Abstract
Exposure to traffic-generated pollution is associated with alterations in blood-brain barrier (BBB) integrity and exacerbation of cerebrovascular disorders. Angiotensin (Ang) II signaling through the Ang II type 1 (AT1) receptor is known to promote BBB disruption. We have previously reported that exposure to a mixture of gasoline and diesel vehicle engine emissions (MVE) mediates alterations in cerebral microvasculature of C57Bl/6 mice, which is exacerbated through consumption of a high-fat (HF) diet. Thus, we investigated the hypothesis that inhalation exposure to MVE results in altered central nervous system microvascular integrity mediated by Ang II-AT1 signaling. Three-month-old male C57Bl/6 mice were placed on an HF or low-fat diet and exposed via inhalation to either filtered air (FA) or MVE (100 μg/m3 PM) 6 h/d for 30 days. Exposure to HF+MVE resulted in a significant increase in plasma Ang II and expression of AT1 in the cerebral microvasculature. Results from a BBB coculture study showed that transendothelial electrical resistance was decreased, associated with reduced expression of claudin-5 and occludin when treated with plasma from MVE+HF animals. These effects were attenuated through pretreatment with the AT1 antagonist, Losartan. Our BBB coculture showed increased levels of astrocyte AT1 and decreased expression of aryl hydrocarbon receptor and glutathione peroxidase-1, associated with increased interleukin-6 and transforming growth factor-β in the astrocyte media, when treated with plasma from MVE-exposed groups. Our results indicate that inhalation exposure to traffic-generated pollutants results in altered BBB integrity, mediated through Ang II-AT1 signaling and inflammation, which is exacerbated by an HF diet.
Keywords: traffic pollution, angiotensin II, AT1 receptor, cerebral microvasculature, tight junction proteins
It is well documented in the literature that signaling of the renin-angiotensin system (RAS) is involved in the pathogenesis of many cardiovascular disease (CVD)-states (Ferrario, 2006), including hypertension and stroke (Volpe et al., 2006). In the vasculature, Angiotensin (Ang) II is known to signal via ligand interactions with the angiotensin receptors, Ang II receptor type 1 (AT1) and/or Ang II receptor type 2 (AT2) (Morawietz et al., 1999; Nickenig and Harrison, 2002). The AT1 receptor is reported to be the major Ang II receptor subtype present in the cardiovascular system, abnormal signaling through which is associated with the pathogenesis of CVD (Ferrario, 2006). In addition, several studies have shown that cross-talk exists between the AT1 receptor and the main receptor for oxidized low-density lipoprotein (oxLDL) in the vascular endothelium, the lectin-like oxLDL receptor (LOX-1); additionally, both of these receptors are reported to be increased in CVDs, including atherosclerosis and stroke (Ferrario, 2006; Pirillo et al., 2013). Although the mechanisms involved have not been fully elucidated, one pathway may be through disruption of the blood-brain barrier (BBB). We have previously reported that both oxLDL and LOX-1 are significantly elevated in the cerebral vasculature of Apolipoprotein E null (ApoE−/−) mice exposed to mixed exhaust; however, very little research has been done studying the effects of air pollution in the brains of healthy wild-type mice and/or whether differences in diet (high fat vs low fat) exacerbate these pathways. Although the role of Ang II signaling can vary in different vascular disease-states, increased Ang II-AT1 signaling is associated with the production of pro-inflammatory mediators, such as interleukin (IL)-6 and transformation growth factor (TGF)-β, adhesion molecules, and reactive oxygen species in the vasculature (Ruiz-Ortega et al., 2001; Skultetyova et al., 2007).
The BBB is a unique structure which is responsible for regulating transport between the blood and the central nervous system (CNS). This specialized barrier contains endothelial cells (ECs) that are sealed by tight junction (TJ) proteins including claudins, occludin, and junction adhesion molecules, in addition to astrocytes and pericytes (Hawkins and Davis, 2005; Pelkonen, 2010). Astrocytes are known to contribute to the integrity of cerebral microvessels, in addition to playing a neuroprotective role (Abbott, 2002). Antioxidant activity is reported to be increased in astrocytes, compared with ECs, in the brain microvasculature (Schroeter et al., 1999). Several antioxidants are found in astrocytes including superoxide dismutase, glutathione, and heat shock protein (Barreto et al., 2012; Dringen, 2000; Sandhu et al., 2009). In addition, signaling through the aryl hydrocarbon receptor (AhR) is also associated with antioxidant mechanisms in the cell (Dietrich, 2016).
In brain microvascular ECs, it has previously been shown that increased Ang II-AT1 signaling results in a decreased transendothelial electrical resistance (TEER) measurement, which is a measurement correlated with (intact) barrier integrity (Fleegal-DeMotta et al., 2009). Conversely, Ang II-AT1 signaling on astrocytes has been reported to decrease BBB permeability to infiltrating leukocytes, suggesting an increase in BBB integrity, after unilateral stereotactic lesion promotion (Wosik et al., 2007). Interestingly, Ang II-AT2 signaling has been reported to have a protective effect on the brain after stroke, as well as mediate neural differentiation in a mouse model (Mogi and Horiuchi, 2013).
