Abstract
Skeletal myogenesis is a coordinated sequence of events associated with dramatic changes in cell morphology, motility, and metabolism, which causes cellular stress and alters proteostasis. Chaperones, such as heat-shock proteins (HSPs), play important roles in limiting cellular stresses and maintaining proteostasis, but whether HSPs are specifically involved in myogenesis is not well understood. Here, we characterized gene and protein expression and subcellular localization of various HSPs in proliferating C2C12 myoblasts and differentiating myotubes under control conditions and in response to heat stress. Hsp25, Hsp40, and Hsp60 protein expression declined by 48, 35, and 83%, respectively, during differentiation. In contrast, Hsp70 protein levels doubled during early differentiation. Hsp25 was predominantly localized to the cytoplasm of myoblasts and myotubes but formed distinct aggregates in perinuclear spaces of myoblasts after heat-shock. Hsp40 was distributed diffusely throughout the cytoplasm and nucleus and, after heat-shock, translocated to the nucleus of myoblasts but formed aggregates in myotubes. Hsp60 localized to the perinuclear space in myoblasts but was distributed more diffusely across the cytoplasm in myotubes. Hsp70 was expressed diffusely throughout the cytoplasm and nucleus and translocated to the nucleus after heat-shock in myoblasts, but not in myotubes. Hsp90 was expressed diffusely across the cytoplasm in both myoblasts and myotubes under control conditions and did not change in response to heat-shock. These findings reveal distinct and different roles for HSPs in the regulation of myogenic cell proliferation and differentiation.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-01001-2) contains supplementary material, which is available to authorized users.
Keywords: Heat-shock proteins, Molecular chaperones, Skeletal muscle, Myogenesis, C2C12, Muscle development
Introduction
Skeletal muscles have high regenerative potential due to their resident population of quiescent adult muscle stem cells (MuSCs), which lie between the sarcolemma of the muscle fiber and the basal lamina (Mauro 1961). In response to injury, MuSCs are activated, re-enter the cell cycle, proliferate, differentiate, and undergo fusion to form multinucleated myotubes that mature into muscle fibers (Bentzinger et al. 2012; Dumo\nt et al. 2015). During myogenesis, dramatic changes occur in cell size, shape, motility, and metabolism, which alter proteostasis and cause cellular stress (Tang and Rando 2014).
HSPs are an evolutionarily conserved group of stress-response proteins that are produced rapidly and robustly after exposure to various stressors, including hyperthermia, ischemia, hypoxia, UV irradiation, heavy metals, oxidative stress, and infection, and provide cytoprotection from stressful stimuli (Macario and Conway de Macario 2005). HSPs are classified into different families based on their molecular weight, which are as follows: small HSPs (≤ 34 kDa), HSP40 (35–54 kDa), HSP60 (55–65 kDa), HSP70 (65–80 kDa), HSP90 (81–99 kDa), and large HSPs (≥ 100 kDa). HSPs vary in their cellular location, expression patterns, molecular characteristics, and functions (Thakur et al. 2018).
Analysis of the whole transcriptome datasets of quiescent and activated MuSCs (Liu et al. 2013; Ryall et al. 2015) and both ex vivo cultured (Ryall et al. 2015) and in vivo activated MuSCs (Liu et al. 2013) revealed differential expression of many HSP genes, including those encoding Hsp25 (hspb1), Hsp40 (dnajb1), Hsp60 (hspd1), Hsp70 (hspa1a), and Hsp90 (hsp90ab1), providing strong preliminary evidence for direct involvement of HSPs in myogenesis. Hsp25, a member of the small HSP family, acts as a potent inhibitor of apoptosis and modulator of cytoskeletal and myofibrillar structure/interactions (Bruey et al. 2000; Koh and Escobedo 2004). Elevated levels of Hsp25 occur during differentiation of C2C12 myoblasts into myotubes (Ito et al. 2001), likely driven by protein kinase cascades through regulation of MyoD and MyoG. Hsp40 belongs to the DnaJ/Hsp40 family of co-chaperones that assist folding of denatured proteins by modulating chaperone activity (Kampinga and Craig 2010). Hsp60 assists folding of mitochondrial proteins and controls mitochondrial biogenesis, processes critical for muscle function (Deocaris et al. 2006). Hsp70, the stress-inducible member of the Hsp70 family, prevents stress-induced protein unfolding/aggregation, inhibits apoptosis, maintains Ca2+ homeostasis, regulates muscle regeneration, and activates immune cell responses as an extracellular ligand (Dybdahl et al. 2002; Gehrig et al. 2012; Giraldo et al. 2010; Jiang et al. 2005; Kovalchin et al. 2006; Mayer and Bukau 2005; Senf et al. 2013). Hsp70 levels are elevated in differentiated myotubes relative to proliferating myoblasts, contributing to the increased resistance of myotubes to staurosporine-induced apoptosis (Xiao et al. 2011). Hsp90 regulates assembly of protein complexes and promotes cellular signaling (Botos et al. 2004; Tago et al. 2004). It may also have a pro-myogenic role since specific inhibition of Hsp90 in differentiating C2C12 cells with geldanamycin impaired myotube formation (Wagatsuma et al. 2011). These studies are plagued by inconsistencies regarding the differential expression of HSPs and the direction/magnitude of the differences reported. Furthermore, variations in experimental conditions make valid comparisons between studies difficult.
The use of controlled hyperthermal therapy to treat skeletal muscle disease/injury has increased as it is considered safe, cheap, reliable, and effective. Induction of localized heat stress promoted skeletal muscle regeneration in rats after crush injury (Hatade et al. 2014; Takeuchi et al. 2014). In C2C12 cells, while heat stress promoted myofibrillogenesis during myogenesis, moderate hyperthermia also activated muscle atrophy signaling pathways and therefore has potential to affect muscle size (Guo et al. 2016). Heat therapy improved insulin sensitivity in patients with type 2 diabetes (Hooper 1999) and improved skeletal muscle structure and function in dystrophic mice (Gehrig et al. 2012). Importantly, injured and diseased muscles contain myogenic cells at different stages of myogenesis, and so characterizing the role of the HSPs in muscle formation and understanding how heat stress affects HSP expression and localization in the different muscle cell types are important for enhancing the therapeutic potential of this intervention. To date, no study has examined the expression profiles and localization patterns of the major HSP family members during myogenesis, which is surprising given that HSPs function cooperatively and in a coordinated manner (Michels et al. 1997; Srikakulam and Winkelmann 2004; Yamane et al. 1995). Here, we performed a detailed characterization of gene and protein expression of Hsp25, Hsp40, Hsp60, Hsp70, and Hsp90 in proliferating and differentiating C2C12 cells during myogenesis and in response to heat stress.
Methods
Cell culture
Proliferating C2C12 murine myoblasts (ATCC, Manassas, VA, USA) were maintained at 60–70% confluency in Dulbecco’s Modified Eagle’s Medium (DMEM, 4.5 g/L D-glucose, 4.0 mM L-glutamine, 1.0 mM sodium pyruvate; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) at 37 °C, in 95% air and 5% CO2. Myogenic differentiation was induced when C2C12 cells reached 100% confluency, by replacing growth media with low serum differentiation media [DMEM, 4.5 g/L D-glucose, 4.0 mM L-glutamine; Thermo Fisher Scientific, supplemented with 2% horse serum (HS; Thermo Fisher Scientific)]. Differentiation media was replaced every 24 h for the duration of differentiation.
