOI] [PubMed] [Google Scholar]
- 6.Goes AC, Pinto FM, Fernandes GC, Barbosa JS, Correia ES, Ribeiro RA, Guimaraes SB, Lima Junior RC, Brito GA, Rodrigues LV. Electroacupuncture ameliorates experimental colitis induced by TNBS through activation of interleukin-10 and inhibition of iNOS in mice. Acta Cir Bras. 2014;29(12):787–793. doi: 10.1590/S0102-86502014001900004. [DOI] [PubMed] [Google Scholar]
- 7.Wu JC, Ziea ET, Lao L, Lam EF, Chan CS, Liang AY, Chu SL, Yew DT, Berman BM, Sung JJ. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16(3):306–314. doi: 10.5056/jnm.2010.16.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin H, Guo J, Liu J, Lyu B, Foreman RD, Yin J, Shi Z, Chen JDZ. Anti-inflammatory effects and mechanisms of vagal nerve stimulation combined with electroacupuncture in a rodent model of TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2017;313(3):G192–G202. doi: 10.1152/ajpgi.00254.2016. [DOI] [PubMed] [Google Scholar]
- 9.Tang Y, Yin HY, Rubini P, Illes P. Acupuncture-induced analgesia: a neurobiological basis in purinergic signaling. Neuroscientist. 2016;22(6):563–578. doi: 10.1177/1073858416654453. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280(15):14981–14988. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- 12.Zuurbier CJ, Koeman A, Houten SM, Hollmann MW, Florijn WJ. Optimizing anesthetic regimen for surgery in mice through minimization of hemodynamic, metabolic, and inflammatory perturbations. Exp Biol Med (Maywood) 2014;239(6):737–746. doi: 10.1177/1535370214524877. [DOI] [PubMed] [Google Scholar]
- 13.Wang SJ, Yang HY, Wang F, Li ST. Acupoint specificity on colorectal hypersensitivity alleviated by acupuncture and the correlation with the brain-gut axis. Neurochem Res. 2015;40(6):1274–1282. doi: 10.1007/s11064-015-1587-0. [DOI] [PubMed] [Google Scholar]
- 14.Luongo L, Petrelli R, Gatta L, Giordano C, Guida F, Vita P, Franchetti P, Grifantini M, de Novellis V, Cappellacci L, Maione S. 5'-Chloro-5'-deoxy-(+/-)-ENBA, a potent and selective adenosine A(1) receptor agonist, alleviates neuropathic pain in mice through functional glial and microglial changes without affecting motor or cardiovascular functions. Molecules. 2012;17(12):13712–13726. doi: 10.3390/molecules171213712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobato KR, Binfare RW, Budni J, Rosa AO, Santos AR, Rodrigues AL. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32(4):994–999. doi: 10.1016/j.pnpbp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Linzer R, Reddy MS, Levine MJ. Structural studies of the serotype-f polysaccharide antigen from Streptococcus mutans OMZ175. Infect Immun. 1987;55(12):3006–3010. doi: 10.1128/iai.55.12.3006-3010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Rocha Lapa F, de Oliveira AP, Accetturi BG, de Oliveira Martins I, Domingos HV, de Almeida Cabrini D, de Lima WT, Santos AR. Anti-inflammatory effects of inosine in allergic lung inflammation in mice: evidence for the participation of adenosine A2A and A 3 receptors. Purinergic Signal. 2013;9(3):325–336. doi: 10.1007/s11302-013-9351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 19.Do A, Reid RC, Lohman R-J, Sweet MJ, Fairlie DP, Iyer A. An HDAC6 inhibitor confers protection and selectively inhibits B-cell infiltration in DSS-induced colitis in mice. J Pharmacol Exp Ther. 2017;360(1):140–151. doi: 10.1124/jpet.116.236711. [DOI] [PubMed] [Google Scholar]
- 20.Jakobsson T, Vedin LL, Hassan T, Venteclef N, Greco D, D'Amato M, Treuter E, Gustafsson JA, Steffensen KR. The oxysterol receptor LXRbeta protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunol. 2014;7(6):1416–1428. doi: 10.1038/mi.2014.31. [DOI] [PubMed] [Google Scholar]
- 21.Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103(4):836–844. doi: 10.1111/j.1365-2672.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 22.Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailon E, Nieto A, Concha A, Olivares M, Zarzuelo A, Xaus J, Galvez J. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007;97(1):96–103. doi: 10.1017/S0007114507257770. [DOI] [PubMed] [Google Scholar]