Several studies have reported that exposure of traffic-generated air pollution is associated with increased occurrence of both ischemic and hemorrhagic stroke (Johnson et al., 2010; Villeneuve et al., 2006), and also altered BBB integrity and permeability (Oppenheim et al., 2013). In addition, chronic traffic-generated pollutant exposure has recently been reported to increased occurrence of hypertension (Fuks et al., 2017). Recent studies also report a strong correlation between air pollution-exposure and increased neuroinflammation and neurodegeneration, which are hallmark pathologies observed in Alzheimer’s and Parkinson’s diseases (Block and Calderon, 2009). Although the correlations of exposure to traffic-generated air pollutant and pathophysiological outcomes in the cerebral microvasculature and CNS have been well documented, contributing mechanistic signaling pathways are still under investigation. Our laboratory has previously reported that subchronic inhalation exposure to MVE results in increased BBB permeability and decreased TJ claudin-5 expression, associated with matrix metalloproteinase (MMP)-9 activity, in the cerebral microvasculature of C57Bl/6 wild-type mice on high-fat (HF) diet (Lucero et al., 2017; Suwannasual et al., 2018). Although Ang II-AT1 signaling is known to mediate BBB disruption (Fleegal-DeMotta et al., 2009), the effects of inhaled traffic-generated air pollution exposure on Ang II-AT1 signaling in the cerebral microvasculature has not yet been fully characterized. Thus, using both in vivo exposures (C57Bl/6 wild-type mice) and in vitro (BBB cocultures) models, we investigated the hypothesis that inhalation exposure to MVE results in altered BBB integrity, associated with Ang II-AT1 signaling. Furthermore, we characterized whether concurrent consumption of an HF diet exacerbated exposure-mediated outcomes in the cerebral microvasculature, as well as astrocytes in the BBB coculture experiments.
MATERIALS AND METHODS
Animals and inhalation exposure protocol
Three-month-old male C57Bl/6 mice were fed either a normal mouse chow or an HF diet (TD88137 Custom Research Diet, Harlan Teklad, Madison, Wisconsin; 21.2% fat content by weight, 42% kcal from fat, 1.5 g/kg cholesterol content) beginning 30 days prior to initiation of the exposure studies. Mice were then randomly grouped to be exposed by whole-body inhalation to a mixture of whole gasoline engine exhaust and diesel engine exhaust (MVE: 30 µg PM/m3 gasoline engine emissions + 70 µg PM/m3 diesel engine, n = 16) or filtered air (FA) (controls, n = 16) for 6 h/d for 30 days, as previously described in Suwannasual et al. (2018). MVE was created by combining exhaust from a 1996 GM gasoline engine and a Yanmar diesel generator system and characterized for chemical and PM components, as previously reported (Lund et al., 2011; McDonald et al., 2004; Oppenheim et al., 2013). Mice were housed in standard shoebox cages within an AAALAC International-approved rodent housing facility (2 m3 exposure chambers) for the entirety of the study, which maintained constant temperature (20°C–24°C) and humidity (30%–60% relative humidity). Mice had access to chow and water ad libitum throughout the study period, except during daily exposures when chow was removed. All procedures were approved by the Lovelace Respiratory Research Institute’s Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Tissue collection
Animals were sacrificed within 14–16 h after the final exposure. Mice were anesthetized with Euthasol (0.1 ml per 30 g mouse) and euthanized by exsanguination, brains carefully dissected, meninges gently removed, and were either (1) embedded in OCT (VWR Scientific) (n = 4–6 per group; midbrain region) and frozen on dry ice or (2) immediately snap frozen in liquid nitrogen (n = 8 per group). Blood was immediately centrifuged for 10 min at 3000 × g at 4°C, plasma was aliquoted, snap frozen in liquid nitrogen, and stored at −80°C until analysis.
ELISA for plasma Ang II and cell culture media endpoints
Angiotensin II was measured in the plasma from study animals using an ELISA kit (Enzo Ang II ELISA kit, No. ADI-900-204), following manufacturer’s protocol. Sample values were derived from a standard curve (generated from serial dilution of known standards) and results expressed as pg/ml. n = 8 samples per group were used for analysis.
Media from astrocyte cultures of BBB cocultures treated with plasma were used to measure for cytokines and angiotensin-converting enzyme (ACE) by ELISA (n = 6). Interleukin-6 (IL-6, Novex, No. KMC0061), transforming growth factor-β (TGF-β) (Invitrogen, No. BMS608-4), and ACE (ThermoFisher, No. EMACE) concentrations were quantified in the astrocyte media using the appropriate ELISA immunoassay kit, following manufacturer protocols.
Mouse BBB coculture
C57Bl/6 mouse primary brain microvascular ECs (Cell Biologics, C57-6023) were passaged in complete Mouse Endothelial Cell Medium and grown to confluence in a gelatin-coated (Cell Biologics, 6950) 75T flask and C57Bl/6 mouse astrocytes (ScienCell, M1800-57) were grown in complete Astrocyte media (ScienCell, 1831) in a poly-l-Lysine-coated (ScienCell, 0413) 75T flask. Both cells were maintained in at 37°C and 5% CO2 humidified atmosphere. They were subcultured every 5–6 days and used for experiments up to passage 4. For the BBB coculture, the astrocytes were seeded at 5 × 103 cell/cm2 into 24- or 6-well plates coated with poly-l-Lysine for 1 or 2 days before the ECs were seeded into the transwell insert at 2 × 104 cell/cm2 (Corning, collagen Types I insert 0.45 Micron) and incubated for 6 days (Supplementary Figure 1). Only passages 2–4 of both primary astrocytes and ECs were utilized for experiments presented herein.
