Abstract
Objective:
To explore the factors influencing adoption of the sentinel lymph node (SLN) technique for endometrial cancer (EC) staging among gynecologic oncologists.
Methods:
A self-administered, web-based survey was sent via email (April 20 through May 21, 2017) to all members of European Society of Gynecologic Oncologists, International Gynecologic Cancer Society, and Society of Gynecologic Oncologists. Surgical and pathologic practices related to SLN and reasons for not adopting this technique were investigated.
Results:
Overall, 489 attending physician or consultants in gynecologic oncology from 69 countries responded: 201 (41.1%), 118 (24.1%), and 117 (23.9%) from Europe, United States, and other countries, respectively (10.8% did not report a country). SLN was adopted by 246 (50.3%) respondents, with 93.1% injecting the cervix and 62.6% using indocyanine green dye. The National Comprehensive Cancer Network SLN algorithm was followed by 160 (65.0%) respondents (United States, 74.4%; Europe, 55.4%; other countries, 71.4%). However, 67.7% completed a backup lymphadenectomy in high-risk patients. When SLN biopsy revealed isolated tumor cells (ITC), 13.8% of respondents recommended adjuvant therapy. This percentage increased to 52% if micrometastases were detected. Among the 243 not adopting SLN, 50.2% cited lack of evidence and 45.3% stated that inadequate instrumentation fueled their decisions.
Conclusions:
SLN with a cervical injection is gaining widespread acceptance for staging of EC among gynecologic oncologists worldwide. Standardization of the surgical approach with the National Comprehensive Care Network algorithm is applied by most users. Management of ITC and the role of backup lymphadenectomy for “high-risk” cases remain areas of investigation.
Keywords: endometrial cancer, lymphadenectomy, minimally invasive surgery, sentinel lymph node, survey
Introduction
Endometrial cancer (EC) is the most frequent gynecologic malignancy in developed countries. Approximately 63,000 women are diagnosed with early stage EC in the United States (US) annually.(1) The cornerstone of surgical treatment is removal of the uterus, fallopian tubes, and ovaries.(2) A minimally invasive approach (laparoscopic or robotic assisted) is preferred over open surgery for apparent early stage disease because it is associated with reduced postoperative pain scores, shorter hospitalizations, less surgical-related morbidity, and faster return to daily activities, with no difference in survival outcomes.(3–6) One of the main independent predictors of survival is the presence of lymph node metastases; as such, its identification influences the administration of adjuvant therapies such as radiotherapy, chemotherapy, or both.(7, 8) A comprehensive dissection of the lymph nodes was traditionally suggested to assess for the presence of extrauterine disease in patients with apparent early stage EC, whereas the recent European guidelines(2) recommend systematic removal of pelvic and para-aortic nodes in patients with high-risk EC.(9) However, when compared with simple hysterectomy, the performance of lymphadenectomy (pelvic alone or pelvic plus para-aortic) has been shown to prolong operative time, increase costs, and cause adverse effects such as lower-extremity lymphedema.(10–12)
With the intention of reducing surgical morbidity, sentinel lymph node (SLN) biopsy has been proposed in the past decade as a feasible option for EC staging.(13) Recently, the accuracy of this strategy has been tested in the FIRES trial, which showed 97.2% sensitivity and a negative predictive value of 99.6%.(14) Although the reductions in adverse effects predicted to occur with this technique have not yet been fully investigated, it seems reasonable to hypothesize that the decreased radicality of SLN biopsy (compared with lymphadenectomy) may result in less morbidity and better quality of life.(15) The National Comprehensive Care Network (NCCN) has included SLN biopsy as a valid method for EC staging in its 2017 guidelines, but this method has not been universally adopted.(16)
The present survey aimed to investigate SLN technique adoption rates and identify factors that influence implementation of the SLN technique for EC staging. In addition, we report surgical and adjuvant treatments commonly offered to patients with metastatic disease discovered during the SLN biopsy.
Material and Methods
The study protocol was approved by the Mayo Clinic Institutional Review Board (Rochester, Minnesota, United States) and by the Society of Gynecologic Oncologists (SGO), International Gynecologic Cancer Society (IGCS), and European Society of Gynecologic Oncologists (ESGO).