- 23.Xiao X, Kim J, Sun Q, Kim D, Park CS, Lu TS, Park Y. Preventive effects of cranberry products on experimental colitis induced by dextran sulphate sodium in mice. Food Chem. 2015;167:438–446. doi: 10.1016/j.foodchem.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Shi LW, Gu H, Liu MJ, Li JG, Zhao X, Jiao JY, Cheng N. Effect of electroacupuncture and manual acupuncture on colonic inflammatory injury, cytokine levels and cell apoptosis in ulcerative colitis rats. Zhen Ci Yan Jiu. 2017;42(1):56–61. [PubMed] [Google Scholar]
- 25.Kim H, Banerjee N, Sirven MA, Minamoto Y, Markel ME, Suchodolski JS, Talcott ST, Mertens-Talcott SU. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1alpha axis in vivo and in vitro. J Nutr Biochem. 2017;43:107–115. doi: 10.1016/j.jnutbio.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Utsumi D, Matsumoto K, Amagase K, Horie S, Kato S. 5-HT3 receptors promote colonic inflammation via activation of substance P/neurokinin-1 receptors in dextran sulphate sodium-induced murine colitis. Br J Pharmacol. 2016;173(11):1835–1849. doi: 10.1111/bph.13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstock JV. Substance P and the regulation of inflammation in infections and inflammatory bowel disease. Acta Physiol (Oxf) 2015;213(2):453–461. doi: 10.1111/apha.12428. [DOI] [PubMed] [Google Scholar]
- 28.Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Sim J, Jang KS. Mast cell number, substance P and vasoactive intestinal peptide in irritable bowel syndrome with diarrhea. Scand J Gastroenterol. 2014;49(1):43–51. doi: 10.3109/00365521.2013.857712. [DOI] [PubMed] [Google Scholar]
- 29.Katsumata R, Ishii M, Lee S, Handa Y, Murao T, Fujita M, Matsumoto H, Otsuki T, Shiotani A. Cytokine profile and immunoglobulin E-mediated serological food hypersensitivity in patients with irritable bowel syndrome with diarrhea. J Neurogastroenterol Motil. 2018;24(3):415–421. doi: 10.5056/jnm17114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu S, Shao QQ, Sun JT, Yang N, Xie Q, Wang DH, Huang QB, Huang B, Wang XY, Li XG, Qu X. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro-Oncology. 2013;15(9):1160–1172. doi: 10.1093/neuonc/not067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 32.Koles L, Furst S, Illes P. Purine ionotropic (P2X) receptors. Curr Pharm Des. 2007;13(23):2368–2384. doi: 10.2174/138161207781368747. [DOI] [PubMed] [Google Scholar]
- 33.Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63(1):1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol. 2001;439(1):46–64. doi: 10.1002/cne.1334. [DOI] [PubMed] [Google Scholar]
- 35.Fornai M, Antonioli L, Colucci R, Ghisu N, Buccianti P, Marioni A, Chiarugi M, Tuccori M, Blandizzi C, Del Tacca M. A1 and A2a receptors mediate inhibitory effects of adenosine on the motor activity of human colon. Neurogastroenterol Motil. 2009;21(4):451–466. doi: 10.1111/j.1365-2982.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 36.Okumura T, Nozu T, Kumei S, Takakusaki K, Miyagishi S, Ohhira M. Adenosine A1 receptors mediate the intracisternal injection of orexin-induced antinociceptive action against colonic distension in conscious rats. J Neurol Sci. 2016;362:106–110. doi: 10.1016/j.jns.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Sjolund KF, Sollevi A, Segerdahl M, Lundeberg T. Intrathecal adenosine analog administration reduces substance P in cerebrospinal fluid along with behavioral effects that suggest antinociception in rats. Anesth Analg. 1997;85(3):627–632. doi: 10.1213/00000539-199709000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Antonioli L, Fornai M, Colucci R, Awwad O, Ghisu N, Tuccori M, Del Tacca M, Blandizzi C. Differential recruitment of high affinity A1 and A2A adenosine receptors in the control of colonic neuromuscular function in experimental colitis. Eur J Pharmacol. 2011;650(2–3):639–649. doi: 10.1016/j.ejphar.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 39.