Abstract
Background:
The function of the left atrium (LA) is reduced in many cardiac diseases even with normal size. The assessment of its compliance could represent an added value in an echocardiographic report in case the gold standard technique (speckle-tracking echocardiography [STE]) is not available. We sought to test a simple and quick method as surrogate of STE: the dynamic measurement of the LA anteroposterior diameter (APD) that we called LA fractional shortening (LAFS).
Materials and Methods:
A total of 153 consecutive patients underwent a transthoracic echocardiography in our echo laboratory between January and June 2017. The only inclusion criteria were the presence of an acoustic window and the informed consent. We chose to not apply exclusion criteria to assess LAFS feasibility. The LAFS was calculated as (maxAPD−minAPD)/(maxAPD) × 100 in parasternal long-axis view. We evaluated the correlation of its value with the peak atrial longitudinal strain (PALS) and the LA emptying fraction (EF).
Results:
Mean execution time was 32.1 ± 5 s for LAFS, 2.3 ± 0.7 min for LAEF, and 2 ± 1 min for PALS. LAFS, with a feasibility of about 97%, was moderately correlated with PALS and LAEF (R between 0.20 and 0.30, P < 0.05). LAFS fractional shortening also emerged as surrogate for PALS via the relationship PALS = 21.07 + 0.364x (LAFS).
Conclusions:
LAFS demonstrated a correlation with PALS, a short execution time, a high feasibility, and the possibility to be used as a surrogate of PALS, applying a specific formula.
Keywords: Echocardiography, function, left atrium, M-mode, strain
INTRODUCTION
Left atrium (LA) is gaining more and more attention, overcoming the role of a simple conduit chamber. During a transthoracic echocardiography (TTE), the study of LA starts from the assessment of its dimension and size with different parameters. The oldest one is the anteroposterior diameter (APD), obtained in parasternal long-axis view with a mono-dimensional (M-mode) o two-dimensional (2D) approach. Despite its common use and high reproducibility, APD is considered inaccurate because it might be not representative of the real LA size[1] and the recommendations suggest not to write it as the only LA-dimensional parameter in an echo report.[2] Also LA area, measured in apical two- or four-chambers views, is quite easy to obtain, but it is scarcely used in common practice. On the contrary, LA volume, better when evaluated as LA volume index (that is, LA volume indexed to BSA, body surface area), is the preferable method, mostly considering its lower dependence on geometric assumptions, with an upper normal value of 34 ml/m2.[2] LA volume has demonstrated to be a strong index of functional capacity,[3] prognosis,[4] and follow-up in patients with different diseases, from atrial fibrillation (AF)[5,6] and mitral regurgitation (MR)[7,8] to heart failure with preserved ejection fraction (HFpEF).[9,10] However, in the last years, another important concept on LA emerged: is fundamental to evaluate its function beyond the lone assessment of size because also a small LA can already hide a dysfunction[11] and its reservoir function is strongly correlated to outcomes.[12] The gold standard for LA function analysis is speckle-tracking echocardiography (STE)[13] mostly considering the QRS as reference point; the peak atrial longitudinal strain (PALS), measured at the peak of the first positive curve, evaluates LA reservoir function.[14] As an alternative, it is possible to use LA volumes, measured during cardiac cycle at different time points (at end-systole, before mitral valve opening, that is maximal volume; at the onset of P wave on electrocardiogram [ECG], that is preatrial contraction volume; at end diastole, before mitral valve closure, that is minimal volume) and calculate the LA emptying fraction (EF)[15] that is much more time-consuming. Therefore, the aim of our study was to find a new parameter that could help the busy cardiologists, who cannot access STE, using APD not as just a static number but in different moment of the cardiac cycle, to quickly assess LA function during a standard TTE, and to include a simple functional index in the echo report.
MATERIALS AND METHODS
Study population
For this study, we enrolled 153 consecutive outpatients who were referred to our laboratory for a TTE for several indications, between June 2017 and January 2018. The only exclusion criteria were: absent acoustic window and refused to participate to the study. We did not want to extend these criteria to test also the feasibility of the new index in everyday real-life clinical practice, independently from the presence or the type of cardiac disease and from the LA size. All procedures were conducted in accordance with the Declaration of Helsinki.