A self-administered, anonymous survey (Supplemental Table) was sent via email to all the members of the SGO, IGCS, and ESGO from April 20 through May 21, 2017. The Mayo Clinic Survey Research Center (Rochester, Minnesota) received membership lists from SGO (n=1,014) and IGCS (n=854). When duplicate members were identified in both societies, the member was randomly assigned to only 1 group, yielding a final sample of 942 for SGO and 731 for IGCS. The Survey Research Center sent individualized emails to SGO and IGCS members such that each member was assigned a unique survey link that permitted the person to complete the survey only once. The use of the unique links for SGO and IGCS members also allowed the Survey Research Center to send 2 reminder emails to nonrespondents.
ESGO was unable to release their member list to the Survey Research Center, and thus an email with a generic survey link (ie, all respondents received the same link) was sent to ESGO members by that society. The number of ESGO members contacted to complete the survey and the number of ESGO members who were possible duplicates in the other 2 societies was not known. Because we could not identify nonrespondents with the generic survey link, 2 subsequent emails were sent to all ESGO members, thanking them if had already responded and encouraging them to respond if they had not.
The intended survey population was gynecologic oncologists affiliated with the above-mentioned societies who were fluent in reading English (the survey was not translated into other languages). Respondents who identified themselves as either medical or radiation oncologists, affiliated physicians in training (eg, residents and fellows), or retired physicians were excluded from the analysis. They also were not considered when calculating the response rates for IGCS and SGO. We calculated the response rates for these societies in 2 ways. The first used the American Association for Public Opinion Research response rate formula 1 (RR1), which assumes all nonrespondents were eligible to complete the survey. The second formula (RR3) assumes the same proportion of eligible members among the nonrespondents as the respondents.(17) The denominator for ESGO was unknown; thus, a response rate could not be calculated for that society.
The survey was designed to assess surgical and pathologic practice details related to SLN biopsy among respondents, specifically to determine the use of the SLN technique as a common practice of surgical staging. Demographic characteristics of the respondents and their institutions included country of institution, type of institution (academic center, nonacademic center), availability of laparoscopic and robotic surgery, yearly number of EC cases surgically treated at each institution, and the availability of a frozen section pathology laboratory in each center. The questionnaire also assessed the awareness and application of the NCCN SLN algorithm developed at Memorial Sloan Kettering Cancer Center.(16, 18) Respondents were asked whether they performed frozen section or ultrastaging on the removed SLNs, and they were also asked about the use and type of adjuvant management in cases with isolated tumor cells (ITCs) (≤0.2 mm), micrometastases (>0.2 mm and ≤2 mm), or macrometastases (>2 mm). Reason(s) for not adopting SLN technique and alternative practices for staging were ascertained.
Statistical Analysis
Results are reported as frequency and percentages. For analytic purposes, respondents were classified into 3 geographic categories based on the location of their institutions (Europe, US, and other). Comparisons of respondents from Europe and the US were evaluated with the Fisher exact test or χ2 test. Factors were evaluated by univariate analysis for an association with SLN adoption for EC staging using the χ2 test for categorical variables and the Cochran-Armitage test for trend for ordinal variables. A multivariable logistic regression model was fit to evaluate combinations of factors for an association with SLN adoption. All calculated P values were 2-sided and P values <.05 were considered statistically significant. Statistical analysis was performed with the SAS software package (v 9.4; SAS Institute Inc).
Results
Survey responses were obtained from 610 respondents, of whom 494 (81.0%) were an attending physician or consultant in gynecologic oncology, 70 (11.5%) were a resident, fellow, or retired physician or consultant in gynecologic oncology, 15 (2.5%) were a radiation or medical oncologist, and 31 (5.1%) were self-declared as “other.” After excluding the ineligible respondents, we calculated response rates for SGO (RR1=14.8%, RR3=14.9%) and IGCS (RR1=17.8%, RR3=21.1%). Among the 494 attending physicians or consultants in gynecologic oncology respondents who were considered for this analysis, 5 (1.1%) answered only the initial items regarding their position and years of experience; their responses were therefore excluded. The reported analyses are based on the remaining 489 respondents (226 [46.2%], 124 [25.4%], and 139 [28.4%] from ESGO, IGCS, and SGO, respectively).