Cell viability test
To test the cell viability of plasma on the ECs in the BBB cocultures, a MTT colorimetric assay was used (Hansen et al., 1989). Endothelial cells were seeded into 96-well plate and maintained until 70%–80% confluency. Then plasma (1:20 dilution) collected from the C57Bl/6 mice on the exposure study (LF+FA, LF+MVE, HF+FA, HF+MVE) was placed in the EC media and incubated for 24 h (n = 3 per diet/exposure group). Tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (final concentration of 0.5 mg/ml) was then added to the cells and incubated in at 37°C and 5% CO2 humidified atmosphere for 4 h. The purple formazan dye was dissolved in 100% DMSO, and 200 µl was placed on the cultured cells to analyze cell viability. The purple solution OD was measured using a Cytation 5 (BioTek, Winooski, Vermont) at an absorbance of 595 nm. The results were shown as the percent of proliferation compared with control (cells with no plasma treatment).
BBB permeability assay in coculture model
Baseline TEER measurements were recorded once both endothelial and astrocyte cultures were 95%–100% confluent. Transendothelial electrical resistance measurements were determined using an EVOM2 Voltammeter (WPI) and EndOhm chamber, across the apical and basal compartments, as previously described; (Supplementary Figure 2). The TEER value was calculated as the measured values minus measurements of coated (cell-free) culture inserts. The difference was multiplied with the area of the culture insert, resulting in a TEER value given as a mean in Ω × cm2. After TEER measurements reached 200–300 Ω × cm2 (indicating structural integrity in the BBB coculture), plasma (1:20 dilution) from mice exposed to either MVE or FA (± HF diet) (n = 6–8 per group) was added to the media of the apical compartment (ECs). Transendothelial electrical resistance measurements were assessed at 24 or 48 h post-treatment.
Angiotensin II-AT1 receptor in BBB coculture barrier integrity
In a subset of experiments using the BBB coculture, AT1 receptors were blocked by pretreating the ECs with AT1-antagonist Losartan (10−6 mol/l) diluted in media (n = 3 per group) for 30 min prior to treatment with plasma from each of the animal exposure groups (LF+FA, LF+MVE, HF+MVE) into the media of the apical EC compartment. Cells were analyzed for TEER and TJ protein expression 48 h after plasma was added to the media.
Immunofluorescent staining of cerebral tissue
Brain sections (10 µm) were prepared for AT1 receptor (Abcam, Cambridge, Massachusetts; No. ab18801, 1:1000 dilution) and von Willebrand factor (vWF, Abcam No. 11713, 1:1000 dilution) double immunofluorescence, using anti-goat Alexa Fluor 647 and Anti-sheep Alexa Fluor 488 secondary antibodies, imaged, and analyzed, as previously described by our laboratory (Oppenheim et al., 2013). Colocalization was determined by quantifying total fluorescence of overlaid signals from a minimum of 3 slides, 2 sections each, 5 regions/section (n = 4 per group).
Fluorescent immunocytochemistry
Plasma from mice exposed to either MVE or FA (± HF diet) (n = 3 per group) was incubated to the media of the apical compartment (ECs) for 24 h Fluorescent immunocytochemistry analysis of TJ proteins (claudin-5 or occludin) in ECs from BBB cocultures occurred via fixation of cells in ice-cold 100% MeOH for 15 min at −20°C, cells were then rinsed 3 times in 300 μl cool PBS for 5 min and then blocked in 3% BSA/Tween buffer for 60 min. The cells were incubated with conjugated antibodies (claudin-5 conjugated to Alexa Flour 488 [1:1000]; Invitrogen No. 352588, or occludin conjugated to Alexa Flour 555 [1:500], Invitrogen, No. 331598) overnight at 4°C. The nuclei were counter-stained with DAPI (1:1000) at room temperature for 1 min (Gillespie et al., 2016). Cells were then imaged on the Cytation 5 BioTek using the appropriate excitation/emission filters (GFP; 469/525, RFP; 531/593) digitally recorded, and analyzed by image densitometry using Cell Profiler (n = 3–5). Images were morphed using open-line operations with pixel-width measurements, masked with an overlay to ensure positive TJ detection by the software, and included a threshold to discard fluorescence outside of line-object mapping. Image intensity measurements analyzed areas where line objects were applied. Images were given values on total fluorescent intensity over total area of the image, provided a mean average of fluorescent intensity per unit area. All images were processed in an identical fashion, to ensure fidelity and the analysis was performed by a blinded individual, to prevent bias when building the CellProfiler module pipeline.