Standard echocardiography
Echocardiography was performed using a high-quality machine (Vivid E9; GE Medical Systems, Milwaukee, WI) equipped with a 1.5-MHz and 3.6-MHz transducer. All the patients were studied in the left lateral recumbent position. Standard left ventricular (LV) diameters were measured in long-axis parasternal view. LV and LA volumes were assessed from apical four-chambers and two-chambers views using the biplane modified Simpson's rule, according to the current ASE recommendations.[2] Maximal, preatrial systole, and minimal LA volumes were measured at end-systole, just before the mitral valve opening (at the beginning of the P wave) and at the mitral valve closure, respectively. LA volumes were subsequently indexed to body surface area; the LA EF was calculated as ([LA maximum volume indexed − LA minimum volume indexed]/[LA maximum volume indexed]) × 100. The new parameter, that we called LA fractional shortening (LAFS), was calculated as ([maximum APD − minimum APD]/maximum APD) × 100 where maximum ADP was obtained at end-systole and minimum APD at the moment of mitral valve closure [Figure 1]. M-mode measurements of mitral annular plane systolic excursion (MAPSE) were performed by placing the cursor perpendicular to the lateral site of the mitral annulus.[16]
Figure 1.
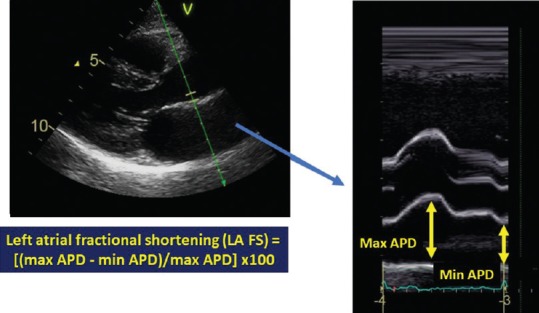
Left atrial fractional shortening measurement. Left atrial fractional shortening is calculated as ([maximum APD − minimum APD]/maximum APD) × 100 in parasternal long axis. It is possible to observe how the excursion of the wall generates a profile comparable to peak atrial longitudinal strain curve (right)
Speckle-tracking echocardiography
For STE analysis, apical four- and two-chamber views images were obtained using conventional 2D grayscale echocardiography, during breath hold and with a stable ECG recording. Care was taken to not foreshorten the LA during the acquisition of the images, allowing a more reliable delineation of the atrial endocardial border. Three consecutive heart cycles were recorded and averaged. The frame rate was set between 60 and 80 frames per second. The analysis of files recorded was performed offline by two single experienced and independent echocardiographer, who was not directly involved in the image acquisition and had no knowledge of hemodynamic measurements, using a commercially available semi-automated 2D strain software (EchoPac, GE, USA). LA endocardial border was manually traced in both four- and two-chamber views, delineating a region of interest (ROI), composed by six segments for each view. Then, after the segmental tracking quality analysis and the eventual manual adjustment of the ROI, the longitudinal strain curves were generated by the software for each atrial segment. PALS, measured at the end of the reservoir phase, was calculated by averaging values observed in all LA segments (global PALS).[17]
Data collection and reproducibility
Patients were randomly selected and a Bland–Altman analysis[18] was performed to assess the reproducibility of LAFS and of LA EF. Regarding LA strain, the reproducibility and the feasibility has been previously reported by studies conducted in our echo laboratory.[17] We also measured the mean execution time for PALS, LA EF, and LA FS.
Statistical analysis
All summary statistics are expressed as means ± standard deviation (for continuous variables) or as numbers and percentages (for binary variables). Continuous variables were checked for normality using the Kolmogorov–Smirnov test.
Pairs of interested were compared using Pearson correlation. Each comparison was performed twice: Once with no confounding variables (model 1) and once with age, sex, and BSA as confounding variables (model 2). The versions with confounders were obtained via partial correlation, which allows for multiple control variables.