Heat-shock treatment
For heat-shock experiments, proliferating myoblasts were grown to 100% confluence and then either treated (MB) or differentiated into multinucleated myotubes at 37 °C, 5% CO2 for 4 days (MT). On the day of treatment, the cells were incubated at 42 °C, 5% CO2 for 2 h to induce heat-shock. Additional cells were maintained at 37 °C, 5% CO2 during this period to serve as a control.
RNA extraction and semi-quantitative real-time PCR
Total RNA was extracted from proliferating C2C12 myoblasts (MB) and C2C12 cells differentiated for 1, 2, 3, or 4 days (D1–4) using a RNeasy mini kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. RNA quality and concentration were determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific) and transcribed into cDNA with a superscript VILO cDNA synthesis kit (Thermo Fisher Scientific) in a Mastercycler PCR express machine (Eppendorf, Hamburg, Germany) for 10 min at 25 °C, 60 min at 42 °C, and 5 min at 85 °C.
Specific primers were designed using Primer-BLAST (NCBI, NIH, Bethesda, MD, USA), and primer efficiency was confirmed by a single melt curve. Reaction mix (10 μL total volume) containing optimal working concentration of forward and reverse primers (Table 1), 1× SYBR green (Thermo Fisher Scientific), and 2.5 ng of template cDNA was subjected to qPCR using an iQ5 Cycler detection system (Bio-Rad Laboratories, Hercules, CA, USA) programmed at 95 °C for 30 s followed by 39 cycles at 95 °C for 5 s and 60 °C for 10 s. Gene expression was quantified using 2−Ct method, and mRNA expression levels for each gene were normalized to total cDNA concentration for each sample as determined using Qubit 2.0 Fluorometer (Thermo Fisher Scientific) as described previously (Swiderski et al. 2016).
Table 1.
List of semi-quantitative real-time PCR (qPCR) primers used
| Gene | Forward (5′…3′) | Reverse (5′…3′) |
|---|---|---|
| pax7 | CGCCATCAACCATGCATCAG | GGGGATAAGGGGACTGAGGT |
| myog | CACTCCCTTACGTCCATCGT | CAGGACAGCCCCACTTAAAA |
| hspb1 | CCTCTTCCCTATCCCCTGAG | GCCTCGAAAGTAACCGGAAT |
| dnajb1 | TTCGACCGCTATGGAGAGGAA | CACCGAAGAACTCAGCAAACA |
| hspd1 | CGCCCCGCAGAAATGCTT | ACAGTTCTTCCCTTTGGCCC |
| hspa1a | GCAAGGAGAAGCAGCAGAGT | TTTGTGTTTGGACTCTCCCC |
| hsp90ab1 | GCGGCAAAGACAAGAAAAAG | CAAGTGGTCCTCCCAGTCAT |
Preparation of total cell extract
Whole cell lysates were prepared from proliferating C2C12 myoblasts and C2C12 cells differentiated for 1, 2, 3, or 4 days, as described previously (Park et al. 2016). Briefly, the cells were lysed at 4 °C in radioimmunoprecipitation assay (RIPA) lysis buffer [50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% Na-deoxycholic acid, and 0.1% sodium dodecyl sulphate (SDS); Millipore, Burlington, MA, USA] containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) using a cell scraper. Cell lysates were sonicated for 15 s using a Microson XL-2000 sonicator (Misonix, Farmingdale, NY, USA), centrifuged for 10 min at 10,000 rpm and 4 °C, and stored at − 80 °C until further steps were performed.
SDS-PAGE and Western immunoblotting
Total protein concentration was determined using a DC protein assay (Bio-Rad Laboratories) by measuring absorbance at 750 nm on a Multiskan Spectrum spectrophotometer (Thermo Fisher Scientific). All samples were equalized to a protein concentration of 1 μg/μl with 4× Laemmli buffer and denatured for 5 min at 95 °C. Lysates (10 μg) were loaded onto 4–15% Criterion TGX Stain-Free Precast Gels (Bio-Rad Laboratories) and resolved for 45 min at 200 V. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes by semi-dry transfer (Trans-blot Turbo, Bio-Rad Laboratories) at 1 amp for 7 min. Membranes were blocked in 3% bovine serum albumin (BSA; Sigma-Aldrich) in TBST (50 mM Tris–HCl, 150 mM NaCl, 0.05% Tween 20) for 2 h at room temperature (RT) and incubated overnight at 4 °C with antibodies diluted in blocking buffer as outlined in Table 2. Membranes were washed (TBST; 1 × 2 min and 3 × 10 min) and incubated with HRP-conjugated secondary antibodies diluted in 3% BSA in TBST (see Table 2). Immunodetection was performed using SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific) on a ChemiDoc System (Bio-Rad Laboratories). Protein bands were analyzed using ImageLab software (Bio-Rad Laboratories), and the intensity of the band of interest was normalized to total protein.
Table 2.
List of antibodies used for Western immunoblotting (WB) and immunofluorescence (IF)
| Antibody | Company | Host species | WB dilution | IF dilution |
|---|---|---|---|---|
| Pax7 | Developmental Studies Hybridoma Bank, University of Iowa, IA, USA | Mouse monoclonal IgG1 | 1:100 | |
| Myogenin | Santa Cruz Biotechnology, Dallas, TX, USA | Mouse monoclonal IgG1 | 1:400 | |
| Sarcomeric MyHC (MF20) | Developmental Studies Hybridoma Bank, University of Iowa, IA, USA | Mouse monoclonal IgG2b | 1:5000 | |
| Hsp25 | Enzo Life Sciences, Farmingdale, NY, USA | Rabbit polyclonal IgG | 1:5000 | 1:100 |
| Hsp40 | Rabbit polyclonal IgG | |||
| Hsp60 | Goat polyclonal IgG | |||
| Hsp70/72 | Rabbit polyclonal IgG | |||
| Hsp90 | Rabbit polyclonal IgG | |||
| Anti-mouse HRP | GE Healthcare Life Sciences, Marlborough, MA, USA | Sheep | 1:5000 | |
| Anti-rabbit HRP | Donkey | 1:5000 | ||
| Anti-goat HRP | Abcam, Cambridge, MA, USA | Donkey | 1:5000 | |
| Alexa Fluor 488 anti-rabbit IgG | Thermo Fisher Scientific, Waltham, MA, USA | Goat | 1:1000 | |
| Alexa Fluor 488 anti-goat IgG | Donkey | 1:1000 |
Immunofluorescence
C2C12 cells were grown on 18 mm round glass coverslips in 12-well cell culture plates. Proliferating myoblasts were grown for 24 h or cells were differentiated until they formed multinucleated myotubes, after which they were subjected to heat-shock treatment. Control and heat-shocked myoblasts and myotubes were fixed with 4% paraformaldehyde (PFA; Aesar, Ward Hill, MA, USA) in PBS for 15 min at room temperature, washed in PBS, and permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. Non-specific binding sites were blocked with 3% BSA in PBS for 45 min at room temperature, and the cells were incubated overnight at 4 °C with primary antibodies diluted in blocking buffer (Table 2). Following PBS washes (3 × 5 min), the cells were incubated with Alexa Fluor 488-conjugated secondary antibodies (Table 2) for 2 h at room temperature in the dark. The cells were then washed with PBS (3 × 5 min) and counterstained with 4′6-diamidino-2-phenylindole (DAPI; 1:1000, Thermo Fisher Scientific) in PBS. Coverslips were mounted onto glass slides using a water-based mounting medium (Fluoro-Gel; ProSciTech Pty. Ltd., Kirwan, QLD, Australia) and sealed with nail polish. Immunofluorescence images were captured on a Zeiss Axio Imager M2 microscope with a monochrome camera (AxioCam 506) using Zen software (Carl Zeiss Pty. Ltd., Oberkochen, Germany).
