Abstract
Rationale:
Schiff base modification of peptides has been shown to facilitate their primary structural characterization via tandem mass spectrometry. However, we have discovered a novel rearrangement reaction via ion trap collisional activation involving the imine of the Schiff base and one of several functional groups, particularly the side-chains of the basic residues lysine, arginine, and histidine, in the peptide.
Methods:
Gas-phase ion/ion reactions involving an aldehyde-containing reagent were used to generate Schiff-base modified model peptides in a hybrid triple quadrupole/linear ion trap tandem mass spectrometer. Subsequent ion trap collisional activation was used to study the rearrangement reaction.
Results:
Schiff-base modified peptide ions were found to undergo a rearrangement reaction that was observed to be either a major or minor contributor to the product ion spectrum, depending upon a variety of factors that include, for example, ion polarity, identity of the nucleophile in the peptide (e.g., side-chains of lysine, histidine, and arginine), and the position of the nucleophile relative to the imine.
Conclusions:
Relatively low energy rearrangement reaction can occur in Schiff base modified peptide ions that involve the imine of the Schiff base and a nucleophile present in the polypeptide. While this rearrangement process does not appear to compromise the structural information that can be generated via collisional activation of Schiff-base-modified peptide ions, it can siphon away signal from the structurally diagnostic processes in some instances.
Keywords: Ion/ion reaction, Schiff-base-modified peptide, Ion trap tandem mass spectrometry
Introduction
Tandem mass spectrometry is widely used to probe ion structures by generating informative product ions via fragmentation.1-3 Fragmentation patterns can be highly sensitive to ion type (e.g., protonated molecule versus radical cation) such that complementary information can be obtained by interrogating different analyte ion types.4, 5 As an approach to the transformation of an analyte from one ion type to another within the mass spectrometer, gas-phase ion/ion reactions have been developed.6, 7 Small particle transfer ion/ion reactions, e.g., proton and electron transfer reactions, change the analyte charge state, which can open up different fragmentation pathways.8, 9 Gas-phase covalent modification ion/ion reactions are functional group specific reactions that selectively target particular functional groups and thereby alter fragmentation pathways.6, 7 Moreover, unique chemistries have been observed via gas-phase ion/ion reactions compared to the solution phase.7, 10 Examples of gas-phase covalent modification reactions include the reactions between N-hydroxysuccinimide (NHS) esters and nucleophiles like primary amines11, guanidine groups12 and carboxylates13; gas-phase oxidation of peptides with periodate14 or persulfate derivatives15; carboxylic acid group labelling with carbodiimide reagents16; and the Schiff base formation between formyl-benzenesulfonic acids and primary amines17.
The gas-phase modification of bio-ions via Schiff base formation was first described in 2009.17 Formation of Schiff base is achieved by the reaction between a primary amine (i.e., the N-terminus or ε-amine group of a lysine residue) in a peptide analyte ion and the formyl group in the 4-formyl-1,3-benzenedisulfonic acid (FBDSA) reagent. Gas-phase collisional activation of the Schiff-base-modified analyte ions has been noted to result in a higher sequence coverage compared to the unmodified peptides in several scenarios.17-20 Cotham et al. took advantage of the benzene chromophore in FBDSA and modified the analyte peptide in solution with this reagent to enhance the ultra-violet photodissociation (UVPD) efficiency.21 They also found that the Schiff base modification of phosphopeptides with FBDSA helps retain the phosphate group during the fragmentation process. Fragmentation of the unmodified phosphopeptide often results in loss of the phosphate functionality and thereby precludes identification of the phosphate position. In the case of Schiff-base-modified peptides, on the other hand, the sulfonate group on the reagent disrupts the phosphate neutral loss process and stabilizes the phosphate group when collision-induced dissociation (CID) is applied.22
Studies of Schiff-base-modified polypeptide ions via tandem mass spectrometry using CID have resulted predominantly in b/y-type fragment ions17-22, in analogy with unmodified ions23. However, in this study, we focus on fragmentation products generated via CID that are unique to the Schiff-base-modified peptide. This work has revealed the existence of a fragmentation pathway that is specific to the Schiff base modification, which should be recognized when using this modification for structural characterization purposes. Possible mechanisms are proposed.
Experimental
Materials.