Quantitative reverse transcription polymerase chain reaction
Gene expression of AhR, Glutathione peroxidase-1 (GPx), AT1, and AT2 in astrocytes were analyzed using the appropriate forward and reverse primers (Table 1), via real-time RT-qPCR, as previously described in Lund et al. (2011). RNA was isolated using an AllPrep DNA/RNA/miRNA kit (Qiagen, Germantown, Maryland) for tissue or using a Tissue Lyser system and RNeasy Mini kit (Qiagen) for cells, following the manufacturer protocol. Quantitative reverse transcription polymerase chain reaction was completed and analyzed in the BIORAD CX (Hercules, California) using the appropriate primers, as previously described by our laboratory (Oppenheim et al., 2013). Glyceraldehyde-3-phosphate dehydrogenase was used as the internal control. Results were analyzed from n = 6 animals from each group.
Table 1.
Primer Sequences Used for Real-time RT-qPCR
| GPx | FP: 5′-AAGCAACCCAGCCTTTTCTC |
| RP: 5′-TGAGCATTCTCCTTTGGAC | |
| AhR | FP: 5′-CGGCTTCTTGCAAAACACAGT |
| RP: 5′-GTAAATGCTCTCGTCCTTCTTCATC | |
| AT1 | FP: 5′-ACTGGATGATGCTGTCTGGC |
| RP: 5′-GAGACACGTGAGCAGGAACA | |
| AT2 | FP: 5′-GGTCTGCTGGGATTGCCTTAATG |
| RP: 5′ACTTGGTCACGGGTAATTCTGTTC | |
| GAPDH | FP: 5′-CATGGCTTCCGTGTTCCTA |
| RP: 5′-GCGGCATCAGATCCA |
Abbreviations: AhR, aryl hydrocarbon receptor; AT1, angiotensin II type 1 receptor; AT2, angiotensin II type 2 receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GPx, glutathione peroxidase.
Statistical analysis
Data are shown as mean ± SEM. A 2-way ANOVA with post hoc Tukey’s test was used to analyze statistical significance between diet/exposure permutations. An independent t test was used for statistical analysis when comparing between 2 groups. Statistical analyses were conducted using Sigma Plot 12.0 (Systat, San Jose, California). A p < .050 was considered statistically significant for all endpoints.
RESULTS
C57Bl/6 Mice Exposed to MVE Exhibit Elevated Plasma Ang II and AT1 Receptor Expression in the Cerebral Microvasculature
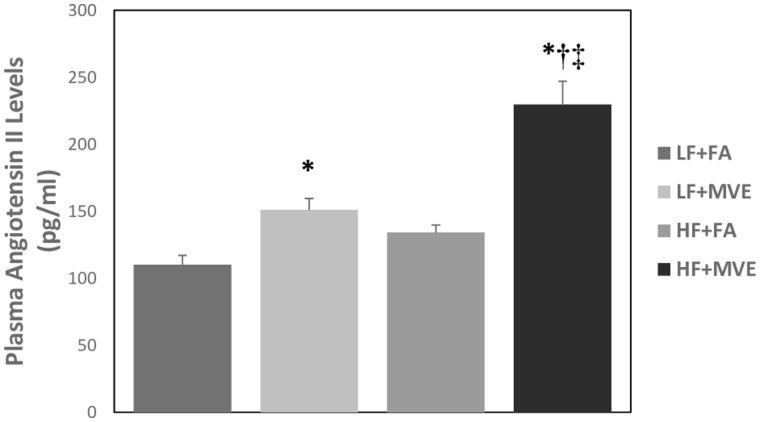
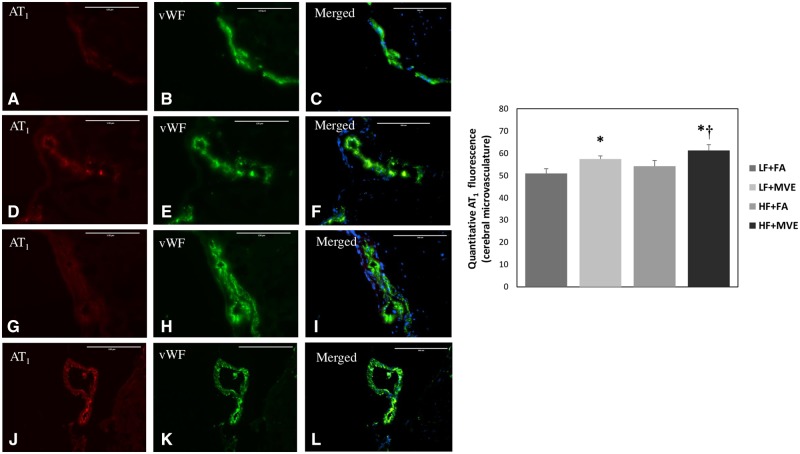
As Ang II-AT1 signaling has previously been reported to mediate alterations in BBB integrity (Fleegal-DeMotta et al., 2009), we quantified plasma Ang II and AT1 receptor expression in the cerebral microvascular in our study animals. We observed a significant increase in plasma Ang II levels in both LF+MVE and HF+MVE exposure groups, compared with LF+FA and HF+FA controls (Figure 1, p < .001). In addition, there was an exacerbated effect on plasma Ang II levels in MVE-exposed animals, when they were concurrently fed an HF diet, compared with MVE+LF (p = .010). The F values for plasma Ang II levels are: exposure = 41.519, diet = 22.303, exposure x diet interaction = 6.157. Correlated with plasma Ang II levels, compared with LF+FA controls (Figs. 2A–C), we observed a significant increase in cerebral microvascular AT1 receptor expression in the LF+MVE (Figs. 2D–F;p = .045), and HF+MVE (Figs. 2J–L;p = .004) exposure groups, F = 9.247 for exposure. AT1 receptor expression in the HF+MVE group was also statistically increased compared with the HF+FA group (Figs. 2G–I;p = .030) but there was no significant differences noted between LF+MVE versus HF + MVE. There was no significant change in either plasma Ang II (Figure 1) or cerebral microvascular AT1 levels in the HF+FA group (Figs. 2G–I) compared with the LF+FA (Figs. 2A–C). The F values for cerebral microvascular AT1 expression are: exposure = 9.247, diet = 2.517, and diet x exposure interaction = 0.0162.
