Abstract
IMPORTANCE
Physical rehabilitation in the intensive care unit (ICU) may improve the outcomes of patients with acute respiratory failure.
OBJECTIVE
To compare standardized rehabilitation therapy (SRT) to usual ICU care in acute respiratory failure.
DESIGN, SETTING, AND PARTICIPANTS
Single-center, randomized clinical trial at Wake Forest Baptist Medical Center, North Carolina. Adult patients (mean age, 58 years; women, 55%) admitted to the ICU with acute respiratory failure requiring mechanical ventilation were randomized to SRT (n=150) or usual care (n=150) from October 2009 through May 2014 with 6-month follow-up.
INTERVENTIONS
Patients in the SRT group received daily therapy until hospital discharge, consisting of passive range of motion, physical therapy, and progressive resistance exercise. The usual care group received weekday physical therapy when ordered by the clinical team. For the SRT group, the median (interquartile range [IQR]) days of delivery of therapy were 8.0 (5.0–14.0) for passive range of motion, 5.0 (3.0–8.0) for physical therapy, and 3.0 (1.0–5.0) for progressive resistance exercise. The median days of delivery of physical therapy for the usual care group was 1.0 (IQR, 0.0–8.0).
MAIN OUTCOMES AND MEASURES
Both groups underwent assessor-blinded testing at ICU and hospital discharge and at 2, 4, and 6 months. The primary outcome was hospital length of stay (LOS). Secondary outcomes were ventilator days, ICU days, Short Physical Performance Battery (SPPB) score, 36-item Short-Form Health Surveys (SF-36) for physical and mental health and physical function scale score, Functional Performance Inventory (FPI) score, Mini-Mental State Examination (MMSE) score, and handgrip and handheld dynamometer strength.
RESULTS
Among 300 randomized patients, the median hospital LOS was 10 days (IQR, 6 to 17) for the SRT group and 10 days (IQR, 7 to 16) for the usual care group (median difference, 0 [95% CI, −1.5 to 3], P = .41). There was no difference in duration of ventilation or ICU care. There was no effect at 6 months for handgrip (difference, 2.0 kg [95% CI, −1.3 to 5.4], P = .23) and handheld dynamometer strength (difference, 0.4 lb [95% CI, −2.9 to 3.7], P = .82), SF-36 physical health score (difference, 3.4 [95% CI, −0.02 to 7.0], P = .05), SF-36 mental health score (difference, 2.4 [95% CI, −1.2 to 6.0], P = .19), or MMSE score (difference, 0.6 [95% CI, −0.2 to 1.4], P = .17). There were higher scores at 6 months in the SRT group for the SPPB score (difference, 1.1 [95% CI, 0.04 to 2.1, P = .04), SF-36 physical function scale score (difference, 12.2 [95% CI, 3.8 to 20.7], P = .001), and the FPI score (difference, 0.2 [95% CI, 0.04 to 0.4], P = .02).
CONCLUSIONS AND RELEVANCE
Among patients hospitalized with acute respiratory failure, SRT compared with usual care did not decrease hospital LOS.
A cute respiratory failure is associated with high mortality and prolonged morbidity, with impaired physical function for many survivors. Interventions directed at attenuating the profound muscle wasting in patients with acute respiratory failure are patient-centered.1 Such therapies designed to improve patient-reported weakness and impaired physical function could reduce recovery time in patients with acute respiratory failure. As well, such interventions could potentially improve long-term health-related quality of life, which for this population is commonly below normal following hospital discharge.2–4 Reports have suggested that a rehabilitation program, delivered by an intensive care unit (ICU) rehabilitation team, may be associated with reduced length of stay (LOS) and improved physical function, although findings to the contrary exist as well.5–11 This randomized clinical trial was designed to test the hypothesis that early delivery of a standardized, multifaceted ICU and hospital rehabilitation program would decrease hospital LOS and improve physical function for patients with acute respiratory failure.
Methods
Study Design and Oversight
The institutional review board at the enrolling hospital approved the clinical trial. Written consent was obtained from participants or their legally authorized representative. Race and ethnicity data were collected per the National Institutes of Health reporting policy and determined by patient or surrogate self-reporting based on fixed categories. The study was a single-center, assessor-blinded, randomized investigation with 2 groups: standardized rehabilitation therapy (SRT) and usual care conducted at Wake Forest Baptist Medical Center in Winston Salem, North Carolina. The SRT group received rehabilitation therapy 7 days a week, from enrollment through hospital discharge, including days spent in a regular floor bed. The usual care group received routine care as dictated by the patient’s attending physician from Monday through Friday. SRT ended at hospital discharge. Both groups underwent testing at ICU and hospital discharge, and at 2, 4, and 6 months after enrollment by research personnel blinded to the randomization assignment.
Study Patients
Inclusion criteria were admission to a medical ICU, being 18 years or older, mechanical ventilation via endotracheal tube or noninvasive ventilation by mask, and an arterial oxygen partial pressure to fractional inspired oxygen (Pao2/FIO2) ratio less than 300. Exclusion criteria were inability to walk without assistance prior to the acute ICU illness (use of cane or walkers were not exclusions), cognitive impairment prior to acute ICU illness described by surrogate, as nonverbal, acute stroke, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) greater than 50, neuromuscular disease impairing weaning from mechanical ventilation, acute hip fracture, unstable cervical spine or pathologic fracture, mechanically ventilate d more than 80 hours or current hospitalization (including transferring hospital) more than 7 days, orders for do not intubate on admission, considered to be moribund by the primary attending, or enrolled in another research study.
Randomization
Patients were randomly assigned, using a computergenerated variably sized approach (in block sizes of 2, 4, 6, or 8), to SRT or usual care.