Statistical analysis
All values presented are mean ± SEM unless indicated otherwise. For HSP gene and protein expression analyses in total cell extracts, a one-way ANOVA with Tukey’s post-hoc test was used to determine statistical differences between timepoints. To examine the effect of heat-shock treatment on protein expression of HSPs, a t test was used. Significance was set at P < 0.05.
Results
Morphological changes and myogenic marker expression during myogenesis
Prior to analyzing HSP expression during myogenesis, gene and protein expression profiles of the myogenic markers were examined in proliferating C2C12 myoblasts (MB) and C2C12 cells differentiated for 1–4 days (Fig. S1a) to confirm the well-characterized sequence of events during myogenesis. The mRNA levels of the paired homeobox transcription factor pax7, a marker of myogenic cell specification, did not change significantly between myoblasts and differentiating C2C12 cells (Fig. S1b). The gene expression of differentiation marker myogenin (myog) was barely detectable in myoblasts, increased progressively during differentiation peaking at day 3 (165-fold compared to MB; P < 0.001), and remained high at D4 (95-fold relative to MB; P < 0.05; Fig. S1c). At the protein level, Pax7 expression was high in myoblasts and decreased during differentiation, becoming undetectable by day 4 (P < 0.05; Fig. S1d). Myogenin protein levels increased progressively during differentiation, peaking at day 3 (55-fold compared to day 1; P < 0.001), and then declined by 17-fold at day 4 relative to day 3 (P < 0.001; Fig. S1e). Sarcomeric MyHC, the contractile component of myotubes, was first detected at day 2, and its levels increased and peaked at day 4 (42-fold compared to day 2; P < 0.05), during myotube maturation and growth (Fig. S1f). Together, these findings imply that the morphological changes and expression of myogenic markers are consistent with the expected temporal sequence during C2C12 cell proliferation and differentiation.
Expression and subcellular localization of Hsp25 during myogenesis
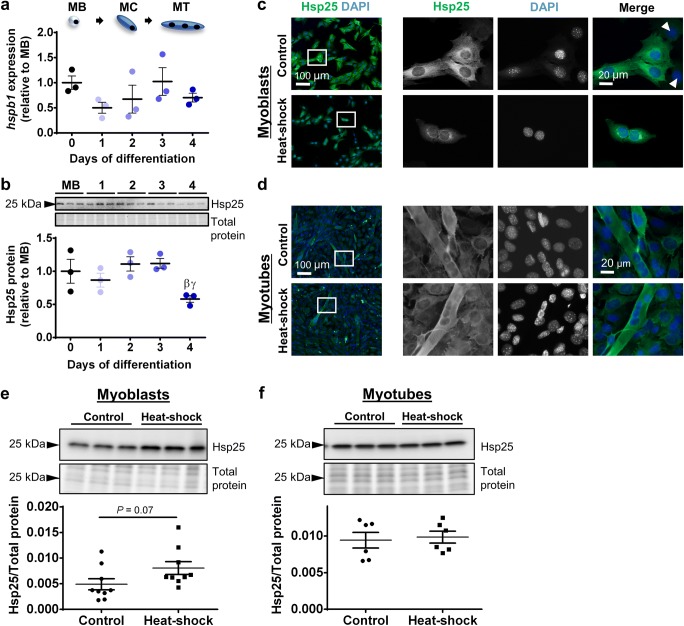
To characterize expression of Hsp25 during myogenesis, transcript levels of the Hsp25-encoding gene (hspb1) were examined in proliferating C2C12 myoblasts (MB) and C2C12 cells differentiated for 1–4 days. hspb1 mRNA expression did not change between proliferating cells and cells undergoing differentiation (Fig. 1a). At the protein level, Hsp25 expression was constant between myoblasts and through differentiation to day 3 and then decreased by 48% at day 4 relative to day 2 and day 3 levels (P < 0.05; Fig. 1b). The localization of Hsp25 was assessed by immunofluorescence in proliferating myoblasts and differentiated myotubes under normal culture conditions or following 2 h heat–shock at 42 °C. In control C2C12 myoblasts, Hsp25 was expressed predominantly in the cytoplasm (Fig. 1c). Interestingly, a subpopulation of cells displayed low levels of Hsp25 expression, indicating heterogeneity in Hsp25 expression. After heat–shock, Hsp25 formed distinct aggregates in the perinuclear space in myoblasts (Fig. 1c). In control C2C12 myotubes, Hsp25 was expressed predominantly in the cytoplasm and its distribution did not change after heat–shock (Fig. 1d). At the whole cell level, Western immunoblotting confirmed that Hsp25 protein expression did not change after heat–shock in either myoblasts (Fig. 1e) or myotubes (Fig. 1f).
Fig. 1.
