Abstract
Objective:
Dietary triglycerides (TG) are partially retained in the intestine within intracellular or extracellular compartments which can be rapidly mobilized in response to several stimuli, including glucose and glucagon-like peptide-2 (GLP-2). To elucidate the mechanism of intestinal lipid mobilization, this study examined the patterns and time course of lymph flow and TG following glucose and GLP-2 treatment in rats.
Approach and Results:
Lymph flow, TG concentration and TG output were assessed in mesenteric lymph cannulated rats in response to an intraduodenal (i.d.) lipid bolus followed 5 hours later by either: 1) i.d. saline + intraperitoneal (i.p.) saline (placebo), 2) i.d. glucose plus i.p. saline, 3) i.d. saline + i.p. GLP-2, or 4) i.d. glucose + i.p. GLP-2. GLP-2 and glucose administered alone or in combination stimulated total TG output to a similar extent, but the timing and pattern of stimulation differed markedly. Whereas GLP-2 rapidly increased lymph flow with no effect on lymph TG concentration or TG:apoB48 ratio (a surrogate marker of chylomicron size) compared to placebo, glucose transiently decreased lymph flow followed by delayed stimulation of lymph flow and increased lymph TG concentration and TG:apoB48 ratio.
Conclusions:
Glucose and GLP-2 robustly enhanced intestinal TG output in rats but with different effects on lymph flow, lymph TG concentration and chylomicron size. GLP-2 stimulated TG output primarily by enhancing lymph flow with no effect on chylomicron size, whereas glucose mobilized intestinal TG, stimulating secretion of larger chylomicrons. This suggests these two stimuli mobilize intestinal lipid by different mechanisms.
Keywords: lymph flow, triglycerides, chylomicrons, glucose, glucagon-like peptide-2
Subject codes: Lipids and Cholesterol, Metabolism, Physiology
Graphical abstract
INTRODUCTION
Hypertriglyceridemia, due to an expanded pool of triglyceride-rich lipoprotein (TRL) particles, is a component of the atherogenic dyslipidemia complex contributing to increased risk of cardiovascular disease1. In recent years we and others have discovered that the secretion of chylomicrons (CM), ie intestinally derived TRLs, is regulated not only by fat ingestion but also by intrinsic and extrinsic signals including lipid and non-lipid luminal nutrients2–4, hormones5,6 and neural activity7. Pharmacological targeting of CM production may present a therapeutic opportunity for ameliorating cases of dyslipidemia, thereby reducing the risk of cardiovascular disease.
During the postprandial phase, dietary TG is hydrolyzed in the intestinal lumen to 2-monoacylglycerol and fatty acids, which are absorbed by enterocytes and re-esterified to TG at the endoplasmic reticulum (ER) membrane8. The majority of TG accumulating in the ER fuse with lipid-poor apolipoprotein B48 (apoB48) during its co-translational translocation into the ER lumen to form prechylomicrons, in a process facilitated by microsomal triglyceride transfer protein (MTP)9. Prechylomicrons undergo further processing in the Golgi apparatus into mature CMs, which are subsequently secreted across the basolateral membrane into the lamina propria of the intestinal mucosa. Secreted CMs migrate across the lamina propria towards the blind-ended villus lacteal via a convective process10. CMs enter lacteals primarily by size exclusion and drain into the mesenteric lymph duct before entering the general circulation at the left subclavian vein.
Alternatively, TG accumulating between ER leaflets can bud off into the cytosol to form cytoplasmic lipid droplets (CLDs)11. CLDs serve as a transient intestinal lipid storage pool, which we have observed in enterocytes up to 10 hours after a meal in humans 4and are postulated to exist at least up to 18 hours post-meal7. Similarly, intestinal lipid stores have been shown to be retained for up to 12 hours post-meal in rodents12. In addition to CLDs, CMs have been identified in the intracellular secretory pathway, lamina propria and mesenteric lacteals in humans3,4 and rodents12,13,14.
Several stimuli have been shown to mobilize intestinal lipid stores including oral glucose3,4, glucagon-like peptide-2 (GLP-2)5, a subsequent meal15, and sham fat feeding7. Oral glucose ingested 5 hours after a high-fat meal decreased jejunal3 and duodenal4 lipid stores in humans. We demonstrated in humans a coincidental increased plasma TG, primarily in the CM fraction, decreased CLD number and a shift in the size distribution of CLDs towards predominantly small CLDs in enterocytes suggesting mobilization of intracellular TG stores4. In healthy men, a single subcutaneous dose of GLP-2 administered during intraduodenal lipid infusion and a pancreatic clamp increased plasma and CM TG as well as apoB485. Importantly, GLP-2 can elicit these effects when administered 7 hours after a high-fat meal, which we attributed to mobilization of an enterocyte pool of apoB48 or preformed CMs5. Similar responses have been seen ex vivo in jejunal tissue, and in vivo in mice and hamsters16,17. In hamsters, GLP-2 increased intestinal lipid absorption and CM secretion, and mobilized intestinal TG stores 5 hours after an oil bolus16,17.GLP-2 receptors are expressed along the gastrointestinal tract most abundantly on subepithelial myofibroblasts, enteroendocrine cells, and enteric neurons but are notably absent from enterocytes18–20. GLP-2 receptors are abundant in circular and longitudinal muscle of the non-grandular stomach and duodenum as well as the duodenal lamina propria of the mucosa layer21. GLP-2 stimulation of mesenteric blood flow in humans22 and pigs23 as well as GLP-2-mediated chylomicron secretion in rodents17 were shown to be nitric oxide dependent suggesting GLP-2 is likely to mobilize intestinal lipid stores via indirect mechanisms.