Study Measurements and Procedures
The SRT protocol contained 3 exercise types: passive range of motion, physical therapy, and progressive resistance exercises, and was administered by a rehabilitation team for a total of 3 separate sessions every day of hospitalization for 7 days per week.6 The team comprised a physical therapist, an ICU nurse, and a nursing assistant. Passive range of motion included 5 repetitions for eachupper and lower extremity joint. Physical therapy included bed mobility, transfer training, and balance training. These exercises included transfer to the edge of the bed; safe transfers to and from bed, chair, or commode; seated balance activities; pregait standing activities (forward and lateral weight shifting, marching in place); and ambulation. Progressive resistance exercise included dorsiflexion, knee flexion and extension, hip flexion, elbow flexion and extension, and shoulder flexion. Resistance was added through the use of elastic resistance bands (TheraBand, Hygienic Corporation). Both the physical therapy and resistance training targeted lower extremity functional tasks and activities of daily living (for further details of the implementation of SRT modalities, see trial protocol in Supplement 1).
The patient’s level of consciousness determined suitability for receipt of physical therapy or progressive resistance exercise, and ability to complete the exercises.12 When patients were unconscious, the 3 sessions consisted of passive range of motion. Once the patient gained consciousness, physical therapy and progressive resistance exercise were introduced. Being free from mechanical ventilation was not a prerequisite for any of the exercise sessions. The usual care group received no rehabilitation per treatment protocol. Physical therapy could be ordered as part of routine care, but only Monday through Friday.
Study Outcomes
The primary end point was hospital LOS, defined to include hospital calendar days (or any portion of a calendar day) at the enrolling hospital and at any long-term acute care facility to which the patient was directly transferred. Research team members were not involved in the decision for hospital discharge (ie, the primary end point). Hospital floor medical teams separate from the ICU teams were responsible for hospital discharge. Study days were days of hospitalization following randomization.
Secondary outcomes included physical function and health-related quality of life. Physical function was measured using both performance-based and self-report instruments. Performance-based tests included the Short Performance Physical Battery (SPPB) and muscular strength as determined by handgrip dynamometer (Jamar, Lafayette Instrument) and from a handheld dynamometer (microFET2, Hoggan Health Industries). SPPB scores were derived from performance of 3 components: a 4-meter walk, chair sit-to-stand, and a balance test.13 Muscular strength of the shoulder flexors, elbow flexors and extensors, hip flexors, knee flexors and extensors, and ankle dorsiflexors was measured thrice bilaterally. The maximum values from each test were averaged to produce a single composite value of muscular strength. Self-report tests consisted of the short form Functional Performance Inventory (FPI),14 and the physical functioning scale of the medical outcomes study 36- Item Short Form Health Survey (SF-36 PFS).15 Health-related quality of life was measured using the SF-36 physical health survey (SF-36 PHS) and mental health survey (SF-36 MHS) component summary scores and Mini-Mental State Examination (MMSE) score. Measures of physical function were obtained at ICU discharge, hospital discharge and 2, 4, and 6 months after enrollment. Health-related quality-of-life measures were obtained at hospital discharge and 2, 4, and 6 months after enrollment. The SF-36 and the FPI were not administered at ICU discharge as they were not considered relevant to the patient at this time. The FPI was not administered at hospital discharge for the same reason. Post-hoc outcomes were the number of days that patients were alive and breathing without ventilator assistance (ventilator-free days), ICU-free and hospital-free days to day 28.16 Adverse events were quantified by deaths, device removals, reintubations, and patient falls during physical therapy (for classification of adverse events, see trial protocol in Supplement 1).
Statistical Analysis
The initial plan was to accrue 326 participants to provide 80% power for detecting a 30% decrease in the median hospital LOS at the 5% 2-sided level of significance assuming an exponential LOS distribution, a 20% in-hospital mortality, and that 5% of the remaining patients would withdraw prior to discharge, resulting in 247 discharges.
The projected 30% decrease in the primary outcome (hospital LOS) is slightly larger than the decrease observed in a previous quality improvement report,6 but, as described below, there was a greater expected effect with the current intervention due to a greater potential for exposure to the SRT after ICU discharge in this study. An important feature of the previous quality improvement report was that the intervention was delivered only in the ICU. Hence, the effect reported was for intervention delivered only in the ICU, not after ICU discharge. Despite the intervention being limited to the ICU, there was a 24% adjusted reduction in hospital LOS (hazard ratio [HR], 1.31). The current study design delivered the SRT from ICU admission through hospital discharge and due to the addition of progressive resistance exercise, there was a much greater clinical effect expected.
The in-hospital mortality and dropout were both less than expected and enrollment was stopped after 300 patients were accrued, 257 of whom were discharged.
Kaplan-Meier methods were used to estimate hospital LOS, and a log-rank test was used to assess the difference between groups. Patients who died or dropped out before discharge were censored in the analyses. A Cox proportional hazards regression model was used to estimate the hazard ratio. Because there were concerns that censoring (particularly from deaths) might be informative, 2 extremes were considered-assuming all the patients who died would have been discharged on the day of their death and that all the patients who died would have had the longest hospital stays. The same assumptions were made regarding the patients who simply withdrew even though there is less reason to believe that those would be informative. Analyses were repeated under the possible combinations of assumptions regarding the deaths and dropouts. For each of these scenarios, unadjusted analyses and analyses adjusted for those variables related to in-hospital death (sex, mean arterial pressure, partial pressure of carbon dioxide [PaCO2], PaO2, FIO2, and Acute Physiology and Chronic Health Evaluation [APACHE] score) were conducted. χ2 Tests were used to assess group differences in-hospital and after discharge deaths and Wilcoxon rank-sum tests were used to assess group differences in ventilator-free and ICU-free days. Median differences of medians and 95% confidence intervals were generated using bootstrap methods with 10 000 bootstrap samples. The significance threshold was P < .05 for each outcome and testing was2-sided. Due to the lack of adjustment for multiple testing, the secondary analyses should be considered exploratory. The statistical software was SAS (SAS Institute), version 9.4.