Normal linear regression was used to determine a linear formula for PALS as a function of LAFS. Again, two models were tested: one with only LAFS and one with the addition of control variables age, sex, and BSA. To use LAFS as surrogate for PALS, confidence intervals were provided for their simple linear relationship.
RStudio (RStudio Boston MA, USA) was used to perform all the analyses.
RESULTS
Table 1 shows the general characteristics of the study population. The mean age was 54.8 ± 20.3 years, with 41.8% of male participants. 53 patients (34.6%) were in therapy with ACE inhibitors, while the 12.4% with beta-blockers. In Table 1, we can also observe standard TTE parameters and PALS value: mean LV ejection fraction was 55.5% ± 9.6%. Regarding LA size: mean APD was 37.07 ± 7.9 mm, mean maximum LA volume indexed 25.7 ± 11.3 ml/m2, mean pre-LA systole volume indexed 15.6 ± 8.7 ml/m2, and mean minimum LA volume indexed 10.8 ± 7.8 ml/m2 with a mean LA EF of 59.3% ± 15.6%. The new LA FS was 25.8% ± 10.0%, regarding deformation analysis, mean four-chamber PALS was 29.7% ± 12.4%, two-chamber PALS 31.2% ± 13.3%, and global PALS 30.5% ± 11.8%. Average execution time was: 32.1 ± 5 s for LA FS, 2.3 ± 0.7 min for LA EF, and 2 ± 1 min for PALS. Hence, LA FS was definitely less time-consuming. Regarding reproducibility, interobserver variability coefficients of LAFS and LA EF were 2.4% and 5.3%, respectively, while for intraobserver variability, we obtained 1.9% and 4.2%. Feasibility was 97% for LA FS and 63% for LA EF.
Table 1.
General characteristics, echocardiographic parameters, and left atrial strain value of the study population (n=153)
| Total (n=153) | |
|---|---|
| Age (years) | 54.8±20.3 |
| Men (%) | 64 (41.8) |
| BSA (m2) | 1.86±0.23 |
| BMI (kg/m2) | 26.1±5.8 |
| Smoker (%) | 13 (8.5) |
| Ex-smoker (%) | 35 (22.9) |
| HR (bpm) | 70.4±11.8 |
| Systolic BP (mmHg) | 130.8±18.3 |
| Diastolic BP (mmHg) | 77.5±10.3 |
| Diabetes mellitus (%) | 37 (25) |
| Hypertensives (%) | 74 (48) |
| Hypercholesterolemia (%) | 44 (28.8) |
| Hypertriglyceridemia (%) | 19 (12.4) |
| Drugs | |
| ACE-inhibitors (%) | 53 (34.6) |
| Beta-blockers (%) | 19 (12.4) |
| Statins (%) | 26 (17.0) |
| E/A | 1.07±0.56 |
| E/e’ | 8.78±4.11 |
| Mitral S’ | 0.09±0.03 |
| LV EDD (mm) | 48.7±8 |
| LV ejection fraction (%) | 55.5±9.6 |
| LA emptying fraction (%) | 59.3±15.6 |
| MAPSE (mm) | 14.5±3.1 |
| PALS (%) | 30.5±11.8 |
| LA fractional shortening (%) | 25.8±10.0 |
Data are expressed as mean±SD. BMI=Body mass index, BP=Blood pressure, BSA=Body surface area, EDD=End-diastolic diameter, HR=Heart rate, LA=Left atrial, LV=Left ventricular, MAPSE=Mitral annulus plane systolic excursion, PALS=Peak atrial longitudinal strain, SD=Standard deviation, ACE=Angiotensin-converting enzyme
Initial tests indicated that LA FS was moderately correlated with PALS, LA EF, and MAPSE, with all correlations being roughly in the range 0.20–0.30 [Table 2]. These correlations were all significant at a 0.05 level.
Table 2.