Figure 1.
Plasma angiotensin II levels in C57Bl/6 mice exposed for 6 h/d for 30 days to either filtered air (FA), mixed engine emissions (MVE; 100 μg/m3 PM) and fed either a low-fat (LF) or high-fat (HF) diet. *p < .05 compared with LF+FA. †p < .05 compared with HF+FA. ‡p < .05 compared with LF+MVE.
Figure 2.
Double immunofluorescence of angiotensin II type 1 receptor (AT1, A, D, G, J) and vWF (B, E, H, K) in the cerebral microvasculature of C57Bl/6 mice exposed to filtered air (FA) on a low-fat diet (A–C); mixed vehicle emissions (MVE; 100 μg/m3 PM of mixed gasoline and diesel engine emissions) for 6 h/d, for 30 days on a LF diet (D–F); FA+HF diet (G–I) or MVE + HF diet (J–L). n = 3–4 per exposure group; 4 sections each, 3–5 vessels per section. Right panels (C, F, I, L) are merged figures of panels for AT1 and vWF. Blue fluorescence is Hoechst stained nuclei. Scale bar = 100 μm. Results represent mean ± SEM. *p < .05 compared with LF+FA. †p < .05 compared with HF+FA.
Plasma From MVE-Exposed C57Bl/6 Mice Alter BBB Coculture TJ Protein Expression and Endothelial Barrier Integrity
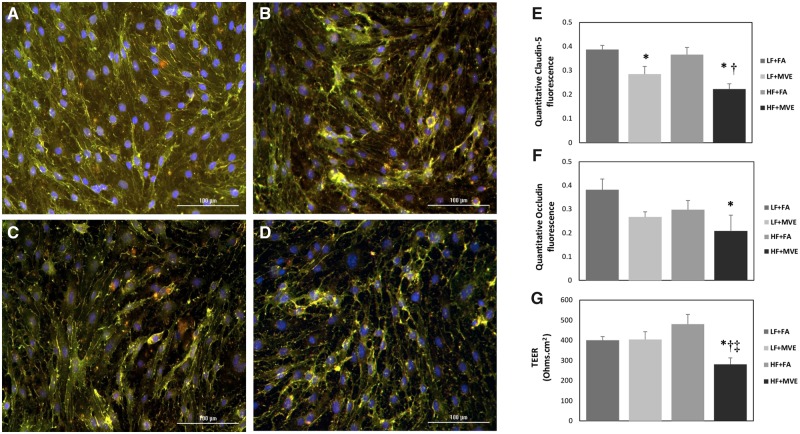
To elucidate the effects of MVE ± fat content in diet on alterations in integrity of the BBB, we utilized a BBB coculture model consisting of ECs (apical compartment) cocultured with astrocytes (basal compartment). Plasma from each of the animals in the exposure study was applied to the EC media, as previously described in Oppenheim et al. (2013). To confirm that application of the plasma (alone) to our BBB coculture did not alter cell viability (eg toxicity), we analyzed changes in cell viability of the ECs, via an MTT assay, prior to experimentation. The MTT assays showed no change in cell viability with plasma treatment-only on the ECs (Supplementary Tables 1 and 2). To analyze alterations in structural integrity of EC layer in the BBB coculture, we assessed expression of TJ proteins claudin-5 and occludin via immunofluorescence staining. Compared with exposure of ECs in coculture to plasma from LF+FA mice (Figure 3A), mice exposed to HF+MVE (Figure 3D), showed a significant reduction in expression of TJ proteins claudin-5 (Figure 3E;p = .002; F = 22.291 for exposure, F = 2.551 for diet, F = 0.641 for diet x exposure interaction) and occludin (Figure 3F;p = .048; F = 5.420 for exposure, F = 2.258 for diet, F = 0.116 for diet x exposure interaction). We also observed a decrease in claudin-5 expression in ECs treated with plasma from LF+MVE (Figure 3B) compared with LF+FA (Figure 3E;p = .024), and also between HF+FA (Figure 3C) and HF + MVE groups (Figure 3E. p = .005).
Figure 3.