For secondary outcomes assessed longitudinally, a mixed- effects repeated measures analysis of variance model was used to assess differences in these measures between the SRT and usual care groups at discharge, 2, 4, and 6 months. An unstructured covariance matrix was used to account for the within-patient correlation over time.
χ2 and Wilcoxon rank-sum tests were used to assess differences in patient characteristics between those patients with and without missing data. Those characteristics predictive of missingness (due either to death or withdrawal) were included in the longitudinal mixed models. These covariates included age, race, BMI, ICU diagnosis, mean arterial pressure, PaCO2, PaO2/FIO2 ratio, APACHE score, and number of comorbid conditions. Multiple imputation was also used to assess the sensitivity of the results to the missing at random assumption. To be conservative, it was assumed that all dropouts would follow a pattern similar to that seen among the control patients (usual care group).17 One hundred data sets were generated using the SAS MI procedure (SAS Institute), a repeated measures mixed model was run on each data set, and results were combined using the SAS MIANALYZE procedure (SAS Institute).18 Covariates related to missing data were included in the imputations and in the adjusted mixed models. The imputation analyses included all patients.
Results
Study Patients
From October 2009 through November 2014, 4804 patients with acute respiratory failure were screened, 618 were eligible, and 300 were randomized (Figure 1) and followed up for up to 6 months after the enrollment date (last follow-up visit, November 2014). There were 84 patients in the SRT group (56%) vs 81 in the usual care group (54%) who completed the 6-month follow-up. There were no clinically important differences in baseline characteristics between the 2 groups (Table 1).
Figure 1. Flow of Patients Through the Study of Rehabiliation for Patients With Acute Respiratory Failure.
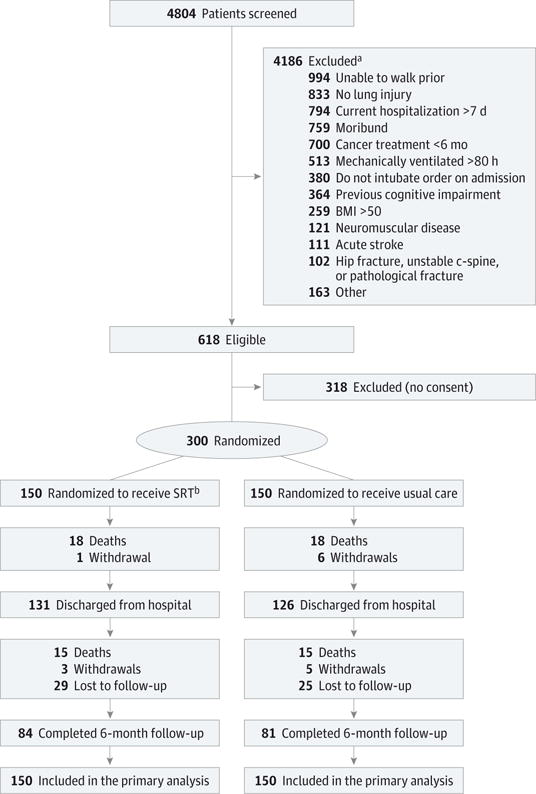
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); SRT, standardized rehabilitation therapy.
aPatients could have more than 1 exclusion. Either patient or surrogate may have provided or refused consent.
bOne patient after completing intervention was deemed technically ineligible; the patient was consented and randomized to SRT but was found to be unable to walk prior to study and included in the primary analysis.
Table 1.
Baseline Characteristics for Patients With Acute Respiratory Failure Receiving Standard Rehabilitation Therapy (SRT) vs Usual Care
| No. (%) | |||
|---|---|---|---|
| All (N = 300) | SRT (n = 150) | Usual Care (n = 150) | |
| Age, mean (SD), y | 56 (15) | 55 (17) | 58 (14) |
| Sex | |||
| Women | 166 (55.3) | 84 (56.0) | 82 (54.7) |
| Men | 134 (44.7) | 66 (44.0) | 68 (45.3) |
| Race/ethnicity | |||
| Hispanic or Latino | 4 (1.3) | 2 (1.3) | 2 (1.3) |
| Black or African American | 64 (21.3) | 33 (22.0) | 31 (20.7) |
| White | 232 (77.3) | 115 (76.7) | 117 (78.0) |
| APACHE III score, mean (SD)a | 76 (27) | 76 (26) | 75 (27) |
| Intensive care unit diagnosis | |||
| Coma | 5 (1.7) | 1 (0.7) | 4 (2.7) |
| Acute respiratory failure | |||
| Without chronic lung disease | 203 (67.7) | 98 (65.3) | 105 (70.0) |
| With chronic lung disease | 92 (30.7) | 51 (34.0) | 41 (27.3) |
| Home oxygen | 59 (19.7) | 32 (21.3) | 27 (18.0) |
| Dialysis prehospital | 24 (8.0) | 13 (8.7) | 11 (7.3) |
| Mean arterial pressure, mean (SD), mm Hg | 75.1 (22.4) | 76.2 (22.3) | 74.1 (22.5) |
| PaCO2, mean (SD), mm Hg | 44.1 (17.2) | 44.4 (18.2) | 43.8 (16.2) |
| PaO2/FIO2 ratio, mean (SD) | 178.6 (83.8) | 182.0 (81.2) | 175.1 (86.4) |
| Noninvasive ventilation | 21 (7.0) | 11 (7.3) | 10 (6.7) |
| Shock | 69 (23.0) | 36 (24.0) | 33 (22.0) |
Abbreviation: APACHE, Acute Physiology and Chronic Health Evaluation.
