Abstract
The goal of this research consortium including Janssen, MSD, Ncardia, FNCR/LBR, and Health and Environmental Sciences Institute (HESI) was to evaluate the utility of an additional in vitro assay technology to detect potential drug-induced long QT and torsade de pointes (TdP) risk by monitoring cytosolic free Ca2+ transients in human stem-cell-derived cardiomyocytes (hSC-CMs). The potential proarrhythmic risks of the 28 comprehensive in vitro proarrhythmia assay (CiPA) drugs linked to low, intermediate, and high clinical TdP risk were evaluated in a blinded manner using Ca2+-sensitive fluorescent dye assay recorded from a kinetic plate reader system (Hamamatsu FDSS/µCell and FDSS7000) in 2D cultures of 2 commercially available hSC-CM lines (Cor.4U and CDI iCell Cardiomyocytes) at 3 different test sites. The Ca2+ transient assay, performed at the 3 sites using the 2 different hSC-CMs lines, correctly detected potential drug-induced QT prolongation among the 28 CiPA drugs and detected cellular arrhythmias-like/early afterdepolarization in 7 of 8 high TdP-risk drugs (87.5%), 6 of 11 intermediate TdP-risk drugs (54.5%), and 0 of 9 low/no TdP-risk drugs (0%). The results were comparable among the 3 sites and from 2 hSC-CM cell lines. The Ca2+ transient assay can serve as a user-friendly and higher throughput alternative to complement the microelectrode array and voltage-sensing optical action potential recording assays used in the HESI-CiPA study for in vitro assessment of drug-induced long QT and TdP risk.
Current preclinical safety assessment models for small molecule drug candidates have been shown to provide adequate evaluation of risk for drug-induced long QT and torsade de pointes (TdP). However, the overall approach taken by different companies is often complex and incorporates different in vitro and in vivo assays involving preparations from multiple animal species, each of which do not always fully translate to humans and so are applied in a matrix to provide a safety net of detection. The need for more human-based translational cardiac models together with the effort to implement the 3R’s (replace, reduce, refine) concept with respect to preclinical animal use has led to intensive investigation of the potential utility of human stem-cell-derived cardiomyocytes (hSC-CMs).
The rapid progress in the field of hSC-CMs has allowed extensive evaluation of the utility of this platform to predict cardiac proarrhythmic liabilities (Kopljar et al., 2018a; Sala et al., 2016). As a result, hSC-CMs are increasingly being used as part of early safety derisking of drug candidates (Authier et al., 2017) and are one of the work stream components of the comprehensive in vitro proarrhythmia assay (CiPA) initiative (Colatsky et al., 2016; Gintant et al., 2016). The objective of this initiative is to evaluate and facilitate the adoption of a new paradigm focused on actual clinical potential for proarrhythmia rather than human ether-à-go-go-related gene (hERG) inhibition and QT prolongation alone (http://cipaproject.org/about-cipa/). As part of the hSC-CMs component of the CiPA initiative, the Myocyte Team, coordinated by Health and Environmental Sciences Institute (HESI) and the FDA, has conducted a phase I pilot study with 8 reference drugs (Millard et al., 2018) and a phase II validation study with 28 drugs selected by the CiPA steering committee using 2 assay platforms: microelectrode array (MEA) and voltage-sensing optical action potential recording (VSO) using voltage-sensitive dyes on hSC-CMs (Blinova et al., 2018). These 2 studies suggest that hSC-CMs are useful to detect potential drug-induced risks for long QT and related TdP.
In addition to the formal assessment of hSC-CM assays as part of the CiPA initiative, in preparation for phase I submission, hSC-CM assays based on calcium-sensitive imaging are also being evaluated and implemented as an early cardiac safety screening model since it is more amenable to a moderate/high-throughput testing format (Abi-Gerges et al., 2017; Bedut et al., 2016; Dempsey et al., 2016; Lu et al., 2015; Neil et al., 2017; Rast et al., 2015; Watanabe et al., 2017; Zeng et al., 2016). Cytosolic-free Ca2+ is the critical intermediary within the excitation-contraction coupling in cardiomyocytes (Fearnley et al., 2011). Application of calcium-sensitive fluorescence dyes in hSC-CM cultures enables imaging of intracellular calcium transients, which reflect the rise and decay of cytosolic Ca2+ during a cardiac action potential. Directly after the depolarization of the plasma membrane (excitation) in phase II of the action potential, Ca2+ is released from sarcoplasmic reticulum into the cytosol, which activates the contractile machinery (Blanchette et al., 2018). Following the contraction, cytosolic Ca2+ is rapidly removed from the cytosol causing relaxation of the cardiomyocyte and ending the excitation-contraction cycle. In hSC-CMs, changes in calcium transient morphology indirectly reflect action potential duration and arrhythmic-like events (eg, early afterdepolarization [EADs]) (Spencer et al., 2014), suggesting that calcium transient duration (CTD) might be a valuable surrogate marker for drug-induced changes in action potential duration and proarrhythmia (Oksana et al., 2017; Pfeiffer et al., 2016; Sirenko et al., 2013). Therefore, calcium-sensitive imaging can be used as a practical medium-to-high-throughput screening assay for detecting drug-induced cardiac liabilities in hSC-CMs.
Here, we present results of an evaluation of the 28 CiPA reference compounds using the calcium transient assay to determine the potential for drug-induced QT prolongation and proarrhythmia risk in 2 different commercial cell types of hSC-CMs performed at 3 different sites. The standard and blinded CiPA protocol was used in these studies. The results indicate that the Ca2+ transient assay in hSC-CMs can be used as an additional assay to detect potential for drug-induced QT prolongation and proarrhythmia risk.
MATERIALS AND METHODS
Study sites and platforms
Three independent laboratories (Safety and Exploratory Pharmacology, Merck Sharp & Dohme Corp., West Point, Pennsylvania: MSD; Janssen Pharmaceutica NV, Belgium: J&J; and Ncardia, AG-Germany) participated in the study using the calcium transient assay—Functional Drug Screening System (FDSS) platform (Hamamatsu, Japan): FDSS7000 (Ncardia), FDSS/µCell (J&J) and MSD. The machines are equipped with the temperature control modules enabling recordings at physiological temperature and robotics for automated application of drugs.
Cell culture and reagents in hSC-CMs
Two commercially available hSC-CM cell lines were used: iCell Cardiomyocytes (Cellular Dynamics International, Madison, Wisconsin) and Cor.4U Cardiomyocytes (Ncardia AG, Cologne, Germany). Human SC-CMs from Ncardia (Cor.4U Cardiomyocytes) were used at Janssen and Ncardia sites, while cells from Cellular Dynamics International (iCells) were used by the MSD site.
Cor.4U Cardiomyocytes were delivered as CardioPlate 96 that contained ready-to-use living and precultured cardiomyocytes in fibronectin-coated 96-well “µClear plates” at a density of 25 000 cells/well. iCell cardiomyocytes were delivered as cryopreserved vials, seeded at 30 000 cells/well into 96-well microtiter plates to form a confluent synchronously beating monolayer after thawing. Cells were cultured according to each cell provider’s instructions.
Calcium transient measurements
The method for the calcium transient assay used in the present study has been described recently (Zeng et al., 2016).
On the day of experiment execution, culture medium in the 96-well plates containing the monolayers of hSC-CMs was replaced with Tyrode’s solution (Sigma, No. T2397) supplemented with 10-mM HEPES together with KCl to yield isokalemic (4.2 mM K+) conditions.
At Janssen and Ncardia, the calcium-sensitive fluorescence dye Cal-520 AM (Cat. no. 21131; AAT Bioquest) was used to capture the intracellular calcium transients in hiPSC-CMs. The protocol used was as described by Kopljar et al., (2018a). Briefly, Cal-520 was incubated for 70 min followed by a washout with supplemented Tyrode’s solution and a 30-min recovery before starting the experiments at 37°C. On the day of experiment, these solutions were further diluted with the supplemented Tyrode’s solution. Compound was added automatically using the Functional Drug Screen System (FDSS/µCell; Hamamatsu, Japan) head stage by adding 100 µl of the 2-fold concentrated compound solution to wells with hSC-CMs already containing a volume of 100 µl of the experimental solution, finally reaching the intended test concentration in 0.1% dimethylsulfoxide (DMSO). At MSD iCells were cultured for 14 days, thus forming a stable and spontaneously beating monolayer, before cells were incubated with the Codex ACTOne Non-Wash Calcium Dye (Cat. no. CB-80500-311) in serum-containing medium for 1 h at 37°C and 5% CO2.