Hsp25 gene expression, protein expression, and subcellular localization in proliferating and differentiating C2C12 cells. a The mRNA expression of gene encoding Hsp25 (hspb1) was examined by semi-quantitative real-time PCR (qPCR) in proliferating myoblasts (MB or day 0) and C2C12 cells differentiated for 1–4 days. Gene transcript levels were normalized to cDNA content and expressed relative to MB levels. b Representative Western blot showing protein expression of Hsp25 in MB and at days 1–4. Densitometry values were normalized to total protein and expressed relative to MB levels. Individual data points and the mean (horizontal line) ± SEM shown; n = 3 replicates/timepoint. βP < 0.05 vs. day 2; γP < 0.05 vs. day 3. c–d Representative immunofluorescence images of C2C12 myoblasts (c) and myotubes (d) stained with DAPI and endogenous Hsp25 in the absence of (control; top row) and after heat-shock treatment at 42 °C for 2 h (bottom row). Arrow heads indicate a subpopulation of cells with low Hsp25 expression. Scale bars as indicated. Boxed regions shown at higher magnification. e–f Western blot analysis showing protein expression of Hsp25 in lysates prepared from control and heat-shock-treated myoblasts (e) and myotubes (f). Densitometry values were normalized to total protein content assessed from the stain-free gel image. Individual data points and the mean (horizontal line) ± SEM are shown; n = 6–9 replicates/timepoint
Expression and subcellular localization of Hsp40 during myogenesis
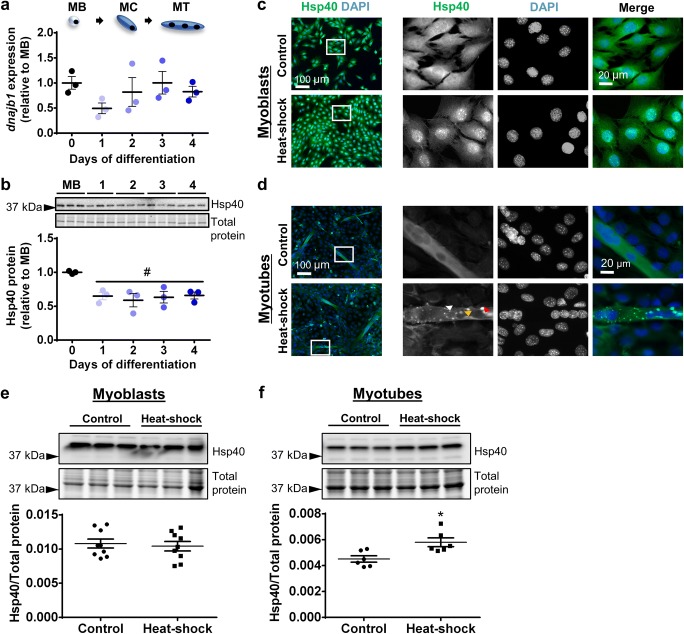
At the transcriptional level, no significant changes were observed in the expression of the Hsp40-encoding dnajb1 gene between proliferating cells and cells undergoing differentiation (Fig. 2a). In contrast, Hsp40 protein expression decreased by 35% at the onset of differentiation (day 1) compared to myoblasts (P < 0.05) and remained at this decreased level to day 4 (Fig. 2b). Immunolocalization analyses showed that Hsp40 was distributed diffusely in both cytoplasm and nucleus of C2C12 myoblasts under control conditions but translocated to the nucleus after heat–shock (Fig. 2c). In C2C12 myotubes, Hsp40 was expressed diffusely in cytoplasm and nucleus under control culture conditions but formed large punctate aggregates, sub-nuclear aggregates, and smaller cytoplasmic aggregates after heat–shock (Fig. 2d). At the whole cell level, Western immunoblotting confirmed that Hsp40 protein expression did not change in myoblasts (Fig. 2e) but increased in myotubes (P < 0.05; Fig. 2f) in response to heat–shock.
Fig. 2.
Hsp40 gene expression, protein expression, and subcellular localization in proliferating and differentiating C2C12 cells. a The mRNA expression of gene encoding Hsp40 (dnajb1) was examined by qPCR in MB and at days 1–4. Gene transcript levels were normalized to cDNA content and expressed relative to MB levels. b Representative Western blot showing protein expression of Hsp40 in MB and at days 1–4. Densitometry values were normalized to total protein and expressed relative to MB levels. Individual data points and the mean (horizontal line) ± SEM shown; n = 3 replicates/timepoint. #P < 0.05 vs. MB. c–d Representative immunofluorescence images of C2C12 myoblasts (c) and myotubes (d) stained with DAPI and endogenous Hsp40 in the absence of (control; top row) and after heat-shock treatment at 42 °C for 2 h (bottom row). In heat-shocked myotubes, Hsp40 formed large punctate aggregates (red arrowhead), submyonuclear aggregates (orange arrowhead), and smaller cytoplasmic aggregates (white arrowhead). Scale bars as indicated. Boxed regions shown at higher magnification. e–f Western blot analysis showing protein expression of Hsp40 in lysates prepared from control and heat-shock-treated myoblasts (e) and myotubes (f). Densitometry values were normalized to total protein content assessed from the stain-free gel image. Individual data points and the mean (horizontal line) ± SEM are shown; n = 6–9 replicates/timepoint. *P < 0.05 vs. control
Expression and subcellular localization of Hsp60 during myogenesis
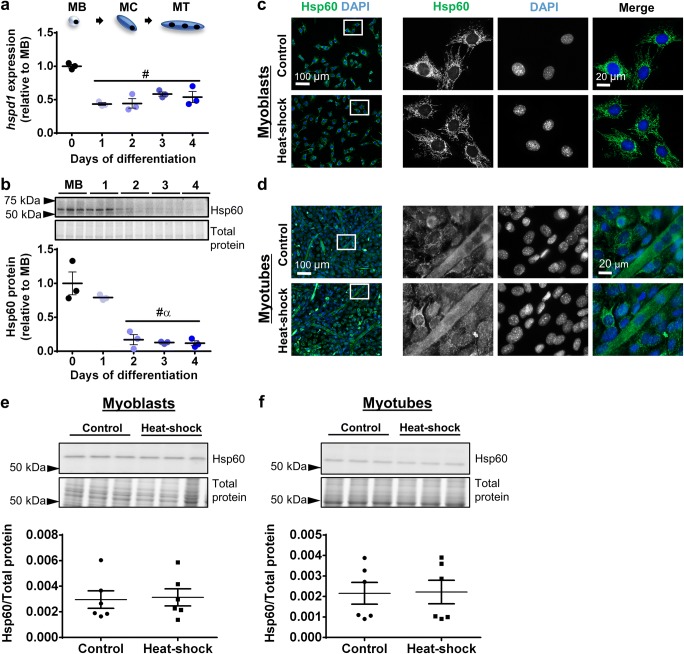
The gene encoding Hsp60 (hspd1) was expressed in myoblasts and decreased by 57% (P < 0.001) at the onset of differentiation (day 1) and remained decreased to day 4 (Fig. 3a). Similarly, Hsp60 protein levels decreased during early differentiation, declining by 83% at day 2 relative to myoblasts (P < 0.001) and remained low to day 4 (Fig. 3b). Immunostaining showed that under control conditions Hsp60 exhibited perinuclear localization in myoblasts (Fig. 3c) while in myotubes it was distributed more diffusely across the cytoplasm (Fig. 3d). Hsp60 localization did not change after heat-shock treatment in either myoblasts or myotubes (Fig. 3c, d). At the whole cell level, Western immunoblotting confirmed that Hsp60 protein expression did not change after heat–shock in either myoblasts (Fig. 3e) or myotubes (Fig. 3f).
Fig. 3.