APACHE III19 score ranged from 0 to 299. A higher score indicates an increased risk of mortality.
Study Interventions
For the SRT group, the median days to first therapy exercise were 1 (interquartile range [IQR], 0–2) for passive range of motion, 3 (IQR, 1–6) for physical therapy, and 4 (IQR, 2–7) for progressive resistance exercise, whereas the days to first therapy exercise for the usual care group were 7 (IQR, 4–10). The mean percentage of study days SRT patients received therapy was 87.1% (SD, 18.4%) for passive range of motion, 54.6% (SD, 27.2%) for physical therapy, and 35.7% (SD, 23.0%) for progressive resistance exercise. The mean percentage of study days usual care patients received physical therapy was 11.7% (SD, 14.5%). For the SRT group, the median days of delivery of therapy per participant was 8.0 (IQR, 5.014.0) for passive range of motion, 5.0 (IQR, 3.0–8.0) for physical therapy, and 3.0 (IQR, 1.0–5.0) for progressive resistance exercise. The median days of delivery of physical therapy for the usual care group was 1.0 (IQR, 0.0–8.0).
Primary Outcomes and Hospital Data
The median hospital LOS was 10 days (IQR, 6 to 17) for the SRT group and 10 days (IQR, 7 to 16) for the usual care group (median difference, 0 [95% CI, −1.5 to 3], P = .41) (Table 2 and Figure 2). The estimated hazard ratio (SRT to usual care) was 1.11 (95% CI, 0.86 to 1.45). There were no differences between groups in the number of days taking a vasopressor, Confusion Assessment Method for the ICU-positive days, days receiving intravenous sedative drugs, days with restraint, or net ICU-related fluid balance (Table 2). Sensitivity analyses were performed for the primary outcome as described in the methods. The assumptions regarding the censored observations made little difference to the outcome, with a median 9 to 10 days in the SRT group and 10 days in the usual care group across the various scenarios. Hazard ratios ranged from 1.03 to 1.11 (with SRT patients more likely to get discharged) unadjusted for covariates and from 1.06 to 1.18 after adjusting for those covariates predictive of in-hospital death. The difference between groups was nonsignificant in each sensitivity analysis (P >.22).
Table 2.
Outcomes for Standard Rehabilitation Therapy (SRT) vs Usual Care Among Patients With Acute Respiratory Failure
| Median (IQR) | Median Difference (95% CI) | P Value | ||
|---|---|---|---|---|
| SRT (n = 150) | Usual Care (n = 150) | |||
| Hospital days (primary outcome) | 10.0 (6 to 17) | 10.0 (7 to 16) | 0 (−1.5 to 3) | .41a |
| Free daysb | ||||
| Hospital | 18 (7 to 22) | 18 (9 to 21) | 0 (−3 to 1.5) | .96c |
| Ventilator | 24 (19 to 26) | 24 (20 to 26) | 0 (−2 to 1) | .59c |
| Intensive care unit | ||||
| Days | 7.5 (4 to 14) | 8.0 (4 to 13) | 0 (−2.5 to 2) | .68a |
| Free daysb | 19 (8 to 23) | 19 (12 to 24) | 0 (−1.5 to 3) | .83c |
| Intravenous sedationd | ||||
| Days | 2 (1 to 5) | 2 (0 to 4) | 0 (0 to 1.5) | .11 |
| Days, % | 30.8 (0.8 to 54.1) | 27.1 (0 to 50.0) | 3.8 (−5.5 to 14.5) | .14 |
| Vasopressor | ||||
| Days | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 0) | >.99 |
| Days, % | 0 (0 to 6.7) | 0 (0 to 8.3) | 0 (0 to 0) | .90 |
| ICU fluid balance, cc | −68.5 (−806.6 to 664.4) | −148.8 (−766.8 to 520.2) | 53.9 (−270.3 to 281.2) | .89 |
| Restraint | ||||
| Days | 1 (0 to 4) | 1 (0 to 3) | 0 (−1 to 1) | .71 |
| Days, % | 25.0 (0 to 55.8) | 25.0 (0 to 50.0) | 0 (−16.7 to 12.3) | .82 |
| CAM-ICUe | ||||
| Negative | ||||
| Days | 2 (0 to 3) | 2 (0 to 4) | 0 (−1 to 1) | .88 |
| Days, % | 24.5 (0 to 44.8) | 20 (0 to 50.0) | 3.4 (−5.0 to 10.1) | .91 |
| Positive | ||||
| Days | 0 (0 to 1) | 0 (0 to 1) | 0 (0 to 0) | .77 |
| Days, % | 0 (0 to 12.5) | 0 (0 to 9.1) | 0 (0 to 0) | .71 |
| RASS score of 4 or 5f | ||||
| Days | 1 (0 to 4) | 1 (0 to 3) | 0 (−1 to 1) | .43 |
| Days, % | 14.6 (0 to 36.9) | 14.3 (0 to 33.3) | 1.8 (−6.7 to 10.5) | .71 |
Abbreviations: CAM-ICU, Confusion Assessment Method for the Intensive Care Unit20; IQR, interquartile range; RASS, Richmond Agitation Sedation Scale.21
Log-rank test.
All free days are based on 28 days.
Wilcoxon ranked sum.
Intravenous sedation days were defined as any part of a day a continuous delivery intravenous occurred of fentanyl, morphine, midazolam, lorazepam, propofol or dexmedetomidine. Percentage of restraint days, CAM-ICU–positive days, CAM-ICU–negative days, and RASS score 4 or 5 days represent the percentage of ventilator days.