HPLC-grade water and methanol were purchased from Fisher Scientific (Waltham, MA, USA). 4-formyl-1,3-benzenedisulfonic acid (FBDSA) and 2-formyl-benzene(mono)sulfonic acid (FBMSA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Model peptides KGAGGKGAGGKL, RARARAA, AHAAAHA and HAHAHAA were synthesized by NeoBioLab (Cambridge, MA, USA); KAKAKAA was synthesized by Pepnome Ltd (Shenzhen, China). All peptide solutions for electrospray were prepared in 50/50 (v/v) methanol/water (~ 0.2 mM). The FBDSA and FBMSA reagent solutions for electrospray were prepared in water (~ 3 mM).
Mass Spectrometry.
All experiments were performed on a QTRAP® 4000 hybrid triple quadrupole/linear ion trap mass spectrometer (SCIEX, Concord, ON, Canada) previously modified for ion/ion reactions24, unless specifically noted. Alternately pulsed nano-electrospray (nESI)25 allowed for sequential injections of reagent and analyte ions, which were sequentially isolated in the Q1-mass filter prior to their injection into the q2 reaction cell. After a defined mutual storage reaction time, the product ions were then transferred to Q3. The ions then underwent further probing via MSn using ion trap collisional activation and mass analysis via mass-selective axial ejection (MSAE)26. The exact mass measurement experiments were performed on a TripleTOF® 5600 mass spectrometer (SCIEX, Concord, ON, Canada) previously modified for ion/ion reactions.24
Results and Discussion
Novel Fragmentation Pathway from Schiff-Base-Modified Lysine-Containing Peptides.
Gas-phase Schiff base formation has been demonstrated as an approach to provide structural information complementary to that derived from unmodified species in the tandem mass spectrometry of polypeptide ions.17-22 It involves the reactivity between a peptide primary amine group, either on the N-terminus or the lysine side chain, and the formyl group on the reagent ion. The reagents used in this study are 4-formyl-1,3-benzenedisulfonic acid (FBDSA) and 2-formyl-benzene(mono)sulfonic acid (FBMSA) (structures shown in Scheme 1(c)). Each reagent contains a benzaldehyde reactive group and one or two sulfonate group(s).
Scheme 1.
Reactions for Schiff base modification and fragmentation in (a) positive mode and (b) negative mode with FBDSA as an example. (c) Structures of Schiff base modification reagents (FBDSA, FBMSA) and proposed neutral loss structures (AMBDSA and AMBMSA). Solid diamonds (♦) denote ion mass shift with Schiff base modification (+248 Da for FBDSA). Hollow diamonds (◊) denote the mass shift of the special fragmentation products (−19 Da).
The Schiff base modification and ion trap CID of the Schiff-base-modified peptide ion are illustrated by the spectra provided in Figure 1. Using a model peptide of sequence KAKAKAA, the doubly protonated peptide, [M + 2H]2+, was formed via positive nESI and reacted with singly deprotonated FBDSA, [FBDSA − H]−, resulting in an electrostatically bound complex, [M + FBDSA + H]+, and a proton transfer product, [M + H]+, as seen in Figure 1(a). Upon CID of the complex (see Figure 1(b)), a signature water loss from the complex reflects the formation of a Schiff-base-modified peptide cation, [M + H + ♦]+, where the black diamond (♦) depicts the mass shift caused by the modification (+248 Da). Further CID of the Schiff-base-modified species (see Figure 1(c)) gives rise to b/y fragment ions, both modified (e.g., b6♦ and y6♦) and unmodified (e.g., b3 and y4), which implies different Schiff base modification sites, either the N-terminus or any one of the lysine side chains. Besides the b/y fragment ions, a highly abundant peak, labelled as [M + H + ◊]+, was observed 267 Da lower in mass than the peak from the Schiff-base-modified peptide [M + H + ♦]+, which is inconsistent with commonly observed neutral losses from peptide ions. The hollow diamond (◊) in Figure 1(c) refers to the mass shift of the novel product, −267 Da from the precursor [M + H + ♦]+, or an apparent −19 Da from the mass of the protonated peptide, [M + H]+. Similarly, there are peaks that are observed −267 Da lower in mass than the modified b ions (b6♦, b5♦, b4♦, and b3♦), which are also labeled with the hollow diamond (as in b6◊, b5◊, b4◊, and b3◊).
Figure 1.