Correlations between left atrium fractional shortening, peak atrial longitudinal strain, and other echocardiographic parameters
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| r | P | R | P | |
| LA fractional shortening vs | ||||
| PALS | 0.31 | <0.0001 | 0.28 | 0.0023 |
| LA emptying fraction | 0.28 | 0.0005 | 0.26 | 0.0047 |
| MAPSE | 0.30 | 0.0001 | 0.30 | 0.0013 |
| PALS vs | ||||
| LA emptying fraction | 0.52 | <0.0001 | 0.58 | <0.0001 |
| LA fractional shortening | 0.31 | <0.0001 | 0.28 | 0.0023 |
| MAPSE | 0.23 | 0.005 | 0.25 | 0.0062 |
LA=Left atrial, MAPSE=Mitral annulus plane systolic excursion, PALS=Peak atrial longitudinal strain
Many parameters were tested against PALS to determine which one was the most predictive. LA EF turns out to have the highest R value at 0.52 [Figure 2], which rises to 0.58 when including the usual control/confounding variables. Furthermore, LA FS was all well correlated with PALS, with correlation being significantly larger than zero (0.05 level) [Table 2].
Figure 2.
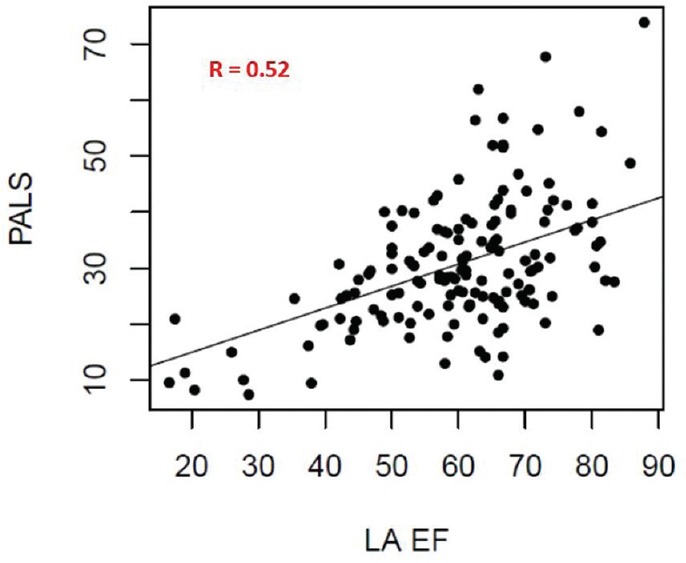
Correlation between peak atrial longitudinal strain and left atrial emptying fraction
In particular, LA FS was correlated with PALS with coefficient R = 0.31 [Figure 3]. A normal linear regression [Figure 4] showed that LA FS can be used as surrogate for PALS via the relationship PALS = 21.07 + 0.364x (LA FS). This relationship is mostly intact when including age, sex, and BSA as control variates, each being nonsignificant in the linear-model t-tests. 95% confidence intervals are provided for all linear-model coefficients, in order to correctly assess the prediction error when using LA FS as surrogate for PALS [Table 3].
Figure 3.
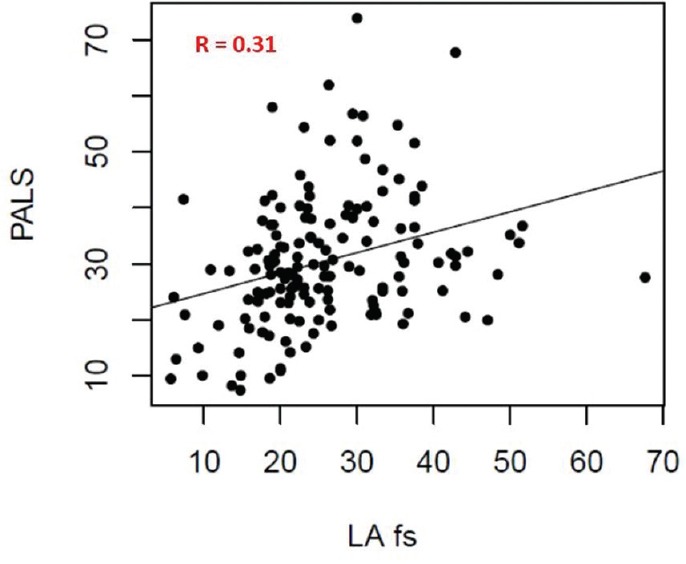
Correlation between peak atrial longitudinal strain and left atrial fractional shortening
Figure 4.