Hsp60 gene expression, protein expression, and subcellular localization in proliferating and differentiating C2C12 cells. a The mRNA expression of gene encoding Hsp60 (hspd1) was examined qPCR in MB and at days 1–4. Gene transcript levels were normalized to cDNA content and expressed relative to MB levels. b Representative Western blot showing protein expression of Hsp60 in MB and at days 1–4. Densitometry values were normalized to total protein and expressed relative to MB levels. Individual data points and the mean (horizontal line) ± SEM shown; n = 3 replicates/timepoint. #P < 0.05 vs. MB; αP < 0.05 vs. day 1. c–d Representative immunofluorescence images of C2C12 myoblasts (c) and myotubes (d) stained with DAPI and endogenous Hsp60 in the absence of (control; top row) and after heat-shock treatment at 42 °C for 2 h (bottom row). Scale bars as indicated. Boxed regions shown at higher magnification. e–f Western blot analysis showing protein expression of Hsp60 in lysates prepared from control and heat-shock-treated myoblasts (e) and myotubes (f). Densitometry values were normalized to total protein content assessed from the stain free gel image. Individual data points and the mean (horizontal line) ± SEM are shown; n = 6 replicates/timepoint
Expression and subcellular localization of Hsp70 during myogenesis
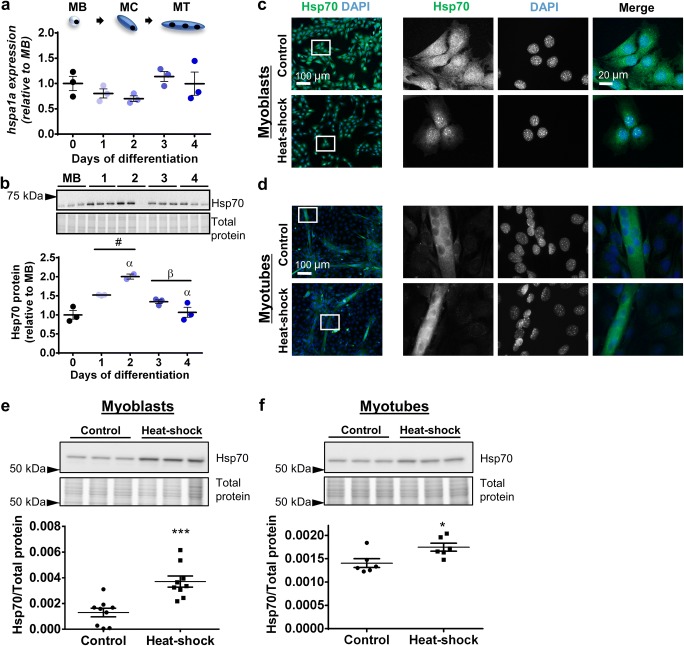
The mRNA levels of the hspa1a gene which encodes Hsp70 did not change significantly between proliferating cells and cells undergoing differentiation (Fig. 4a). However, at the protein level, Hsp70 expression increased following initiation of differentiation (52% at day 1 compared to MB; P < 0.05), peaked at day 2 (two-fold greater at day 2 compared to MB; P < 0.001), and then progressively returned to myoblast levels by day 4 (Fig. 4b). Immunostaining showed that Hsp70 expression was diffused throughout the cytoplasm and nucleus of C2C12 myoblasts under normal culture conditions and translocated to the nucleus after heat–shock (Fig. 4c). In contrast, in C2C12 myotubes, Hsp70 was distributed across the cytoplasm and nucleus under control conditions and after heat–shock (Fig. 4d). At the whole cell level, Western immunoblotting confirmed that Hsp70 protein expression increased in both myoblasts (P < 0.01; Fig. 4e) and myotubes (P < 0.05; Fig. 4f) in response to heat–shock.
Fig. 4.
Hsp70 gene expression, protein expression, and subcellular localization in proliferating and differentiating C2C12 cells. a The mRNA expression of gene encoding Hsp70 (hspa1a) was examined by qPCR in MB and at days 1–4. Gene transcript levels were normalized to cDNA content and expressed relative to MB levels. b Representative Western blot showing protein expression of Hsp70 in MB and at days 1–4. Densitometry values were normalized to total protein and expressed relative to MB levels. Individual data points and the mean (horizontal line) ± SEM shown; n = 3 replicates/timepoint. #P < 0.05 vs. MB; αP < 0.05 vs. day 1; βP < 0.05 vs. day 2. c–d Representative immunofluorescence images of C2C12 myoblasts (c) and myotubes (d) stained with DAPI and endogenous Hsp70 in the absence of (control; top row) and after heat-shock treatment at 42 °C for 2 h (bottom row). Scale bars as indicated. Boxed regions shown at higher magnification. e–f Western blot analysis showing protein expression of Hsp70 in lysates prepared from control and heat-shock-treated myoblasts (e) and myotubes (f). Densitometry values were normalized to total protein content assessed from the stain free gel image. Individual data points and the mean (horizontal line) ± SEM are shown; n = 6–9 replicates/timepoint. *P < 0.05, ***P < 0.001 vs. control
Expression and subcellular localization of Hsp90 during myogenesis
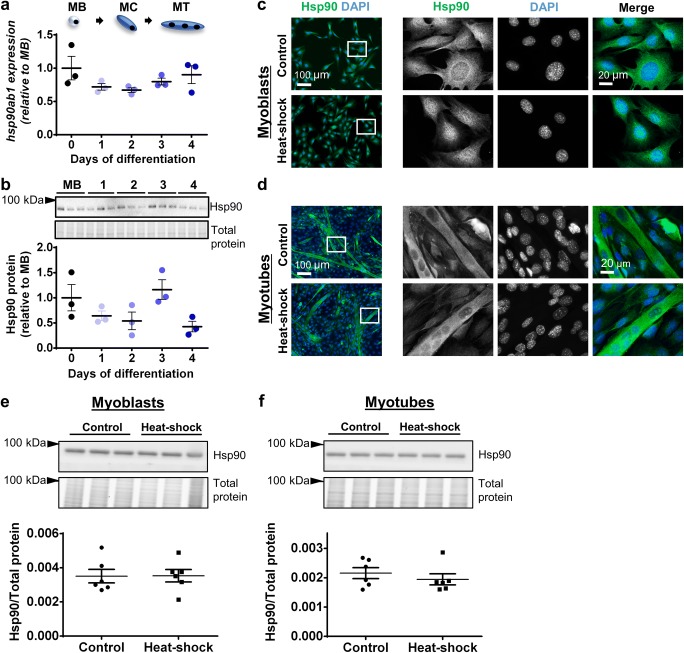
Neither transcript levels of the hsp90ab1 gene nor Hsp90 protein expression changed significantly between proliferating and differentiating C2C12 cells (Fig. 5a, b). Immunostaining revealed that Hsp90 localized diffusely across the cytoplasm and to a lesser extent in the nucleus of both myoblasts and myotubes under control conditions and after heat–shock (Fig. 5c, d). At the whole cell level, Western immunoblotting confirmed that Hsp90 protein expression did not change after heat–shock in either myoblasts (Fig. 5e) or myotubes (Fig. 5f).
Fig. 5.