Spectra illustrating the gas-phase Schiff base modification of KAKAKAA with FBDSA and its special fragmentation pathway. Positive mode (a) ion/ion reaction between doubly protonated peptide cation [M+2H]2+ and singly deprotonated FBDSA [FBDSA−H]−, (b) CID of the ion/ion complex [M+FBDSA+H]+ forming a Schiff-base-modified peptide [M+H+♦]+, (c) Ion-trap CID of [M+H+♦]+. Negative mode (d) ion/ion reaction between singly protonated peptide cation [M+H]+ and doubly deprotonated FBDSA [FBDSA−2H]2−, (e) CID of the ion/ion complex [M+FBDSA−H]− forming a Schiff-base-modified peptide [M−H+♦]−, (f) ion-trap CID of [M−H+♦]−. Degree symbols (°) denote water losses (−18 Da). Asterisks (*) denote ammonia losses (−17 Da). Solid diamonds (♦) denote ion mass shift with Schiff base modification (+248 Da). Hollow diamonds (◊) denote the special fragmentation products (−19 Da) and all products in the special fragmentation pathway are labeled in red.
Evidence for an analogous reaction is noted in the negative mode (Figure 1(d)-(f)). The singly protonated peptide was charge inverted by the doubly deprotonated FBDSA to form a negatively charged complex, [M + FBDSA − H]− (Figure 1(d)). CID of the electrostatically bound complex generates a Schiff-base-modified peptide anion [M − H + ♦]− (Figure 1(e)). As above, the solid diamond (♦) indicates the mass shift of the Schiff base modification (+248 Da). CID of the Schiff-base-modified peptide anion [M − H + ♦]− mainly leads to backbone fragment ions, including modified b/y and c fragment ions (e.g., b5♦, y5♦ and c2♦) and unmodified backbone fragment ions (e.g., a5) (Figure 1(f)). In previous negative mode Schiff base modification studies [18-20], another common CID fragmentation product from [M − H + ♦]− is deprotonated FBDSA [FBDSA − H]− (m/z 265 Da). The precursor [M − H + ♦]− population can consist of both Schiff-base-modified peptides and the unreacted electrostatic complex of FBDSA and peptide with one water loss from somewhere else in the peptide. The latter type of [M − H + ♦]− ion can give rise to the [FBDSA − H]− CID product. In Figure 1(f), however, no [FBDSA − H]− peak (m/z 265) was observed. Rather, there are two dominant singly negatively charged peaks of m/z 266 (i.e., the peak labeled with [NL − H]− in red) and m/z 249 (the peak labeled with * in red) in the lower mass region. These two fragments are inconsistent with any common peptide fragment pathways. Considering that the neutral loss (NL), i.e., the mass difference between the unidentified peak and the Schiff-base-modified peptide, is 267 Da, the m/z 266 product ion was assigned as [NL − H]−, and the m/z 249 product was assigned as [NL − H − NH3]− due to the 17 Da difference. Activation of the m/z 266 Da ion ([NL − H]−) formed in the CID spectrum of the Schiff-base-modified KAKAKAA anion with FBDSA leads exclusively to ammonia loss, as shown in the Supplemental Figure S2(c), which implies that the neutral loss structure contains an amine group.
Analogous results were obtained using singly deprotonated FBMSA in reaction with doubly protonated KAKAKAA. The Schiff-base-modified peptide product, [M + H + ♦]+ (♦ = +168 Da), was directly formed upon ion/ion reaction along with the proton transfer product, [M + H]+ (Supplemental Figure S1(a)). Upon activation of the [M + H + ♦]+ product, the resulting product ion spectrum (Supplemental Figure S1(b)) contained a dominant [M + H + ◊]+ product that is −187 Da from the [M + H + ♦]+ ion, and b/y ions with the same mass shift (b3◊, b4◊, b5◊, and b6◊), all of which are labeled with the hollow diamond (◊ = −19 Da). Other backbone fragmentation products were observed in the spectrum in relatively low abundances. The 187 Da mass loss in the case of the FBMSA reagent is 80 Da less than that observed from the reactions with the FBDSA reagent, which is consistent with the difference in mass between FBMSA and FBDSA.
The [M + H + ◊]+ ions generated from Schiff-base-modified KAKAKAA cations with FBDSA and FBMSA were subjected to CID (Supplemental Figure S2(a) and (b)). The essentially identical dissociation patterns for the two spectra indicates that the [M + H + ◊]+ ions generated from modified peptides with different reagents are of the same structure or mixture of structures. The fragment peaks with the same mass shift (◊ = −19 Da) are mostly b ions (b2◊, b3◊, b4◊, b5◊, b6◊) and some y ions (y5◊, y6◊). Their fragmentation sites and abundance ratios are similar to the modified b/y fragment ions (b2♦, b3♦, b4♦, b5♦, b6♦, y5♦, y6♦) in Figure 1(c), which indicates that the mass shift is related to the Schiff base modification. Unmodified b/y fragment ions were also observed.