Representative images of tight junction (TJ) proteins, claudin-5 (green) and occludin (red), fluorescence on endothelial cells (ECs) treated with plasma from mice exposed to (A) filtered air (FA) on a low-fat (LF) diet, (B) exposed to mixed vehicle exhaust (MVE: 100 µg/m3 PM) for 6 h/d, for 30 days on a LF diet, (C) FA + high-fat (HF) diet, or (D) MVE+ HF diet. The cells were fixed and stained for TJ proteins at 24 h post-plasma treatment. Fluorescence was quantified and represented as mean ± SEM (E, F) from n = 3. Blue fluorescence = Hoechst stained nuclei. Scale bar = 100 µm. Trans endothelial electrical resistance measurements in BBB coculture treated with a 1:20 dilution of mouse plasma on the EC (apical) layer from C57Bl/6 mice (G). Transendothelial electrical resistance values were measured 24 h post-plasma treatment. *p < .05 compared with LF+FA. †p < .05 compared with HF+FA. ‡p < .05 compared with LF+MVE.
The resistance across the monolayer of ECs was assessed by TEER measurement, which provides a measure of membrane integrity. When the apical EC layer was treated with plasma from animals exposed to MVE+HF, we observed a significant decrease in TEER values (approximately 30%) at 24 h post exposure, compared with TEER measurement in ECs treated with plasma from LF+FA animals (Figure 3G;p = .027), LF + MVE (Figure 3G, p = .042), and also HF+FA (Figure 3G;p = .005). The F value for exposure = 7.310, the F value for diet = 0.371, whereas the F value for diet x exposure interaction = 7.869. Interestingly, we saw no significant change in TEER values resulting from treatment of EC layers in BBB cocultures with plasma from either the LF+MVE or HF+FA groups, compared with LF+FA controls (Figure 3G).
AT1 Mediated by MVE Exposure Induced Decreasing BBB Integrity and Claudin-5 Expression
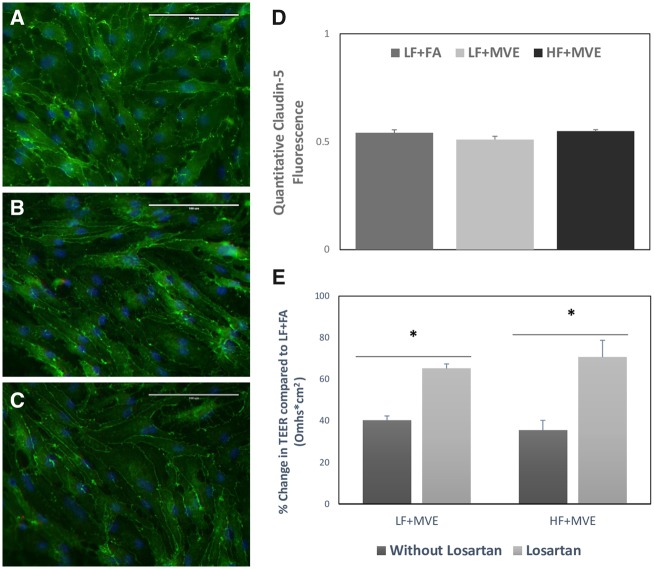
To determine whether AT1 signaling mediates the observed alterations in TJ protein expression and TEER measurements in the BBB cocultures treated with plasma from HF+MVE animals, we utilized the AT1 receptor antagonist, Losartan. For this experiment, TEER values and TJ protein levels were assessed at 48 h post-plasma treatment (vs post 24 h plasma treatment for the previous experiment) because we wanted to determine whether a longer exposure of the BBB coculture to the plasma from our study animals had a time-dependent effect. Pretreatment of the BBB coculture EC (apical) cells with Losartan prior to applying plasma from study animals resulted in an attenuation of the observed MVE-mediated reduction in EC expression of the TJ protein, claudin-5, as noted by no change in expression of claudin-5 across the LF+FA, LF+MVE, nor HF+MVE plasma-treated groups (Figs. 4A–D), compared with the MVE-mediated reduction in claudin-5 observed in Figures 3A–E. Interestingly, when normalized to LF+FA control EC layer integrity (TEER) measurements, we observed a significant reduction in TEER measurements in BBB cocultures treated with plasma from both LF+MVE and HF+MVE groups (Figure 4E; bars indicated as without Losartan), which was not observed at the 24 h post-treatment time point (Figure 3E). Such findings suggest there is likely a time-dependent effect from the plasma-treatment on EC layer integrity in the MVE+LF group. Thus, we chose the 48 h time point to use for our AT1 inhibitor (Losartan) studies to see if we could normalize altered TEER across both groups. Losartan pretreatment of the ECs resulted in an increase in normalized integrity (TEER) measurements in LF+MVE and HF+MVE treated cocultures (Figure 4E, bars indicated with Losartan), 42% (p = .0014) and 54% (p = .0058) respectively, compared with that measured in the same animals/samples without Losartan (Figure 4E, bars indicated without Losartan). Importantly, we did not analyze changes in TEER with Losartan treatment in the HF+FA animals because the previous experiments showed no significant change in TEER compared with LF+FA groups (Figure 3G).
Figure 4.