Hsp90 gene expression, protein expression, and subcellular localization in proliferating and differentiating C2C12 cells. a The mRNA expression of gene encoding Hsp90 (hsp90ab1) was examined by qPCR in MB and at days 1–4. Gene transcript levels were normalized to cDNA content and expressed relative to MB levels. b Representative Western blot showing protein expression of Hsp90 in MB and at days 1–4. Densitometry values were normalized to total protein and expressed relative to MB levels. Individual data points and the mean (horizontal line) ± SEM shown; n = 3 replicates/timepoint. c–d Representative immunofluorescence images of C2C12 myoblasts (c) and myotubes (d) stained with DAPI and endogenous Hsp90 in the absence of (control; top row) and after heat-shock treatment at 42 °C for 2 h (bottom row). Scale bars as indicated. Boxed regions shown at higher magnification. e–f Western blot analysis showing protein expression of Hsp90 in lysates prepared from control and heat-shock-treated myoblasts (e) and myotubes (f). Densitometry values were normalized to total protein content assessed from the stain-free gel image. Individual data points and the mean (horizontal line) ± SEM are shown; n = 6 replicates/timepoint
Discussion
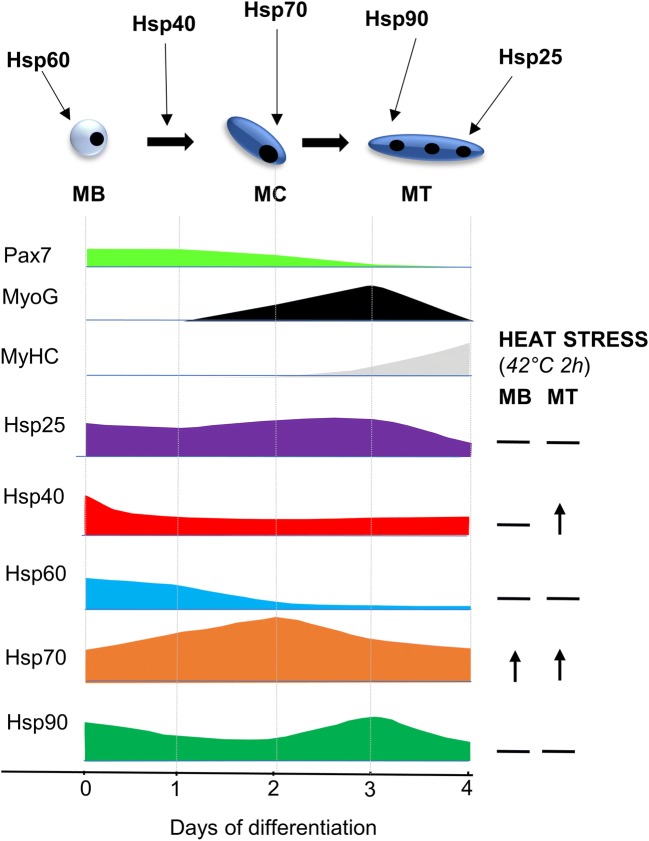
Although a potential role for HSPs in the regulation of myogenesis has been identified through the whole transcriptome analyses (Liu et al. 2013; Ryall et al. 2015; Tomczak et al. 2004) and studies have implicated individual HSPs in regulating specific aspects of myogenesis (Fan et al. 2018; Ito et al. 2001; Senf et al. 2013; Wagatsuma et al. 2011), there is a lack of information regarding overall changes in HSP expression that coordinate these events. In this study, we showed differential expression, cellular localization, and response to heat stress of Hsp25, Hsp40, Hsp60, Hsp70, and Hsp90 during changes in myogenic cell state from proliferation to differentiation, fusion, and maturation, indicating specific roles for different HSPs during myogenesis.
The changes in expression pattern and subcellular localization of Hsp25, Hsp40, Hsp60, Hsp70, and Hsp90 appear to demonstrate a coordinated network of HSPs that influence the transition through each cellular phase of myogenesis (Fig. 6). At the protein level, Hsp25 expression was maintained during the first three days of differentiation and then decreased at day 4. As this coincides with the increase in myosin expression, it indicates a potential role in myotube maturation and myofibrillar organization. In support of this contention, Hsp25 is known to stabilize cytoskeletal and contractile proteins in multiple cell types and models (Koh and Escobedo 2004; Mounier and Arrigo 2002) and has been implicated in the initial organization of myofibril assembly in developing myotubes, via association with actin microfilaments (Dubinska-Magiera et al. 2014). Furthermore, Hsp25, during both development and after maximal eccentric exercise, localizes to specific sarcomeric structures including the Z-disks and I-bands, indicating a role for Hsp25 in the maintenance of myofibrillar organization and protection of myofibrils (Paulsen et al. 2007). In contrast to Hsp25, Hsp70 protein levels increased during the early stages of C2C12 differentiation, peaking at day 2, and then returning progressively to baseline. The peak expression level at day 2 precedes the increase in myogenin expression, suggesting a potential role for Hsp70 in myogenic differentiation and the formation of nascent myotubes. Previously, siRNA-mediated Hsp70 knockdown was shown to impair myoblast differentiation by decreasing the number of myogenin positive myoblasts, resulting in shorter and thinner MyHC-positive myotubes with fewer nuclei (Fan et al. 2018). Together, these findings strongly implicate a role for Hsp70 in early myogenic differentiation.
Fig. 6.
Coordinated expression of HSPs during myogenesis under control and heat-shock conditions. Protein expression profile of Hsp25, Hsp40, Hspo60, Hsp70, and Hsp90 in relation to myogenic markers and each other in myoblasts (MB) and during days 1–4 of cell differentiation. Figure depicts phases of myogenesis with proposed Hsp involvement and changes in Hsps with heat stress
Protein expression of both Hsp40 and Hsp60 decreased during the early stages of differentiation. The decline in Hsp40 protein expression at the onset of C2C12 differentiation suggests a role in terminal cell cycle withdrawal of myoblasts, like that observed in other cell types (Cheng et al. 2005; Tsai et al. 2006; Zhang et al. 2008). The Hsp40 family proteins may therefore regulate cell cycle withdrawal of myoblasts by controlling localization of cell cycle regulators and muscle-specific regulatory factors. Hsp60 protein levels declined during the early stages of C2C12 differentiation and exhibited perinuclear staining in myoblasts and diffuse cytoplasmic staining in myotubes, suggesting divergent roles during myogenesis. The perinuclear expression in myoblasts suggests mitochondrial localization, consistent with well-known roles of Hsp60 in mitochondria-resident processes, such as protein folding and mitochondrial biogenesis (Deocaris et al. 2006). The presence of Hsp60 in the cytoplasm of myotubes might reflect a pro-survival mechanism responsible for protecting against apoptosis, since cytoplasmic Hsp60 inhibits caspase-3 activation (Kirchhoff et al. 2002; Lin et al. 2001; Zhang et al. 2004).
In contrast to all other HSPs examined, we observed no differences in Hsp90 protein expression, although Hsp90 was localized to the cytoplasm in both myoblasts and myotubes. A potential role for Hsp90 in myotube formation has been suggested (Wagatsuma et al. 2011), despite the Hsp90 inhibitor geldanamycin being unable to reduce Hsp90 expression. Further in vitro and in vivo studies are needed to conclusively determine the role of Hsp90 during myogenesis.
Interestingly, except for Hsp60 (hspd1) whose transcript levels at the onset of differentiation preceded a reduction in Hsp60 protein levels during early differentiation, no changes were observed in mRNA expression of other HSPs during myogenesis, despite distinct changes in protein levels. This suggests that the protein changes observed may be attributed to post-transcriptional and post-translational modifications regulating HSP expression. The expression of both Hsp60 and Hsp70 is regulated by the muscle-specific microRNAs (MyomiRs) miR-1 and miR-206 (Kukreti et al. 2013; Shan et al. 2010). Additionally, the expression and localization of many HSPs is regulated by post-translational modification, such as phosphorylation, methylation, acetylation, and ubiquitination (Cloutier and Coulombe 2013). How HSP expression and localization is affected by post-transcriptional and/or post-translational modification remains to be investigated.