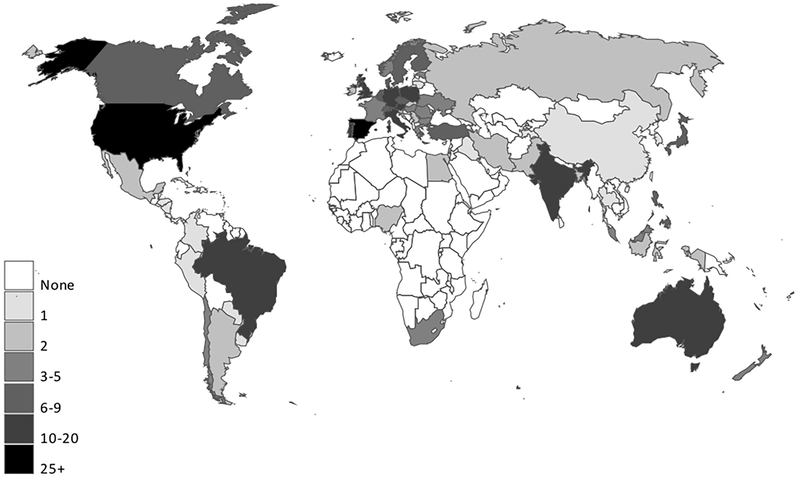
Overall, 436 of the 489 respondents (89.2%) reported the country where they practiced (Europe, n=201 [41.1%]; US, n=118 [24.1%]; other, n=117 [23.9%]; not reported, n=53 [10.8%]). Sixty-nine countries (28 in Europe) were represented among these 436 respondents: 340 (78.0%) were from a high-income country, 51 (11.7%) were from an upper-middle-income country, 44 (10.1%) were from a lower-middle-income country, and 1 (0.2%) was from a low-income country. (Country income level was based on the World Bank list of economies as of March 2017) (19). A map reporting the institutional countries of the respondents is shown in Figure 1.
Figure 1.
Map of Respondents’ Institutions. Countries were determined from answers to the question “What country are you from?” (489 respondents).(19)
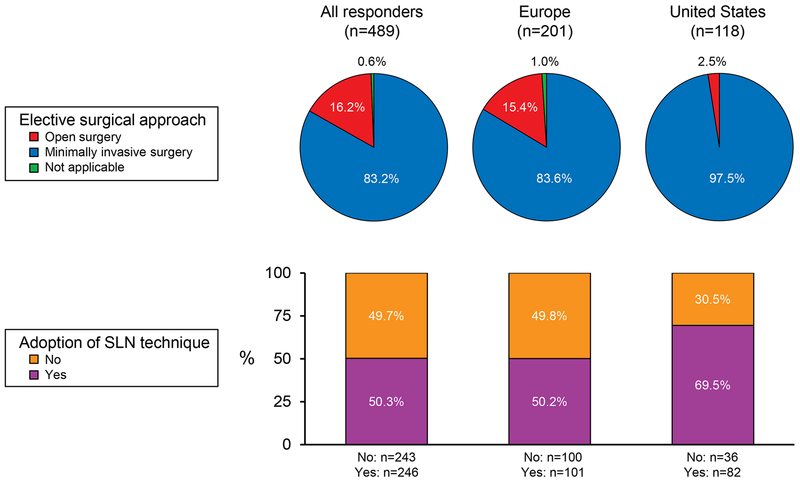
SLN technique was used by 246 (50.3%) respondents: 101 (50.2%) in Europe, 82 (69.5%) in the US, 42 (35.9%) in other countries, and 21 (39.6%) who did not report their institutional country. When the SLN technique was used, indocyanine green (ICG) was the most common dye (overall, 62.6%; US, 87.8%; Europe, 56.4%), and the uterine cervix was the site most commonly injected (overall, 93.1%; US, 97.6%; Europe, 90.1%). Despite the majority of the respondents reporting that they were applying the NCCN SLN algorithm (overall, 65.0%; US, 74.4%; Europe, 55.4%; other countries, 71.4%), a backup lymphadenectomy in high-risk patients was still performed by 66.7% of respondents (US, 63.4%; Europe, 68.3%), whereas only 16.3% (US, 25.6%; Europe, 11.9%) stopped the dissection after SLN removal. For those who used SLN techniques, frozen section was part of the pathologic evaluation for 30.5% of the respondents (US, 28.0%; Europe, 34.7%), and ultrastaging was performed by 78.9% (US, 84.1%; Europe, 79.2%).
Among the 243 not adopting SLN techniques, the majority (n=126 [51.9%]) pursued surgical staging, performing a selective pelvic lymphadenectomy, with or without para-aortic lymphadenectomy, on the basis of tumor risk factors (US, 69.4%; Europe, 55.0%). Overall, 50.2% of respondents (US, 61.1%; Europe, 61.0%) cited lack of evidence and 45.3% (US, 30.6%; Europe, 37.0%) cited inadequate instrumentation as fueling their decisions not to use SLN biopsy. Detailed results are shown in Table 1.
