Abstract
Introduction:
The current pharmacological and psychotherapeutic approaches have limited benefit in symptom management of obsessive-compulsive disorder (OCD) urging clinicians and researchers to seek newer avenues of management. Transcranial direct current stimulation (tDCS) has shown promise in this aspect from a neuromodulatory perspective. The current study aims to study the response to tDCS as an adjunctive treatment in patients with treatment-resistant OCD.
Materials and Methods:
This open-label study was conducted among 20 patients with treatment-resistant OCD. All participants received 20 sessions of tDCS with the cathode at the supplementary motor area (SMA) and the anode at right occipital area. The primary outcome measure was the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) and the secondary outcome was evaluated on the clinical global impression (CGI) and side effect checklist for tDCS.
Results:
There was a significant improvement from baseline in the mean scores of Y-BOCS and CGI scales after tDCS intervention. An improvement of >35% Y-BOCS score change was observed in 15% of participants. Short-lasting side effects were reported as mild headache and localized tingling sensation.
Conclusion:
Cathodal tDCS at SMA may be a useful approach to manage treatment-resistant OCD. The use of tDCS was not associated with any significant harmful consequence to the participants.
Keywords: Brain stimulation, neuromodulation, obsessive-compulsive disorder, transcranial, transcranial direct current stimulation
INTRODUCTION
With a prevalence rate in the general population of approximately 2%–3%, obsessive-compulsive disorder (OCD) is considered one of the most disabling medical conditions with considerable direct and indirect economical costs on society.[1,2] Even with the currently available pharmacological and psychotherapeutic options, there are still about 40%–60% of individuals with OCD that do not experience satisfactory outcomes.[3,4] Thus, researchers yearn for newer avenues of approach toward management of such treatment nonresponsive patients.
Evidence suggests that OCD is associated with dysfunctions in the cortico-striatal-thalamic-cortical (CSTC) circuitry including the dorsolateral prefrontal cortex (DLPFC), the orbitofrontal cortex (OFC), medial prefrontal cortices such as the supplementary motor area (SMA), anterior cingulate cortex, and the Basal Ganglia.[5,6] Multiple modalities of structural and functional neuroimaging have identified various regions involved in the pathophysiology of OCD.[7] Neuromodulatory approach utilizes this aspect of the disorder to modulate the neural functioning in this circuit to generate positive outcomes. While some of these approaches are limited in applicability due to their invasiveness like deep brain stimulation (DBS) and vagal nerve stimulation, others have utilized more feasible noninvasive approaches such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). Studies have depicted variable levels of improvement with these varied brain stimulation techniques in OCD.
tDCS is a relatively novel nonpharmacological intervention that has been increasingly investigated in the treatment of mental disorders and it has important advantages over other brain stimulation interventions, particularly for low cost, ease of use, portability, safety, and tolerability.[8,9,10] For all these reasons, tDCS has gained increased interest in clinical psychiatry over the past decade and has subsequently showed promising results in the treatment of several neuropsychiatric disorders.[11,12,13] In this procedure, a weak, direct current is applied on the scalp through flat electrodes, the current flows from the anode to the cathode with a fraction of the current entering the brain. Depending on the polarity of the active electrode, the current can increase (on anodal stimulation) or decrease (on cathodal stimulation) the cortical excitability and regional cerebral blood flow,[14,15] by producing low-intensity electric field (<1 V/m) in the brain.[16] This leads to small changes (<1 mV) in the neuron's membrane potential,[17] which does not induce any action potential rather leads to the development of neuromodulatory effect and thus influences the frequency of spikes and modify the net cortical excitability.[8,14,18]
With the limitability of DBS being a highly invasive procedure and rTMS being costly and having a long duration of treatment, tDCS may provide a viable option of treatment in patients of OCD. Previous positive results in randomized controlled trials (RCT) of DBS and rTMS in OCD have identified certain brain regions to be focused for neuromodulation.[9] Few cases of successful management of OCD with tDCS have also been reported in the literature. The current study aims to study the response to tDCS as an add-on treatment in patients with treatment-resistant OCD.