In response to heat stress, protein expression of Hsp70 increased in both myoblasts and myotubes, Hsp25, Hsp60, and Hsp90 were unchanged, and Hsp40 expression was differentially regulated in myoblasts compared to myotubes. Regarding localization, in both myoblasts and myotubes, Hsp25, Hsp60, and Hsp90 localization did not change after heat–shock, whereas, in agreement with previous reports (Velazquez and Lindquist 1984; Welch and Feramisco 1984), Hsp70 translocated to the nucleus. Hsp25 formed distinct speckled aggregates in the cytoplasm of myoblasts after heat stress, which potentially contain Hsp25 oligomers that prevent aggregation of denatured proteins through refolding or accelerated degradation (Ehrnsperger et al. 1997). Hsp40 translocated to the nucleus of myoblasts but not myotubes after heat–shock. In multiple mammalian cell lines with similar kinetics of nuclear translocation, Hsp40 has been shown to colocalize with Hsp70 (Hattori et al. 1993; Yamane et al. 1995) and regulate Hsp70 function (Fan et al. 2003). As Hsp40 lacks a nuclear localization sequence, the two may form a complex that translocates to the nucleus after heat stress (Ohtsuka 1993). This suggests that Hsp40 and Hsp70 may function together in myoblasts but not myotubes, indicating distinct roles in response to heat stress in different myogenic states.
In conclusion, the differential expression of Hsp25, Hsp40, Hsp60, Hsp70, and Hsp90 during myogenesis implicates specific roles for individual HSPs in this process. Our characterization of HSP expression during muscle fiber development and observation that some HSPs respond differently to heat stress at different stages of myogenesis also highlights the potential for therapeutic manipulation or specific modification of HSPs at crucial times during differentiation and proliferation, worthy of further investigation. Successful approaches would find broad clinical application for conditions where muscle regeneration is compromised, including muscular diseases and related muscle wasting conditions, hastening rehabilitation after injury, orthopedic and reconstructive surgeries, with aging, and in sports medicine.
Electronic supplementary material
Analysis of myogenic markers confirmed expected pattern of gene and protein expression in proliferating and differentiating C2C12 cells. a Representative bright field images showing proliferating C2C12 myoblasts (MB) and C2C12 cells differentiated for 1–4 days. Scale bar: 100 μm. b–c The mRNA expression of pax7 (b) and myog (c) was examined using semi-quantitative real-time PCR (qPCR) in MB and at days 1–4. Gene transcript levels were normalized to cDNA content and expressed relative to MB levels. d–f Representative Western blots showing protein expression of Pax7 (d), myogenin (e), and sarcomeric myosin heavy chain (MyHC; f) in MB and at days 1–4. Densitometry values were normalized to total protein and expressed relative to MB. Individual data points and the mean (horizontal line) ± SEM shown; n = 3 replicates/timepoint. #P < 0.05 vs. MB; αP < 0.05 vs. day 1; βP < 0.05 vs. day 2; γP < 0.05 vs. day 3 (tif 7347 kb) (PNG 1186 kb)
Acknowledgements
This project was supported by grants from the National Health and Medical Research Council of Australia (NHMRC; GNT1065456) and the Muscular Dystrophy Association, USA (MDA255153). SST is supported by the Research Training Program Scholarship, the June Opie Fellowship, and the Lionel Murphy Endowment Scholarship. KS and JGR were supported by the Early Career Fellowships from the NHMRC
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botos J, Xian W, Smith DF, Smith CL. Progesterone receptor deficient in chromatin binding has an altered cellular state. J Biol Chem. 2004;279:15231–15239. doi: 10.1074/jbc.M309718200. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cenciarelli C, Nelkin G, Tsan R, Fan D, Cheng-Mayer C, Fidler IJ. Molecular mechanism of hTid-1, the human homolog of Drosophila tumor suppressor l(2)Tid, in the regulation of NF-kappaB activity and suppression of tumor growth. Mol Cell Biol. 2005;25:44–59. doi: 10.1128/MCB.25.1.44-59.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier P, Coulombe B. Regulation of molecular chaperones through post-translational modifications: decrypting the chaperone code. Biochim Biophys Acta. 2013;1829:443–454. doi: 10.1016/j.bbagrm.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones. 2006;11:116–128. doi: 10.1379/CSC-144R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinska-Magiera M, Jablonska J, Saczko J, Kulbacka J, Jagla T, Daczewska M. Contribution of small heat shock proteins to muscle development and function. FEBS Lett. 2014;588:517–530. doi: 10.1016/j.febslet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5:1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OFM, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Lee S, Cyr DM. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones. 2003;8:309–316. doi: 10.1379/1466-1268(2003)008<0309:MFROHF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Gao XK, Rao XS, Shi YP, Liu XC, Wang FY, Liu YF, Cong XX, He MY, Xu SB, Shen WL, Shen Y, Yan SG, Luo Y, Low BC, Ouyang H, Bao Z, Zheng LL, Zhou YT. Hsp70 interacts with MAPK-activated protein kinase 2 to regulate p38MAPK stability and myoblast differentiation during skeletal muscle regeneration. Mol Cell Biol. 2018;38:e00211–e00218. doi: 10.1128/MCB.00211-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, Lamon S, Russell AP, Davies KE, Febbraio MA, Lynch GS. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484:394–398. doi: 10.1038/nature10980. [DOI] [PubMed] [Google Scholar]
- Giraldo E, Martin-Cordero L, Garcia JJ, Gehrmann M, Multhoff G, Ortega E. Exercise-induced extracellular 72 kDa heat shock protein (Hsp72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2. Eur J Appl Physiol. 2010;108:217–225. doi: 10.1007/s00421-009-1201-8. [DOI] [PubMed] [Google Scholar]
- Guo Q, Miller D, An H, Wang H, Lopez J, Lough D, He L, Kumar A. Controlled heat stress promotes myofibrillogenesis during myogenesis. PLoS One. 2016;11:e0166294. doi: 10.1371/journal.pone.0166294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatade T, Takeuchi K, Fujita N, Arakawa T, Miki A. Effect of heat stress soon after muscle injury on the expression of MyoD and myogenin during regeneration process. J Musculoskeletal Neuronal Interact. 2014;14:325–333. [PubMed] [Google Scholar]
- Hattori H, Kaneda T, Lokeshwar B, Laszlo A, Ohtsuka K. A stress-inducible 40 kDa protein (hsp40): purification by modified two-dimensional gel electrophoresis and co-localization with hsc70(p73) in heat-shocked HeLa cells. J Cell Sci. 1993;104:629–638. doi: 10.1242/jcs.104.3.629. [DOI] [PubMed] [Google Scholar]
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341:924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp Cell Res. 2001;266:213–221. doi: 10.1006/excr.2001.5220. [DOI] [PubMed] [Google Scholar]
- Jiang B, Xiao W, Shi Y, Liu M, Xiao X. Heat shock pretreatment inhibited the release of Smac/DIABLO from mitochondria and apoptosis induced by hydrogen peroxide in cardiomyocytes and C2C12 myogenic cells. Cell Stress Chaperones. 2005;10:252–262. doi: 10.1379/CSC-124R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA. The Hsp70 chaperone machinery: J-proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. doi: 10.1161/01.CIR.0000019403.35847.23. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Escobedo J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am J Physiol Cell Physiol. 2004;286:C713–C722. doi: 10.1152/ajpcell.00341.2003. [DOI] [PubMed] [Google Scholar]
