Abstract
No abstract available.
Keywords: intranuclear inclusions, nuclear cleft, nuclear invagination, FTLD-TDP, neurodegeneration, Lamin B1
Intranuclear inclusions, in addition to intracytoplasmic inclusions, are frequent findings in some sporadic and/or genetic neurodegenerative diseases [1]. TDP-43 and FUS are nuclear proteins with multiple functions in mRNA processing, which have been involved in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) [2, 3, 4]. TDP-43 and FUS proteins shuttle between nucleus, and cytoplasm and defects in nucleocytoplasmic transport can contribute to these pathologies [5]. For example, in sporadic ALS and FTLD-FUS/FET (fused in sarcoma/Ewing sarcoma/TAF15) an accumulation of nuclear import protein Transportin 1 has been observed [6, 7]; and proteins involved in nucleocytoplasmic transport are major components of TDP-43 aggregates [8]. On the other hand, alterations of nuclear membrane morphology and formation of clefts or invaginations are a frequent finding in FTLD and other neurodegenerative diseases [9]. In FTLD-TDP, the lentiform morphology of some nuclear inclusions (Figure 1C) – also named cat-eye inclusion – is reminiscent of nuclear clefts or invaginations (Figure 1A, D). Similarly, the nuclear vermiform inclusions observed in aFTLD-U, a subtype of FTLD-FET, also mimic the morphology of nuclear clefts. This suggests that a fraction of TDP-43 and FUS proteins may accumulate within the nuclear clefts or invaginations and form a subtype of intranuclear inclusions characteristic of the disease, that may result into a cell-specific and disease-characteristic transcriptomic pattern.
Figure 1. A: Hematoxylin-Eosin staining of a large neuron with a central basophilic cleft crossing the nucleus. B: Immunohistochemistry for lamin B1 nicely depicts the nuclear envelop and enhances the nuclear clefts. C: Cat-eye lentiform intranuclear inclusion in a patient with FTLD-TDP. D: Immunohistochemistry for Transportin 1 shows small lanceolated or vermiform intranuclear inclusions in granular neurons of the dentate gyrus of the hippocampus in a patient with aFTLD-U/ FTLD-FET. These inclusions are also immunoreactive for FUS (F). E: Double immunofluorescence for TDP43 and lamin B1 reveals that the cat-eye inclusion is not surrounded by the nuclear membrane. Instead, lamin B1 immunoreactivity shows an irregular distribution throughout the nucleus.
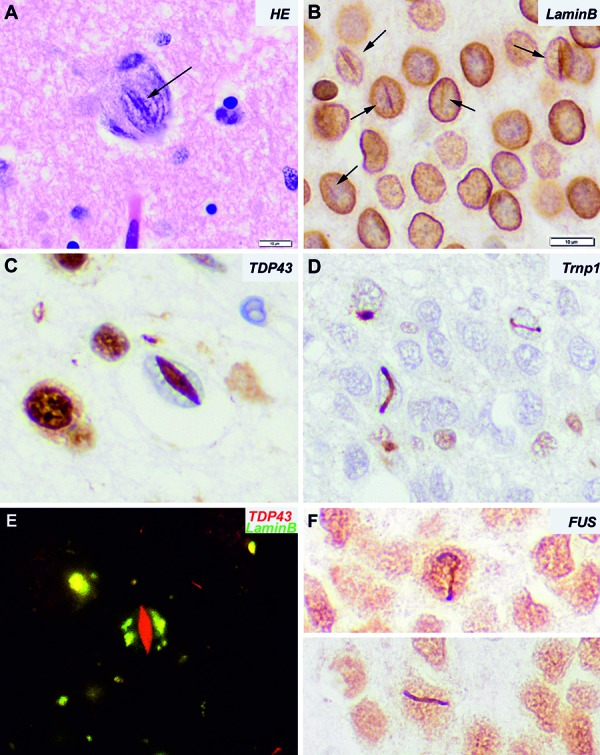
Nuclear clefts or invaginations are visible on HE-stained sections but are better identified by immunohistochemistry for the nuclear membrane protein lamin B1 (Figure 1B). However, TDP-43 cat-eye type nuclear inclusions do not necessarily contain nor are surrounded by the nuclear membrane on double immunofluorescence with lamin B1 in the case illustrated here (Figure 1E). This finding might suggest an alteration of the nuclear transport structure in neurons harboring apparently intranuclear inclusions. Alternatively, although defects in nucleocytoplasmic transport can contribute to this pathology, nuclear TDP-43 inclusions may develop independently of the nuclear membrane clefts.