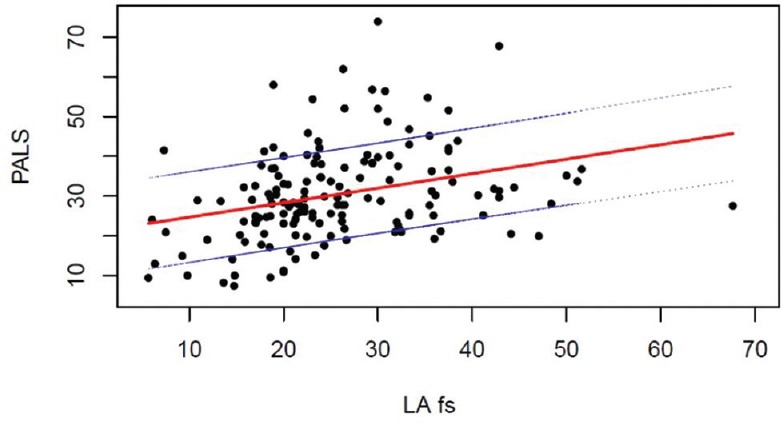
Peak atrial longitudinal strain prediction using left atrial fractional shortening as surrogate. The graph includes the regression line and ± 1DS interval
Table 3.
Linear models of peak atrial longitudinal strain versus left atrial fractional shortening
| Coefficient | P | 2.50% | 97.50% | |
|---|---|---|---|---|
| Model 1 | ||||
| Intercept | 21.07 | <0.0001 | 16.12 | 26.02 |
| LA fractional shortening | 0.364 | <0.0001 | 0.185 | 0.543 |
| Model 2 | ||||
| Intercept | 15.46 | 0.17 | −6.67 | 37.59 |
| LA fractional shortening | 0.348 | 0.0023 | 0.127 | 0.568 |
| Age | 0.041 | 0.47 | −0.071 | 0.154 |
| Male | −0.832 | 0.74 | −5.861 | 4.198 |
| BSA | 2.259 | 0.67 | −8.094 | 12.611 |
BSA=Body surface area, LA=Left atrial
DISCUSSION
Main findings of the study
In our study, we sought to find a 2D echocardiographic index that could allow the busy cardiologists to fast and accurately evaluate LA function. 153 patients underwent standard echocardiography and STE, and we analyzed LA reservoir function by PALS, LA EF, and a new parameter, that we called LA FS calculated as ([maximum APD-minimum APD]/maximum APD) × 100, comparing execution time and with successive statistical analysis, searching for a correlation between PALS, that can be considered as the gold standard, and the other two indexes.
We found that: (1) in a population with normal values of LA size (mean APD 37.07 ± 7.9 mm, mean maximum LA volume indexed 25.7 ± 11.3 ml/m2), the mean value of the new LA FS was 25.8 ± 10.0%; (2) beside normal M-mode and 2D LA dimensions, PALS was reduced in the study population that was composed also by diabetic and hypertensive patients, confirming previous studies; (3) LA FS moderately correlated with PALS and LA EF but with moderate correlations (between 0.20 and 0.30); (4) the most predictive parameter of PALS was LA EF, also including different confounding variables but with an execution time significantly higher that LA FS; and (5) the linear regression analysis demonstrated that LA FS can be used as a surrogate of PALS by the formula PALS = 21.07 + 0.364x (LA FS).