Table 1.
Descriptive Analysis of Staging Procedures for Endometrial Cancer Among Gynecologic Oncologists, Stratified by Geographic Region
| Standard Practice of Staging for Endometrial Cancer | Overall (N=489)a | United States (n=118) | Europe (n=201) | Other Countries (n=117) |
|---|---|---|---|---|
| SLN Technique | ||||
| SLN technique used, No. (%) | 246 (50.3) | 82 (69.5) | 101 (50.2) | 42 (35.9) |
| Tracer most commonly injected, No. (%) | ||||
| Indocyanine green | 154 (62.6) | 72 (87.8) | 57 (56.4) | 12 (28.6) |
| Blue dye | 40 (16.3) | 3 (3.7) | 14 (13.9) | 19 (45.2) |
| Combination (indocyanine + blue) | 20 (8.1) | 6 (7.3) | 7 (6.9) | 6 (14.3) |
| Technetium Tc 99 | 4 (1.6) | 0 (0) | 4 (4.0) | 0 (0) |
| Technetium Tc 99 combined with other tracers | 24 (9.8) | 0 (0) | 19 (18.8) | 4 (9.5) |
| Other or item not answered | 4 (1.6) | 1 (1.2) | 0 (0) | 1 (2.4) |
| Site of injection, No. (%) | ||||
| Cervix | 229 (93.1) | 80 (97.6) | 91 (90.1) | 41 (97.6) |
| Uterine body | 3 (1.2) | 0 (0) | 2 (2.0) | 0 (0) |
| Cervix and uterine body | 10 (4.1) | 1 (1.2) | 7 (6.9) | 1 (2.4) |
| Other or item not answered | 4 (1.6) | 1 (1.2) | 1 (1.0) | 0 (0) |
| Backup lymphadenectomy after SLN biopsy performed, No. (%) | ||||
| In all cases | 37 (15.0) | 9 (11.0) | 18 (17.8) | 5 (11.9) |
| Only in “high risk” patients | 164 (66.7) | 52 (63.4) | 69 (68.3) | 30 (71.4) |
| Never | 40 (16.3) | 21 (25.6) | 12 (11.9) | 7 (16.7) |
| Item not answered | 5 (2.0) | 0 (0) | 2 (2.0) | 0 (0) |
| Pathologic evaluation of the SLNs removed, No. (%) | ||||
| Frozen section | 75 (30.5) | 23 (28.0) | 35 (34.7) | 12 (28.6) |
| Ultrastaging | 194 (78.9) | 69 (84.1) | 80 (79.2) | 33 (78.6) |
| Non-SLN Technique | ||||
| Non-SLN technique used, No. (%) | 243 (49.7) | 36 (30.5) | 100 (49.8) | 75 (64.1) |
| Reason for not adopting SLNb | ||||
| Not enough evidence | 122 (50.2) | 22 (61.1) | 61 (61.0) | 25 (33.3) |
| Lack of instrumentation | 110 (45.3) | 11 (30.6) | 37 (37.0) | 50 (66.7) |
| Cost | 30 (12.3) | 2 (5.6) | 9 (9.0) | 13 (17.3) |
| Standard practice for staging, No. (%) | ||||
| No lymph node assessment | 5 (2.1) | 0 (0) | 3 (3.0) | 0 (0) |
| Selective pelvic lymphadenectomy c | 26 (10.7) | 2 (5.6) | 8 (8.0) | 14 (18.7) |
| Systematic pelvic lymphadenectomy | 25 (10.3) | 0 (0) | 9 (9.0) | 14 (18.7) |
| Selective pelvic ± para-aortic lymphadenectomyc | 126 (51.9) | 25 (69.4) | 55 (55.0) | 29 (38.7) |
| Systematic pelvic + para-aortic lymphadenectomy | 52 (21.4) | 8 (22.2) | 22 (22.0) | 15 (20.0) |
| Other or not answered | 9 (3.7) | 1 (2.8) | 3 (3.0) | 3 (4.0) |
Abbreviations: SLN, sentinel lymph node; ±, with or without.
Geographic region was not reported by 53 respondents (10.8%).
Multiple answers were allowed.
Procedure was performed only if tumor-related risk factors for lymph node metastasis were present.