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177(5):2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 40.Dal Ben D, Antonioli L, Lambertucci C, Fornai M, Blandizzi C, Volpini R. Purinergic ligands as potential therapeutic tools for the treatment of inflammation-related intestinal diseases. Front Pharmacol. 2018;9:212. doi: 10.3389/fphar.2018.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Rocha Lapa F, da Silva MD, de Almeida Cabrini D, Santos AR. Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal. 2012;8(4):693–704. doi: 10.1007/s11302-012-9299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonioli L, Csoka B, Fornai M, Colucci R, Kokai E, Blandizzi C, Hasko G. Adenosine and inflammation: what’s new on the horizon? Drug Discov Today. 2014;19(8):1051–1068. doi: 10.1016/j.drudis.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117(1):123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, Auer H, Palatini J, Hassanain HH, Cardounel AJ, Javed A, Grants I, Wunderlich JE, Christofi FL. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis. 2006;12(8):766–789. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, Salzman A, Szabo C. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5'-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003;466(3):323–329. doi: 10.1016/S0014-2999(03)01570-X. [DOI] [PubMed] [Google Scholar]
- 46.Ravani A, Vincenzi F, Bortoluzzi A, Padovan M, Pasquini S, Gessi S, Merighi S, Borea PA, Govoni M, Varani K (2017) Role and function of A2A and A(3) adenosine receptors in patients with ankylosing spondylitis, psoriatic arthritis and rheumatoid arthritis. Int J Mol Sci 18(4). 10.3390/ijms18040697 [DOI] [PMC free article] [PubMed]
- 47.Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62(22):2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolachala V, Ruble B, Vijay-Kumar M, Wang L, Mwangi S, Figler H, Figler R, Srinivasan S, Gewirtz A, Linden J, Merlin D, Sitaraman S. Blockade of adenosine A2B receptors ameliorates murine colitis. Br Aust J Pharm. 2008;155(1):127–137. doi: 10.1038/bjp.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182(8):4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135(3):861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental design timeline. Abbreviations: EA, electroacupuncture; CLM, colon length measurement; WB, Western blotting. a Design timeline of experiment 1. The mechanical thresholds, body weight and diarrhoea scores were tested for 3 days before TNBS/vehicle injection and the average of which was calculated as the baseline, behaviour tests (mechanical thresholds, body weight and diarrhoea scores) were observed once daily for 7 consecutive days 1 day after vehicle/TNBS injection. In the TNBS+EA/ sham EA group, EA/sham EA was applied once daily for 7 consecutive days 1 day after TNBS injection. Behaviour tests (mechanical thresholds, body weight and diarrhoea scores) were observed after EA/sham EA treatment every day. After the last time of behaviour tests, mice were deeply anaesthetised and their descending colon tissues were removed for colon length measurement and Western blotting. b Design timeline of experiment 2. The mechanical thresholds, body weight and diarrhoea scores were tested for 3 days before TNBS/vehicle injection and the average of which was calculated as the baseline, behaviour tests (mechanical thresholds, body weight and diarrhoea scores) were observed once daily for 7 consecutive days 1 day after vehicle/TNBS injection.In the TNBS+EA/ sham EA group, EA/sham EA was applied once daily for 7 consecutive days 1 day after TNBS injection. In the TNBS+EA + DPCPX/ZM241385/PSB603/MRS3777 group, 4 kinds of adenosine receptor antagonists were injected 30 min before EA treatment every day, respectively. Behaviour tests were observed after EA treatment every day. After the last time of behaviour tests, mice were deeply anaesthetised and their descending colon tissues were removed for colon length measurement and Western blotting. ○, EA treatment; ●, sham EA treatment; △, EA treatment + A1R antagonist; ▲, EA treatment + A2aR antagonist; ◊, EA treatment + A2bR antagonist; ♦, EA treatment + A3R antagonist. (PNG 502 kb)