MATERIALS AND METHODS
Study participants
The study was an open-label study conducted among patients with treatment-resistant OCD attending the outpatient clinic in the Department of Psychiatry, All India Institute of Medical Sciences, New Delhi, India, between March 2016 and July 2017. Nonrandom, nonstratified, purposive sampling was done to include 20 right-handed patients with OCD (diagnosed using tenth-revision of the International Classification of Diseases-10 criteria) of either gender, aged between 18 and 45 years, meeting criteria for treatment-resistant as per Level 1 nonresponse of the International Treatment-Refractory OCD Consortium (ITROC), providing written informed consent and completing the tDCS intervention. Participants were excluded if the OCD was not of at least moderate severity (Yale-Brown Obsessive Compulsive Scale [Y-BOCS] score ≥16) or there was a score of ≥17 on the Hamilton Depression Rating Scale (HAM-D). Participants were also excluded if there was any comorbid psychiatric/neurological/medical illness including substance abuse/dependence (except caffeine or nicotine), history of seizure or receiving rTMS or electroconvulsive therapy treatment, imminent suicide risk, any implanted device or metals in the brain/head or cardiac pacemaker, known or suspected pregnancy or were currently lactating.
Ethical clearance was obtained from the Institute Ethics Committee (Ref. No. IECPG/151/27.01.2016, RT-28/24.02.2016) and the Trial registration was done with the Clinical Trial Registry-India before the study initiation (CTRI/2017/02/007775).
Instruments
Semi-structured interview sheet
It consisted of sociodemographic and clinical details of participants.
Mini-international neuropsychiatric interview 5.0
It was used as a screening tool used for evaluating the presence of any comorbid psychiatric diagnoses.[19] The scale has a sensitivity of 0.70 and specificity of 0.80.
Edinburgh handedness inventory
This 10-item inventory was used to aid in classification of handedness of participants as right, left, or ambidextrous.[20] It is the most commonly used handedness questionnaire in various neuroimaging studies. The test-retest correlation coefficient is 0.75–0.86.
Clinical global impression
With a sensitivity of 0.90 and specificity of 0.94, the clinical global impression (CGI) measures symptom severity, treatment response, and the efficacy of treatments in treatment studies on a 7-point rating scale.[21]
Yale-brown obsessive compulsive scale
The widely used clinician administered scale assess the severity of OCD symptoms and is used to monitor progress of treatment in five domains – time, distress, interference, resistance, and control.[22] The severity of illness is graded as 0–7 (subclinical), 8–15 (mild), 16–23 (moderate), 24–31 (severe), and 32–40 (extreme). The scale has a sensitivity of 0.90 and a specificity of 0.94 with inter-rater reliability of 0.95. A reduction of 35% in Y-BOCS score is considered optimal to indicate clinical response.[23]
Hamilton depression rating scale
This 21-item scale assess the severity of major depression with a focus on somatic symptoms.[24] The severity of disease is graded as 0–7 (normal), 8–16 (mild depression), 17–23 (moderate depression), and ≥24 (severe depression).[25] The scale has a sensitivity of 0.86 and a specificity of 0.92.
Hamilton anxiety rating scale
It consists of 14 items and is used to assess the severity of anxiety.[26] Each item is rated from 0 to 4. The optimal Hamilton anxiety rating scale (HAM-A) score ranges are: mild anxiety = 8–14, moderate = 15–23, and severe ≥24 (scores ≤7 are considered to represent no/minimal anxiety).[27]
Side effect checklist for transcranial direct current stimulation
Literature reports several mild adverse effects of tDCS treatment including moderate fatigue (35%), mild headache or dizziness (11.8%), nausea (2.9%), and a transient itching sensation in area of stimulation.[8,28] Phosphene, i.e., brief flashes of light have been reported if the electrode is placed near the eye. No cases of seizure have been reported in relation to the use of tDCS as a clinical intervention.[8,28] A checklist was prepared to record any previously reported or newer side effect of tDCS during the current study.
Transcranial direct current stimulation protocol
Stimulation through tDCS was delivered by a constant-current battery-operated stimulator unit (HDC Kit, Magstim, Whitland, UK) through two electrodes in conductive silicone (5 cm × 5 cm) kept under the sponge holding bag of plant cellulose (6 cm × 7.5 cm) soaked in a saline solution (NaCl 0.9%). The active electrode “Cathode” was placed over the SMA and the reference electrode “Anode” was placed over the right occipital area, using the International 10–20 EEG system. A 2 mA of DC current was applied for 20 min in one session with two sessions of tDCS per day separated by duration of at least 3 h. Total 20 sessions of tDCS were completed in 10 days (5 days/week) for each subject.
