In 2005, national expenditure for back pain approached $86 billion; meanwhile, patients with back pain reported worse physical and social function, worse mental health, and worse ability to work than in 1996 when spending was nearly 40% less [1]. Over the same period, disability attributed to musculoskeletal pain, with back pain as a major contributor, rose from 20% to 25% [2]. More recently and more disturbingly, there were 16,000 deaths in 2013 related to misuse of prescription opioid medications, many of which were prescribed to treat back pain [3].
Because the status quo is unsustainable, we sought to improve value for back pain care through a meticulous redesign process. Our process, previously described in Stroke in 2014 by Kalanithi et al., includes four components: 1) literature review and discussion with authors of peer-reviewed publications that examine attempts to improve the value of back pain care; 2) consideration of advances in biomedical and behavioral science-based technologies; 3) on-site investigations of efforts to deliver back pain care more cost-effectively; and 4) qualitative interviews with patients and clinicians to understand their discontent with current care delivery [4]. The latter consisted of informal, semistructured interviews with patients, families, health care providers, and support staff. Conversations focused on the day-to-day experience in their current role (secretary, nurse, physical therapist, physician, patient, family member). These conversations occurred with individuals from across the United States, from various institutions—public, private, academic—and across the adult age range. All information was de-identified and discussed in aggregate by the design team to inform new care model opportunities.
Using these techniques, we identified the most promising opportunities for improvement and translated them into a testable care delivery innovation to provide better quality of care at lower cost.
Approximately 90% of back pain episodes resolve within six weeks regardless of treatment type. In the interim, many such patients receive unnecessary imaging, specialist referrals, procedures, or opioids [2]. During our site visits, providers cited confusion over guidelines and concerns about litigation as reasons for prematurely ordering expensive diagnostics and treatments. Similarly, many patients expressed discomfort with conservative care and had the perception that more aggressive approaches during the first six weeks would lead to better outcomes.
Among the 10% of patients who do not improve by six weeks, a subset has complex anatomic or medical problems that require procedures or specialty referral. However, many others have risk factors including life stressors, maladaptive behaviors, or mental health disorders that are frequently unaddressed. These factors increase the probability of treatment failure and pain chronicity [5], both of which are associated with increasing use of the health system including costly, and often unnecessary, imaging studies and interventions [2]. Our discussions with health care providers revealed a lack of resources and time to address the behavioral and social needs of these patients.
Those seeking relief from back pain can access numerous providers. While the majority of initial encounters are with primary care physicians and chiropractors, many self-refer to specialists, seek complementary alternative medicine, or present to emergency rooms. Subsequently, patients are often referred to provider after provider in no particular order. Complicating matters are the more than 200 treatment modalities available for back pain [6]. Rather than a logical pathway toward relief, patients articulate a maze-like experience characterized by confusion, long wait times, and poor coordination.
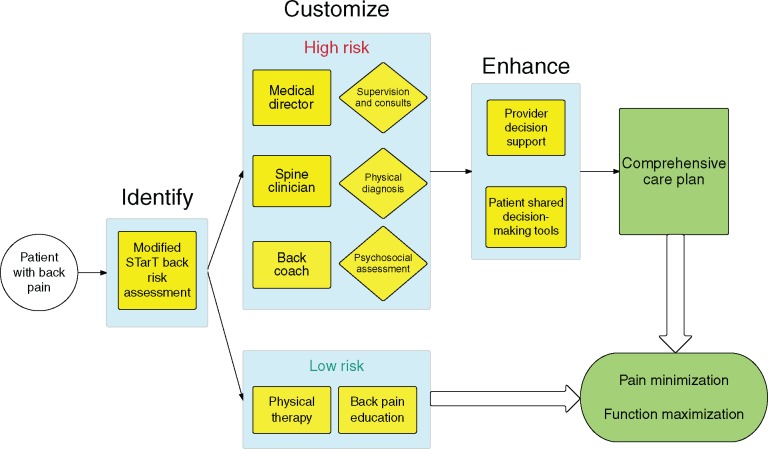
Having identified three major opportunities to improve back pain care, we created matching solutions. First, providers need a rapid method of identifying patients who are likely to improve quickly and a conservative care plan that augments their natural course of healing. The STartBack tool, a nine-question survey created and validated at Keele University, allows such patients to be identified and triaged at their first encounter with the health system [7]. Next, managing the physical and psychosocial needs of patients at high risk of developing chronic pain requires specially trained personnel. Recent publications on health coaching and care teams for chronic disease provide a starting point for solutions [8]. Finally, navigating the maze of treatment options can be made significantly easier for patients and providers by deploying cognitive aids that enhance decision-making in real time. Such aids are effective in reducing overutilization of medical resources at institutions like Virginia Mason, where creating streamlined care pathways is a priority [9]. We combined these three approaches to create the Identify, Customize, and Enhance (ICE) model for back pain care (Figure 1).
Figure 1.
Identify, Customize, Enhance (ICE) model for back pain care.