Acknowledgment
We wish to thank Brain Donors of the Neurological Tissue Bank of the Biobanc-Hospital Clinic-IDIBAPS and their families for their generosity, and Sara Charif and Veronica Santiago for their technical assistance.
Ethics
All tissue samples were obtained according to Spanish Legislation. The informed consents for postmortem studies were signed by the patients or by their legal representatives in their name, as approved by local ethics committees and allowed by Spanish law.
Funding
There was no specific funding for this study.
Conflict of interest
The authors report no conflict of interest.
References
- 1. Woulfe J Kertesz A Munoz DG Frontotemporal dementia with ubiquitinated cytoplasmic and intranuclear inclusions. Acta Neuropathol. 2001; 102: 94–102. [DOI] [PubMed] [Google Scholar]
- 2. Neumann M Sampathu DM Kwong LK Truax AC Micsenyi MC Chou TT Bruce J Schuck T Grossman M Clark CM McCluskey LF Miller BL Masliah E Mackenzie IR Feldman H Feiden W Kretzschmar HA Trojanowski JQ Lee VM Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006; 314: 130–133. [DOI] [PubMed] [Google Scholar]
- 3. Kwiatkowski TJ Bosco DA Leclerc AL Tamrazian E Vanderburg CR Russ C Davis A Gilchrist J Kasarskis EJ Munsat T Valdmanis P Rouleau GA Hosler BA Cortelli P de Jong PJ Yoshinaga Y Haines JL Pericak-Vance MA Yan J Ticozzi N Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009; 323: 1205–1208. [DOI] [PubMed] [Google Scholar]
- 4. Neumann M Rademakers R Roeber S Baker M Kretzschmar HA Mackenzie IR A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009; 132: 2922–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim HJ Taylor JP Lost in transportation: nucleocytoplasmic transport defects in ALS and other neurodegenerative diseases. Neuron. 2017; 96: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neumann M Bentmann E Dormann D Jawaid A DeJesus-Hernandez M Ansorge O Roeber S Kretzschmar HA Munoz DG Kusaka H Yokota O Ang LC Bilbao J Rademakers R Haass C Mackenzie IR FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain. 2011; 134: 2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borrego-Écija S Cortés-Vicente E Cervera-Carles L Clarimón J Gámez J Batlle J Ricken G Molina-Porcel L Aldecoa I Sánchez-Valle R Rojas-García R Gelpi E Does ALS-FUS without FUS mutation represent ALS-FET? Report of three cases. Neuropathol Appl Neurobiol. 2018; Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 8. Chou CC Zhang Y Umoh ME Vaughan SW Lorenzini I Liu F Sayegh M Donlin-Asp PG Chen YH Duong DM Seyfried NT Powers MA Kukar T Hales CM Gearing M Cairns NJ Boylan KB Dickson DW Rademakers R Zhang YJ TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci. 2018; 21: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paonessa F Evans LD Solanki R Larrieu D Wray S Hardy J Jackson SP Livesey FJ Microtubules Deform the Nuclear Membrane and Disrupt Nucleocytoplasmic Transport in Tau-Mediated Frontotemporal Dementia. Cell Reports. 2019; 26: 582–593.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