Outcome assessment
All the baseline assessments (HAM-D, HAM-A, Y-BOCS, CGI, and side effect checklist for tDCS [SECT]) were conducted within 1 week before the first tDCS session and were repeated within 1 week following the last session. The recruited participants continued to receive the medications in the same pattern and dosage as previously prescribed without any change during the course of tDCS intervention. Y-BOCS scores were kept as primary outcome measure and others (HAM-D, HAM-A, CGI, and SECT) were kept as secondary outcome measures.
Statistical analysis
The data analysis was performed using Statistical Package for the Social Sciences (SPSS) software version 21.0 (IBM, SPSS Inc., Chicago, USA). Descriptive statistics were used to understand the demographic and clinical profile of the group. Data is presented as mean ± standard deviation for quantitative variables and frequencies (percentage) for categorical variables. Normalcy distribution of data was assessed using histogram, skewness and kurtosis values, and Shapiro–Wilk test. Scores of the scales applied were found to be normally distributed with a skewness of −0.412 (standard error [SE] = 0.524) and a kurtosis of −0.115 (SE = 1.014), following which no data transformation was attempted and parametric inferential statistical methods were used for data analysis.
Paired t-test was conducted to evaluate the differences before (baseline) and after (20 sessions of tDCS) intervention for all the dependent variables (outcome measures). The P values were two tailed and probability level for significant difference was set at P < 0.05 for all the analysis. The percentage change in scores was calculated by computing variables using the formula ([Baseline score − end score] × 100/baseline score). Correlation analysis was done to analyze relation between sociodemographic categorical variables and percentage change score of Y-BOCS, CGI, HAM-D, and HAM-A using Spearman's test. Pearson's test was used to analyze relation between continuous variables and percentage change score of Y-BOCS, CGI, HAM-D, and HAM-A.
RESULTS
Of the 29 individuals assessed for the study, all of the 20 individuals recruited in the study completed the tDCS intervention and were analyzed.
Sociodemographic parameters
The details of the sociodemographic profile of the participants are presented in Table 1. The mean age of patients included in the study was (31.25 ± 7.72 years). There was near equal representation of the gender distribution with eleven being male and nine of female gender (χ2 = 0.2, P = 0.65).
Table 1.
Demographic characteristics of the participants (n=20)
| Variable | Frequency (%) | χ2, P | |
|---|---|---|---|
| Gender | Male | 11 (55) | χ2=0.2, P=0.65 |
| Female | 09 (45) | ||
| Marital status | Never married | 07 (35) | χ2=6.10, P=0.05 |
| Married | 11 (55) | ||
| Divorced | 02 (10) | ||
| Educational status | Illiterate | 01 (05) | χ2=13.0, P=0.02 |
| Primary | 01 (05) | ||
| Middle | 03 (15) | ||
| Intermediate | 07 (35) | ||
| Graduate | 07 (35) | ||
| Postgraduate | 01 (05) | ||
| Occupational status | Professional | 01 (05) | χ2=9.0, P=0.06 |
| Skilled worker | 03 (15) | ||
| Housewife | 09 (45) | ||
| Student | 04 (20) | ||
| Business | 03 (15) | ||
| Monthly income (in Indian Rupees) | 5000-9999 | 03 (15) | χ2=8.5, P=0.07 |
| 10000-19999 | 08 (40) | ||
| 20000-39999 | 06 (30) | ||
| 40000-59999 | 02 (10) | ||
| >60000 | 01 (05) | ||
| Religion | Hindu | 19 (95) | χ2=16.2, P<0.001 |
| Islam | 01 (05) | ||
| Background | Urban | 14 (70) | χ2=3.2, P=0.07 |
| Rural | 06 (30) | ||
| Family history of psychiatric illness | BPAD | 01 (05) | χ2=32.4, P<0.001 |
| Psychosis | 02 (10) | ||
| OCD | 01 (05) | ||
| None | 16 (80) |
BPAD: Bipolar affective disorder; OCD: Obsessive compulsive disorder; χ2: Chi square
Comparing the change in the clinical variables at baseline and after intervention
The mean age of illness onset was 21.60 ± 7.64 years with mean total illness duration of 115.20 ± 65.63 months. There was a significant improvement in the mean scores of Y-BOCS, CGI, HAM-D, and HAM-A scales after tDCS intervention from baseline scores [Table 2].