Although most of the analyzed variables (univariate analysis) were significantly associated with adoption of the SLN technique (Table 2), after adjusting for the use of minimally invasive surgery (ie, laparoscopy, robotic surgery) in a multivariable model, none of the other variables attained statistical significance. Regarding the approach of choice for EC, minimally invasive surgery was reported by 83.2% of respondents (US, 97.5%; Europe, 83.6%; Figure 2).
Table 2.
Factors Associated With SLN Adoption
| Factor | Used SLN Technique for EC Staging, No. (%) | P Valuea |
|---|---|---|
| Years since completing training | .23 | |
| <3 (n=40) | 15 (37.5) | |
| 3-10 (n=135) | 68 (50.4) | |
| >10 (n=314) | 163 (51.9) | |
| Country | <.001 | |
| Europe (n=201) | 101 (50.2) | |
| US (n=118) | 82 (69.5) | |
| Other than US or Europe (n=117) | 42 (35.9) | |
| Not reported (n=53) | 21 (39.6) | |
| Country’s income categoryb | <.001 | |
| High income (n=340) | 196 (57.6) | |
| Upper middle income (n=51) | 20 (39.2) | |
| Lower middle income (n=44) | 9 (20.5) | |
| Low or country not reported (n=54) | 21 (38.9) | |
| Type of institution | .04 | |
| Academic center (n=346) | 186 (53.8) | |
| Nonacademic center (n=122) | 51 (41.8) | |
| Other (n=19) | 7 (36.8) | |
| Item not answered (n=2) | 2 (100) | |
| No. of ECs surgically treated at institution per year | <.001 | |
| <10 (n=15) | 3 (20.0) | |
| 10-20 (n=44) | 8 (18.2) | |
| 21-50 (n=111) | 54 (48.6) | |
| 51-100 (n=144) | 75 (52.1) | |
| 101-200 (n=115) | 65 (56.5) | |
| >200 (n=58) | 40 (69.0) | |
| Item not answered (n=2) | 1 (50.0) | |
| Type of MIS available at institution | <.001 | |
| Laparoscopic and robotic (n=250) | 87 (34.8) | |
| Laparoscopic only (n=211) | 133 (63.0) | |
| Robotic only (n=5) | 3 (60.0) | |
| Neither (n=23) | 20 (87.0) | |
| MIS as first-line choice for the treatment of apparent early stage EC at institution | <.001 | |
| Yes (n=407) | 234 (57.5) | |
| No (n=79) | 11 (13.9) | |
| Item not answered (n=3) | 1 (33.3) | |
| Frozen-section pathology commonly used (>50% of cases) to assess uterine risk | .03 | |
| Yes (n=238) | 132 (55.5) | |
| No (n=250) | 114 (45.6) | |
| Item not answered (n=1) | 0 (0) |
Abbreviations: EC, endometrial cancer; MIS, minimally invasive surgery; SLN, sentinel lymph node; US, United States.
Two-sided χ2 test for categorical variables and the Cochran-Armitage test for trend for the number of ECs treated at the institution per year. The “not reported” or “item not answered” categories were ignored in the statistical comparisons between categories.
The country’s income category is based on the World Bank list of economies as of March 2017.
Figure 2.
Suggested Surgical Approach for Apparent Early Stage Endometrial Cancer and Adoption of SLN Technique. Results were based on the answers to the questions “Is minimally invasive surgery (laparoscopy and/or robotic surgery) the approach of choice for the treatment of apparent early stage endometrial cancer at your institution?” and “Do you use SLN technique for endometrial cancer staging?” Answers were stratified by geographic area. SLN indicates sentinel lymph node.
Of physicians who reported performing SLN biopsy as their common practice, 13.8% (US, 22.0%; Europe, 10.9%) stated that they would always suggest adjuvant treatment when removed SLNs revealed ITCs, whereas 58.9% reported that they would recommend adjuvant therapy on the basis of tumor risk factors. In cases of micrometastasis in the SLNs, the majority of respondents suggested adjuvant treatment should always be given, regardless of the presence of risk factors (overall, 52.0%; US, 59.8%; Europe, 49.5%). A majority (89.4%) supported the use of adjuvant therapy when macrometastasis was detected, regardless of the presence or absence of other risk factors (US: 98.8%; Europe 85.1%). The combination of chemotherapy and radiotherapy was reported to be the primary option when adjuvant treatment was prescribed (Table 3).