Left atrial function: Go beyond dimensions
TTE represents the most used and suitable-for-all tool for the evaluation of LA. A correct assessment of this chamber starts from an accurate measurement of LA size. APD is a quick method, but it allows to study LA diameter only along one spatial plane, thus not considering the possible geometrical abnormalities on the other planes and resulting poorly sensible. The latest recommendations for cardiac chamber quantification[2] suggest to always include LA volume in the echo report, better if indexed for the BSA, that is the more accurate parameter. The normality upper cutoff value is 34 ml/m2. In the last few years, the role of the LA gained great relevance, mostly in HFpEF and AF patients.[19,20] In these settings, a fundamental concept has emerged: a complete evaluation of atrial chamber cannot disregard also the assessment of its function because the finding of a small LA is not indicative of a correctly functional LA. Thanks to the diffusion of STE in clinical practice, the application of strain analysis to the LA allows a rapid, accurate, feasible, and reproducible[17] evaluation of its longitudinal function both regarding reservoir and boost function. A normal LA strain curve is composed by two positive peaks: the first, that is also the highest, is called PALS and investigate the maximum compliance of the atrium (reservoir), while the second (PACS) represents the capacity of the LA to actively contract and participate to LV filling (LA systole). Patients with HFpEF typically have a clinical history of arterial hypertension and diabetes mellitus. In these diseases, emerged how LA deformation mechanics assessed by PALS are early impaired, before the appearance of a macroscopically visible atrial remodeling.[11] Moreover, LA strain well correlated with LV filling pressure,[21,22,23] NT-proBNP values,[24] peak oxygen consumption (peak VO2) at cardiopulmonary exercise testing,[25] hospital readmission rate,[26] major cardiovascular events and mortality[12] in HFpEF, with increasing relevance as added value in this setting. Regarding AF, LA strain can be considered a noninvasive method to optimize the management of the patients, from diagnosis to treatment.[27] In fact, the value of PALS has been correlated to the extent of LA fibrosis assessed by 3D cardiac magnetic resonance,[28] to the risk of developing an ischemic stroke,[29] to the maintenance of sinus rhythm after electrical cardioversion,[30] and the recurrence of the arrhythmia after a catheter ablation.[31,32] All these data make us understand how evaluating LA function is more important that measuring its size, and this aspect should be applied not only for research purpose but also in the daily practice. In everyday life, the software for the analysis of LA strain is rarely available in peripheral hospitals and private echo laboratories where the cardiologist has also to deal with many echo examinations in short periods of time.
How to use standard two-dimensional echocardiography to assess left atrium function
In every cardiac cycle, the LA physiology can be portraited in three different moments: the reservoir phase, following mitral valve closure, where the LA fills up to a maximum volume; the conduit phase, after mitral valve opening, when, thanks to the potential energy previously stored in its walls; and the ventricular suction effect, LA allows the transfer of blood from the pulmonary circulation to the LV; the atrial systole, where, only in patients with sinus rhythm, LA actively contracts completing the LV diastolic filling. The LA function can be evaluated by measuring the chamber volumes in these time points (at end-systole, before mitral valve opening, that is maximum volume; at the onset of P wave on ECG, that is preatrial contraction volume; and at end-diastole, before mitral valve closure, that is minimum volume).[15] LA EF, is obtained by the formula ([LA maximum volume indexed − LA minimum volume indexed]/[LA maximum volume indexed]) × 100. Our statistical analysis confirmed a good correlation between LA EF by indexed volumes and PALS, also considering confounding variables, but with the highest execution time (2.3 ± 0.7 min). The aim of our study was to find an easy and fast method to include a measure of LA function in our echocardiographic report too. Therefore, we gave new light to an old simple parameter, the APD, and we started to approach it not as a static measure but in its dynamic meaning. Applying the formula ([maximum APD − minimum APD]/maximum APD) × 100, where maximum ADP was obtained at end-systole and minimum APD at mitral valve closure, we defined a new parameter that we called LAFS. We found the presence of a correlation, although with moderate values, with LA EF (model 1: R 0.28, P = 0.0005; model 2: R 0.26, P = 0.0047) and PALS (model 1: R 0.31, P < 0.0001; model 2: R 0.28, P = 0.0023) that remains the gold standard. The execution time was only 32.1 ± 5 s, so it is highly usable. Moreover, the successive linear regression showed that LAFS can be used as surrogate for PALS through the relationship PALS = 21.07 + 0.364x (LAFS).