Table 2.
Outcome scale scores as changed from baseline to posttranscranial direct current stimulation intervention
| Outcome scales | Mean±SD | Comparisons of paired differences | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Postintervention | Mean±SD | 95% CI | T | P | ||
| Lower | Upper | ||||||
| Total Y-BOCS | 31.65±4.92 | 26.35±8.62 | 5.30±8.06 | 1.52 | 9.07 | 2.93 | 0.008** |
| Y-BOCS obsessions | 16.85±1.63 | 14.10±4.14 | 2.75±4.09 | 0.83 | 4.66 | 3.01 | 0.007** |
| Y-BOCS compulsions | 14.80±4.11 | 12.25±4.92 | 2.55±4.17 | 0.59 | 4.50 | 2.73 | 0.013* |
| CGI S | 4.70±0.57 | 4.30±0.87 | 0.40±0.75 | 0.05 | 0.75 | 2.37 | 0.028* |
| CG I | 3.95±0.22 | 3.05±0.95 | 0.90±0.85 | 0.50 | 1.29 | 4.72 | 0.000** |
| CGI E | 12.85±0.67 | 9.20±3.78 | 3.65±3.48 | 2.02 | 5.28 | 4.68 | 0.000** |
| Total HAM-D | 7.35±4.03 | 3.85±3.60 | 3.50±3.13 | 2.03 | 4.96 | 4.98 | 0.000** |
| Total HAM-A | 7.00±5.39 | 4.70±5.14 | 2.30±2.73 | 1.02 | 3.58 | 3.76 | 0.001** |
*P value significant at <0.05; **P value significant at <0.01. CI – Confidence Interval of the difference; CGI – Clinical Global Impression (S – Severity; I – Improvement and E – Efficacy index scale); HAM-D – Hamilton Depression Rating Scale; HAM-A – Hamilton Anxiety Rating Scale; Y-BOCS – Yale-Brown Obsessive Compulsive Scale; SD – Standard deviation
There was a significant decrease in mean scores of total Y-BOCS (5.30 ± 8.06; 95% confidence interval [CI]: 1.52–9.07; t (19): 2.93; P = 0.008), Y-BOCS obsession subscale (2.75 ± 4.09; 95% CI: 0.83-4.66; t [19]: 3.01; P = 0.007) and Y-BOCS compulsion subscale (2.55 ± 4.17; 95% CI: 0.59–4.50; t [19]: 2.73; P = 0.013) as depicted in Figure 1. A significant decrease was also observed in mean scores of CGI-S (0.40 ± 0.75; 95% CI: 0.05-–0.75; t [19]: 2.37; P = 0.028), CGI-I (0.90 ± 0.85; 95% CI: 0.50–1.29; t [19]: 4.72; P < 0.001) and CGI-E (3.65 ± 3.48; 95% CI: 2.02–5.28; t [19]: 4.68; P < 0.001) shown in Figure 2. Reduction in mean scores was also observed in HAM-D and HAM-A scales [Figure 3].
Figure 1.
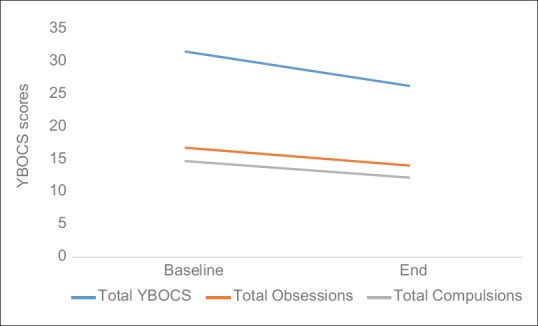
Mean change in Yale Brown Obsessive Compulsive Scale scores (total, obsession and compulsion sub-scales) over 20 transcranial direct current stimulation sessions
Figure 2.
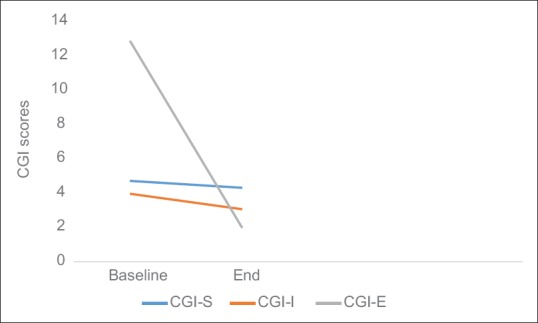
Mean change in clinical global impression scores over 20 transcranial direct current stimulation sessions
Figure 3.