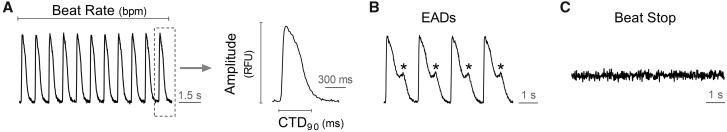
The spontaneous beating activity of hSC-CMs was assessed through measurement of the Ca2+ fluorescence signal integrated over the whole well. Ten minutes before starting the recordings, the experimental plates were put into the FDSS system to stabilize. Next, a baseline recording was run for 4 min followed by compound addition. The effect of compounds was recorded around 30 min after compound addition for 5 min. The Ca2+ transient signals were recorded at a sampling every 16 ms (frame rate of 62.5 Hz) in hSC-CMs maintained at 37°C during the acquisition time. Calcium transient duration at 90% of repolarization (CTD90) is considered to be a surrogate for action potential duration or QT interval of the electrocardiogram (Spencer et al., 2014). In addition, beat rate (BR = peak count/min) and amplitude (amp = difference between max. and min. of every beat) were measured and incidence of cellular arrhythmias, eg, EAD-like activity (considered a surrogate of TdP) and cessation of beating (= stop beating) were also recovered (Figure 1).
Figure 1.
Parameters were recorded in Ca2+ transient assay using hSC-CMs. CDT90: calcium transient duration at 90% of decay following the peak amplitude (in ms).
Drug dilution and addition
Blinded samples of neat drug were sent to all sites by the Chemotherapeutic Agents Repository of the National Cancer Institute (USA), and stored at −20°C until the day of testing. Four concentrations of each drug were studied (Table 1). Four DMSO stocks for each drug concentration were prepared and either used on the same day or aliquoted and frozen. Concentrated (2×) testing solutions (50× for sequential dosing) for each concentration were prepared freshly on the day of testing by diluting DMSO stocks into experimental medium. Two-fold dilution was done when drugs were added to the experimental well to achieve targeted concentration. For sequential dosing, DMSO concentrations were adjusted sequentially up to 0.1% at the highest concentration to achieve targeted concentration of each drug. The 28 CIPA compounds and test concentrations are listed in Table 1. Each compound concentration was tested in 6 replicates.
Table 1.
The 28 CIPA Compounds Categorized Based on High, Intermediate, and Low Risk for Clinical TdP Were Used in the Present Study
| Generic Name | Free Cmax | Drug Concentration in µM | ||||
|---|---|---|---|---|---|---|
| High risk | Azimilide | 0.07 | 0.01 | 0.1 | 1 | 10 |
| Bepridil | 0.032 | 0.01 | 0.1 | 1 | 10 | |
| d,l-Sotalol | 15 | 0.1 | 1 | 10 | 100 | |
| Disopyramide | 0.7 | 0.1 | 1 | 10 | 100 | |
| Dofetilide | 0.002 | 0.0003 | 0.001 | 0.003 | 0.01 | |
| Ibutilide | 0.1 | 0.0001 | 0.001 | 0.01 | 0.1 | |
| Quinidine | 3 | 1 | 3 | 9.5 | 30 | |
| Vandetanib | 0.3 | 0.01 | 0.1 | 1 | 10 | |
| Intermediate risk | Astemizole | 0.0003 | 0.0001 | 0.001 | 0.01 | 0.1 |
| Chlorpromazine | 0.0345 | 0.1 | 0.3 | 0.9 | 3 | |
| Cisapride | 0.00258 | 0.003 | 0.01 | 0.03 | 0.1 | |
| Clarithromycin | 1.21 | 0.1 | 1 | 10 | 100 | |
| Clozapine | 0.071 | 0.1 | 0.3 | 0.9 | 3 | |
| Domperidone | 0.02 | 0.003 | 0.03 | 0.3 | 3 | |
| Droperidol | 0.016 | 0.03 | 0.1 | 0.3 | 1 | |
| Ondansetron | 0.37 | 0.03 | 0.3 | 3 | 30 | |
| Pimozide | 0.00043 | 0.001 | 0.003 | 0.01 | 0.03 | |
| Risperidone | 0.0018 | 0.003 | 0.01 | 0.03 | 0.1 | |
| Terfenadine | 0.00029 | 0.001 | 0.01 | 0.1 | 1 | |
| Low risk | Diltiazem | 0.13 | 0.01 | 0.1 | 1 | 10 |
| Loratadine | 0.00045 | 0.001 | 0.003 | 0.01 | 0.03 | |
| Metoprolol | 1.8 | 3 | 10 | 32 | 100 | |
| Mexiletine | 2.5 | 0.1 | 1 | 10 | 100 | |
| Nifedipine | 0.0077 | 0.001 | 0.01 | 0.1 | 1 | |
| Nitrendipine | 0.0030 | 0.01 | 0.03 | 0.1 | 0.3 | |
| Ranolazine | 1.95 | 0.1 | 1 | 10 | 100 | |
| Tamoxifen | 0.021 | 0.1 | 0.3 | 1 | 3 | |
| Verapamil | 0.045 | 0.001 | 0.01 | 0.1 | 1 | |
The free Cmax level and concentrations were selected based on the HESI/CIPA stem-cell group studies (Blinova et al., 2018).
Dofetilide was used for the positive control: 3 nM of dofetilide was used by Janssen and Ncardia, while 10 nM of dofetilide was used by MSD according to the recommendation of the cell provider.
Statistical analysis
For all individual experiments, delta percent (Δ%) at 30 min with respect to the baseline value was calculated (eg, Δ% for CTD90 = CTD90 30 min − CTD90 0 min)/CTD90 0 min × 100). The following 2 statistical approaches were used to make decisions about a compound’s effect.
First, the tolerance interval (TI)-based categorization, where the Δ% values of all (pooled) DMSO wells (experiments in this study) were centered around zero (corrected with the mean) and the parametric 90%–95% (p = .90 and 1−α = 0.95) TI was calculated (Liao et al., 2005). TIs indicate an interval where, with a certain confidence level (1−α = 0.95), a specified proportion (p = .90) of a sampled population falls. The lower and upper limits of the TI values were used as cutoff for the vehicle corrected net effect (ΔΔ%) values of the compounds to categorize them. These ΔΔ% net effects were the values obtained after normalization for baseline (Δ% versus baseline) per well and the aggregated compound treated wells are vehicle corrected by subtraction with the aggregated Δ% of the corresponding DMSO from the same plate (eg, ΔΔ% = mean Δ% drug – mean ΔΔ% DMSO). The categorization was made first for each concentration and then aggregated per compound and test site. If a net effect (ΔΔ%) at a given concentration of a compound was below the lower limit of the TI, then it was categorized as “decreased”; if the net effect was within the DMSO TI then it was categorized as “no effect”; and if the net effect was above the upper limit of the TI then it was categorized as “increased”. At compound level, a compound was categorized as “increased” if it was in a category “increased” in at least at 1 of the 4 concentrations. Similarly, a compound was categorized as decreased if it was in a category “decreased” in at least 1 of the 4 concentrations.
Second, statistical significance testing against DMSO (of the same plate) using a nonparametric 2-sided test (Wilcoxon’s Mann Whitney) (Ellison, 1964) was performed, and p values below .05 are considered significant. Otherwise, it was declared nonsignificant (NS). Data are expressed as means ±SEM. All statistical analyses were performed using the SAS software. Copyright 2002-2012 SAS Institute Inc.
RESULTS
As described in the CiPA study (Blinova et al., 2018), the potential for drug-induced APD/QT prolongation and TdP are the most important endpoints for the evaluation of the 28 CiPA compounds, which are linked to different proarrhythmic risk levels in the clinic. Therefore, our results are mainly focused on drug-induced changes in CTD90, a surrogate for action potential duration on hSC-CMs and incidence of EAD-like activity (considered to be a surrogate of TdP).