Table 3.
Clinical Management of Positive SLN, Stratified by Geographic Region
| SLN Status | Overalla (N=246) | US (n=82) | Europe (n=101) | Other Countries (n=42) | P Valueb |
|---|---|---|---|---|---|
| Macrometastases (>2 mm) | |||||
| Suggested management | .005 | ||||
| Adjuvant treatment always | 220 (89.4) | 81 (98.8) | 86 (85.1) | 37 (88.1) | |
| Adjuvant treatment based on tumor risk factors | 14 (5.7) | 1 (1.2) | 9 (8.9) | 3 (7.1) | |
| No adjuvant treatment suggested | 2 (0.8) | 0 (0) | 1 (1.0) | 1 (2.4) | |
| Item not answered | 10 (4.1) | 0 (0) | 5 (5.0) | 1 (2.4) | |
| Type of treatment suggested, No. (%) | (n=234) | (n=82) | (n=95) | (n=40) | <.001 |
| Chemotherapy only | 21 (9.0) | 5 (6.1) | 13 (13.7) | 2 (5.0) | |
| Radiotherapy only | 22 (9.4) | 0 (0) | 14 (14.7) | 8 (20.0) | |
| Combined chemoradiation therapy | 178 (76.1) | 69 (84.1) | 65 (68.4) | 28 (70.0) | |
| Other or item not answered | 13 (5.6) | 8 (9.8) | 3 (3.2) | 2 (5.0) | |
| Micrometastases (>0.2 to ≤2 mm) | |||||
| Suggested management | .08 | ||||
| Adjuvant treatment always | 128 (52.0) | 49 (59.8) | 50 (49.5) | 18 (42.9) | |
| Adjuvant treatment based on tumor risk factors | 108 (43.9) | 33 (40.2) | 46 (45.5) | 23 (54.8) | |
| No adjuvant treatment suggested | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Item not answered | 10 (4.1) | 0 (0) | 5 (5.0) | 1 (2.4) | |
| Type of treatment suggested, No. (%) | (n=236) | (n=82) | (n=96) | (n=41) | <.001 |
| Chemotherapy only | 27 (11.4) | 6 (7.3) | 15 (15.6) | 5 (12.2) | |
| Radiotherapy only | 49 (20.8) | 3 (3.7) | 28 (29.2) | 15 (36.6) | |
| Combined chemoradiation therapy | 136 (57.6) | 59 (72.0) | 46 (47.9) | 18 (43.9) | |
| Other or item not answered | 24 (10.2 ) | 14 (17.1) | 7 (7.3) | 3 (7.3) | |
| Isolated Tumor Cells (≤0.2 mm) | |||||
| Suggested management, No. (%) | .02 | ||||
| Adjuvant treatment always | 34 (13.8) | 18 (22.0) | 11 (10.9) | 3 (7.1) | |
| Adjuvant treatment based on tumor risk factors | 145 (58.9) | 46 (56.1) | 55 (54.5) | 30 (71.4) | |
| No adjuvant treatment suggested | 56 (22.8) | 18 (22.0) | 29 (28.7) | 8 (19.0) | |
| Item not answered | 11 (4.5) | 0 (0) | 6 (5.9) | 1 (2.4) | |
| Type of treatment suggested, No. (%) | (n=179) | (n=64) | (n=66) | (n=33) | .006 |
| Chemotherapy only | 31 (17.3) | 10 (15.6) | 17 (25.8) | 3 (9.1) | |
| Radiotherapy only | 61 (34.1) | 14 (21.9) | 28 (42.4) | 14 (42.4) | |
| Combined chemoradiation therapy | 67 (37.4) | 32 (50.0) | 15 (22.7) | 11 (33.3) | |
| Other or item not answered | 20 (11.2) | 8 (12.5) | 6 (9.1) | 5 (15.2) | |
Abbreviations: SLN, sentinel lymph node; US, United States.
Geographic region was not reported by 21 respondents (8.5%).
Comparing respondents from Europe and the US only. Comparisons were evaluated with the Fisher exact for the distribution of suggested management and the χ2 test for the distribution of type of treatment suggested.