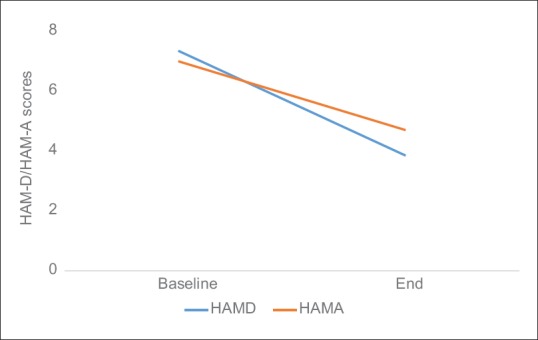
Mean change in Hamilton depression rating scale and Hamilton anxiety rating scale scores over 20 transcranial direct current stimulation sessions
Percentage change in clinical scale scores
The mean percentage change in total Y-BOCS scores was 16.47% (±23.42); total obsession scores was 16.02% (±23.28); total compulsion scores was 16.24% (±24.75); CGI-S scores was 8.25% (±15.32); CGI-I scores was 23.33% (±22.71); and CGI-E scores was 29.11% (±28.80).
For assessing number of treatment responders, it was observed that an improvement of more than 35% score change was observed in 15% of individuals assessed over Y-BOCS [Table 3]. Among the subjects, 25th percentile participants had no change in total Y-BOCS scores after the intervention, while 50th percentile and 75th percentile participants had 7.29% and 20% reductions of total Y-BOCS scores.
Table 3.
Percentage change in Yale-Brown Obsessive Compulsive Scale parameters after transcranial direct current stimulation intervention
| Scale | Mean | Median | Study sample percentiles | Percentage study population having >35% score change | ||
|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||
| Total Y-BOCS score | 16.47 | 7.29 | 0 | 7.29 | 20.00 | 15 |
| Total obsessions score | 16.02 | 6.06 | 0 | 6.06 | 21.81 | 15 |
| Total compulsions score | 16.24 | 5.26 | 0 | 5.26 | 23.52 | 15.8 |
Y-BOCS – Yale-Brown Obsessive Compulsive Scale
Correlation among clinical variables and sociodemographic parameters
There was no significant correlation among any clinical scores and sociodemographic variables.
Intervention related side effects
The common side effects reported were mild headache (which was relieved with oral analgesics), mild heaviness in head and tingling sensation at the site of stimulation which were relieved on its own by 3rd day. Headache was reported by two participants (10%), tingling sensation at the site of stimulation by three participants (15%), and mild heaviness in head by two participants (10%). None of the participants left the study due to side effects.
DISCUSSION
With encouraging results with neuromodulation modalities in treatment-resistant OCD keeping the neurobiological model of OCD as the pathogenesis process, the current open label pilot study assessed the effect (safety and efficacy) of adjunctive tDCS with cathode over SMA and anode over right Occipital area in patients with treatment-resistant OCD. There were no serious side effects and tDCS was well-tolerated by all subjects. There was significant improvement in the scale score of Y-BOCS, CGI, HAM-D, and HAM-A after 20 sessions of tDCS. In 15% of subjects, there was more than 35% improvement in the Y-BOCS score and were labeled as treatment responders according to the ITROC criteria of response.
Stimulation parameters
Dosage of tDCS delivered depends on the current dosage (measured in milliamperes (mA)); duration of stimulation; and electrode montage (size and position of all electrodes). The most common tDCS parameters used in different psychiatric illnesses are 1–2 mA of currents with electrode sizes of 25–35 cm2 for 20–40 min.[8] tDCS parameters used in the studies involving OCD have used 2 mA of current, 20–30 min daily to twice daily sessions (with 2–3 h gap) ranging from total 5 to 20 sessions with electrode size ranging from 25 to 35 cm2.
The current density is calculated as the stimulation intensity divided by the area of the electrode through which the current is applied. It is strongly recommended not to exceed the threshold of 0.05 mA/cm2 that could cause a painful sensation and tissue damages.[29] The current density for the study was 2 mA/45 cm2 = 0.04 mA/cm2. Thus, it was well within the safety limits and robust stimulation parameters were selected in line with the literature to the best of our knowledge.