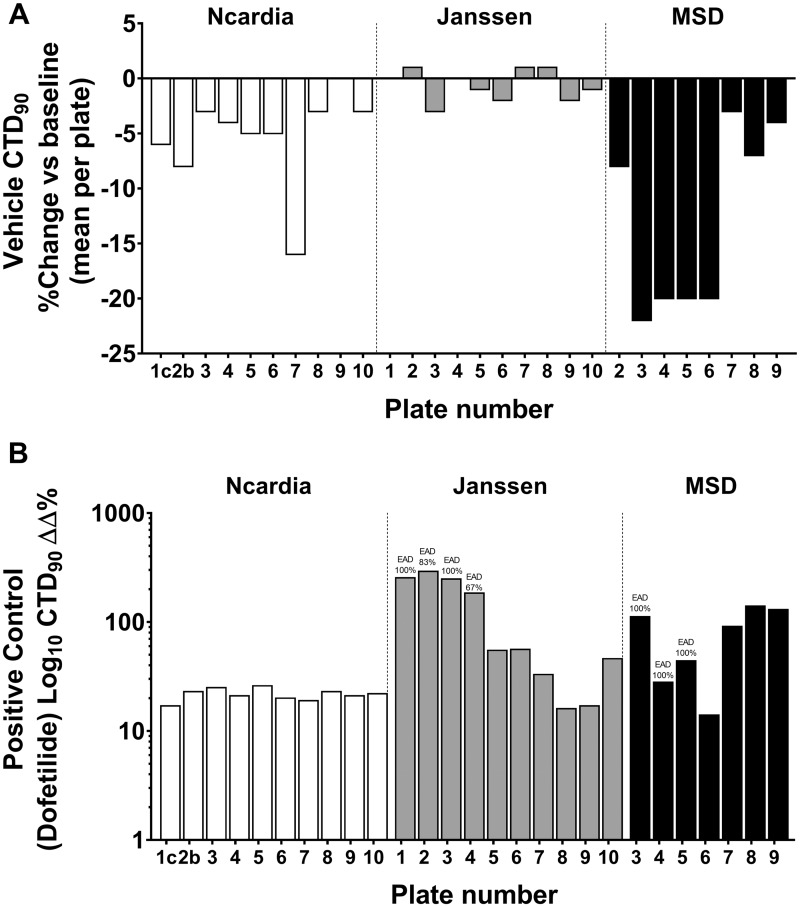
Vehicle treatments showed relatively low variability in Δ% for CTD90, beat rate (BR), and amplitude (Amp) with respect to baseline across the 3 sites (an example of variability in CTD90 in the DMSO controls is shown in Figure 2A), although it was higher in MSD site than other 2 sites. To define the cutoffs (boundaries) between the different effect zones for CTD90, we used vehicle controls and the positive control dofetilide from 3 test sites (Figure 2B). The “no effect” zone represents changes in CTD90 that are likely within vehicle variability, whereas “effect” zones for CTD90 are differentiated bidirectionally (shortening = decrease or prolongation = increase), although, in the present study we are mainly focusing on “prolongation = increase” for these 28 CiPA drugs. The variabilities of the BR and amplitude with DMSO and positive control-dofetilide in 3 sites are provided in Supplementary Figures 1 and 2.
Figure 2.
Plate-to-plate and site-to-site variability on CTD90 (mean/plate/site) expressed in Δ% for vehicle (A) and in ΔΔ% for dofetilide (as positive control) in hSC-CMs (B), measured from the Ca2+ transient in different test sites.
The TIs (Table 2) indicate the interval where, at a certain confidence level, a specified proportion of a sampled population falls. Cutoffs were determined by correcting the TIs for each parameter (CTD90, BR, and amplitude) with the centralized TI windows of vehicles (Table 1). The positive control dofetilide (tested at 3 nM at Janssen and Ncardia, and at 10 nM at MSD [Kenilworth, New Jersey] clearly showed prolongation of CTD90 [Figure 2B]).
Table 2.
Tolerance Intervals (TI 90%–95%) for CTD90, Fridericia’s Rate-Corrected CTD90 (CTD90c F), Beat Rate, and Amplitude of the Calcium Transient in hSC-CMs at Each Test Site
| Parameter | Site |
|||||
|---|---|---|---|---|---|---|
| Ncardia |
Janssen |
MSD |
||||
| Lower | Upper | Lower | Upper | Lower | Upper | |
| CTD90 | −5 | 5 | −4 | 4 | −10 | 10 |
| CTD90c F | −4 | 4 | −3 | 3 | −8 | 8 |
| Beat rate | −8 | 8 | −7 | 7 | −16 | 16 |
| Amplitude | −5 | 5 | −7 | 7 | −14 | 14 |
Rate-corrected action potential duration of CTD90 based on QTc formulas such as Fridericia () have been used in other studies (Blinova et al., 2018). As we recently reported (Lu et al., 2017), QTc formulas used for in vivo human ECG correction have limitations when applied to rate correction of action potential duration for in vitro hiPSC-CMs, and there is no significant or relevant difference between using the rate correction formula for drug-induced changes in repolarization duration compared with noncorrected duration in hSC-CMs (Kopljar et al., 2018b). Our current data also indicate that changes in the rate-corrected and noncorrected CTD90 are qualitatively similar to the 28 CiPA drugs (Supplementary Table 2).
Effects of the 28 CiPA Drugs Using Ca2+ Transient Assay in Two Types of hSC-CMs at 3 Sites
Next, we analyzed the changes in CTD90 and incidence of EADs for the 28 CIPA drugs using the standard protocol with blinded testing at 3 sites and 2 hSC-CMs cell lines (Cor.4U Cardiomyocytes for Janssen and Ncardia site, and iCells for MSD [Kenilworth, New Jersey]). Compounds in the 28 CiPA drugs list are grouped by risk of manifesting human TdP, and are ranked as high, intermediate, and low risk as shown in the CiPA studies (Blinova et al., 2018). The concentration-dependent effects of the 28 compounds on CTD90 and incidence of EAD on Ca2+ transient assay from the 3 sites are provided in detail in Supplementary Table 1.
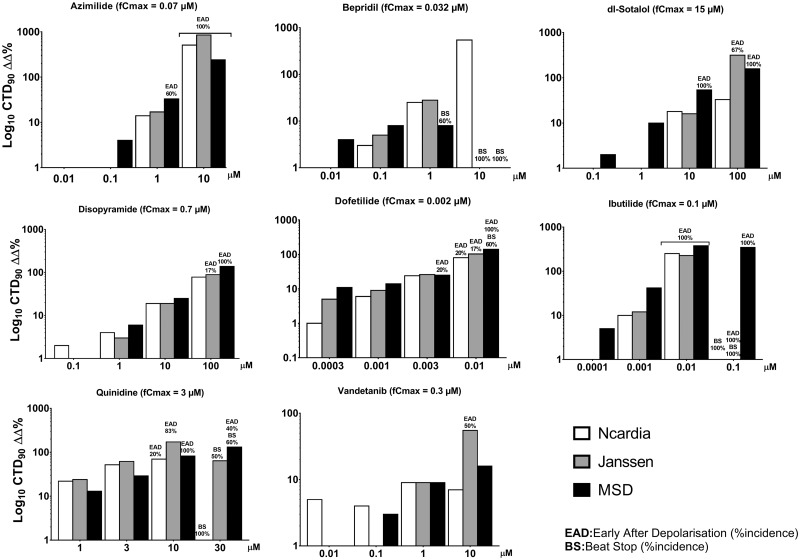
The 8 high-risk drugs (dofetilide, azimilide, bepridil, d,l-sotalol, disopyramide, ibutilide, quinidine, and vandetanib) all prolonged CTD90 (Table 3 and Figure 3) at all sites with 1 exception being bepridil, which only did not prolong CTD90 in the MSD site except with cessation of beating at the top dose (Table 3). Although bepridil is a hERG blocker with propensity to prolong repolarization, it also possesses Ca2+ and Na+ antagonist properties (Yatani et al., 1986), which tends to offset the degree of prolongation of repolarization and can alter rate and potentially shorten repolarization. At 10 µM, bepridil resulted in cessation of beating in all wells at the MSD site, and at Janssen, which precluded assessment of possible changes in CTD90. In the HESI/FDA CiPA study, similar results with bepridil were observed using MEA and VSO assays (Blinova et al., 2018). Importantly, at all 3 sites the 8 high TdP-risk drugs elicited high incidence of EADs (Table 3), which are cellular arrhythmias that are considered to be a surrogate for TdP.