The mechanisms by which intestinal lipid stores are mobilized are unclear. We hypothesized that glucose and GLP-2 access distinct lipid pools in the intestine for mobilization, likely through differing mechanisms. Therefore, these two stimuli administered alone or in combination could provide insights into mechanisms of intestinal TG mobilization. We examined this with the conscious rat mesenteric lymph duct cannulation model, which allows measurement of lymph flow rate, TG concentration and TG output. We did not demonstrate additive or synergistic effects of glucose and GLP-2. Instead, we showed strikingly different patterns of lymph TG dynamics by GLP-2 and glucose, supporting the existence of differing mechanisms of intestinal lipid mobilization.
MATERIALS & METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
Male Sprague-Dawley rats weighing 250–300g (Harlan Laboratories, Indianapolis, IN) were housed in pairs and acclimatized for two weeks in a temperature- and humidity-controlled facility prior to the experiment. Rats were maintained on a standard rodent diet (LM-485 Mouse/Rat Sterilizable Diet; Harlan Laboratories, Madison, WI) with ad libitum access to water and a 12-hour light/dark cycle at the University of Cincinnati Laboratory Animal Medical Services. All animal procedures were approved by University of Cincinnati Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Mesenteric Lymph Duct & Duodenal Cannulation
Following an overnight fast, the mesenteric lymph duct and duodenum were cannulated under inhalant isoflurane anaesthesia, as previously described24. Briefly, the mesenteric lymph duct was cannulated according to procedures described by Bollman et al.,25 in which a polyvinyl chloride (PVC) tubing (0.2mm ID; 0.50mm ID) was advanced into the mesenteric lymph duct and secured by a drop of cyanoacrylate glue. The same tubing material was placed in the intraperitoneal cavity to facilitate intraperitoneal delivery of experimental treatments. Silicone tubing (0.5mm ID, 0.8mm OD) was advanced approximately 1 cm into the duodenum via a fundal incision in the stomach which was secured by purse-string suture. Pre-operative buprenorphine (Buprenex, Reckitt Bencksizer Inc., Slough, UK) was provided as analgesic. Following surgery, rats were housed in a Bollman restraint cage with air temperature maintained at 26°C. Immediately following surgery, duodenal infusion of saline containing 5% glucose was commenced at a rate of 3 ml/hour for 5–8 hours. To simulate an overnight fast, saline without glucose was infused intraduodenally at a rate of 3ml/hour and continued throughout the remainder of the study.
Study Protocol
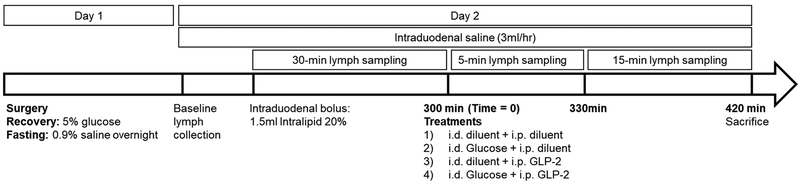
Following an overnight fast with intraduodenal saline infusion, a fasted lymph sample was collected. A bolus of 1.5 ml Intralipid 20% (Sigma Aldrich, St. Louis, MO) was administered via the intraduodenal cannula. Intraduodenal saline infusion at a rate of 3 ml/hr was continued for the duration of the study. At 300 minutes post-Intralipid bolus (time = 0), rats were randomized to receive one of four treatments: 1) saline (1 ml, i.d.) + phosphate buffered saline (PBS, 0.5 ml, i.p.) (Placebo, n=10); 2) glucose (0.2 g in 1 ml saline, i.d.) + PBS (0.5 ml, i.p.) (GLC, n=11); 3) saline (1 ml, i.d.) + GLP-2 (75 ug in 0.5 ml PBS, i.p.) (GLP-2, n=10); 4) glucose (0.2 g in 1 ml saline, i.d.) + GLP-2 (75 ug in 0.5 ml PBS, i.p.) (GLP-2 + GLC, n=10) (Figure 1). GLP-2 was synthesized at >90% purity as a DPP-IV resistant analog [Gly2]-GLP-2 (Pepceuticals, Inc., Leichestershire, UK). Lymph samples were collected on ice for five hours prior to treatment and two hours post-treatment in 5-, 15- and 30-minute intervals (Figure 1). Euthanasia was performed at 120 minutes post-treatment by lethal intraperitoneal pentobarbital (100 mg/kg Euthanyl, Vetoquinol Inc., Lure, France).
Figure 1.
Study protocol.mesenteric lymph fluid was collected over 420 minutes following an intraduodenal lipid bolus
Sample Analyses
Lymph samples were refrigerated at 4°C and analyzed for TG concentration on the day of collection using a commercial kit (Randox, Crumlin, Northern Ireland). Western blots for apoB48 were performed by loading 7 ul of lymph fluid per well on 4–20% SDS-PAGE gels for separation and transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 hour with 5% non-fat milk in 1% Tween-20 in Tris-buffered saline. Membranes were incubated with primary antibody goat anti-mouse apoB (1:8000) at 4° C for overnight, followed by incubation with secondary rabbit anti-goat (1:10,000, Agilent, Cat no. P0449) for 1 hour at room temperature. Bands were visualized with Immobilon Western Chemiluminescent HRP substrate (EMD Millipore, Billerica, MA). Total Lab Quant Analysis Software (Fotodyne, Hartland, WI) was used to determine band density.