Clinical effectiveness
The evidence derived from the clinical efficacy of inhibitory rTMS and from the neurophysiological measures of altered motor cortex excitability in OCD, which is normalized after “inhibitory” low-frequency (1 Hz) rTMS applied to the pre-SMA/SMA, suggests that the premotor/motor system is abnormally hyperactive in OCD, and that there is some pathophysiological link between such hyperexcitability and OCD symptoms.[30]
There are limited studies assessing the effect of tDCS in resistant OCD patients. The earlier case reports had varied findings. Cathodal stimulation of left DLPFC was reported to cause improvement in depressive and anxiety symptoms but not obsessive-compulsive symptoms (OCS).[10] While anodal stimulation of left DLPFC and cathodal stimulation of right DLPFC reported improvement in OCS, depressive and anxiety symptoms.[31] Others have reported improvement in OCS utilizing cathodal stimulation over left OFC.[32,33] Cases with positive outcomes have been reported even using cathodal stimulation of right supraorbital area and anodal stimulation at SMA/pre-SMA.[34,35] On the contrary, it has been reported that cathodal stimulation at pre-SMA is beneficial in reducing OCS while anodal stimulation at the same site worsened the symptoms.[36] Few other case reports suggest positive results with cathodal stimulation of SMA.[37]
One open-label study utilized left OFC as the cathodal site and found positive results with significant improvement in OCD symptoms.[38] Another open-label trial used cathode at right OFC and the anode at left DLFC and found reduction in frequency and distress associated with OCD symptoms following 3 weeks, which were not maintained at 1-month follow-up.[39] A cross-over design RCT reported improvement with cathodal stimulation of pre-SMA similar to our findings.[40] Improvement in OCS has also been reported by a RCT utilizing cathodal stimulation of left DLPFC while providing anodal stimulation at right DLPFC.[41] However, the study subjects had lower baseline Y-BOCS scores (19.5 and 18.6 in active and sham groups, respectively) as compared to other studies.
Overall, studies using tDCS have shown promising results targeting OFC, SMA, and DLPFC (part of the CSTC circuitry) in concordance to the results obtained using rTMS literature.[42] While the cathodal stimulation of SMA can decrease cortical excitability of the CSTC circuitry producing improvement in OCS as reported by majority of currently available literature,[42] few case reports have also observed improvement with anodal stimulation at SMA.[34,35] Researchers have suggested that polarity effects may not translate to other cortical areas and in fact, may be modified or even inverted according to other factors such as stimulation intensity or baseline cortical activity.[43,44] In consortium with case reports, RCT also suggest that anodal stimulation at SMA with extra-cephalic positioning of cathode may lead to worsening of OCS.[36,40] Higher baseline Y-BOCS score has been predictive of higher dropout rate when anodal stimulation is done at SMA.[40] Evidence also suggests that that there is sustainability of effects as well as a progressively increasing positive effect of tDCS after intervention completion when cathodal stimulation of left OFC or SMA is done.[37,38,40] Studies conducted using tDCS in OCD have evaluated outcome only over Y-BOCS and not over CGI scale.
Tolerability of transcranial direct current stimulation
tDCS is relatively safe with mild and transient adverse effects. All patients in the current study tolerated the intervention with no serious adverse event or other life-threatening event reported and none of the participants left the study due to side-effects. In a systematic review of 209 studies only 117 (56%) studies reported of any adverse event due to tDCS stimulation. Most common side effects reported were itching (active group = 39.3% and sham group = 32.9%) and tingling sensation (active group = 22.2% and sham group = 18.3%) followed by headache (active group = 14.8% and sham group = 16.2%), burning sensation (active group = 8.7% and sham group = 10%), and discomfort (active group = 10.4% and sham group = 13.4%).[8]
Limitations
The strength of the study included the use of well validated instruments, stringent selection criteria for enrolment of only treatment-resistant cases, and robust stimulation parameters. Small sample size and subject enrolment from a single center compounded with the lack of a control arm, however, limits the generalizability of study findings. Study has no follow-up data, so the prospective effects of tDCS over time could not be commented in terms of decline, stabilization, or amplification. Placebo effect by care giving, change in environment of patients and interventional effects in hopeless patient may have played role in the improvement of symptom. Concomitant medication with tDCS sessions may act like confounding factor which may have modulated the effects. However, the participants were receiving stable course of ongoing medications from more than 12 weeks before initiating tDCS intervention.