Discussion
The present investigation revealed that gynecologic oncologists consider SLN biopsy to be a feasible and valid option for the surgical staging of patients with apparent early stage EC. Consistent with a review of published studies,(15) our respondents indicated that the cervix was the preferred site of dye inoculation. Among respondents in the US, ICG was the dye most commonly used. As expected from prior analyses,(20–22) we found that cervical injection of ICG was the preferred technique for SLN biopsy. This method is associated with a higher SLN detection rate than hysteroscopic endometrial injection, without influencing the anatomic nodal distribution in patients with EC.(23) Although the decision to use this dye is supported by current evidence,(15) only one-half of European respondents adopting the SLN technique reported ICG as their first option.
The use of SLN technique is gaining widespread acceptance worldwide; however, the results of the present survey confirmed strong differences regarding EC staging among gynecologic oncologists. For example, 15% of our survey respondents still indicated that they preferred the abdominal (open) surgical approach, despite clear evidence that minimally invasive techniques should be standard in this population.(16)
Although conclusions from the FIRES trial(14) and a recent meta-analysis(20) support the use of SLN biopsy for identifying lymphatic metastases in EC staging, respondents from Europe and the US cited a low level of evidence as the primary reason for not adopting the SLN technique. The lack of confidence in the SLN technique is also confirmed by the fact that for high-risk patients, complete lymphadenectomy is performed by almost 70% of respondents. This practice contradicts NCCN guidelines for SLN mapping in EC staging, which suggest side-specific lymphadenectomy only for cases of unsuccessful mapping, but it is in accordance with the ESGO recommendations regarding lymphadenectomy in apparent early stage EC.(2,16) Results of ongoing prospective randomized controlled trials comparing SLN with lymphadenectomy will help define the role of systematically removing regional lymph nodes for EC staging.(24, 25)
As we predicted, the survey showed extensive discrepancies in the indications for adjuvant treatment, especially for patients with ITCs and micrometastases, thus emphasizing the need for a consensus on postoperative management of these patients. The recommendation for adjuvant chemotherapy plus radiotherapy was much more uniform for patients with macrometastases, consistent with guidelines from Europe and the US.(2, 16)
Some limitations of the present survey need to be addressed. First, despite the high number of gynecologic oncologists who participated in this survey, our results may be affected by nonresponse bias. We were unable to determine overlap between ESGO vs SGO or IGCS member respondents. It is possible that some responses were received from the ESGO direct mailing; if these responses were submitted from oncologists with multiple society memberships, it could lead us to underestimate the response rate for the other 2 societies. Second, the survey was created with closed-ended questions that may not capture the full range of practice behaviors and attitudes. However, this method was necessary to avoid the plausible heterogeneity of the answers from open-text questions that may yield richer data but complicate analysis and interpretation of the results. Third, response rates ranged from about 15% to 21%, depending on the society and formula used. Thus, a bias due to survey nonresponses is possible; that is, individuals who chose to respond may differ with respect to the study variables from those who did not respond. Fourth, grouping different geographic areas into the category of “other countries” and receiving information only from the members of the ESGO, SGO, and IGCS might not reflect the actual practice of individual regions.
Although a subset of patients might not benefit from lymph node removal, it is extremely difficult to identify these patients preoperatively because of the uncontrollable variable of change in grade and depth of invasion on final histopathology.(26, 27) We believe that the application of the SLN technique following the Memorial Sloan Kettering Cancer Center algorithm(16, 18) is a valid alternative to lymphadenectomy in patients with early stage disease.
SLN mapping with cervical injection is gaining widespread acceptance for staging of EC among gynecologic oncologists worldwide. However, the standardization of this surgical approach (including the role of backup lymphadenectomy for “high-risk” cases) and a general consensus on postoperative management in patients with micrometastases and ITC are still controversial. The present survey highlights a marked heterogeneity in the management of patients with early stage EC among gynecologic oncologists worldwide. We hope our data will stimulate further prospective studies of adjuvant therapy in case of micrometastases and ITC and a move toward standardization of the management of uterine cancer.
Supplementary Material
Acknowledgments
Role of the Funding Source: This study was not externally funded.
Abbreviations
- EC
endometrial cancer
- ESGO
European Society of Gynecologic Oncologists
- ICG
indocyanine green
- IGCS
International Gynecologic Cancer Society
- ITC
isolated tumor cells
- NCCN
National Comprehensive Care Network
- RR1, RR3
response rate formula 1 or 3
- SGO
Society of Gynecologic Oncologists
- SLN
sentinel lymph node
- US
United States
Footnotes
Presented at the 20th International Meeting of the European Society of Gynaecological Oncology, Vienna, Austria, November 4-7, 2017.