Table 3.
Overview Summary of the Effects of the 28 CIPA Drugs on the CTD90 Prolongation and Incidence of Early Afterdepolarizations (EADs), Measured From the Ca2+ Transient Imaging on hSC-CMs, at the 3 Test Sites
| Concentration (µM) |
CTD90 |
EAD Incidence |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Free Cmax | Tested Range | Ncardia | Janssen | MSD | Ncardia | Janssen | MSD | |
| High | Azimilide | 0.07 | 0.01–10 | Prolong | Prolong | Prolong | + | + | + |
| Bepridil | 0.032 | 0.01–10 | Prolong | Prolong | A | − | − | − | |
| d,l-Sotalol | 15 | 0.1–100 | Prolong | Prolong | Prolong | − | + | + | |
| Disopyramide | 0.7 | 0.1–100 | Prolong | Prolong | Prolong | − | + | + | |
| Dofetilide | 0.002 | 0.0003–0.01 | Prolong | Prolong | Prolong | + | + | + | |
| Ibutilide | 0.1 | 0.0001–0.1 | Prolong | Prolong | Prolong | + | + | + | |
| Quinidine | 3 | 1–30 | Prolong | Prolong | Prolong | + | + | + | |
| Vandetanib | 0.3 | 0.01–10 | Prolong | Prolong | Prolong | − | + | − | |
| Intermediate | Astemizole | 0.0003 | 0.0001–0.1 | Prolong | NE | Prolong | − | − | + |
| Chlorpromazine | 0.0345 | 0.1–3 | Prolong | Prolong | Prolong | − | − | − | |
| Cisapride | 0.00258 | 0.003–0.1 | Prolong | Prolong | Prolong | − | − | + | |
| Clarithromycin | 1.21 | 0.1–100 | Prolong | Prolong | NE | − | + | − | |
| Clozapine | 0.071 | 0.1–3 | Prolong | Short | Prolong | − | − | − | |
| Domperidone | 0.02 | 0.003–3 | Prolong | Prolong | Prolong | − | + | + | |
| Droperidol | 0.016 | 0.03–1 | Prolong | Prolong | Prolong | − | + | − | |
| Ondansetron | 0.37 | 0.03–30 | Prolong | Prolong | Prolong | − | + | + | |
| Pimozide | 0.00043 | 0.001–0.03 | NE | NE | Prolong | − | − | − | |
| Risperidone | 0.0018 | 0.003–0.1 | Prolong | Prolong | NE | − | − | − | |
| Terfenadine | 0.00029 | 0.001–1 | Prolong | Prolong | Prolong | − | − | − | |
| Low | Diltiazem | 0.13 | 0.01–10 | Short | Short | A | − | − | − |
| Loratadine | 0.00045 | 0.001–0.03 | NE | NE | NE | − | − | − | |
| Metoprolol | 1.8 | 3–100 | Prolong | Prolong | Short | − | − | − | |
| Mexiletine | 2.5 | 0.1–100 | Prolong | Prolong | Prolong | − | − | − | |
| Nifedipine | 0.0077 | 0.001–1 | Short | NE | NE | − | − | − | |
| Nitrendipine | 0.0030 | 0.01–0.3 | Short | Short | Short | − | − | − | |
| Ranolazine | 1.95 | 0.1–100 | Prolong | Prolong | A | − | − | − | |
| Tamoxifen | 0.021 | 0.1–3 | Prolong | NE | Short | − | − | − | |
| Verapamil | 0.045 | 0.001–1 | Short | Short | Short | − | − | − | |
NE, no effect (within vehicle tolerance interval range); A, no value because of the cell with beating stop or EAD at the dose; +, EAD presence. Prolong or short is determined by comparing for each concentration the ΔΔ% (net effect) versus the corresponding centered vehicle TI interval and then aggregate across the 4 tested concentrations per compound and per test site.
Figure 3.
Concentration-dependent effects of the high TdP-risk drugs on CTD90 and incidence of early afterdepolarizations (EADs) and beat stop (BS) in hSC-CMs, measured from the Ca2+ transient assay from the 3 sites.
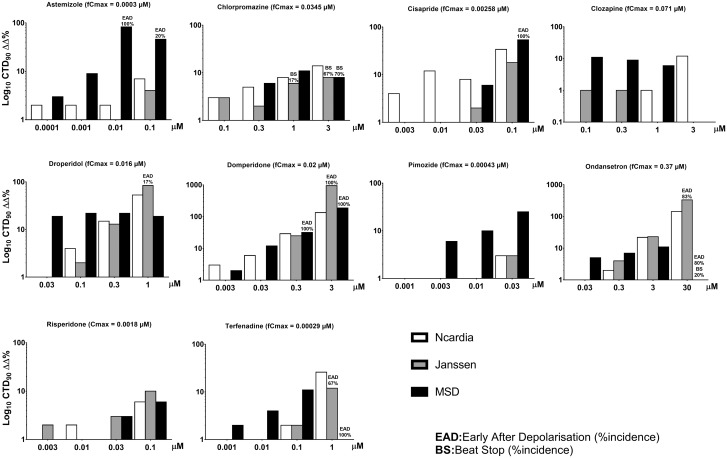
The 11 drugs in the intermediate TdP-risk category were terfenadine, astemizole, chlorpromazine, cisapride, clarithromycin, clozapine, domperidone, droperidol, ondansetron, pimozide, and risperidone. hSC-CM responses to terfenadine for the 3 sites are shown in Table 3 and corresponding figures for its dose-dependent effects along with the other intermediate risk drugs are shown in Supplementary Figure 4. With respect to prolongation of CTD90, the majority of compounds were detected and 6 of these drugs elicited EAD as well. Although astemizole did not prolong CTD90 at the Janssen site, there are several reasons that might explain this variability in response to this in vitro assay. For example, in vitro, it was reported that detection of action potential prolongation at 0.1–3 µM astemizole required 180-min incubation (Adamantidis et al., 1995) and the compounds might stick to the plastic. The drug has low concentration recovery in in vitro assays, which might cause inconsistencies in results (internal personal communication at Janssen). Clarithromycin and risperidone caused CTD90 prolongation at 2 of 3 sites. On the other hand, pimozide did not prolong CTD90 at 2 sites but at the MSD site it moderately prolonged CTD90 at the highest dose of 0.03 µM (∼70-fold free therapeutic Cmax). Overall, the assay performed reasonably well in detecting the potential risk of these 11 intermediate TdP-risk category drugs. In general, these drugs are categorized as intermediate risk based on requirement for other concurrent risk factors in order to prolong QT interval and induce TdP in humans. These risk factors may include pharmacokinetic drug-drug interactions that may raise plasma levels, pharmacodynamic drug-drug interactions when use in combination with another drug with propensity to prolong QT interval and the patient conditions such as with LQTs (Figure 4).
Figure 4.
Concentration-dependent effects of the 11 intermediate TdP-risk drugs on CTD90 and incidence of early afterdepolarizations (EADs) and beat stop (BS) in hSC-CMs, measured using the Ca2+ transient assay at the 3 sites. fCmax, free therapeutic plasma level in humans.