CAM-ICU scores were positive or negative for delirium.
RASS score ranged from −3 (moderate sedation) to 4 (combative).
Figure 2. Length of Stay for Patients With Acute Respiratory Failure Receiving SRT vs Usual Care.
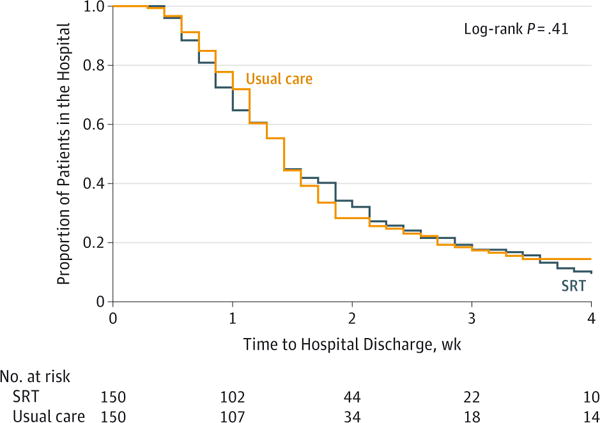
SRT indicates standardized rehabilitation therapy. Time zero indicates time of randomization.
Secondary Outcomes
Performance-based and self-reported measures of physical function are shown in Table 3. None of the scores were significantly different between groups at either ICU or hospital discharge. Strength values from handgrip and from handheld dynamometer did not differ between treatment groups at any of the measurement time points. The SPPB, SF-36 PFS, and FPI scores were not significantly different between groups at 2 or 4 months. However, each of these outcomes was significantly greater in the SRT group at the 6-month follow-up visit. At hospital discharge there was no difference in the proportion of SRT patients who could perform the 4-meter walk vs usual care (71% vs 61%, P = .15). By 6 months, those percentages had increased to 96% for the SRT group vs 88% for the usual care group (P = .037).
Table 3.
Secondary Outcomes: Physical Function Measures and Health-Related Quality of Life for Patients With Acute Respiratory Failure, by Group
| Measurement | Group | ICU Discharge | Hospital Discharge | 2-Month Follow-up | 4-Month Follow-up | 6-Month Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Least Square | No. of Patients | Least Square | No. of Patients | Least Square | No. of Patients | Least Square | No. of Patients | Least Square | No. of Patients | ||
| Means (95% Cl) | Providing Data | Means (95% Cl) | Providing Data | Means (95% Cl) | Providing Data | Means (95% Cl) | Providing Data | Means (95% Cl) | Providing Data | ||
| Physical Function | |||||||||||
| Short Physical Performance Battery scorea | SRT | 1.6 (1.0 to 2.2) | 86 | 4.7 (4.0 to 5.4) | 106 | 8.7 (8.1 to 9.4) | 88 | 8.9 (8.2 to 9.6) | 88 | 9.0 (8.3 to 9.7) | 84 |
| Usual care | 1.9 (1.3 to 2.4) | 98 | 4.7 (4.0 to 5.4) | 98 | 7.8 (7.1 to 8.5) | 76 | 8.0 (7.2 to 8.7) | 79 | 8.0 (7.2 to 8.7) | 81 | |
| Difference | −0.3 (−1.1 to 0.5) | −0.01 (−1.0 to 0.9) | 0.9 (−0.01 to 1.9) | 1.0 (−0.03 to 1.9) | 1.1 (0.04 to 2.1) | ||||||
| P Valueb | .46 | .97 | .05 | .06 | .04 | ||||||
| Dynamometer strength, lb | SRT | 20.3 (17.9 to 22.8) | 67 | 23.7 (21.6 to 25.8) | 100 | 28.5 (26.3 to 30.8) | 84 | 28.8 (26.5 to 31.0) | 85 | 31.1 (28.8 to 33.4) | 78 |
| Usual care | 22.8 (20.4 to 25.1) | 77 | 23.9 (21.7 to 26.2) | 86 | 28.0 (25.6 to 30.4) | 73 | 29.6 (27.2 to 31.9) | 75 | 30.8 (28.4 to 33.1) | 77 | |
| Difference | −2.4 (−5.8 to 1.0) | −0.2 (−3.3 to 2.9) | 0.5 (−2.8 to 3.8) | −0.8 (−4.1 to 2.5) | 0.4 (−2.9 to 3.7) | ||||||
| P Valueb | .16 | .90 | .76 | .63 | .82 | ||||||
| Handgrip strength, kg | SRT | 20.0 (17.8 to 22.3) | 78 | 22.6 (20.6 to 24.6) | 104 | 27.2 (25.1 to 29.2) | 87 | 29.0 (26.8 to 31.2) | 87 | 29.3 (26.9 to 31.6) | 83 |
| Usual care | 20.9 (18.7 to 23.1) | 88 | 24.3 (22.2 to 26.4) | 94 | 26.0 (23.8 to 28.1) | 74 | 27.2 (24.9 to 29.4) | 77 | 27.2 (24.8 to 29.6) | 81 | |
| Difference | −0.8 (−4.0 to 2.3) | −1.7 (−4.6 to 1.2) | 1.2 (−1.8 to 4.2) | 1.8 (−1.3 to 5.0) | 2.0 (−1.3 to 5.4) | ||||||
| P Valueb | .60 | .25 | .43 | .25 | .23 | ||||||
| SF-36 physical functioning scale scorec | SRT | 38.4 (33.2 to 43.7) | 108 | 47.4 (41.8 to 53.1) | 89 | 52.2 (46.7 to 57.7) | 86 | 55.9 (50.0 to 61.7) | 82 | ||
| Usual care | 38.3 (32.8 to 43.8) | 100 | 43.0 (37.0 to 49.0) | 77 | 47.2 (41.4 to 53.0) | 77 | 43.6 (37.5 to 49.7) | 79 | |||
| Difference | 0.1 (−7.6 to 7.8) | 4.4 (−3.9 to 12.7) | 5.0 (−3.0 to 13.0) | 12.2 (3.8 to 20.7) | |||||||
| P Valueb | .97 | .29 | .22 | .001 | |||||||
| Functional Performance Inventory scored | SRT | 2.0 (1.9 to 2.1) | 89 | 2.2 (2.1 to 2.3) | 86 | 2.2 (2.1 to 2.4) | 83 | ||||
| Usual care | 2.0 (1.9 to 2.1) | 75 | 2.1 (1.9 to 2.2) | 77 | 2.0 (1.9 to 2.2) | 79 | |||||