Calculations and Statistical Analysis
Ten animals per treatment provides 80% power to detect a 13% difference in treatment means given a standard deviation of 10%. TG output was calculated as the product of lymph flow and TG concentration. Similarly, relative apoB48 output was calculated as the product of lymph flow and apoB48 abundance. Area under the curve (AUC) following treatment was calculated by trapezoid method. Fold-change was calculated at each time point post-treatment as a fraction of time zero. Slope of rise was calculated from nadir to peak, slope of decline was calculated from peak to post-peak minimum. Time courses post-treatment were analyzed by PROC MIXED of SAS (version 9.4, SAS Institute Inc., Cary, NC) with time as a repeated measure, where time, treatment and time x treatment interaction were considered fixed effects and block was considered a random effect. First-order autoregressive covariance structure was assumed, and least-square means were separated by the PDIFF option of SAS. The difference between each pair of means was adjusted for multiple comparisons by Tukey adjustment. Characteristics of time-courses were analyzed by block and treatment using PROC MIXED of SAS with least-square means separated by Tukey adjustment. Data are presented as least-square means ± SEM, unless otherwise stated. Significance was declared at p < 0.05, trends were declared at 0.05 < p ≤ 0.10.
RESULTS
Lymph flow
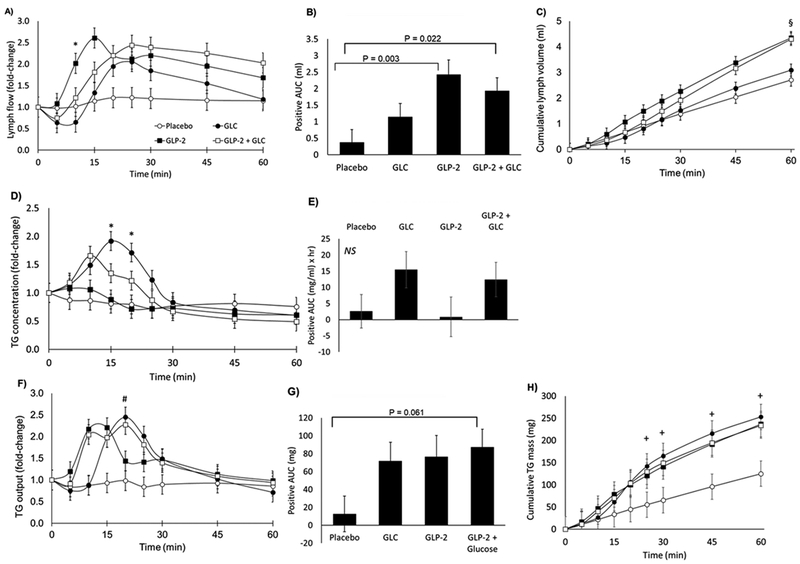
Over the initial 5 hours of observation prior to administration of i.d. glucose and/or i.p. GLP-2 or corresponding placebo, lymph flow was approximately 2.5 ml/hr (Figure S1A). Intraduodenal glucose transiently decreased lymph flow by 51% over the first 15 minutes before increasing (Figure 2A), resulting in net AUC of lymph flow after one hour that did not differ between glucose and placebo (Figure 2B). GLP-2 increased lymph flow within 5 minutes, with flow remaining significantly elevated by the end of the first hour (Table 1). At 10 minutes, lymph flow fold-change was significantly greater for GLP-2 compared to glucose (p = 0.019, Figure 2A). GLP-2 + GLC did not differ significantly from GLP-2 or glucose alone at 10 minutes.
Figure 2.
A) Fold-change in lymph flow rate B) Positive area-under-the-curve of lymph flow (ml); C) Cumulative lymph volume (ml); D) Fold-change in triglyceride (TG) concentration; E) Positive area-under-the-curve of TG concentration (mg/ml) × hr); F) Fold-change in TG output; G) Positive area-under-the-curve of TG output (mg); H) Cumulative TG mass (mg)
Table 1:
Descriptive characteristics of fold-change time-courses for lymph flow, triglyceride (TG) concentration, and TG output following administration of the treatments at 300mins
| Placebo | GLC | GLP-2 | GLP-2 + GLC | SEM | P-value | |
|---|---|---|---|---|---|---|
| Lymph flow | ||||||
| Time to peak (min) | 28.8ab | 32.7ab | 14.6b | 35.1a | 5.50 | 0.023 |
| Maximal fold change | 1.39b | 2.44ab | 2.68a | 2.73ab | 0.38 | 0.033 |
| Slope of rise (fold change/hr) | 1.45 | 3.93 | 6.54 | 5.01 | 1.67 | 0.125 |
| Slope of decline (fold change/hr) | −1.24 | −2.21 | −1.43 | −1.31 | 0.49 | 0.334 |
| Fold change at 60 minutes | 1.15b | 1.18b | 1.68ab | 2.03a | 0.20 | 0.009 |
| Cumulative lymph volume at 60 minutes (ml) | 2.72b | 3.27ab | 4.40a | 4.23a | 0.39 | 0.002 |
| Triglyceride concentration | ||||||
| Time to peak (min) | 19.4 | 12.0 | 9.46 | 11.45 | 3.26 | 0.169 |
| Maximal fold change | 0.98b | 2.21a | 1.19ab | 1.76ab | 0.29 | 0.019 |
| Slope of rise (fold change/hr) | 1.45 | 5.64 | 1.91 | 4.29 | 1.86 | 0.228 |
| Slope of decline (fold change/hr) | −0.94b | −2.77a | −1.32ab | −2.22ab | 0.44 | 0.018 |
| Fold change at 60 minutes | 0.75 | 0.60 | 0.62 | 0.49 | 0.08 | 0.131 |
| Cumulative TG mass at 60 minutes (mg) | 129 | 230 | 239 | 228 | 35.8 | 0.058 |
| TG output | ||||||
| Time to peak (min) | 19.9 | 22.9 | 13.9 | 18.4 | 3.87 | 0.435 |
| Maximal fold change | 1.18b | 3.05a | 2.67ab | 3.17a | 0.48 | 0.021 |
| Slope of rise (fold change/hr) | 1.21b | 8.07a | 6.55ab | 10.4a | 2.59 | 0.049 |
| Slope of decline (fold change/hr) | −0.67b | −3.87a | −2.14ab | −3.30a | 0.64 | 0.005 |
| Fold change at 60 minutes | 0.778 | 0.753 | 0.973 | 0.979 | 0.15 | 0.260 |
Superscripts of differing letters within a row indicate significant differences as identified by Tukey separation
Calculated total lymph volume as AUC of lymph volume (Figure 2B) and cumulative lymph volume (Figure 2C) over one hour indicated significantly greater lymph volume with GLP-2 and GLP-2 + GLC compared to placebo, with no difference from glucose (Table 1). Other analyses of lymph flow, including the slope of the rise to peak and decline from peak did not differ by treatment (Table 1).