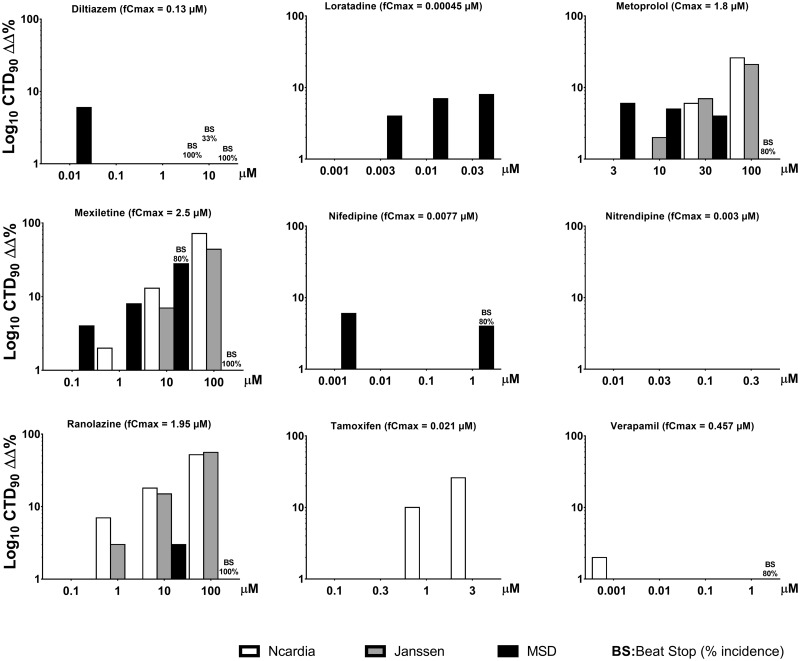
The 9 drugs in the low TdP-risk category were verapamil, diltiazem, loratadine, metoprolol, mexiletine, nifedipine, nitrendipine, ranolazine, and tamoxifen. hSC-CMs responses to verapamil for all 3 sites are shown in Table 3 and Figure 5. The corresponding figures for its concentration-dependent effects along with the effects of the other low risk drugs are shown in Supplementary Tables. Importantly, none of the 9 low TdP-risk drugs elicited EADs. Loratadine did not prolong repolarization or induce EADs at any concentrations at all 3 sites. The Ca2+ antagonists: diltiazem, nifedipine, nitrendipine, and verapamil showed mostly shortenings of CTD90.
Figure 5.
Concentration-dependent effects of the 9 low TdP-risk drugs on CTD90 and incidence of early afterdepolarizations (EADs) and beat stop (BS) in hSC-CMs, measured using the Ca2+ transient assay at the 3 sites.
The remaining 4 drugs (ranolazine, metoprolol, tamoxifen, and d-mexiletine) showed the same or only slightly different effects on CTD90 across the 3 test sites. Ranolazine is a known mixed ion channel blocker including hERG blocking activities (Schram et al., 2004) at clinical concentrations and produces QT prolongation. Ranolazine caused CTD90 prolongation starting at 10 and 100 µM at 2 sites, and elicited cessation of beating (beat stop: BS) in all wells at 100 µM (>50-fold free Cmax) at the MSD site.
Metoprolol is a selective β-1 blocker that slows heart rate reduction clinically and is not associated with TdP risk. However, many β-blockers including metoprolol are known to have membrane-stabilizing activities, which affect different ion currents such as hERG channel at high concentrations (Klara et al., 2001). In hSC-CMs, where β-blockade does not occur due to the absence of sympathetic innervation, metoprolol moderately prolonged CTD90 only at the very high concentration of 100 µM (55-fold Cmax) at 2 test sites and did not prolong CTD90 at the other test sites. At 10-µM mexiletine (∼4-fold Cmax) slightly prolonged CTD90 at all 3 test sites and at the highest concentration (100 µM, ∼40-fold Cmax). Mexiletine moderately prolonged CTD90 all sites and induced cessation of spontaneous beating (BS) in all wells at the MSD site. These data are similar to that in HESI/CIPA study (Blinova et al., 2018). Mexiletine is a Na+ channel blocker which inhibits hERG with an IC50 of 3.7 µM (Gualdani et al., 2015). Tamoxifen, only at the extremely high concentration of 3 µM (143-fold free Cmax), showed slightly shortened CTD90 at 2 sites. All other doses of tamoxifen (up to 3 µM ∼48-fold free Cmax) had no effect on CTD90.
The effects of 28 CIPA compounds on the BR and the amplitude of the Ca2+ transient of hSC-CMs are presented in Supplementary Tables 3 and 4. Additionally, the outcome of the compound per dose using statistical hypothesis testing (versus vehicle control) was similar to the outcome of the 28 CIPA drugs using the TI analysis (Supplement Table 1). Examples of tracings of Ca2+ transients from 3 different TdP-risk group drugs are presented in Figure 6.
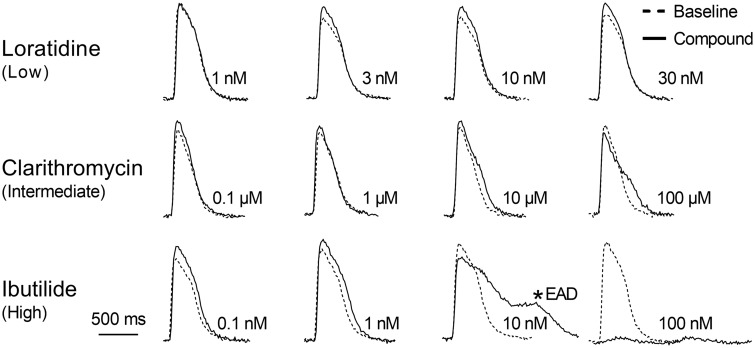
Figure 6.
Representative calcium transients showing the 30-min effect of loratadine (a low TdP-risk drug), clarithromycin (an intermediate TdP-risk drug) and ibutilide (a high TdP-risk drug) compared with their respective baseline recording (black tracing) in hSC-CMs. EAD, early afterdepolarization.
Additionally, we applied modeling of drug proarrhythmic potential on the 28 blinded compounds which was used in FDA/HESI myocyte Subteam (Blinova et al., 2018). The results are similar to these in FDA/HSEI Study and JiCSA (Kanda et al., 2018) (Supplementary Figures 3 and 4).
DISCUSSION
hSC-CMs are becoming an established platform for the evaluation of cardiac liabilities within early derisking and preclinical drug development. Screening of drug-induced effects on hSC-CMs is possible with different technologies eg, based on measurements of extracellular membrane potentials (Ando et al., 2017), impedance/MEA (Bot et al., 2018; Zhang et al., 2016), video motion imaging (Hayakawa et al., 2014; Kopljar et al., 2017), voltage-sensitive dyes (Bedut et al., 2016; Lu et al., 2017), and calcium-sensitive dyes (Abi-Gerges et al., 2017; Bedut et al., 2016; Dempsey et al., 2016; Lu et al., 2015; Rast et al., 2015; Watanabe et al., 2017; Zeng et al., 2016). With the aim of reconsidering ICHS7B and E14 guidelines, a new in vitro assay system using hSC-CMs has been subjected to worldwide validation using MEA and VSO recordings in order to establish a better prediction of potential drug-induced QT prolongation and TdP risk, as part of the HESI/FDA’s CiPA initiative (Blinova et al., 2018; Nozaki et al., 2017). In the present study, we evaluated an additional technology using calcium-sensitive dyes to study the 28 CiPA reference drugs in a blinded fashion to assess their potential to prolong QT and predict TdP risk. Calcium transient morphology has been shown to be a good surrogate/marker for changes in action potential duration, shape (eg, EAD) and occurrence of arrhythmic events in hSC-CMs (Spencer et al., 2014). Our data from 3 different test sites indicate that the calcium transient assay offers an alternative and user-friendly approach, with medium to high throughput screening capability for detection of drug-induced QT prolongation and proarrhythmic risk.
The 28 CiPA reference drugs have been assigned to low, intermediate, and high proarrhythmic risk based on published reports, analysis of the FDA adverse event report system (AERS) database, other data sources, and expert opinions (Colatsky et al., 2016). With 8 high TdP-risk drugs, the assay detects not only the CTD90 prolongation but most importantly indicates high proarrhythmic risk based on incidence of EADs, which are generally considered as potential cellular electrophysiological precursors of TdP. The only exception in this high-risk category was for bepridil: slightly prolonged CTD90 at J&J and Ncardia sites and did not prolong up to the third dose but elicited cessation of beating at the top dose at MSD site. These results are consistent with the result of the CiPA study of bepridil using MEA and VSO assay (Blinova et al., 2018). Bepridil is a Ca2+ antagonist with mixed ion channel actions that include hERG and Na+ channel inhibitory activities (Obejero-Paz et al., 2015) at therapeutic free Cmax of 32 nM (Blinova et al., 2018). In fact, bepridil did prolong CTD90 in studies at all 3 sites. In comparison, other pure Ca2+ antagonists (diltiazem and nitrendipine) markedly shortened CTD90. The 1 site not showing prolongation of CTD90 had beating arrest which precluded assessment of CTD90. It is known that in in vitro assays, the Ca2+ antagonists shorten CTD90/action potential duration/QT interval while in vivo, the same Ca2+ antagonists do not shorten QT interval. The reason for this difference between in vivo and in vitro outcome might relate to their effect on the vascular system to reduce blood pressure with heart rate changes in vivo after oral administration, while bepridil only blocks Ca2+ channel in cardiomyocytes to shorten action potential durations in vitro.