- Kovalchin JT, Wang R, Wagh MS, Azoulay J, Sanders M, Chandawarkar RY. In vivo delivery of heat shock protein 70 accelerates wound healing by up-regulating macrophage-mediated phagocytosis. Wound Repair Regen. 2006;14:129–137. doi: 10.1111/j.1743-6109.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Kukreti H, Amuthavalli K, Harikumar A, Sathiyamoorthy S, Feng PZ, Anantharaj R, Tan SLK, Lokireddy S, Bonala S, Sriram S, McFarlane C, Kambadur R, Sharma M. Muscle-specific microRNA1 (miR1) targets heat shock protein 70 (HSP70) during dexamethasone-mediated atrophy. J Biol Chem. 2013;288:6663–6678. doi: 10.1074/jbc.M112.390369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103:1787–1792. doi: 10.1161/01.CIR.103.13.1787. [DOI] [PubMed] [Google Scholar]
- Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, Brunet A, Rando TA. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario AJ, Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med. 2005;353:1489–1501. doi: 10.1056/NEJMra050111. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels AA, Kanon B, Konings AW, Ohtsuka K, Bensaude O, Kampinga HH. Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J Biol Chem. 1997;272:33283–33289. doi: 10.1074/jbc.272.52.33283. [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:ACASHS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K. Cloning of a cDNA for heat-shock protein hsp40, a human homologue of bacterial DnaJ. Biochem Biophys Res Commun. 1993;197:235–240. doi: 10.1006/bbrc.1993.2466. [DOI] [PubMed] [Google Scholar]
- Park SY, Yun Y, Lim JS, Kim MJ, Kim SY, Kim JE, Kim IS. Stabilin-2 modulates the efficiency of myoblast fusion during myogenic differentiation and muscle regeneration. Nat Commun. 2016;7:10871. doi: 10.1038/ncomms10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Vissing K, Kalhovde JM, Ugelstad I, Bayer ML, Kadi F, Schjerling P, Hallén J, Raastad T. Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R844–R853. doi: 10.1152/ajpregu.00677.2006. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, Sartorelli V. The NAD+-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16:171–183. doi: 10.1016/j.stem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf SM, Howard TM, Ahn B, Ferreira LF, Judge AR. Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS One. 2013;8:e62687. doi: 10.1371/journal.pone.0062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan ZX, Lin QX, Deng CY, Zhu JN, Mai LP, Liu JL, Fu YH, Liu XY, Li YX, Zhang YY, Lin SG, Yu XY. miR-1/miR-206 regulate Hsp60 expression contributing to glucose-mediated apoptosis in cardiomyocytes. FEBS Lett. 2010;584:3592–3600. doi: 10.1016/j.febslet.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Chaperone-mediated folding and assembly of myosin in striated muscle. J Cell Sci. 2004;117:641–652. doi: 10.1242/jcs.00899. [DOI] [PubMed] [Google Scholar]
- Swiderski K, Thakur SS, Naim T, Trieu J, Chee A, Stapleton DI, Koopman R, Lynch GS. Muscle-specific deletion of SOCS3 increases the early inflammatory response but does not affect regeneration after myotoxic injury. Skelet Muscle. 2016;6:36. doi: 10.1186/s13395-016-0108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago K, Tsukahara F, Naruse M, Yoshioka T, Takano K. Regulation of nuclear retention of glucocorticoid receptor by nuclear Hsp90. Mol Cell Endocrinol. 2004;213:131–138. doi: 10.1016/j.mce.2003.10.057. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Hatade T, Wakamiya S, Fujita N, Arakawa T, Miki A. Heat stress promotes skeletal muscle regeneration after crush injury in rats. Acta Histochem. 2014;116:327–334. doi: 10.1016/j.acthis.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 2014;33:2782–2797. doi: 10.15252/embj.201488278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur SS, Swiderski K, Ryall JG, Lynch GS. Therapeutic potential of heat shock protein induction for muscular dystrophy and other muscle wasting conditions. Philos Trans R Soc Lond Ser B Biol Sci. 2018;373:20160528. doi: 10.1098/rstb.2016.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomczak KK, Marinescu VD, Ramoni MF, et al. Expression profiling and identification of novel genes involved in myogenic differentiation. FASEB J. 2004;18:403–405. doi: 10.1096/fj.03-0568fje. [DOI] [PubMed] [Google Scholar]
- Tsai MF, Wang CC, Chang GC, Chen CY, Chen HY, Cheng CL, Yang YP, Wu CY, Shih FY, Liu CC, Lin HP, Jou YS, Lin SC, Lin CW, Chen WJ, Chan WK, Chen JJW, Yang PC. A new tumor suppressor DnaJ-like heat shock protein, HLJ1, and survival of patients with non-small-cell lung carcinoma. J Natl Cancer Inst. 2006;98:825–838. doi: 10.1093/jnci/djj229. [DOI] [PubMed] [Google Scholar]
- Velazquez JM, Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984;36:655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- Wagatsuma A, Shiozuka M, Kotake N, Takayuki K, Yusuke H, Mabuchi K, Matsuda R, Yamada S. Pharmacological inhibition of HSP90 activity negatively modulates myogenic differentiation and cell survival in C2C12 cells. Mol Cell Biochem. 2011;358:265–280. doi: 10.1007/s11010-011-0977-0. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Feramisco JR. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984;259:4501–4513. [PubMed] [Google Scholar]
- Xiao R, Ferry AL, Dupont-Versteegden EE. Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts. Apoptosis. 2011;16:221–234. doi: 10.1007/s10495-010-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane M, Hattori H, Sugito K, Hayashi Y, Tohnai I, Ueda M, Nishizawa K, Ohtsuka K. Cotranslocation and colocalization of hsp40 (DnaJ) with hsp70 (DnaK) in mammalian cells. Cell Struct Funct. 1995;20:157–166. doi: 10.1247/csf.20.157. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pelech S, Uitto VJ. Bacterial GroEL-like heat shock protein 60 protects epithelial cells from stress-induced death through activation of ERK and inhibition of caspase 3. Exp Cell Res. 2004;292:231–240. doi: 10.1016/j.yexcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang Z, Cao Y, Zhang S, Li H, Huang Y, Ding YQ, Liu X. The Hsp40 family chaperone protein DnaJB6 enhances Schlafen1 nuclear localization which is critical for promotion of cell-cycle arrest in T-cells. Biochem J. 2008;413:239–250. doi: 10.1042/BJ20071510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of myogenic markers confirmed expected pattern of gene and protein expression in proliferating and differentiating C2C12 cells. a Representative bright field images showing proliferating C2C12 myoblasts (MB) and C2C12 cells differentiated for 1–4 days. Scale bar: 100 μm. b–c The mRNA expression of pax7 (b) and myog (c) was examined using semi-quantitative real-time PCR (qPCR) in MB and at days 1–4. Gene transcript levels were normalized to cDNA content and expressed relative to MB levels. d–f Representative Western blots showing protein expression of Pax7 (d), myogenin (e), and sarcomeric myosin heavy chain (MyHC; f) in MB and at days 1–4. Densitometry values were normalized to total protein and expressed relative to MB. Individual data points and the mean (horizontal line) ± SEM shown; n = 3 replicates/timepoint. #P < 0.05 vs. MB; αP < 0.05 vs. day 1; βP < 0.05 vs. day 2; γP < 0.05 vs. day 3 (tif 7347 kb) (PNG 1186 kb)