Experiments analogous to those performed for the KAKAKAA peptide were also performed with the peptide KGAGGKGAGGKL and reagent FBDSA (see Supplemental Figures S1(c), S1(d), S2(d), and S2(e)) leading to similar results. For example, the same loss of 267 Da from activation of Schiff-base-modified peptide cation, [M + H + ♦]+ (♦ = +248 Da), leading to a dominant [M + H + ◊]+ product (◊ = −19) was noted (Supplemental Figure S1(c)). Activation of Schiff-base-modified peptide anion [M − H + ♦]− (♦ = +248 Da) gave rise primarily to modified backbone fragment ions (e.g., b11♦, y11♦), as shown in Supplemental Figure S1(d). However, the m/z 266 ion (labeled as [NL − H]− in red) was also observed, but in much lower relative abundance compared to those in the CID spectrum of Schiff-base-modified KAKAKAA anion (Figure 1(f)). The results for the KGAGGKGAGGKL peptide ions indicate that the unusual products noted here arise from a process with at least some generality.
Proposed Reaction and Mechanism.
The general experimental steps and observations in positive mode and negative mode with FBDSA as the reagent are summarized in Scheme 1(a) and Scheme 1(b), respectively. The formation of the unusual product ions and the neutral losses implies a previously unidentified reaction and fragmentation pathway when at least some Schiff-base-modified peptides are subjected to ion trap collisional activation. Considering that the neutral losses for Schiff-base-modified KAKAKAA with FBDSA and FBMSA are 267 Da and 187 Da, respectively, which has the same 80 Da mass difference as the two reagents, it is clear that the neutral loss has the partial structure of the reagents. As mentioned above, the existence of singly negatively charged ions at m/z 266 ([NL − H]−) and m/z 249 ([NL − H − NH3]−) in the product ion spectra of the modified KAKAKAA and KGAGGKGAGGKL anions generated with FBDSA (Figure 1(f) and Supplemental Figure S1(d)) indicates the presence of a relatively acidic site on the 267 Da species. The loss of 17 Da in the CID of [NL−H]− (Supplemental Figure S2(c)) suggests the presence of an amino group. Considering these observations collectively, the neutral loss is proposed to be 4-(aminomethyl)benzene-1,3-disulfonic acid (AMBDSA, 267 Da) for FBDSA or 2-(aminomethyl)benzene(mono)sulfonic acid (AMBMSA, 187 Da) for FBMSA (structures in Scheme 1(c)). An exact mass measurement of the m/z 266 ([NL − H]−) and m/z 249 ([NL − H − NH3]−) ions using a quadrupole/time-of-flight (QTOF) instrument verified the elemental composition of the proposed product. We note that while the m/z 249 ion can be generated from ammonia loss from deprotonated AMBDSA, a mechanism for the direct formation of the product at m/z 249 (Supplemental Scheme S1) might also contribute to at least some of the m/z 249 signal.
Based on the structures of the neutral loss species, proposed mechanisms for the rearrangement of lysine-containing Schiff-base-modified peptides are shown in Scheme 2. The amino groups on the N-terminus and on the ε-amino group of the lysine side chain are used here as an example. Either amino group could be modified with the imine bond. The lone electron pair on the nitrogen of the amine attacks the electrophilic carbon of imine to form an eight-membered ring. Further proton transfer and ring rearrangements generate a more stable six-membered ring. Finally, the leaving group is eliminated with a proton to form the neutral loss amine and thus the rearranged peptide product with a ring imine.
Scheme 2.

Proposed mechanism for the rearrangement of the lysine-containing Schiff-base-modified peptides between an amino group and (a) a neutral imine group, or (b) a protonated imine group. N-terminal lysine residue is used here as an example.
Based on the proposed mechanism for the rearrangement reaction, the imine (on the N-terminus or the lysine side chains) could react with amino groups (unmodified N-terminus or lysine side chain amino groups), which is consistent with the CID spectra of [M + H + ◊]+ and [M − H + ◊]− ions derived from the peptides KAKAKAA and KGAGGKGAGGKL (Supplemental Figure S2). Note that analogous experiments with the model peptide AKAAAKA (Supplemental Figure S3) showed the rearrangement reaction taking place at significantly lower levels than with the N-terminal lysine-containing model peptides, which suggests that a lysine at the N-terminal position is particularly reactive.