For the 11 intermediate TdP-risk drugs, the Ca2+ transient assay detected drug-induced CTD90 prolongation (QT prolongation) and EADs in 6 out of 11 drugs. In 3 sites, in general prolongation of CTD90 were similar within 3 sites, and incidence of EADs are similar between Janssen and MSD while absent in Ncardia. One exception was pimozide, where CTD90 was only slightly prolonged at 0.03 µM (∼70-fold free Cmax) at the MSD site. It is known that pimozide requires much higher doses both in vivo (Redfern et al., 2003) and in vitro to detect QT prolongation (De Bruin et al., 2005). Pimozide prolonged the action potential duration only at ∼230-fold of its free Cmax level in isolated ventricular myocytes (Drolet et al., 2001). In general, all antipsychotic drugs require very high concentrations to detect QT/APD prolongation in in vitro assays. Findings were similar with other antipsychotic drugs tested in the present study (chlorpromazine, clozapine, and risperidone) and similar to results observed in the MEA and VSO recordings in the CiPA studies (Blinova et al., 2018). Additionally, much higher concentrations than their respective free Cmax are needed to detect QT/APD prolongation in earlier studies with other antipsychotic drugs such as sertindole and olanzapine (Redfern et al., 2003). The clinical QT prolongation and TdP incidence with these intermediate TdP-risk drugs, especially for these antipsychotic drugs, are rare and mostly observed in patients with other conditions such as heart disease, hypokalemia/magnesemia/calcemia, bradycardia, and comedications. The basis for the lack of response at 1 site is unknown but there were 2 different hSC-CM types used in this study. This was an outlier drug on the ILSI HESI paper in cross-site comparison on hERG where the IC%’s for most compounds were within 3-fold but 40-fold for Pimozide thus suggesting a difficult drug to deal with (Hanson et al., 2006).
It is very important to note that in the present study using the Ca2+ transient assay in hiPSC-CMs, there were no incidences of EADs in the 9 low TdP-risk drugs. Among the 9 low TdP-risk drugs, there are 7 cardiac active drugs and 2 noncardiac drugs (loratadine and tamoxifen). Loratadine (an antihistamine) and tamoxifen (an antibreast cancer drug) are not known to have TdP risk in humans and had no effects on CTD90 in hSC-CMs. Four low TdP-risk drugs are the Ca2+ antagonists (diltiazem, nifedipine, nitrendipine, and verapamil) which shortened CTD90 as was observed in the other CiPA study using MEA and VSO recording (Blinova et al., 2018).
The remaining 4 low TdP-risk drugs (ranolazine, metoprolol, tamoxifen, and mexiletine) showed some or slight differences in effects on CTD90 among the 3 test sites. Ranolazine is a known mixed ion channel blockers (including hERG inhibition) at clinical concentrations and produces QT prolongation (Schram et al., 2004). Ranolazine caused CTD90 prolongation starting at 10 and 100 µM at 2 sites (JNJ and Ncardia), and elicited beat stop in all wells at 100 µM (>50-fold free Cmax) at the MSD site. Metoprolol is a selective β-1 blocker with membrane-stabilizing activities which affect different ion currents such as hERG at high concentrations (Klara et al., 2001). Metoprolol moderately prolonged CTD90 only at the very high concentration of 100 µM (55-fold Cmax) at 2 test sites (JNJ and Ncardia). Mexiletine is a Na+ channel blocker that also inhibits hERG with an IC50 of 3.7 µM (Gualdani et al., 2015). Mexiletine (at 10 µM ∼4-fold Cmax) slightly prolonged CTD90 at all 3 test sites and at the highest concentration (100 µM, ∼40-fold Cmax), it moderately prolonged CTD90 and induced all cessation of spontaneous beating (BS) at the MSD site without incidence of EADs. Our findings are similar to those from the MEA and VSO data in the HESI/CiPA study (Blinova et al., 2018). With respect to cardiac electrophysiology, calcium channel antagonists evoke very strong responses in beating properties of hSC-CMs in vitro, in which marked shortening of CTD90 was observed while less QT prolongation was observed with Ca2+ antagonists after oral administration in vivo (working mainly on the systemic vascular system). Furthermore, internal calcium from intracellular stores into the cytosol of cardiomyocytes is triggered by cardiac L-type calcium currents during phase II of the action potential which could be measured directly and the effects of INa channel blockers can only be evaluated indirectly in the calcium transient assay eg, by slowing the spontaneous BR and even the beating stop.
In the present study, we focused on drug-induced QT prolongation (CTD90 prolongation) and TdP risk (incidence of EADs) among the 28 CiPA compounds using a Ca2+ transient assay in hiPSC-CMs. Our data indicate that the assay is very useful for detecting drug-induced QT prolongation and TdP risk. However, other cardiac liabilities, such as bradycardia, QT shortening, non-TdP like ventricular tachycardia and fibrillation, can also impede the development of a drug (Lu et al., 2008, 2010). The Ca2+ transient assay in iPSC-CMs can also allow identification of other cardiac hazards for compounds with different pharmacological mechanisms beyond hERG inhibition or QT prolongation as reported recently (Kopljar et al., 2018b) and previously in primary cardiomyocytes (Qian and Guo, 2010). These attributes position hSC-CMs as a versatile early preclinical safety derisking screening tool. As expected, Ca2+ antagonists induced strong responses in hiPSC-CMs, showing largely decreased amplitude, accompanied by CTD90 shortening and pronounced BR increase. Drug-induced beating stop (cessation of beating) in hSC-CMs is often observed with hERG inhibitors at relative low concentrations, Ca2+ antagonists and Na+ channel blockers, and is not necessarily a specific indicator of a potential cardiac effect when the compound is given in vivo, and therefore may not directly translate to clinical cardiac risks as QT prolongation and TdP risk does, but at increased risk for other cardiac effects (Yang et al., 2014). Strongly decreased BR can be related to a bradycardic action or (indirect) effects on action potential propagation (Kopljar et al., 2018a), and BR changes seem to be secondary to changes on CTD90 or amplitude of the calcium transient. However, changes in BR may be more relevant for pharmacological classes such as adrenergic agonists, which increase BR in hSC-CM.
Advantages and Limitations of the Assay
The Ca2+ transient measurement in hSC-CMs using calcium-sensitive fluorescent dyes can readily reveal basic parameters such as BR, CTD90, and amplitude, which correlate well with some electrophysiological parameters recorded either in vitro in isolated cardiomyocytes, ex vivo (ie, cardiac tissue, and heart) or in vivo with ECG recordings. Furthermore, CTD90 is comparable to the QT interval from clinical ECGs and allows us to investigate the potential for drugs to induce long QT interval and proarrhythmia. In particular, the Ca2+ transient assay in hSC-CMs, like other assays using different detection technologies (MEA or VSO recording), is sensitive to drugs that inhibit IKr (hERG) blockers. Additionally, using Ca2+-sensitive fluorescent dyes provides an intermediate to high throughput assay that does not heavily depend on the skills of the technician compared with other electrophysiological recordings like manual patch clamp/MEA. However, calcium dyes can affect the cell physiology, especially with long-term incubation periods with the dye (Bootman et al., 2018). However, protocols can be optimized for calcium transient measurements for hSC-CMs to minimize such impact (Kopljar et al., 2018a). Unpublished data at Janssen indicate that hSC-CMs did not change the viability of any biomarkers such as lactate dehydrogenase, cardiac troponin, or fatty acid binding protein during 5 days of incubation with the dye when the optimized protocol was applied. Also, the strong light required for intracellular Ca2+ transient detection with fluorescent dyes may degrade or alter light-sensitive compounds resulting in potential false negative results (eg, nifedipine). Therefore, drugs should always be tested as freshly prepared solutions protected from the light. In this study, we do not include in-depth discussion on the variabilities and the differences between sites, different cell types, and different dyes, which could be important but not on the scope of this study.