Representative images of claudin-5 fluorescence of endothelial cells (ECs) pretreated with 1 μM Losartan (AT1 receptor antagonist) for 1 h and then treated for 48 h with a 1:40 dilution of mouse plasma from C57Bl/6 mice exposed to either (A) filter air (FA) on a low-fat (LF) diet, or animals exposed to mixed vehicle exhaust (MVE: 100 μg/m3 PM) for 6 h/d for 30 days on either a (B) LF or (C) high-fat (HF) diet. Data represented as mean ± SEM from n = 3–5, blue fluorescence = Hoechst stained nuclei. Scale bar = 100 µm. E, Transendothelial electrical resistance (TEER) measurements in BBB coculture pre-incubated with 1 μM Losartan for 1 h and then treated with a 1:40 dilution of mouse plasma on the EC (apical) layer from C57Bl/6 mice exposed in each group. Transendothelial electrical resistance values were measured 48 h post-plasma treatment. Data are represented as the mean of % change in TEER normalized to mean LF+FA TEER measurements ± SEM from n = 3–5. *p < .05 compared with without treated by Losartan.
MVE-Exposure-Mediated Alterations in Expression of Ang II Receptors and ACE Production From Astrocytes in BBB Co-culture Model
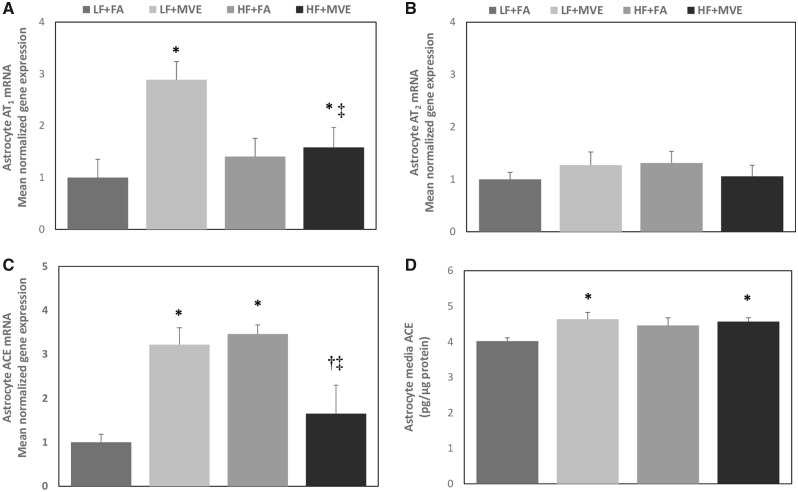
To investigate whether alterations in EC barrier integrity in BBB cocultures treated with plasma from MVE-exposed animals resulted in changes in signaling in astrocyte (basal) cells, we analyzed astrocyte mRNA expression of Ang II receptors AT1 and AT2 and ACE, in addition to secreted ACE levels in the astrocyte media. Plasma-treatment of the apical EC layer from both LF+MVE (Figure 5Ap = .001) and HF+MVE groups (Figure 5A, p = .010) resulted in increased AT1 receptor mRNA expression in astrocytes in the basal compartment when compared with LF+FA and also a significant reduction in AT1 expression in HF+MVE group compared with LF+MVE (Figure 5A, p = .022). The F values for astrocyte AT1 mRNA expression are: exposure = 8.194, diet = 1.550, and diet x exposure interaction = 5.622. However, no change in astrocyte AT2 expression was observed across any of the exposure/diet groups (Figure 5B). Astrocyte ACE mRNA was significantly increased in the LF+MVE and HF+FA groups, compared with the LF+FA group (Figure 5C). In addition, the production of ACE from in the BBB cocultures was significantly elevated in the astrocyte media of the LF+MVE (Figure 5D, p = .016) and HF+MVE-plasma-treated groups (Figure 5D, p = .040) compared with LF+FA controls. The F values for astrocyte media ACE quantification are: exposure = 5.019, diet = 1.301, and diet x exposure interaction = 2.475.
Figure 5.
Normalized gene expression of (A) angiotensin II receptor type 1, (B) angiotensin II receptor type 2, and (C) angiotensin converting enzyme (ACE) mRNA, as determined by real-time qPCR, in BBB coculture astrocytes treated with mouse plasma (1:20 dilution in media) on the endothelial (apical) layer from C57Bl/6 mice exposed to either filtered air (FA) or mixed vehicle exhaust (MVE: 100 μg/m3 PM) for 6 h/d for 30 days on either a low-fat (LF) or high-fat (HF) diet. D, Secreted ACE levels were also assessed in the media of the same astrocyte cultures by ELISA. Data represented as mean ± SEM from n = 6. *p < .05 compared with LF+FA. †p < .05 compared with HF+FA. ‡p < .05 compared with LF+MVE.