| Difference | −0.03 (−0.2 to 0.1) | 0.1 (−0.03 to 0.3) | 0.2 (0.04 to 0.4) | ||||||||
| P Valueb | .74 | .11 | .02 | ||||||||
| Health-Related Quality of Life | |||||||||||
| SF-36 physical health summary scoree | SRT | 30.2 (28.4 to 32.1) | 108 | 33.4 (31.4 to 35.5) | 89 | 36.0 (33.8 to 38.2) | 86 | 36.9 (34.6 to 39.3) | 82 | ||
| Usual care | 30.3 (28.4 to 32.2) | 100 | 32.2 (31.0 to 34.4) | 77 | 33.7 (31.4 to 36.0) | 77 | 33.5 (31.1 to 36.0) | 79 | |||
| Difference | −0.1 (−2.8 to 2.7) | 1.2 (−1.8 to 4.3) | 2.3 (−0.9 to 5.5) | 3.4 (−0.02 to 7.0) | |||||||
| P Valueb | .96 | .43 | .16 | .05 | |||||||
| SF-36 mental health summary scoree | SRT | 43.6 (41.5 to 45.7) | 108 | 46.3 (43.8 to 48.8) | 89 | 47.8 (45.5 to 50.2) | 86 | 48.8 (46.3 to 51.3) | 82 | ||
| Usual care | 43.3 (41.2 to 45.5) | 100 | 46.2 (43.6 to 48.8) | 77 | 47.7 (45.2 to 50.1) | 77 | 46.4 (43.8 to 49.0) | 79 | |||
| Difference | 0.3 (−2.7 to 3.3) | 0.1 (−3.5 to 3.7) | 0.2 (−3.2 to 3.6) | 2.4 (−1.2 to 6.0) | |||||||
| P Valueb | .86 | .96 | .91 | .19 | |||||||
| Mini-Mental State Examination scorec | SRT | 25.4 (24.7 to 26.1) | 114 | 26.7 (25.9 to 27.5) | 88 | 27.6 (27.0 to 28.2) | 86 | 27.6 (27.0 to 28.2) | 84 | ||
| Usual care | 25.1 (24.3 to 25.8) | 104 | 26.8 (26.0 to 27.7) | 75 | 27.2 (26.5 to 27.8) | 78 | 27.0 (26.4 to 27.6) | 81 | |||
| Difference | 0.3 (−0.7 to 1.3) | −0.1 (−1.3 to 1.1) | 0.4 (−0.5 to 1.3) | 0.6 (−0.2 to 1.4) | |||||||
| P Valueb | .55 | .86 | .37 | .17 | |||||||
Abbreviations: ICU, intensive care unit; SRT, standardized rehabilitation therapy. Metric conversion factor: To convert pounds to kilograms, divide by 0.45.
Treatment effect at the given visit.
SF-36 physical functioning scale and Mini-Mental State Examination were performed on hospital discharge and following appointments.
Functional Performance Inventory was performed starting at first outpatient follow-up. Self-report mechanisms use higher scores to indicate greater levels of functioning.
SF-36 mental and physical health summary minimal clinically important differences are 3 to 5 units.15
Health-related quality-of-life measures are shown in Table 3. SF-36 PHS, SF-36 MHS, and MMSE scores were not significantly different between groups at any time points.
The estimated intervention effects when analyses were repeated using multiple imputation assuming conservatively that all dropouts followed the pattern seen in the control group were decreased by approximately 40%. For example, the intervention effects at 6 months decreased from 1.06 to 0.60 for SPPB, 12.2 to 7.3 for SF-36 PFS, 0.21 to 0.12 for FPI, and 3.39 to 2.12 for SF-36 PHS. Only the SF-36 PFS effect remained significant (P = .04); the other P values were .11 for FPI, .16 for SPPB, and .19 for SF-36 PHS.
Outpatient physical therapy was not an intervention per treatment protocol; there was no difference in the number of patients (self-reported at each follow-up visit) who received outpatient or home physical therapy between hospital discharge and the 6-month follow-up visit (41 SRT patients vs 39 usual care patients, P = .69).
There were no differences in discharge destination between the SRT group and the usual care group (ie, home, longterm acute care, skilled nursing, or rehabilitation hospital) (eTable 1 in Supplement 2). Similarly, there were no differences between groups in post-index hospitalization readmissions or discharge emergency department visits without a hospital readmission. The percentage of each study group discharged from the hospital who were alive and hospital readmission-free at 6 months was 48.7% for the SRT group and 44.7% for the usual care group (P = .63). Post-hoc analyses indicated that the median number of ventilator-free days was 24 for both groups (median difference, 0 [95% CI, −2 to 1], P = .59), and the median number of ICU-free days was 19 for both groups (median difference, 0 [95% CI, −1.5 to 3], P = .83).