Limitations of the study and future perspectives
PALS was analyzed applying the software for the LV, still lacking a dedicated GE one. Main limitations of LA strain include frame rate dependence and possible errors in epicardial/endocardial border tracing due to LA thin walls that can alter real values. The recent consensus document by the EACVI/ASE gives all the indication to standardize left atrial deformation imaging using 2D STE, in order to avoid common pitfalls.[33] It should be also considered that PALS is an index of intrinsic LA function and is not dependent from loading conditions, while LAFS correlated with LA geometrical remodeling. However, we think that the new LAFS should be tested in a larger population of participants also with pathological values of LA volume to assess if it is able to correlate with PALS in markedly remodeled atria and in different subsets of patients too (e.g., with MR, AF, HFpEF). The assessment of LA function by STE is obviously the gold standard, but the crucial message of our article is trying to get the more information we can about LA compliance with the available tools of everyday clinical practice, which can also be represented by a simple ADP, going beyond mere static parameters. For example, the evaluation of LA function is crucial in AF setting before ablation to better select the candidates, and this should be obtained even if STE is not usable in that center or the cardiologist has a short time to perform the echocardiographic examination. Considering the confirmed value of PALS as a prognostic index, LA FS should also be tested and correlated to the outcomes and the developing of major adverse cardiovascular events at short and long follow-up. This could also strengthen the results of the present study.
CONCLUSIONS
TTE allows to evaluate LA size, but a real complete assessment of this cardiac chamber should also consider its function. STE-derived strain analysis represents the gold standard for this purpose but is not widely available in daily practice. In this preliminary study, the new parameter LAFS demonstrated, during a single echocardiographic examination, a correlation with PALS, a short execution time, and the possibility to use it as a surrogate of PALS, applying a specific formula, in a population of 153 patients with normal LA dimension.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wade MR, Chandraratna PA, Reid CL, Lin SL, Rahimtoola SH. Accuracy of nondirected and directed M-mode echocardiography as an estimate of left atrial size. Am J Cardiol. 1987;60:1208–11. doi: 10.1016/0002-9149(87)90434-6. [DOI] [PubMed] [Google Scholar]
- 2.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 3.Ratanasit N, Karaketklang K, Chirakarnjanakorn S, Krittayaphong R, Jakrapanichakul D. Left atrial volume as an independent predictor of exercise capacity in patients with isolated diastolic dysfunction presented with exertional dyspnea. Cardiovasc Ultrasound. 2014;12:19. doi: 10.1186/1476-7120-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung DY, Chi C, Allman C, Boyd A, Ng AC, Kadappu KK, et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol. 2010;105:1635–9. doi: 10.1016/j.amjcard.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Parkash R, Green MS, Kerr CR, Connolly SJ, Klein GJ, Sheldon R, et al. The association of left atrial size and occurrence of atrial fibrillation: A prospective cohort study from the Canadian registry of atrial fibrillation. Am Heart J. 2004;148:649–54. doi: 10.1016/j.ahj.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang J, Wang Y, Tang K, Li X, Peng W, Liang C, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: A systematic review and meta-analysis of observational studies. Europace. 2012;14:638–45. doi: 10.1093/europace/eur364. [DOI] [PubMed] [Google Scholar]
- 7.Rusinaru D, Tribouilloy C, Grigioni F, Avierinos JF, Suri RM, Barbieri A, et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: Results from a large international multicenter study. Circ Cardiovasc Imaging. 2011;4:473–81. doi: 10.1161/CIRCIMAGING.110.961011. [DOI] [PubMed] [Google Scholar]
- 8.Le Tourneau T, Messika-Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010;56:570–8. doi: 10.1016/j.jacc.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 9.Donal E, Lund LH, Oger E, Bosseau C, Reynaud A, Hage C, et al. Importance of combined left atrial size and estimated pulmonary pressure for clinical outcome in patients presenting with heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2017;18:629–35. doi: 10.1093/ehjci/jex005. [DOI] [PubMed] [Google Scholar]