Reactivity of Histidine- or Arginine-Containing Schiff-Base-Modified Peptides.
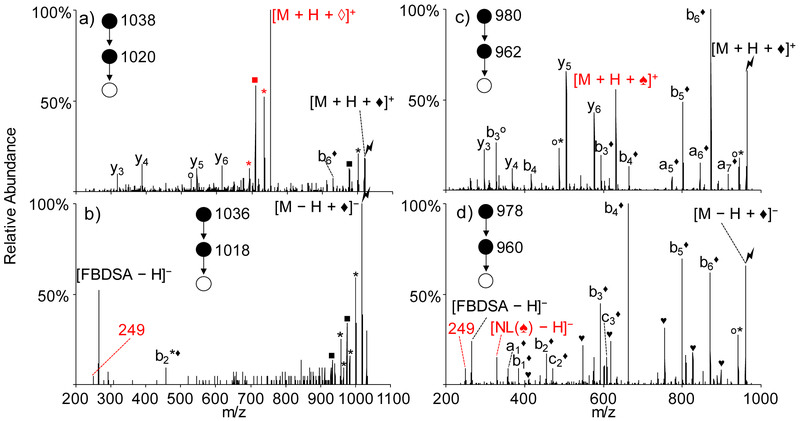
Previous studies have indicated that initial Schiff base formation takes place primarily at neutral primary amines to generate the imine. However, the other common basic residues (i.e., nucleophiles), histidine and arginine, might also be able to engage in a rearrangement process involving the imine via mechanisms analogous to those provided in Scheme 2. To compare with KAKAKAA results, model peptides RARARAA and HAHAHAA were chosen to study the effect of amino acid residue type. The experimental data are summarized in Figure 2.
Figure 2.
Ion trap CID of the gas-phase Schiff-base-modified peptides formed using (a) singly deprotonated FBDSA and doubly protonated RARARAA, (b) doubly deprotonated FBDSA and singly protonated RARARAA, (c) singly deprotonated FBDSA and doubly protonated HAHAHAA, and (d) doubly deprotonated FBDSA and singly protonated HAHAHAA. Degree symbols (°) denote water losses (−18 Da). Asterisks (*) denote ammonia losses (−17 Da). Black solid diamonds (♦) denote ions with Schiff base modification (+248 Da). Solid squares (■) denote the arginine side chain methanediimine (H2N=C=NH2) losses to form ornithine (−42Da). Solid hearts (♥) denote carbon dioxide losses (−44 Da). Hollow diamonds (◊) denote peptide rearrangement products (−19 Da) with the formation of the AMBDSA neutral loss. Solid spades (♠) denote histidine-specific rearrangement products (−82 Da) with the formation of the 330 Da neutral loss. All rearrangement related products are labeled in red.
For positive mode Schiff base modification of RARARAA, doubly protonated RARARAA was subjected to ion/ion reaction with deprotonated FBDSA anions to generate an ion/ion complex of the form [M + H + FBDSA]+. Activation of this complex resulted in the signature water loss to generate the covalently modified product [M + H + ♦]+. Further activation of this product yielded dominant formation of the rearrangement product, viz., [M + H + ◊]+, indicated in red in Figure 2(a), which is similar to the results from Schiff-base-modified peptide KAKAKAA (Figure 1(c)). Since methanediimine loss from the arginine side-chain (labeled with solid square) generates an ornithine residue27, which contains an amino group, the rearrangement products with ornithine-containing fragments could also arise from reactions with the ornithine side-chain. Other fragments derived from neutral losses (e.g., ammonia and water) as well as backbone cleavages (e.g., y3, y4, y5, y6, and b6♦) were observed at lesser abundances. Furthermore, the presence of the modified b6♦ ion and lack of any modified y-ions indicate that the FBDSA is covalently attached to the N-terminal region of the peptide, which is consistent with Schiff-base reactivity at a primary amine. The neutral arginine side chain could react with the imine bond through a similar mechanism, as shown in Scheme 3(a). In the example of arginine in the N-terminus position, the lone pair on the nitrogen of the guanidine group attacks the imine carbon and forms a nine-membered ring. Rearrangement of the ring and further proton transfer leads to the formation of the imine ring structure along with the loss of neutral AMBDSA or AMBMSA. When the arginine side chain is protonated, a similar process could occur, as shown in Scheme 3(b).