Missing Data
Death during the hospital stay was less than expected (12% observed vs 20% expected) as was death during the follow-up period (12% observed vs 15% expected). Dropout during the hospital stay was also less than expected (2% observed vs 5% expected). However, dropout during follow-up was greater than expected (24% observed from discharge to 6-month follow-up vs 10% expected). Neither dropout nor mortality differed between the study groups. Characteristics of those with and without missing data and those who did and did not drop out are shown in eTable 2 and eTable 3 in Supplement 2. Characteristics were fairly well balanced for those patients who remained in the study. Of the patients included in the follow-up analyses, APACHE III scores were lower (better) in the usual care group.
Adverse Events
There were no differences in adverse event reporting between study groups (eTable 4 in Supplement 2). The majority of adverse events captured were not specifically related to SRT delivery. Specific to SRT, there were no untoward events such as endotracheal tube removal, vascular access device removal, patient near-fall or fall, or cardiac arrest. However, there was an episode of asymptomatic bradycardia during a progressive resistance exercise session lasting less than 1 minute, with the patient completing the session afterwards.
Discussion
In this randomized, assessor-blinded study of SRT vs usual care for patients with acute respiratory failure, there was no difference in hospital LOS between groups. Similarly, SRT did not affect ventilator-free days or ICU-free days. Functional-related and health-related quality-of-life outcomes were similar for the 2 study groups at hospital discharge.
The amount of exercise delivered and performed while inhospital was substantially different between SRT and usual care groups. The usual care group received physical therapy for only 12% of the study days and never received resistance training. In contrast, in the SRT group, passive range of motion occurred in 87% of study days, physical therapy in 55%, and progressive resistance exercise in 36%, with no significant hospital- based outcome differences observed. The volume of exercise delivered to SRT patients was delivered with 7 days per week availability. This structure may differ from the current practice in many US ICUs.23 Others have also reported on the real- life delivery of ICU-related exercise being less than expected by ICU practitioners.24–26 In view of these data, it is unclear what ICU exercise dose is required to affect outcomes by hospital discharge for patients with acute respiratory failure.
Following discharge, handgrip strength or strength measured by handheld dynamometer and health-related quality of life remained similar for the 2 groups. But from these exploratory analyses, the physical function measures (SPPB, SF-36 PFS, and FPI) were different at 6 months. The separation of the 2 groups’ self-reported and objectively measured functional data over 6 months of follow-up contrasts with the lack of difference for hospital-centered outcomes.
These findings from the exploratory analyses may highlight the emerging role of placing long-term outcomes within critical care clinical trial design not only as a secondary outcome, but possibly as the primary outcome.27–30 In view of the SPPB, SF-36 PFS, and FPI data at 6 months, the SRT group demonstrated a potential signal of improvement compared with the usual care group that was not evident at hospital discharge. It is not obvious what aspect of the SRT may have accounted for the differences at 6 months; however, both the physical therapy and the progressive resistance training emphasized lower extremity function. The exposure in the hospital may have inclined the SRT group to have greater movement while in the outpatient setting.
The findings from this study contrast with the outcomes of the study by Schweickert and colleagues,7 which found greater improvements in activities of daily living at hospital discharge in an early ICU rehabilitation group than the control group, but no difference in hospital LOS either. The study by Walsh and colleagues31 reported post-ICU hospital-based rehabilitation, including increased physical and nutritional therapy, did not improve physical recovery or quality-of-life scores at 3 months after enrollment. Outpatient-focused patient-level functional outcome differences were not detected in the study by Denehy and colleagues,9 which linked an inpatient rehabilitation exercise repertoire with outpatient exercise instructions for a cohort of patients who were critically ill. Moss and colleagues32 found that an intensive physical therapy program compared with a standard physical therapy program in which the intensive program continued for up to 28 days from randomization, including the outpatient setting, did not improve long-term physical functional performance at 6 months.
Study limitations include a higher than expected dropout (lost to follow-up and withdrawals, 24%) following hospital discharge. Also, there was no intervention following discharge; future study of ICU-initiated rehabilitation programs may need to include a bridge program of some outpatient exercise content to further optimize outcomes.31,33
Another potential limitation was that there was no explicit sedation protocol; the lack of a sedation protocol may have allowed patients in both groups to spend unnecessary days either unconscious or with a positive Confusion Assessment Method score.34,35 Given that the intervention group had approximately 30% of ventilator days associated with intravenous continuous drip medications, and patients were unarousable on 15% of ventilator days, sedation may have been a barrier to receipt of early exercise. These data indicate the challenge of delivering a treatment modality requiring a conscious, engaged patient. Other modalities have been proposed such as functional electrical stimulation for the unconscious patient.36 Additionally, multiple tests may have led to a spurious significant finding for the functional tests.
Conclusions
Among patients hospitalized with acute respiratory failure, SRT compared with usual care did not decrease hospital LOS.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by the National Institutes of Health, National Institute of Nursing Research, and National Heart, Lung, and Blood Institute.
Role of the Funder/Sponsor: The sponsors did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jama.com
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00976833
Author Contributions: Dr Morris had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Morris, Berry, Hauser, Dhar, Chmelo, Case, Hite, Nicklas.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Morris, Berry, Thompson, Hauser, Case, Parry, Mote, Chatterjee. Critical revision of the manuscript for important intellectual content: Berry, Files, Flores, Dhar, Chmelo, Lovato, Case, Bakhru, Sarwal, Parry, Campbell, Winkelman, Hite, Nicklas, Chatterjee, Young.
Statistical analysis: Lovato, Case, Chatterjee.
Obtained funding: Morris, Berry, Hite.
Administrative, technical, or material support: Morris, Berry, Files, Thompson, Hauser, Flores, Dhar, Bakhru, Sarwal, Parry, Mote, Nicklas, Chatterjee.