Lymph TG concentration and TG output
Fasting lymph TG concentration was approximately 20 mg/ml and rose to ~80mg/ml following the lipid bolus (Figure S1B). It is interesting to note that lymph TG concentration and output increased after the duodenal lipid bolus administered at 0 min whereas lymph flow did not increase appreciably in response to the lipid bolus. Glucose induced a maximum 2.21-fold increase in TG concentration, which differed significantly from placebo (Table 1). Fold-change was significantly greater for glucose compared to GLP-2 at 15 minutes (p = 0.008) and 20 minutes (p = 0.014) post-treatment (Figure 2D).
For TG concentration, the time-to-peak and slope of the rise to peak did not differ by treatment. At one-hour post-treatment TG concentration was below baseline for all treatments with no difference between treatments (Table 1).
TG output (mg/hr) was calculated as the product of lymph flow (ml/hr) and TG concentration (mg/ml) at each time point (Figure 2D). At 20 minutes, TG output was significantly greater for glucose compared to placebo (p = 0.03, Figure 2F). AUC of TG output (Figure 2G) tended to be greater for GLP-2 + GLC compared to placebo. Active treatments increased lymph TG output, thus cumulative TG mass in GLC, GLP-2 and GLC+GLP-2 were higher than in placebo at all time points after 25 minutes (p<0.05) (Figure 2H). No significant differences were observed among the three active treatments at any time point.
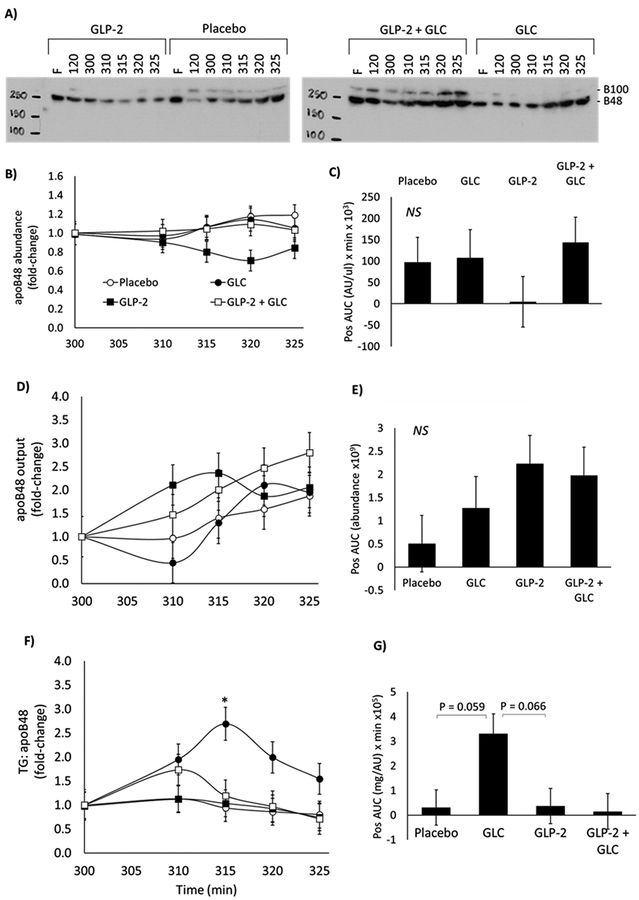
Lymph apolipoprotein B48 abundance, output and ratio of TG: apoB48
Lymph apoB48 abundance was quantified by Western blot (Figure 3A,B). Output was calculated as the product of lymph flow (μl/hr) and apoB48 abundance (AU/ul) and presented in Figure 3D as fold-change relative to time zero. AUC of apoB48 abundance (Figure 3C) and output (Figure 3E) up to 25 minutes post-treatment did not differ by treatment. As a surrogate measure of particle size, the ratio of lymph TG: apoB48 was calculated at each time-point, with a larger ratio indicative of larger particle size and vice versa. Glucose increased TG: apoB48 compared to GLP-2 at 15 minutes post-treatment (p= 0.044) (Figure 3F). There was a tendency for increased TG: apoB48 AUC with glucose compared to placebo (p=0.059) and compared to GLP-2 (p=0.066) (Figure 3G).
Figure 3.