CONCLUSION
Over the past decades, various nonpharmacological techniques have been searched for management of treatment resistant OCD with promising results. tDCS is one of the noninvasive neuromodulation techniques with promising results and added benefits of relatively low cost, ease of use, portability, safety, and tolerability. The current openlabel trial found beneficial effects in primary and secondary outcome measures after using cathodal tDCS at SMA. Use of tDCS was not associated with any significant harmful consequence to the subjects.
Overall, tDCS was found to be a relatively safe and effective treatment modality in improving OC symptoms as an add-on therapy for treatment-resistant OCD patients. Such promising findings warrant more research in the field of utilizing tDCS in OCD. Further research with larger sample size, comparing stimulation protocols, control design, and follow-up assessment is required for providing more definitive conclusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Ms. Komal Gupta and Mr. Vikas Gupta for their kind help during tDCS delivery in TMS laboratory.
REFERENCES
- 1.Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman MM, Bland RC, Canino GJ, Greenwald S, Hwu HG, Lee CK. The cross national epidemiology of obsessive compulsive disorder. The cross national collaborative group. J Clin Psychiatry. 1994;55(Suppl 3):5–10. [PubMed] [Google Scholar]
- 3.Björgvinsson T, Hart J, Heffelfinger S. Obsessive-compulsive disorder: Update on assessment and treatment. J Psychiatr Pract. 2007;13:362–72. doi: 10.1097/01.pra.0000300122.76322.ad. [DOI] [PubMed] [Google Scholar]
- 4.Pallanti S, Quercioli L. Treatment-refractory obsessive-compulsive disorder: Methodological issues, operational definitions and therapeutic lines. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:400–12. doi: 10.1016/j.pnpbp.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Shah DB, Pesiridou A, Baltuch GH, Malone DA, O'Reardon JP. Functional neurosurgery in the treatment of severe obsessive compulsive disorder and major depression: Overview of disease circuits and therapeutic targeting for the clinician. Psychiatry (Edgmont) 2008;5:24–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Stein DJ, Hollander E, Rothbaum BO, editors. Textbook of Anxiety Disorders. Washington: American Psychiatric Pub; 2009. [Google Scholar]
- 7.Whiteside SP, Port JD, Abramowitz JS. A meta-analysis of functional neuroimaging in obsessive-compulsive disorder. Psychiatry Res. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14:1133–45. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 9.Senço NM, Huang Y, D’Urso G, Parra LC, Bikson M, Mantovani A, et al. Transcranial direct current stimulation in obsessive-compulsive disorder: Emerging clinical evidence and considerations for optimal montage of electrodes. Expert Rev Med Devices. 2015;12:381–91. doi: 10.1586/17434440.2015.1037832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpato C, Piccione F, Cavinato M, Duzzi D, Schiff S, Foscolo L, et al. Modulation of affective symptoms and resting state activity by brain stimulation in a treatment-resistant case of obsessive-compulsive disorder. Neurocase. 2013;19:360–70. doi: 10.1080/13554794.2012.667131. [DOI] [PubMed] [Google Scholar]
- 11.Brunelin J, Poulet E, Bediou B, Kallel L, Dalery J, D’amato T, et al. Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res. 2006;81:41–5. doi: 10.1016/j.schres.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs. Electrical current therapy for treating depression clinical study: Results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–91. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 13.Kuo MF, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage. 2014;85(Pt 3):948–60. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- 14.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58:26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–7. doi: 10.1016/j.brs.2009.03.005. 207.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2:215–28. doi: 10.1016/j.brs.2009.03.007. 228.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purpura DP, Mcmurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–85. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 20.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 21.Guy WB. EC-DEU Assessment Manual for Psychopharmacology. Rockville, MD, USA: U.S. Department of Health, Education, and Welfare; 1976. CGI clinical global impressions; pp. 76–338. [Google Scholar]