Scheme 3.

Proposed mechanism for rearrangement reaction of arginine-containing Schiff-base-modified peptides between an imine group and a) a neutral guanidine group or b) a protonated guanidinium group. N-terminal arginine residue is used here as an example.
As for negative mode Schiff base modification, singly protonated RARARAA reacted with doubly deprotonated FBDSA anions to form an electrostatic complex [M − H + FBDSA]−, which upon activation generates the signature water loss peak [M − H + ♦]−. Activation of [M − H + ♦]− species forms mainly neutral loss fragments (e.g., ammonia and methanediimine losses) and deprotonated FBDSA [FBDSA − H]− (Figure 2(b)), which indicates that the water loss from the electrostatic complex is partly due to water loss from the peptide itself and not coming from the formation of the imine bond. [18-20] The m/z 249 Da peak was also seen (labeled with 249 in red). No abundant peptide rearrangement product ions were seen, which is consistent with the low signal for the m/z 249 ion. The attack from the imine to the guanidinium group in the positive mode may be more facile than that from the neutral guanidine group to the neutral imine group in the negative mode (Scheme 3) but further studies would be required to make such a generalization. We note, however, for the limited set of peptide ions examined in this work, peptide anions generally showed notably less contribution from ions generated by the rearrangement reactions described here than the corresponding positive ions.
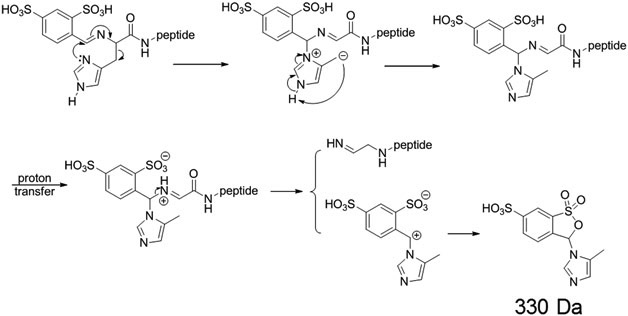
The Schiff-base-modified histidine-containing peptides generated by reaction with FBDSA ions showed a different neutral loss associated with a rearrangement (330 Da) than the lysine and arginine containing peptides (267 Da) upon activation (Figure 2(c) and (d)). The doubly protonated peptide HAHAHAA reacted with singly deprotonated FBDSA in the positive mode to form an ion/ion complex [M + H + FBDSA]+, which upon CID gave the signature water loss indicating formation of Schiff base modification product [M + H + ♦]+. Further activation of the Schiff-base-modified peptide generated backbone cleavages (e.g., b6♦ and y5) as well as a peak labeled as [M + H + ♠]+ (♠ = −82 Da) that is 330 Da lower in mass than the precursor ion [M + H + ♦]+ (Figure 2(c)). In the negative mode, mutual storage of the singly protonated peptide HAHAHAA and the doubly deprotonated FBDSA led to the formation of a complex [M − H + FBDSA]− and upon CID of the complex the signature water loss peak [M − H + ♦]− was generated. Activation of the ion generated by water loss (Figure 2(d)) gave rise predominantly to modified backbone fragment ions (e.g., b6♦) and neutral losses (e.g., CO2 losses). [FBDSA − H]− ions were also observed, along with the m/z 249 Da ion (labeled as 249 in red) and an m/z 329 ion (labeled as [NL(♠) − H]− ), which presumably is the deprotonated species derived from the 330 Da molecule that was observed to be lost in the positive mode experiment described above. The m/z 249 ion can also be generated from the m/z 329 ion through a mechanism shown in the Supplemental Scheme S1.