A) Representative western blots of apolipoprotein B in lymph; B) apoB48 abundance normalized to time of treatment; C) Positive area-under-the-curve of apoB48 abundance; D) Fold-change in apoB48 output; E) Positive area-under-the-curve of apoB48 output; F) Fold-change in the ratio of lymph triglyceride (TG) to apoB48 relative to time of treatment; G) Positive area-under-the-curve of lymph triglyceride to apoB48 ratio
DISCUSSION
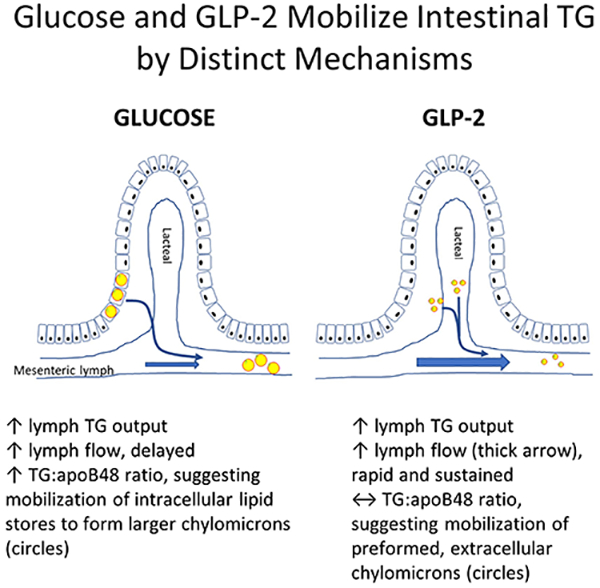
Enteral lipid stores are present in rodents and humans for 18 hours (and possibly longer) after a meal and can be mobilized in response to various stimuli2–5, 17. Here we assessed whether glucose and GLP-2 administered alone or in combination 5 hours after a high-fat bolus have differing or additive effects on intestinal TG mobilization in a conscious lymph-cannulated rat model. We did not find evidence of an additive effect, rather we demonstrated that intraperitoneal GLP-2 and intraduodenal glucose administered alone or together stimulated TG output to a similar extent but by different mechanisms and time course of stimulation. Lymph flow was rapidly and robustly stimulated by GLP-2 and remained elevated for up to one hour post-treatment. In contrast, glucose induced a transient reduction in lymph flow followed by stimulation. GLP-2 had no effect on lymph TG concentration or TG: apoB48 ratio compared to placebo, whereas glucose increased lymph TG concentration and TG:apoB48 ratio, the latter suggestive of an increase in chylomicron particle size. These results suggest that GLP-2 stimulated TG output primarily by enhancing lymph flow with its cargo of secreted chylomicrons.
Intraduodenal glucose administered 5 hours after a high-fat bolus transiently decreased lymph flow over the first 15 minutes. Previous studies in rats have shown no effect26, decrease10 or increase 27 in lymph flow with luminal glucose. This discrepancy could be due to differing concentrations and/or osmolarity of the solution. Isotonic glucose in the jejunal lumen did not affect lymph flow but increased motility26. Intraduodenal administration of low glucose concentrations (5.6–28 mM vs 1100 mM in the current study) increased lymph flow, reaching a plateau with 28 mM glucose. Stimulation of lymph flow was reportedly due to enhanced absorption of water, sodium or both 27. The relatively high concentration and hyper-osmolarity of glucose in the current study likely drove the initial reduction in lymph flow. In contrast to isotonic saline, which has an osmolarity of 0.3 osm/L, the intraduodenal glucose bolus had an osmolarity of 1.41 osm/L. This may have transiently attenuated intestinal water absorption and thus decreased lymph flow. Hyperosmolar intraduodenal lipid infusion containing 20 mM maltose, a disaccharide of glucose hydrolyzed by intestinal maltase, induced interstitial dehydration within the jejunal lamina propria and decreased mesenteric lymph flow to a nadir of 0.17 ml/hr in rats 10. In the current study, glucose decreased lymph flow to a nadir of 1.5 ml/hr at 10 minutes post-treatment suggesting a transient, less drastic dehydration of the interstitial space. Tso et al., 1985 demonstrated that interstitial dehydration doubled the time required for CM appearance in lymph compared to normal hydration. In particular, when lymph flow dropped below 2.4 ml/hr with infusion of a hyperosmolar solution, time required for CM appearance was inversely correlated to lymph flow 10. In the current study, lymph flow decreased to 1.5 ml/hr with glucose treatment. Whether the coincidental 2.2-fold increase in TG concentration was an artifact of decreased lymph flow and/or due to CM mobilization is unclear. Compared to placebo or GLP-2, time-to-peak of apoB48 abundance was not affected by glucose in the current study, therefore the plausible modest interstitial dehydration likely did not hinder CM movement through the lamina propria and lacteals.
Area-under-the-curve of lymph apoB48 abundance and apoB48 output one hour after treatment did not differ between treatments, suggesting that the total quantity of secreted chylomicrons was not affected by treatment. Glucose increased the lymph TG:apoB48 ratio, suggesting an increase in chylomicron size. In humans, we have recently demonstrated that glucose reduced the total lipid content of enterocytes as visualized by electron microscopy, coinciding with an increase in plasma TG, primarily in CM TG4. The distribution of enterocyte CLDs shifted towards predominance of small CLDs suggesting mobilization of intracellularly stored TG4. Therefore, the greater TG:apoB48 ratio may indicate that intraduodenal glucose mobilized CLDs leading to expansion of chylomicron particles. The cellular mechanisms of glucose-mediated CM mobilization are not known but one possibility may involve enhanced Golgi processing of pre-chylomicrons in the secretory pathway4. Intestinal metabolic flexibility and fuel switching in response to altered glucose and lipid supply has been demonstrated in vivo in rodents and swine models28–31. This has been suggested to be independent of gene expression changes32. Therefore, in the current study, it is possible that the intraduodenal glucose bolus partially spared enterocyte fatty acid oxidation and favoured incorporation into CMs, contributing to the formation of larger CMs. Although not assessed, gene expression was likely not a major regulator of this effect as larger CMs were apparent by 15 minutes post-treatment.
We can not completely rule out a contribution of post-enterocyte mechanisms of glucose-mediated chylomicron mobilization. For example, glucose ingestion and subsequent hyperinsulinemia induced sympathetic activation and vasodilation in humans33 and enhanced intestinal blood flow in rats34. Luminal glucose induces endogenous secretion of GLP-1 and GLP2, therefore we cannot rule out a role for endogenous GLP-1 and GLP-2 in modulating glucose-stimulated lymph TG and apoB48 appearance. However, endogenous GLP-1 and GLP-2 likely have modest contributions to plasma TG and apoB48 as observed in humans35.