As with other assays like MEA and VSO, pharmacological responses in 2D monolayers of hSC-CMs may differ compared with tissue models such as isolated cardiac tissues or in vivo studies. For example, calcium channel antagonists that directly reduce blood pressure shorten CTD/APD but generally have minimal effects on the QT interval in vivo. Although the effects of sodium channel blockers on conduction are difficult to detect in cell monolayers, they have clear effects on prolongation of the QRS duration and conduction time in ECGs from isolated cardiac tissue and heart, and in in vivo studies. In fact, Na+ channel blockers tend to arrest beating, which precludes measurement of other parameters. Additionally, like other in vitro assays, the current technology does not detect potential effects of metabolites of a drug, which would only be detected in an in vivo study or by directly testing the activity of the metabolite in the in vitro assay. Finally, similar to any in vitro assay using hSC-CMs, although they express key genes and have comparable function of most cardiac ion channels and receptors, they are not fully adult, which might influence drug responses. Our current data with 28 CIPA showed similar readout prediction for drug-induced potential QT prolongation and arrhythmias to those in the CIPA paper measured with MEA and VSO (Blinova et al., 2018) (Supplementary Figures 3 and 4).
In conclusion, based on the current investigation, the Ca2+ transient assay can serve as a reliable alternative to complement the other 2 assay systems (MEA and VSO recording) used in the HESI-CiPA studies for detecting drug-induced long QT and potential TdP risk, as demonstrated by using 2 cell lines across different sites and a large set of clinically known compounds covering a range of cardiac risks. Moreover, the assay system is user-friendly and can be readily adapted as a medium/high throughput assay for early detection of proarrhythmic risk and other cardiac liabilities within drug discovery and development.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank Danny Geyskens and Eddy Vlaminckx for their technical assistance and Dr Bruce Damiano for the scientific discussions and review. They also thank Dr Stephen L. White, Drug Synthesis & Chemistry Branch at the Division of Cancer Treatment and Diagnosis (DCTD), NIH/National Cancer Institute (NCI) for managerial support on compound supply.
Funding
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract no. HHSN261200800001E. This research was supported (in part) by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute. This fund was only used to provide the 28 blind compounds to 3 test sites.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors certify that all research involving human subjects was done under full compliance with all government policies and the Helsinki Declaration
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- Abi-Gerges N., Pointon A., Oldman K. L., Brown M. R., Pilling M. A., Sefton C. E., Garside H., Pollard C. E. (2017). Assessment of extracellular field potential and Ca2 + transient signals for early QT/pro-arrhythmia detection using human induced pluripotent stem cell-derived cardiomyocytes. J Pharmacol. Toxicol. Methods 83, 1–15. [DOI] [PubMed] [Google Scholar]
- Adamantidis M. M., Lacroix D. L., Caron J. F., Dupuis B. A. (1995). Electrophysiological and arrhythmogenic effects of the histamine type 1-receptor antagonist astemizole on rabbit Purkinje fibers: clinical relevance. J. Cardiovasc. Pharmacol. 26, 319–327. [DOI] [PubMed] [Google Scholar]
- Ando H., Yoshinaga T., Yamamoto W., Asakura K., Uda T., Taniguchi T., Ojima A., Shinkyo R., Kikuchi K., Osada T., et al. (2017). A new paradigm for drug-induced torsadogenic risk assessment using human iPS cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 84, 111–127. [DOI] [PubMed] [Google Scholar]
- Authier S., Pugsley M. K., Koerner J. E., Fermini B., Redfern W. S., Valentin J.-P., Vargas H. M., Leishman D. J., Correll K., Curtis M. J. (2017). Proarrhythmia liability assessment and the comprehensive in vitro proarrhythmia assay (CiPA): an industry survey on current practice. J. Pharmacol. Toxicol. Methods 86, 34–43. [DOI] [PubMed] [Google Scholar]
- Bedut S., Seminatore-Nole C., Lamamy V., Caignard S., Boutin J. A., Nosjean O., Stephan J. P., Coge F. (2016). High-throughput drug profiling with voltage- and calcium-sensitive fluorescent probes in human iPSC-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 311, H44–H53. [DOI] [PubMed] [Google Scholar]
- Blanchette A. D., Grimm F. A., Dalaijamts C., Hsieh N.-H., Ferguson K., Luo Y.-S., Rusyn I., Chiu W. A. (2018). Thorough QT/QTc in a dish: an in vitro human model that accurately predicts clinical concentration-QTc relationships. Clin. Pharmacol. Therap. 105, 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinova K., Dang Q., Millard D., Smith G., Pierson J., Guo L., Brock M., Lu H. R., Kraushaar U., Zeng H., et al. (2018). International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment. Cell Rep. 24, 3582–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M. D., Allman S., Rietdorf K., Bultynck G. (2018). Deleterious effects of calcium indicators within cells; an inconvenient truth. Cell Calcium 73, 82–87. [DOI] [PubMed] [Google Scholar]
- Bot C. T., Juhasz K., Haeusermann F., Polonchuk L., Traebert M., Stoelzle-Feix S. (2018). Cross-site comparison of excitation-contraction coupling using impedance and field potential recordings in hiPSC cardiomyocytes. J. Pharmacol. Toxicol. Methods 93,46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colatsky T., Fermini B., Gintant G., Pierson J. B., Sager P., Sekino Y., Strauss D. G., Stockbridge N. (2016). The comprehensive in vitro proarrhythmia assay (CiPA) initiative—update on progress. J. Pharmacol. Toxicol. Methods 81, 15–20. [DOI] [PubMed] [Google Scholar]
- De Bruin M. L., Pettersson M., Meyboom R. H. B., Hoes A. W., Leufkens H. G. M. (2005). Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur. Heart J. 26(6), 590–597. [DOI] [PubMed] [Google Scholar]
- Dempsey G. T., Chaudhary K. W., Atwater N., Nguyen C., Brown B. S., McNeish J. D., Cohen A. E., Kralj J. M. (2016). Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharmacol. Toxicol. Methods 81, 240–250. [DOI] [PubMed] [Google Scholar]
- Drolet B., Rousseau G., Daleau P., Cardinal R., Simard C., Turgeon J. (2001). Pimozide (Orap®) prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current in native cardiac myocytes. J. Cardiovasc. Pharmacol. Therap. 6, 255–260. [DOI] [PubMed] [Google Scholar]
- Ellison B. E. (1964). On two-sided tolerance intervals for a normal distribution. Ann. Math. Statist. 35, 762–772. [Google Scholar]
- Fearnley C. J., Roderick H. L., Bootman M. D. (2011). Calcium signaling in cardiac myocytes. Cold Spring Harbor Perspect. Biol. 3, a004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintant G., Sager P. T., Stockbridge N. (2016). Evolution of strategies to improve preclinical cardiac safety testing. Nat. Rev. Drug Discov. 15, 457–471. [DOI] [PubMed] [Google Scholar]
- Gualdani R., Tadini-Buoninsegni F., Roselli M., Defrenza I., Contino M., Colabufo N. A., Lentini G. (2015). Inhibition of hERG potassium channel by the antiarrhythmic agent mexiletine and its metabolite m-hydroxymexiletine. Pharmacol. Res. Perspect. 3, e00160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L. A., Bass A. S., Gintant G., Mittelstadt S., Rampe D., Thomas K. (2006). ILSI-HESI cardiovascular safety subcommittee initiative: evaluation of three non-clinical models of QT prolongation. J. Pharmacol. Toxicol. Methods 54, 116–129. [DOI] [PubMed] [Google Scholar]
- Hayakawa T., Kunihiro T., Ando T., Kobayashi S., Matsui E., Yada H., Kanda Y., Kurokawa J., Furukawa T. (2014). Image-based evaluation of contraction–relaxation kinetics of human-induced pluripotent stem cell-derived cardiomyocytes: correlation and complementarity with extracellular electrophysiology. J. Mol. Cell Cardiol. 77, 178–191. [DOI] [PubMed] [Google Scholar]