The ICE Model for Back Pain Care
Identify Patients at Low Risk for Developing Chronic Pain and Treat Conservatively
At each point in which the patient encounters the health system with a complaint of spine pain, two forms of critical assessment are performed. First, a thorough history and physical examination is done to rule out red flags. If any clinical red flags are identified, patients are rapidly treated with the appropriate specialty referral, imaging, or intervention as indicated. (Red flags include patients at the extremes of age or who are immunosuppressed or any clinical signs of concern for underlying malignancy, infection, cord compression, or cauda equine.) The second form of risk assessment performed is done to distinguish which patients are at highest risk of developing chronic problems with pain. Risk of chronicity is assessed using a modified STarTBack survey that adds questions regarding adverse childhood events and educational level—two strong predictors of general health outcomes. Conservative treatment for lower-risk individuals then consists of a brief course of physical therapy and education regarding the likely self-limited nature of the patient’s symptoms. This combination of simple but effective approaches shields individuals from the hazards of overdiagnosis and overtreatment.
Customize Care for Patients at High Risk of Chronic Pain with a Dedicated Team
The team consists of a spine clinician (specially trained physical therapist or chiropractor), a health coach with a focus on back pain (“back coach”), and a supervising physician. The spine clinician spearheads the medical plan while the back coach’s work focuses on behavior change interventions to promote adherence to physical therapy, promote physical activity, and aid in transferring self-management skills. The back coach either has demonstrated expertise with motivational interviewing and techniques for chronic disease self-management or receives supplementary training in these techniques. The physician supervises multiple clinician/coach pairs, providing consultative support for complex medical management and referral to specialty services such as surgery if indicated.
Enhance Treatment Decisions with Shared Decision-Making Tools for Patients and Electronic Decision Support Aids for Providers
Patients who may have struggled to understand their treatment options use shared decision-making tools, such as videos, to identify their preferences. Decision aids reduce rates of preference-sensitive testing and interventions [10]. A preference-sensitive condition is one in which the decision as to how to treat a condition (or even whether or not to test or treat) is highly subject to the physician or patient’s proclivity for a given choice. In addition to shared decision-making tools for patients, physicians are also given prompt access to guideline-based information as they decide which treatment options best match their patients’ needs.
Forward-thinking health systems have experimented with individual elements of our approach, but we are unaware of any that have achieved the synergistic effects that come with deploying all three simultaneously.
Studies have shown that, for patients with various medical conditions, implementation of either comprehensive, high-touch care teams or decision support tools has been associated with reductions in the use of unnecessary diagnostic testing and preference-sensitive procedures [8,9,11]. Many of these interventions have also been associated with a reduction in health system encounters [8]. It is possible that implementation of the ICE model could lead to increased capacity in primary care clinics and emergency rooms by reducing their backlog of patients with back pain. Thus, depending on the area of spending, we estimated that this model could save between 18.6% to 25.3% (net of implementation cost) within three years of implementation [10–13]. Most importantly, patients will encounter a more coordinated experience and an increased likelihood of regaining normal function. There is an ongoing, multicenter, randomized clinical trial currently being done with implementation of this care model. We urge all value-driven institutions to consider adoption and implementation of the ICE model as they seek to improve care for their patients.
Funding sources: This work was supported by the Stanford Clinical and Translational Sciences Award, UL 1 TR001085, awarded to Dr. Matula for the 2014-2015 academic year.
References
- 1. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008;299(6):656–64. [DOI] [PubMed] [Google Scholar]
- 2. Deyo RA, Mirza SK, Turner JA, Martin BI.. Overtreating chronic back pain: Time to back off? J Am Board Fam Med 2009;22(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. National Vital Statistics System mortality data. 2015. Available at: http://www.cdc.gov/nchs/deaths.htm.
- 4. Kalanithi L, Tai W, Conley J, et al. Better health, less spending: Delivery innovation for ischemic cerebrovascular disease. Stroke 2014;45(10): 3105–11. [DOI] [PubMed] [Google Scholar]
- 5. Burns JW, Glenn B, Bruehl S, Harden RN, Lofland K.. Cognitive factors influence outcome following multidisciplinary chronic pain treatment: A replication and extension of a cross-lagged panel analysis. Behav Res Ther 2003;41(10):1163–82. [DOI] [PubMed] [Google Scholar]
- 6. Haldeman S, Dagenais S.. A supermarket approach to the evidence informed management of chronic low back pain. Spine J 2008;8(1):1–7. [DOI] [PubMed] [Google Scholar]
- 7. Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): A randomised controlled trial. Lancet 2011;378(9802):1560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandopulle R. Learning to fly: Building de novo medical home practices to improve experience, outcomes, and affordability. J Ambul Care Manage 2013;36(2):121–5. [DOI] [PubMed] [Google Scholar]
- 9. Blackmore CC, Mecklenburg RS, Kaplan GS.. Effectiveness of clinical decision support in controlling inappropriate imaging. JACR J Am Coll Radiol 2011;8(1):19–25. [DOI] [PubMed] [Google Scholar]
- 10. Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol 2002;39: 471–80. [DOI] [PubMed] [Google Scholar]
- 11. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at group health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood) 2012;31(9): 2094–104. [DOI] [PubMed] [Google Scholar]
- 12. Ip IK, Gershanik EF, Schneider LI, et al. Impact of IT-enabled intervention on MRI use for back pain. Am J Med 2014;127(6):512–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. LaCaille RA, DeBerard MS, Masters KS, Colledge AL, Bacon W.. Presurgical biopsychosocial factors predict multidimensional patient: Outcomes of interbody cage lumbar fusion. Spine J 2005;5(1):71–8. [DOI] [PubMed] [Google Scholar]