In contrast to glucose, intraperitoneal GLP-2 rapidly and robustly increased lymph flow with negligible effects on TG concentration. Saline was infused intraduodenally at a rate of 3 ml/hr. After one hour, GLP-2 alone or in combination with glucose increased cumulative lymph volume beyond the volume of saline (3ml) delivered by continuous intraduodenal infusion, demonstrating contribution of fluid exceeding absorption from the intestinal lumen. The mechanism of lymph flow stimulation is not known but is consistent with activation of GLP-2 receptors on subepithelial myofibroblasts in the mucosal lamina propria and in muscle layers of the gastrointestinal tract 36,37 which may have induced rapid contraction of lacteals and lymphatic ducts. Lymph flow, rather than concentration/abundance, is the primary factor driving TG and apoB48 outputs in response to GLP-2. We speculate that output, which incorporates lymph flow and concentration, is a better indicator of lipid delivery to plasma and more closely reflects previous studies which sampled the plasma compartment. Although GLP-2 did not increase lymph TG concentration or apoB48 abundance, output was increased. This is in accordance with previous studies in humans which demonstrated rapid increases in plasma TRL-TG and TRL-apoB48 following GLP-2 administration 7 hours after a meal 5.Similarly, GLP-2 increased TRL-TG in hamsters 5 hour after a lipid bolus and increased postprandial TRL-TG and TRL-apoB48 in mice18 36,37
We speculate that GLP-2 predominantly mobilized preformed CMs residing in post-enterocyte pools38. Firstly, the rapid stimulation of lymph flow reaching a peak at 10 minutes did not coincide with a dilution of TG concentration or apoB48 abundance to levels below that seen with placebo. This indicates that the additional lymph fluid was not lipid- or CM-poor. Secondly, TG output and apoB48 output increased along with lymph flow within 10 minutes of treatment, suggesting rapid mobilization of apoB48-containing particles. The subsequent decline in apoB48 abundance and output after 15 minutes despite sustained elevated lymph flow may be indicative of depletion of the post-enterocyte CM pool. Lastly, GLP-2 did not elicit any change in the ratio of TG:apoB48 suggesting the size of the particles was not altered and thus mobilization of CLDs or increase in fatty acid uptake were likely not significant contributors to TG appearance. Future studies will employ transmission electron microscopy to visualize enterocytes, as previously described 4 and lymph fluid for precise quantification of lipid depots in specific compartments.
We can not fully exclude a contribution of intra-enterocyte lipid pools to the GLP-2 response. In mice, stimulation of intestinal lipoprotein production in response to GLP-2 was dependent on luminal CD36/fatty acid translocase demonstrating GLP-2 regulation of dietary fatty acid uptake. Similarly, GLP-2 treatment of ex vivo jejunal fragments increased glycosylation of enterocyte CD36/fatty acid translocase16. In hamsters, intraperitoneal GLP-2 administered 5 hours after an oral fat load induced rapid appearance of preformed lipid-poor chylomicrons as indicated by a 2-fold increase in TRL-apoB48 mass within 30 minutes with no change in TRL-TG. In addition, the increase in TRL-apoB48 observed in hamsters occurred with no change in jejunal lipid content or MTP activity suggesting stimulation of fatty acid uptake and CM assembly may not be required for the early mobilization of lipid-poor chylomicrons17. Consistent with this, in our current study in rats, GLP-2 administered 5 hours after an intraduodenal lipid load resulted in a rapid increase in lymph apoB48 output with no effect on TG:apoB48 ratio suggestive of minimal effect on CM particle size. In hamsters, by 90 minutes post-treatment, GLP-2 increased TRL-TG and MTP activity suggesting eventual increased lipidation of chylomicrons in response to GLP-217. Interestingly, these affects were blocked by pre-treatment with the nitric oxide synthase inhibitor L-NAME, implicating a NO-dependent mechanism, involving modulation of mesenteric blood flow, in GLP-2-mediated CM mobilization. Whether GLP-2 mediates CM mobilization by similar mechanisms in humans is unknown. Preliminary data suggests NO-dependent increase in mesenteric blood flow may not be required for GLP-2 mediated CM mobilization in humans 22.
The pharmacological dose of GLP-2 administered in this study may have altered endogenous GLP-1 and GLP-2 secretion. A negative feedback loop has been proposed for GLP-1 regulation of L-cell function to inhibit peptide YY secretion39. It is plausible that GLP-2 administration could alter endogenous peptide secretion; however, the pharmacological GLP-2 dose given here would likely overshadow any minor contribution of altered peptide secretion.
The lack of additive effect was surprising due to the speculation that glucose and GLP-2 act on differing sites to mobilize TG stores. We speculate that there may be a “mass action” effect with both stimulants. Although glucose’s main effect is to mobilize intracellular lipid stores, the increased secretion of chylomicrons into the lamina propria may push extracellularly stored chylomicrons into the lacteals. Similarly, if GLP-2 mainly acts extracellularly, a feedback signal may stimulate further chylomicron secretion from the enterocyte into the lamina propria when this space has been cleared of lipid stores, although this has not been directly proven. Therefore, it is possible that total TG output is not additive as illustrated in this study because both stimulants ultimately mobilize all lipid stores despite differing primary sites of action.
In summary, interestingly and somewhat unexpectedly we demonstrate here that enteral lipid stores retained 5 hours after a high-fat bolus are mobilized by intraduodenal glucose and intraperitoneal GLP-2 with similar potency but by differing mechanisms. Together with our findings in humans, glucose increases TG concentration and intestinal TG output perhaps by mobilizing intracellular lipid pools whereas GLP-2 acts by rapidly increasing lymph flow to mobilize secreted chylomicrons residing in the lamina propria or lacteals4,5. Further studies are required to elucidate the molecular mechanisms of GLP-2 and glucose-mediated CM secretion, which may reveal crucial regulatory sites that can be pharmacologically targeted to reduce atherosclerotic risk.