- Kanda Y., Yamazaki D., Osada T., Yoshinaga T., Sawada K. (2018). Development of torsadogenic risk assessment using human induced pluripotent stem cell-derived cardiomyocytes: Japan iPS Cardiac Safety Assessment (JiCSA) update. J. Pharmacol. Sci. 138, 233–239. [DOI] [PubMed] [Google Scholar]
- Klara B., Andreas B., Birgit B., Mehlhorn U., Schwinger R. H. G. (2001). Nebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardium. Br. J. Pharmacol. 133, 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopljar I., De Bondt A., Vinken P., Teisman A., Damiano B., Goeminne N., Van den Wyngaert I., Gallacher D. J., Lu H. R. (2017). Chronic drug-induced effects on contractile motion properties and cardiac biomarkers in human induced pluripotent stem cell-derived cardiomyocytes. Br. J. Pharmacol. 174, 3766–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopljar I., Hermans A. N., Teisman A., Gallacher D. J., Lu H. R. (2018a). Impact of calcium-sensitive dyes on the beating properties and pharmacological responses of human iPS-derived cardiomyocytes using the calcium transient assay. J. Pharmacol. Toxicol. Methods 91, 80–86. [DOI] [PubMed] [Google Scholar]
- Kopljar I., Lu H. R., Van Ammel K., Otava M., Tekle F., Teisman A., Gallacher D. J. (2018b). Development of a human iPSC cardiomyocyte-based scoring system for cardiac hazard identification in early drug safety de-risking. Stem Cell Rep. 11, 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. T., Lin T. Y., Iyer H. K. (2005). One- and two-sided tolerance intervals for general balanced mixed models and unbalanced one-way random models. Technometrics 47, 323–335. [Google Scholar]
- Lu H. R., Hortigon-Vinagre M. P., Zamora V., Kopljar I., De Bondt A., Gallacher D. J., Smith G. (2017). Application of optical action potentials in human induced pluripotent stem cells-derived cardiomyocytes to predict drug-induced cardiac arrhythmias. J. Pharmacol. Toxicol. Methods 87, 53–67. [DOI] [PubMed] [Google Scholar]
- Lu H. R., Rohrbacher J., Vlaminckx E., Ammel K. V., Yan G. X., Gallacher D. J. (2010). Predicting drug‐induced slowing of conduction and pro‐arrhythmia: identifying the ‘bad’ sodium current blockers. Br. J. Pharmacol. 160, 60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. R., Vlaminckx E., Hermans A. N., Rohrbacher J., Van Ammel K., Towart R., Pugsley M., Gallacher D. J. (2008). Predicting drug-induced changes in QT interval and arrhythmias: qT-shortening drugs point to gaps in the ICHS7B guidelines. Br. J. Pharmacol. 154, 1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. R., Whittaker R., Price J. H., Vega R., Pfeiffer E. R., Cerignoli F., Towart R., Gallacher D. J. (2015). High throughput measurement of Ca++ dynamics in human stem cell-derived cardiomyocytes by kinetic image cytometery: a cardiac risk assessment characterization using a large panel of cardioactive and inactive compounds. Toxicol. Sci. 148, 503–516. [DOI] [PubMed] [Google Scholar]
- Millard D., Dang Q., Shi H., Zhang X., Strock C., Kraushaar U., Zeng H., Levesque P., Lu H.-R., Guillon J.-M., et al. (2018). Cross-site reliability of human induced pluripotent stem cell-derived cardiomyocyte based safety assays using microelectrode arrays: results from a blinded CiPA pilot study. Toxicol. Sci. 164, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J. D., Radleigh S., Joseph V., Pinar K., Tetsuro W. (2017). Calcium transient assays for compound screening with human iPSC-derived cardiomyocytes: evaluating new tools. J. Evol. Stem Cell Res. 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Honda Y., Watanabe H., Saiki S., Koyabu K., Itoh T., Nagasawa C., Nakamori C., Nakayama C., Iwasaki H., et al. (2017). CSAHi study-2: validation of multi-electrode array systems (MEA60/2100) for prediction of drug-induced proarrhythmia using human iPS cell-derived cardiomyocytes: assessment of reference compounds and comparison with non-clinical studies and clinical information. Regul. Toxicol. Pharmacol. 88, 238–251. [DOI] [PubMed] [Google Scholar]
- Obejero-Paz C. A., Bruening-Wright A., Kramer J., Hawryluk P., Tatalovic M., Dittrich H. C., Brown A. M. (2015). Quantitative profiling of the effects of vanoxerine on human cardiac ion channels and its application to cardiac risk. Sci. Rep. 5, 17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksana S., Hancock M. K., Carole C., Matthew H., Sean K., Britt Carlson C., Grischa C. (2017). Phenotypic assays for characterizing compound effects on induced pluripotent stem cell-derived cardiac spheroids. Assay Drug Dev. Technol. 15, 280–296. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. R., Vega R., McDonough P. M., Price J. H., Whittaker R. (2016). Specific prediction of clinical QT prolongation by kinetic image cytometry in human stem cell derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 81, 263–273. [DOI] [PubMed] [Google Scholar]
- Qian J.-Y., Guo L. (2010). Altered cytosolic Ca2+ dynamics in cultured Guinea pig cardiomyocytes as an in vitro model to identify potential cardiotoxicants. Toxicol. .Vitro 24, 960–972. [DOI] [PubMed] [Google Scholar]
- Rast G., Weber J., Disch C., Schuck E., Ittrich C., Guth B. D. (2015). An integrated platform for simultaneous multi-well field potential recording and Fura-2-based calcium transient ratiometry in human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 75, 91–100. [DOI] [PubMed] [Google Scholar]
- Redfern W. S., Carlsson L., Davis A. S., Lynch W. G., MacKenzie I., Palethorpe S., Siegl P. K., Strang I., Sullivan A. T., Wallis R., et al. (2003). Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58, 32–45. [DOI] [PubMed] [Google Scholar]
- Sala L., Bellin M., Mummery C. L. (2016). Integrating cardiomyocytes from human pluripotent stem cells in safety pharmacology: has the time come?. Br. J. Pharmacol. 174,3749–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram G., Zhang L., Derakhchan K., Ehrlich J. R., Belardinelli L., Nattel S. (2004). Ranolazine: ion-channel-blocking actions and in vivo electrophysiological effects. Br. J. Pharmacol. 142, 1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko O., Cromwell E. F., Crittenden C., Wignall J. A., Wright F. A., Rusyn I. (2013). Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol. Appl. Pharmacol. 273, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C. I., Baba S., Nakamura K., Hua E. A., Sears M. A. F., Fu C.-c., Zhang J., Balijepalli S., Tomoda K., Hayashi Y., et al. (2014). Calcium transients closely reflect prolonged action potentials in iPSC models of inherited cardiac arrhythmia. Stem Cell Rep. 3, 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Honda Y., Deguchi J., Yamada T., Bando K. (2017). Usefulness of cardiotoxicity assessment using calcium transient in human induced pluripotent stem cell-derived cardiomyocytes. J. Toxicol. Sci. 42, 519–527. [DOI] [PubMed] [Google Scholar]
- Yang X., Pabon L., Murry C. E. (2014). Engineering adolescence: maturation of human pluripotent stem cell–derived cardiomyocytes. Circ. Res. 114, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Brown A. M., Schwartz A. (1986). Bepridil block of cardiac calcium and sodium channels. J. Pharmacol. Exp. Therap. 237, 9–17. [PubMed] [Google Scholar]
- Zeng H., Roman M. I., Lis E., Lagrutta A., Sannajust F. (2016). Use of FDSS/μCell imaging platform for preclinical cardiac electrophysiology safety screening of compounds in human induced pluripotent stem cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 81, 217–222. [DOI] [PubMed] [Google Scholar]
- Zhang X., Guo L., Zeng H., White S. L., Furniss M., Balasubramanian B., Lis E., Lagrutta A., Sannajust F., Zhao L. L., et al. (2016). Multi-parametric assessment of cardiomyocyte excitation-contraction coupling using impedance and field potential recording: a tool for cardiac safety assessment. J. Pharmacol. Toxicol. Methods 81, 201–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.