- 22.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 23.Lewin AB, De Nadai AS, Park J, Goodman WK, Murphy TK, Storch EA. Refining clinical judgment of treatment outcome in obsessive-compulsive disorder. Psychiatry Res. 2011;185:394–401. doi: 10.1016/j.psychres.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. J Affect Disord. 2013;150:384–8. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Matza LS, Morlock R, Sexton C, Malley K, Feltner D. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psychiatr Res. 2010;19:223–32. doi: 10.1002/mpr.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol. 2009;120:1033–4. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg BD, Ziemann U, Corá-Locatelli G, Harmon A, Murphy DL, Keel JC, et al. Altered cortical excitability in obsessive-compulsive disorder. Neurology. 2000;54:142–7. doi: 10.1212/wnl.54.1.142. [DOI] [PubMed] [Google Scholar]
- 31.Palm U, Leitner B, Kirsch B, Behler N, Kumpf U, Wulf L, et al. Prefrontal tDCS and sertraline in obsessive compulsive disorder: A case report and review of the literature. Neurocase. 2017;23:173–7. doi: 10.1080/13554794.2017.1319492. [DOI] [PubMed] [Google Scholar]
- 32.Goradel AJ, Pouresmali A, Mowlaie M, Sadeghi MF. The effects of transcranial direct current stimulation on obsession-compulsion, anxiety, and depression of a patient suffering from obsessive-compulsive disorder. Pract Clin Psycholo. 2016;4:75–80. [Google Scholar]
- 33.Mondino M, Haesebaert F, Poulet E, Saoud M, Brunelin J. Efficacy of cathodal transcranial direct current stimulation over the left orbitofrontal cortex in a patient with treatment-resistant obsessive-compulsive disorder. J ECT. 2015;31:271–2. doi: 10.1097/YCT.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 34.Hazari N, Narayanaswamy JC, Chhabra H, Bose A, Venkatasubramanian G, Reddy YC. Response to transcranial direct current stimulation in a case of episodic obsessive compulsive disorder. J ECT. 2016;32:144–6. doi: 10.1097/YCT.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 35.Narayanaswamy JC, Jose D, Chhabra H, Agarwal SM, Shrinivasa B, Hegde A, et al. Successful application of add-on transcranial direct current stimulation (tDCS) for treatment of SSRI resistant OCD. Brain Stimul. 2015;8:655–7. doi: 10.1016/j.brs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 36.D'Urso G, Brunoni AR, Anastasia A, Micillo M, de Bartolomeis A, Mantovani A. Polarity-dependent effects of transcranial direct current stimulation in obsessive-compulsive disorder. Neurocase. 2016;22:60–4. doi: 10.1080/13554794.2015.1045522. [DOI] [PubMed] [Google Scholar]
- 37.Silva RM, Brunoni AR, Miguel EC, Shavitt RG. Transcranial direct current stimulation for treatment-resistant obsessive-compulsive disorder: Report on two cases and proposal for a randomized, sham-controlled trial. Sao Paulo Med J. 2016;134:446–50. doi: 10.1590/1516-3180.2016.0155010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bation R, Poulet E, Haesebaert F, Saoud M, Brunelin J. Transcranial direct current stimulation in treatment-resistant obsessive-compulsive disorder: An open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:153–7. doi: 10.1016/j.pnpbp.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Dinn WM, Aycicegi-Dinn A, Göral F, Karamursel S, Yildirim EA, Hacioglu-Yildirim M, et al. Treatment-resistant obsessive-compulsive disorder: Insights from an open trial of transcranial direct current stimulation (tDCS) to design a RCT. Neurol Psychiatry Brain Res. 2016;22:146–54. [Google Scholar]
- 40.D'Urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress Anxiety. 2016;33:1132–40. doi: 10.1002/da.22578. [DOI] [PubMed] [Google Scholar]
- 41.Yekta M, Rostami R, Fayyaz E. Transcranial direct current stimulation of dorsolateral prefrontal cortex in patients with obsessive compulsive disorder to improve decision making and reduce obsession symptoms. Pract Clin Psychol. 2015;3:185–94. [Google Scholar]
- 42.Singh S, Kumar S, Gupta A, Verma R, Kumar N. Effectiveness and predictors of response to 1-hz repetitive transcranial magnetic stimulation in patients with obsessive-compulsive disorder. J ECT. 2019;35:61–6. doi: 10.1097/YCT.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 43.Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, et al. Cellular effects of acute direct current stimulation: Somatic and synaptic terminal effects. J Physiol. 2013;591:2563–78. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]