The rearrangement reaction between the histidine side chain and the imine bond differs from those of the primary amine and guanidine groups because of the ring structure of the imidazole group. The AMBDSA neutral loss observed with lysine and arginine could not form through a similar process for histidine. Instead, the 330 Da neutral loss could be explained by the reaction between the imine bond and the histidine side chain, as shown in Scheme 4. With the rearrangement, the histidine side chain moiety transfers to the original imine carbon, and the imine bond shifts to the other carbon connected to the imine nitrogen. Further activation of the rearranged structure could lead to the 330 Da neutral loss. This mechanism is especially favored if the histidine residue is located on the N-terminus of the peptide, since that location enables the rearrangement to occur with a 6-membered ring transition state structure. When histidine is not present at the N-terminus, the mechanism just mentioned is apparently not particularly competitive, as indicated with the analogous experiment performed with doubly protonated AHAAAHA. Activation of the Schiff-base-modified peptide AHAAAHA in the positive mode (Supplemental Figure S4) yielded abundant backbone cleavages (e.g., b6♦ and y4) but no discernable 330 Da neutral loss, in contrast with the modified HAHAHAA ion (Figure 2(c)). Interestingly, the AMBDSA neutral loss rearrangement product was seen in low abundance in the spectrum of Supplemental Figure S4, which could implicate the rearrangement reaction involving the imine and amide nitrogen (Supplemental Scheme S2), albeit with lower probability due to the lesser nucleophilicity of an amide nitrogen relative to a primary amine.
Scheme 4.
Proposed mechanism for rearrangement reaction of histidine-containing Schiff-base-modified peptides between an imine group and a neutral imidazole group. N-terminal histidine residue is used here as an example.
Conclusions
Rearrangement of gas-phase Schiff-base-modified peptides has been observed and characterized using tandem mass spectrometry. Evidence for the reaction is provided by the appearance of a product ion that is nominally 19 Da lower in mass than that of the protonated or deprotonated peptide upon collisional activation of the Schiff-base-modified peptide. Sequence ions with the same −19 Da mass difference may also be observed at relatively low abundance. Upon casual inspection, these products could be mistaken as arising from a water loss but the mass is off by 1 Da. The results are consistent with a nucleophilic attack on the imine site generated via Schiff base formation. The side chains of lysine, arginine, and histidine all show evidence for participation in the rearrangement reaction. Based on the experimental results, Schiff-base-modified peptides of positive polarity more readily undergo the rearrangement reaction compared to the corresponding negatively charged ions. Plausible mechanisms were proposed for the rearrangement reactions between the imine bond and the amine, guanidine and histidine side chain groups. The possible contribution from this rearrangement reaction, at least under ion trap collisional activation conditions, should be taken into account in any work flow involving the Schiff base modification of polypeptide ions.
Supplementary Material
Acknowledgements:
The authors acknowledge support for this work by the National Institutes of Health under grant GM R37-45372.
References
- 1.McLafferty FW. Tandem mass spectrometry. Science 1981;214(4518), 280–287. doi: 10.1126/science.7280693. [DOI] [PubMed] [Google Scholar]
- 2.Santos LS, Pavam CH, Almeida WP, Coelho F, Eberlin MN. Probing the mechanism of the Baylis-Hillman reaction by electrospray ionization mass and tandem mass spectrometry. Angew Chem Int Ed 2004;43(33):4330–4333. doi: 10.1002/anie.200460059. [DOI] [PubMed] [Google Scholar]
- 3.Aebersold R, Mann M. Mass-spectrometric exploration of proteome structure and function. Nature 2016;537(7620):347–355. doi: 10.1038/nature19949. [DOI] [PubMed] [Google Scholar]
- 4.Reid GE, Roberts KD, Simpson RJ, O’Hair RA. Selective identification and quantitative analysis of methionine containing peptides by charge derivatization and tandem mass spectrometry. J Am Soc Mass Spectrom 2005;16(7):1131–1150. doi: 10.1016/j.jasms.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Kinter M, Sherman NE. Protein Sequencing and Identification Using Tandem Mass Spectrometry. New York, NY: John Wiley & Sons; 2005. [Google Scholar]
- 6.McLuckey SA, Mentinova M. Ion/neutral, ion/electron, ion/photon, and ion/ion interactions in tandem mass spectrometry: do we need them all? Are they enough? J Am Soc Mass Spectrom 2011;22(1):3–12. doi: 10.1007/s13361-010-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prentice BM, McLuckey SA. Gas-phase ion/ion reactions of peptides and proteins: acid/base, redox, and covalent chemistries. Chem Commun 2013;49(10):947–965. doi: 10.1039/c2cc36577d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson JL Jr, McLuckey SA. Ion/ion proton transfer reactions for protein mixture analysis. Anal Chem 1996;68(22):4026–4032. doi: 10.1021/ac9605657. [DOI] [PubMed] [Google Scholar]