Study supervision: Morris, Berry, Files, Thompson, Dhar, Chatterjee.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bakhru reports consulting for Hill Rom Company. Dr Chatterjee reports part-time employment with the US Department of Veterans Affairs and the US Navy. No other disclosures were reported.
Disclaimer: The opinions contained in this article are those of the authors and do not represent the opinions of the US Department of Veterans Affairs nor the US Department of Defense.
Previous Presentation: Selected data in this article were presented at the Australian Physiotherapy Association meeting; October 3–6, 2015; Brisbane, Australia.
Contributor Information
Peter E. Morris, Division of Pulmonary, Critical Care, and Sleep Medicine, University of Kentucky, Lexington.
Michael J. Berry, Department of Health and Exercise Science, Wake Forest University, Winston Salem, North Carolina.
D. Clark Files, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
J. Clifton Thompson, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
Jordan Hauser, Department of Health and Exercise Science, Wake Forest University, Winston Salem, North Carolina.
Lori Flores, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
Sanjay Dhar, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
Elizabeth Chmelo, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
James Lovato, Department of Biostatistical Sciences, Wake Forest University, Winston Salem, North Carolina.
L. Douglas Case, Department of Biostatistical Sciences, Wake Forest University, Winston Salem, North Carolina.
Rita N. Bakhru, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
Aarti Sarwal, Department of Neurology, Wake Forest University, Winston Salem, North Carolina.
Selina M. Parry, Physiotherapy Department, University of Melbourne, Melbourne, Australia.
Pamela Campbell, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
Arthur Mote, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
Chris Winkelman, Francis Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio.
Robert D. Hite, Department of Critical Care, Respiratory Institute, Cleveland Clinic, Cleveland, Ohio.
Barbara Nicklas, Division of Geriatrics, Wake Forest University, Winston Salem, North Carolina.
Arjun Chatterjee, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
Michael P. Young, Section on Pulmonary, Critical Care, Allergy, and Immunologic Disease, Wake Forest University, Winston Salem, North Carolina.
References
- 1.Hermans G, Van Mechelen H, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 2.Herridge MS, Tansey CM, Matté A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 3.Fan E, Dowdy DW, Colantuoni E, et al. Physical complications in acute lung injury survivors: a 2-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, et al. BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 6.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 9.Denehy L, Skinner EH, Edbrooke L, et al. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17(4):R156. doi: 10.1186/cc12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness [published correction appears in JAMA. 2014;311(6):625] JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 11.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 12.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–286. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 14.Leidy NK, Hamilton A, Becker K. Assessing patient report of function: content validity of the Functional Performance Inventory-Short Form (FPI-SF) in patients with chronic obstructive pulmonary disease (COPD) Int J Chron Obstruct Pulmon Dis. 2012;7:543–554. doi: 10.2147/COPD.S32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 16.The Acute Respiratory Distress Syndrome Network. Ventilation with lowertidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 17.Ratitch B, O’Kelly M. Implementation of pattern-mixture models using standard SAS/STAT procedures. http://pharmasug.org/proceedings/2011/SP/PharmaSUG-2011-SP04.pdf. Accessed May26, 2016.
- 18.SAS Institute. SAS/STAT 9.3 SAS User’s Guide. Cary, NC: SAS Institute; 2011. [Google Scholar]
- 19.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation ofthe Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J RespirCrit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 22.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 23.Hodgin KE, Nordon-Craft A, McFann KK, Mealer ML, Moss M. Physical therapy utilization in intensive care units: results from a national survey. Crit Care Med. 2009;37(2):561–566. doi: 10.1097/CCM.0b013e3181957449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nydahl P, Ruhl AP, Bartoszek G, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42(5):1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson C, Bellomo R, Berney S, et al. TEAM Study Investigators Early mobilization and recovery in mechanically ventilated patients in the ICU: a binational, multicentre, prospective cohort study. Crit Care. 2015;19:81. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berney SC, Harrold M, Webb SA, et al. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15(4):260–265. [PubMed] [Google Scholar]
- 27.Hudson LD, Lee CM. Neuromuscular sequelae of critical illness. N Engl J Med. 2003;348(8):745–747. doi: 10.1056/NEJMe020180. [DOI] [PubMed] [Google Scholar]
- 28.Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of postintensive care syndrome therapy and care: engagement of noncritical care providers and survivors in a second stakeholders meeting. Crit Care Med. 2014;42(12):2518–2526. doi: 10.1097/CCM.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 29.Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186(4):302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper DJ, Rosenfeld JV, Murray L, et al. DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 31.Walsh TS, Salisbury LG, Merriweather JL, et al. RECOVER Investigators Increased hospital-based physical rehabilitation and information provision afterintensive care unit discharge: the RECOVER randomized clinical trial. JAMA Intern Med. 2015;175(6):901–910. doi: 10.1001/jamainternmed.2015.0822. [DOI] [PubMed] [Google Scholar]
- 32.Moss M, Nordon-Craft A, Malone D, et al. A Randomized Trial of an Intensive Physical Therapy Program for Patients with Acute Respiratory Failure. Am J Respir Crit Care Med. 2016;193(10):1101–1110. doi: 10.1164/rccm.201505-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brummel NE, Girard TD, Ely EW, et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med. 2014;40(3):370–379. doi: 10.1007/s00134-013-3136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–147. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 35.Barr J, Fraser GL, Puntillo K, et al. American College of Critical Care Medicine Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 36.Parry SM, Berney S, Koopman R, et al. Early rehabilitation in critical care (eRiCC): functional electrical stimulation with cycling protocol for a randomised controlled trial. BMJ Open. 2012;2(5):e001891. doi: 10.1136/bmjopen-2012-001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