Author Disclosure Statement: The authors have nothing relevant to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Colombo N, Creutzberg C, Amant F et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int J Gynecol Cancer. 2016;26: 2–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Corrado G, Cutillo G, Pomati G et al. Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Eur J Surg Oncol. 2015;41: 1074–81. [DOI] [PubMed] [Google Scholar]
- [6].Casarin J, Multinu F, Ubl DS et al. Adoption of Minimally Invasive Surgery and Decrease in Surgical Morbidity for Endometrial Cancer Treatment in the United States. Obstet Gynecol. 2018;131: 304–11. [DOI] [PubMed] [Google Scholar]
- [7].Fleming ND, Soliman PT, Westin SN et al. Impact of Lymph Node Ratio and Adjuvant Therapy in Node-Positive Endometrioid Endometrial Cancer. Int J Gynecol Cancer. 2015;25: 1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dowdy SC, Borah BJ, Bakkum-Gamez JN et al. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012;127: 5–10. [DOI] [PubMed] [Google Scholar]
- [9].Mariani A, Dowdy SC, Cliby WA et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109: 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Achouri A, Huchon C, Bats AS et al. Complications of lymphadenectomy for gynecologic cancer. Eur J Surg Oncol. 2013;39: 81–6. [DOI] [PubMed] [Google Scholar]
- [11].Yost KJ, Cheville AL, Al-Hilli MM et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. 2014;124: 307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bogani G, Multinu F, Dowdy SC et al. Incorporating robotic-assisted surgery for endometrial cancer staging: Analysis of morbidity and costs. Gynecol Oncol. 2016;141: 218–24. [DOI] [PubMed] [Google Scholar]
- [13].Abu-Rustum NR. Sentinel lymph node mapping for endometrial cancer: a modern approach to surgical staging. J Natl Compr Canc Netw. 2014;12: 288–97. [DOI] [PubMed] [Google Scholar]
- [14].Rossi EC, Kowalski LD, Scalici J et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18: 384–92. [DOI] [PubMed] [Google Scholar]
- [15].Holloway RW, Abu-Rustum NR, Backes FJ et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol. 2017;146: 405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koh WJ, Abu-Rustum NR, Bean S et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16: 170–99. [DOI] [PubMed] [Google Scholar]
- [17].American Association For Public Opinion Research. Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 9th ed: AAPOR; 2016. [Google Scholar]
- [18].Leitao MM Jr., Khoury-Collado F, Gardner G et al. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol Oncol. 2013;129: 38–41. [DOI] [PubMed] [Google Scholar]
- [19].The World Bank. Databank. 2018.
- [20].Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216: 459–76 e10. [DOI] [PubMed] [Google Scholar]
- [21].Tanner EJ, Sinno AK, Stone RL et al. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015;138: 542–7. [DOI] [PubMed] [Google Scholar]
- [22].Cormier B, Rozenholc AT, Gotlieb W et al. Sentinel lymph node procedure in endometrial cancer: A systematic review and proposal for standardization of future research. Gynecol Oncol. 2015;138: 478–85. [DOI] [PubMed] [Google Scholar]
- [23].Rossi EC, Jackson A, Ivanova A, Boggess JF. Detection of sentinel nodes for endometrial cancer with robotic assisted fluorescence imaging: cervical versus hysteroscopic injection. Int J Gynecol Cancer. 2013;23: 1704–11. [DOI] [PubMed] [Google Scholar]
- [24].Baiocchi G (2017). Sentinel Node Mapping Versus Sentinel Node Mapping With Systematic Lymphadenectomy in High Risk Endometrial Cancer: a Open Label, Non-inferiority, Randomized Trial. ClinicalTrials.gov identifier (NCT number): . [DOI] [PubMed]
- [25].Leblanc E; Gauthier H (2015). Randomized Study Comparing Sentinel Node (SN) Policy to Current French Initial Staging Protocols in Early Stage Endometrial Carcinomas at Intermediate and High Risk of Recurrence. ClinicalTrials.gov identifier (NCT number):
- [26].Antonsen SL, Jensen LN, Loft A et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer - a multicenter prospective comparative study. Gynecol Oncol. 2013;128: 300–8. [DOI] [PubMed] [Google Scholar]
- [27].Leitao MM Jr., Kehoe S, Barakat RR et al. Accuracy of preoperative endometrial sampling diagnosis of FIGO grade 1 endometrial adenocarcinoma. Gynecol Oncol. 2008;111: 244–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.