- 9.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci U S A 2004;101(26):9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilo AL, McLuckey SA. Selective gas-phase ion/ion reactions: enabling disulfide mapping via oxidation and cleavage of disulfide bonds in intermolecularly-linked polypeptide ions. Anal Chem 2016;88(18):8972–8979. doi: 10.1021/acs.analchem.6b01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mentinova M, McLuckey SA. Covalent modification of gaseous peptide ions with N-hydroxysuccinimide ester reagent ions. J Am Chem Soc 2010;132(51):18248–18257. doi: 10.1021/ja107286p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGee WM, Mentinova M, McLuckey SA. Gas-phase conjugation to arginine residues in polypeptide ions via N-hydroxysuccinimide ester-based reagent ions. J Am Chem Soc 2012;134(28):11412–11414. doi: 10.1021/ja304778j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Z, McGee WM, Bu J, Barefoot NZ, McLuckey SA. Gas phase reactivity of carboxylates with N-hydroxysuccinimide esters. J Am Soc Mass Spectrom 2015;26(1):174–180. doi: 10.1007/s13361-014-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilo AL, Bu J, McLuckey SA. Gas-phase oxidation of neutral basic residues in polypeptide cations by periodate. J Am Soc Mass Spectrom 2016;27(12):1979–1988. doi: 10.1007/s13361-016-1491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilo AL, Bu J, McLuckey SA. Transformation of [M+2H]2+ peptide cations to [M−H]+, [M+H+O]+, and M+• cations via ion/ion reactions: reagent anions derived from persulfate. J Am Soc Mass Spectrom 2015;26(7):1103–1114. doi: 10.1007/s13361-015-1125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prentice BM, Gilbert JD, Stutzman JR, Forrest WP, McLuckey SA. Gas-phase reactivity of carboxylic acid functional groups with carbodiimides. J Am Soc Mass Spectrom 2013;24(1):30–37. doi: 10.1007/s13361-012-0506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H, McLuckey SA. Selective covalent bond formation in polypeptide ions via gas-phase ion/ion reaction chemistry. J Am Chem Soc 2009;131(36):12884–12885. doi: 10.1021/ja904812d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassell KM, Stutzman JR, McLuckey SA. Gas-phase bioconjugation of peptides via ion/ion charge inversion: Schiff base formation on the conversion of cations to anions. Anal Chem 2010;82(5):1594–1597. doi: 10.1021/ac902732v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stutzman JR, Luongo CA, McLuckey SA. Covalent and non-covalent binding in the ion/ion charge inversion of peptide cations with benzene-disulfonic acid anions. J Mass Spectrom 2012;47(6):669–675. doi: 10.1002/jms.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stutzman JR, Hassell KM, McLuckey SA. Dissociation behavior of tryptic and intramolecular disulfide-linked peptide ions modified in the gas phase via ion/ion reactions. Int J Mass Spectrom 2012;312:195–200. doi: 10.1016/j.ijms.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotham VC, Shaw JB, Brodbelt JS. High-throughput bioconjugation for enhanced 193 nm photodissociation via droplet phase initiated ion/ion chemistry using a front-end dual spray reactor. Anal Chem 2015;87(18):9396–9402. doi: 10.1021/acs.analchem.5b02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotham VC, McGee WM, Brodbelt JS. Modulation of phosphopeptide fragmentation via dual spray ion/ion reactions using a sulfonate-incorporating reagent. Anal Chem 2016;88(16):8158–8165. doi: 10.1021/acs.analchem.6b01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodbelt JS. Ion activation methods for peptides and proteins. Anal Chem 2016;88(1):30–51. doi: 10.1021/acs.analchem.5b04563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia Y, Wu J, McLuckey SA, Londry FA, Hager JW. Mutual storage mode ion/ion reactions in a hybrid linear ion trap. J Am Soc Mass Spectrom 2005;16(1):71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Liang X, Xia Y, McLuckey SA. Alternately pulsed nanoelectrospray ionization/atmospheric pressure chemical ionization for ion/ion reactions in an electrodynamic ion trap. Anal Chem 2006;78(9):3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Londry FA, Hager JW. Mass selective axial ion ejection from a linear quadrupole ion trap. J Am Soc Mass Spectrom 2003;14(10):1130–1147. doi: 10.1016/S1044-0305(03)00446-X.. [DOI] [PubMed] [Google Scholar]
- 27.McGee WM, McLuckey SA. Gas phase dissociation behavior of acyl-arginine peptides. Int J Mass Spectrom 2013;354–355:181–187. doi: 10.1016/j.ijms.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.