- 10.Issa O, Peguero JG, Podesta C, Diaz D, De La Cruz J, Pirela D, et al. Left atrial size and heart failure hospitalization in patients with diastolic dysfunction and preserved ejection fraction. J Cardiovasc Echogr. 2017;27:1–6. doi: 10.4103/2211-4122.199064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mondillo S, Cameli M, Caputo ML, Lisi M, Palmerini E, Padeletti M, et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24:898–908. doi: 10.1016/j.echo.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, et al. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264–9. doi: 10.1016/j.amjcard.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 14.Cameli M, Lisi M, Righini FM, Mondillo S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc Ultrasound. 2012;10:4. doi: 10.1186/1476-7120-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, et al. Left atrial function: Physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12:421–30. doi: 10.1093/ejechocard/jeq175. [DOI] [PubMed] [Google Scholar]
- 16.Mondillo S, Galderisi M, Ballo P, Marino PN. Study Group of Echocardiography of the Italian Society of Cardiology. Left ventricular systolic longitudinal function: Comparison among simple M-mode, pulsed, and M-mode color tissue doppler of mitral annulus in healthy individuals. J Am Soc Echocardiogr. 2006;19:1085–91. doi: 10.1016/j.echo.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 2009;7:6. doi: 10.1186/1476-7120-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 19.Di Salvo G, Caso P, Lo Piccolo R, Fusco A, Martiniello AR, Russo MG, et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387–95. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 20.Caso P, Ancona R, Di Salvo G, Comenale Pinto S, Macrino M, Di Palma V, et al. Atrial reservoir function by strain rate imaging in asymptomatic mitral stenosis: Prognostic value at 3 year follow-up. Eur J Echocardiogr. 2009;10:753–9. doi: 10.1093/ejechocard/jep058. [DOI] [PubMed] [Google Scholar]
- 21.Cameli M, Mandoli GE, Loiacono F, Dini FL, Henein M, Mondillo S. Left atrial strain: A new parameter for assessment of left ventricular filling pressure. Heart Fail Rev. 2016;21:65–76. doi: 10.1007/s10741-015-9520-9. [DOI] [PubMed] [Google Scholar]
- 22.Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, et al. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010;8:14. doi: 10.1186/1476-7120-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cameli M, Sparla S, Losito M, Righini FM, Menci D, Lisi M, et al. Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. 2016;33:398–405. doi: 10.1111/echo.13094. [DOI] [PubMed] [Google Scholar]
- 24.Kurt M, Tanboga IH, Aksakal E, Kaya A, Isik T, Ekinci M, et al. Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging. 2012;13:524–30. doi: 10.1093/ejechocard/jer283. [DOI] [PubMed] [Google Scholar]
- 25.Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: Importance of left atrial strain. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.003754. pii: e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: Longitudinal data from the heart and soul study. J Am Coll Cardiol. 2012;59:673–80. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S. Left atrial strain: A useful index in atrial fibrillation. Int J Cardiol. 2016;220:208–13. doi: 10.1016/j.ijcard.2016.06.197. [DOI] [PubMed] [Google Scholar]
- 28.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–9. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 29.Shih JY, Tsai WC, Huang YY, Liu YW, Lin CC, Huang YS, et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr. 2011;24:513–9. doi: 10.1016/j.echo.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Shaikh AY, Maan A, Khan UA, Aurigemma GP, Hill JC, Kane JL, et al. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: A prospective study. Cardiovasc Ultrasound. 2012;10:48. doi: 10.1186/1476-7120-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324–31. doi: 10.1016/j.jacc.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 32.Machino-Ohtsuka T, Seo Y, Ishizu T, Yanaka S, Nakajima H, Atsumi A, et al. Significant improvement of left atrial and left atrial appendage function after catheter ablation for persistent atrial fibrillation. Circ J. 2013;77:1695–704. doi: 10.1253/circj.cj-12-1518. [DOI] [PubMed] [Google Scholar]
- 33.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]