Supplementary Material
Table 2:
Descriptive characteristics of fold-change time-courses for lymph apolipoprotein (apoB48) abundance, apoB48 output and ratio of lymph TG: apoB48
| Placebo | GLC | GLP-2 | GLP-2 + GLC | SEM | P-value | |
|---|---|---|---|---|---|---|
| apoB48 abundance | ||||||
| Time to peak (min) | 22a | 14ab | 2b | 10ab | 3.22 | 0.004 |
| Maximal fold change | 1.35 | 1.19 | 1.02 | 1.31 | 0.113 | 0.201 |
| apoB48 output | ||||||
| Time to peak (min) | 24 | 18 | 17 | 18 | 2.87 | 0.329 |
| Maximal fold change | 1.99 | 2.36 | 2.48 | 2.95 | 0.62 | 0.746 |
| TG: apoB48 | ||||||
| Time to peak (min) | 12.2 | 13.9 | 12.2 | 10.9 | 3.375 | 0.898 |
| Maximal fold change | 1.29 | 2.73 | 1.15 | 1.73 | 0.401 | 0.056 |
Superscripts of differing letters within a row indicate significant differences as identified by Tukey separation
HIGHLIGHTS.
Triglycerides are retained for several hours after a meal as storage pools in the intestine which can be mobilized by stimuli
Glucose and GLP-2 mobilize intestinal lipid stores with differential effects on lymph flow and lymph TG concentration
The underlying mechanisms of glucose- and GLP-2-mediated lipid mobilization may help identify therapeutic targets for treatment of hypertriglyceridemia
ACKNOWLEDGEMENTS
The authors wish to acknowledge the technical assistance of Mack Yang and Chih-Wei Ko (University of Cincinnati).
Sources of funding
This work was supported by an operating grant from Canadian Institute of Health Research. G.F.L. holds the Drucker Family Chair in Diabetes Research and the Sun Life Financial Chair in Diabetes. P.S. is the recipient of a Diabetes Action Canada Post-Doctoral Fellowship award. This study was supported by the Cincinnati Mouse Metabolic Phenotype Center supported by a grant from NIH DK059630
Abbreviations:
- CLD
cytoplasmic lipid droplet
- CM
chylomicron
- GLP-2
glucagon-like peptide-2
- MTP
microsomal triglyceride transfer protein
- TG
triglyceride
- TRL
triglyceride-rich lipoprotein
Footnotes
Conflicts on Interest
The authors have nothing to declare
REFERENCES
- 1.Lewis GF, Xiao C, Hegele RA. Hypertriglyceridemia in the genomic era: A new paradigm. Endocr Rev. 2015;36:131–147. [DOI] [PubMed] [Google Scholar]
- 2.Jackson KG, Robertson MD, Fielding BA, Frayn KN, Williams CM. Olive oil increases the number of triacylglycerol-rich chylomicron particles compared with other oils: an effect retained when a second standard meal is fed. Am J Clin Nutr. 2002;76:942–949. doi: 10.1093/ajcn/76.5.942 [DOI] [PubMed] [Google Scholar]
- 3.Robertson MD, Parkes M, Warren BF, et al. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut. 2003;52:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao C, Stahel P, Carreiro AL, et al. Oral glucose mobilizes triglyceride stores from the human intestine. Cell Mol Gastroenterol Hepatol. 2019;7:313–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dash S, Xiao C, Morgantini C, Connelly PW, Patterson BW, Lewis GF. Glucagon-Like Peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology. 2014;147:1275–1284. doi: 10.1053/j.gastro.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hein GJ, Baker C, Hsieh J, Farr S, Adeli K. GLP-1 and GLP-2 as Yin and Yang of Intestinal Lipoprotein Production: Evidence for Predominance of GLP-2-Stimulated Postprandial Lipemia in Normal and Insulin-Resistant States. Diabetes. 2013;62:373–381. doi: 10.2337/db12-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez–Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139:1538–1548. doi: 10.1053/j.gastro.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansbach CM, Siddiqi SA. The biogenesis of chylomicrons. Annu Rev Physiol. 2010;72:315–333. doi: 10.1146/annurev-physiol-021909-135801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White DA, Bennett AJ, Billett MA, Salter AM. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br J Nutr. 1998;80:219–229. [PubMed] [Google Scholar]
- 10.Tso P, Pitts V, Granger DN. Role of lymph flow in intestinal chylomicron transport. Am J Physiol-Gastrointest Liver Physiol. 1985;249:G21–G28. [DOI] [PubMed] [Google Scholar]
- 11.D’Aquila T, Hung Y-H, Carreiro A, Buhman KK. Recent discoveries on absorption of dietary fat: Presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta. 2016;1861:730–747. doi: 10.1016/j.bbalip.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Lee B, Buhman KK, Cheng J-X. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahara E, Mantani Y, Udayanga KGS, et al. Ultrastructural demonstration of the absorption and transportation of minute chylomicrons by subepithelial blood capillaries in rat jejunal Villi. J Vet Med Sci. 2013;75:1563–1569. doi: 10.1292/jvms.13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung Y-H, Carreiro AL, Buhman KK. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochim Biophys Acta BBA -Mol Cell Biol Lipids. 2017;1862:600–614. doi: 10.1016/j.bbalip.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans K, Kuusela PJ, Cruz ML, Wilhelmova I, Fielding BA, Frayn KN. Rapid chylomicron appearance following sequential meals: effects of second meal composition. Br J Nutr. 1998;79:425. doi: 10.1079/BJN19980072 [DOI] [PubMed] [Google Scholar]
- 16.Hsieh J, Longuet C, Maida A, et al. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. doi: 10.1053/j.gastro.2009.05.051 [DOI] [PubMed] [Google Scholar]
- 17.Hsieh J, Trajcevski KE, Farr SL, et al. Glucagon-like peptide 2 (GLP-2) stimulates postprandial chylomicron production and postabsorptive release of intestinal triglyceride storage pools via induction of nitric oxide signaling in male hamsters and mice. Endocrinology. 2015;156:3538–3547. doi: 10.1210/EN.2015-1110 [DOI] [PubMed] [Google Scholar]
- 18.Pedersen J, Pedersen NB, Brix SW, et al. The glucagon-like peptide 2 receptor is expressed in enteric neurons and not in the epithelium of the intestine. Peptides. 2015;67:20–28. doi: 10.1016/j.peptides.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 19.Guan X, Karpen HE, Stephens J, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 20.Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. Localization and activation of glucagon-Like peptide-2 receptors on vagal afferents in the rat. Endocrinology. 2007;148:1954–1962. doi: 10.1210/en.2006-1232 [DOI] [PubMed] [Google Scholar]
- 21.Wismann P, Barkholt P, Secher T, et al. The endogenous preproglucagon system is not essential for gut growth homeostasis in mice. Mol Metab. 2017;6:681–692. doi: 10.1016/j.molmet.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao C, Stahel P, Dash S, Morgantini C, Lewis GF. Investigation of the role of enteric blood flow in mediating GLP-2 stimulation of triglyceride mobilization and chylomicron secretion in the human intestine. Atheroscler Suppl. 2018;32:29. [Google Scholar]
- 23.Guan X, Stoll B, Lu X, et al. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets. Gastroenterology. 2003;125:136–147. doi: 10.1016/S0016-5085(03)00667-X [DOI] [PubMed] [Google Scholar]
- 24.Kohan AB, Yang Q, Xu M, Lee D, Tso P. Monosodium glutamate inhibits the lymphatic transport of lipids in the rat. Am J Physiol - Gastrointest Liver Physiol. 2016;311:G648–G654. doi: 10.1152/ajpgi.00342.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med. 1948;33:1349–1352. [PubMed] [Google Scholar]
- 26.Lee JS. Relationship between intestinal motility, tone, water absorption and lymph flow in the rat. J Physiol. 1983;345:489–499. doi: 10.1113/jphysiol.1983.sp014991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JS. Luminal and plasma glucose concentrations on intestinal fluid absorption and lymph flow. Am J Physiol-Gastrointest Liver Physiol. 1987;252:G568–G573. doi: 10.1152/ajpgi.1987.252.4.G568 [DOI] [PubMed] [Google Scholar]
- 28.de Wit NJW, Boekschoten MV, Bachmair E-M, et al. Dose-dependent effects of dietary fat on development of obesity in relation to intestinal differential gene expression in C57BL/6J mice. PLoS ONE. 2011;6:e19145. doi: 10.1371/journal.pone.0019145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petit V, Arnould L, Martin P, et al. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res. 2007;48:278–287. doi: 10.1194/jlr.M600283-JLR200 [DOI] [PubMed] [Google Scholar]
- 30.Habold C, Foltzer‐Jourdainne C, Maho YL, Lignot J-H, Oudart H. Intestinal gluconeogenesis and glucose transport according to body fuel availability in rats. J Physiol. 2005;566:575–586. doi: 10.1113/jphysiol.2005.085217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Der Schoor SRD, Van Goudoever JB, Stoll B, et al. The pattern of intestinal substrate oxidation is altered by protein restriction in pigs. Gastroenterology. 2001;121:1167–1175. doi: 10.1053/gast.2001.29334 [DOI] [PubMed] [Google Scholar]
- 32.Sinha N, Suarez-Diez M, Hooiveld GJEJ, Keijer J, Martin dos Santos V, van Schothorst EM. A constraint-based model analysis of enterocyte mitochondrial adaptation to dietary interventions of lipid type and lipid load. Front Physiol. 2018;9. doi: 10.3389/fphys.2018.00749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott EM, Greenwood JP, Vacca G, Stoker JB, Gilbey SG, Mary DASG. Carbohydrate ingestion, with transient endogenous insulinaemia, produces both sympathetic activation and vasodilatation in normal humans. Clin Sci. 2002;102:523–529. doi: 10.1042/cs1020523 [DOI] [PubMed] [Google Scholar]
- 34.Bohlen HG, Unthank JL. Rat intestinal lymph osmolarity during glucose and oleic acid absorption. Am J Physiol-Gastrointest Liver Physiol. 1989;257:G438–G446. doi: 10.1152/ajpgi.1989.257.3.G438 [DOI] [PubMed] [Google Scholar]
- 35.Matikainen N, Björnson E, Söderlund S, et al. Minor Contribution of Endogenous GLP-1 and GLP-2 to Postprandial Lipemia in Obese Men Maedler K, ed. PLOS ONE. 2016;11:e0145890. doi: 10.1371/journal.pone.0145890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Jamal N, Erdual E, Neunlist M, et al. Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. AJP Gastrointest Liver Physiol. 2014;307:G274–G285. doi: 10.1152/ajpgi.00389.2012 [DOI] [PubMed] [Google Scholar]
- 37.Yusta B, Huang L, Munroe D, et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119:744–755. doi: 10.1053/gast.2000.16489 [DOI] [PubMed] [Google Scholar]
- 38.Xiao C, Stahel P, Lewis GF. Regulation of chylomicron secretion: Focus on post assembly mechanisms. Cell Mol Gastroenterol Hepatol. 2019;7:487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Näslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol-Regul Integr Comp Physiol. 1999;277:R910–R916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.