Inflammatory Markers Were Increased and Antioxidant Pathways Decreased in BBB Cocultures Treated With Plasma From MVE-Exposed Animals
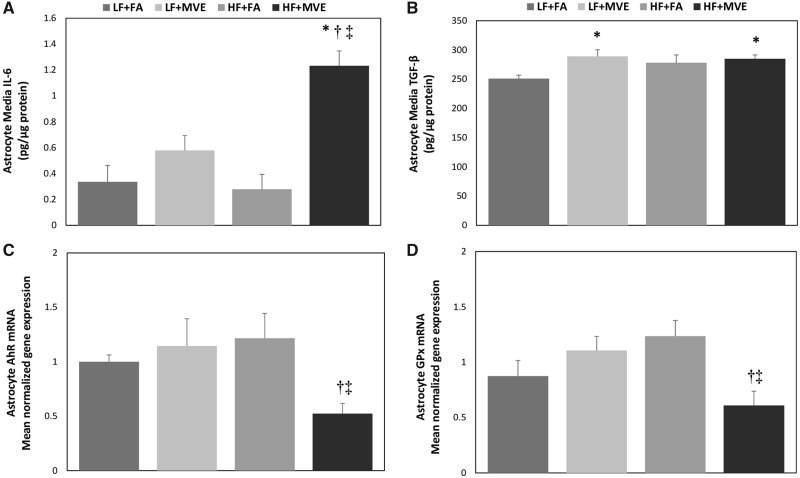
To investigate whether alterations in EC barrier integrity in BBB cocultures treated with plasma from MVE-exposed animals resulted in increased expression of mediators of inflammation in astrocytes, we examined IL-6 and TGF-β levels in the astrocyte media. When the apical ECs were treated with plasma from HF+MVE animals, we observed a significant increase in IL-6 levels in the astrocyte media, compared with LF+FA controls (Figure 6A, p < .001), compared with LF+MVE (Figure 6A, p < .001), and compared with HF+FA (Figure 6A, p < .001). The respective F values are: exposure = 25.805, diet = 6.474, diet x exposure interaction = 9.130. Similarly, TGF-β levels were also observed to be statistically elevated in the astrocyte media from BBB cocultures treated with plasma from both LF+MVE (Figure 6B, p = .016) and HF+MVE (Figure 6B, p = .040), compared with LF+FA controls. The respective F values are: exposure = 5.014, diet = 1.305, diet x exposure interaction = 2.484.
Figure 6.
Quantification of secreted (A) interleukin-6 (IL-6) and (B) transforming growth factor-beta (TGF-β) levels, as assessed by ELISA in astrocyte media from BBB coculture astrocytes treated with mouse plasma (1:20 dilution in media) on the endothelial (apical) layer from C57Bl/6 mice exposed to either filtered air (FA) or mixed vehicle exhaust (MVE: 100 μg/m3 PM) for 6 h/d for 30 days on either a low-fat (LF) or high-fat (HF) diet. Normalized gene expression of astrocyte (C) aryl hydrocarbon receptor and (D) glutathione peroxidase mRNA, as determined by real-time qPCR, from the same conditions listed above for panels A and B. Data represented as mean ± SEM from n = 6. *p < .05 compared with LF+FA. †p < .05 compared with HF+FA. ‡p < .05 compared with LF+MVE.
To characterize the role of decreased BBB integrity on protective properties of astrocytes at the BBB, we analyzed the resulting effects of plasma treatment from the MVE and FA groups on astrocyte AhR and GPx gene expression. We found that astrocyte expression of AhR mRNA in coculture exposed with mouse plasma of HF+MVE was suppressed significantly, compared with the HF+FA group (Figure 6C, p = .012) and compared with LF+MVE (Figure 6C, p = .023). The respective F values are: exposure = 2.368, diet = 1.302, diet x exposure interaction = 5.541. When compared with LF+FA groups, expression of AhR mRNA in coculture exposed with mouse plasma of HF+MVE was decreased significantly (approximately 48%) (Figure 6C, p = .002 by t test). In a similar manner, GPx mRNA expression was decreased in HF+MVE group by 31% (Figure 6D, p = .057 by t test), compared with the LF+FA group.
DISCUSSION
In 2012, the World Health Organization published a report that estimated approximately 7 million people died as a result of air pollution exposure in that year. Multiple epidemiologic studies have shown a positive correlation between exposure to air pollution, progression of CVD, and onset of clinical events including stroke (Alimohammadi et al., 2016; Chi et al., 2016; Du et al., 2016). In addition, the consequences of traffic-generated air pollution in the CNS are associated with neuroinflammation, microglial activation, and alteration of BBB integrity and permeability (Block and Calderon, 2009; Levesque et al., 2011, 2013).
The BBB is a specialized barrier that regulates transport into and out of the CNS. The consequences of BBB dysfunction are associated with many CNS and neurovascular disorders including multiple sclerosis, Alzheimer’s, and stroke (Daneman and Prat, 2015). We have previously reported that inhalation exposure to traffic-generated emissions results in alterations in BBB integrity and permeability, associated with increased inflammation in the CNS in mouse models (Lucero et al., 2017; Oppenheim et al., 2013; Suwannasual et al., 2018); however, the underlying mechanisms have not yet been fully elucidated. Although oxLDL-LOX-1 signaling appears to mediate some of the detrimental effects of inhaled MVE-exposure in both Apo E−/− and C57Bl/6 mice, concurrent treatment with a LOX-1 Ab did not fully attenuate altered permeability, transport, or structural integrity in the cerebral microvasculature (Lucero et al., 2017; Suwannasual et al., 2018). At least a few studies have shown that cross-talk exists between oxLDL and Ang II ligand binding and signaling through the LOX-1 and AT1 receptors in vascular cells (Li et al., 1999, 2000; Morawietz et al., 1999, 2009); oxLDL has also been reported to upregulate AT1 (Yamamoto et al., 2015). Furthermore, both AT1 and LOX-1 have been shown to signal through similar ligand-induced intracellular signaling pathways, including NADPH oxidase-derived superoxide (Chen et al., 2007), which has been confirmed through studies that show oxLDL-mediated induction of oxidative stress is attenuated via AT1 blockade (Yamamoto et al., 2015). We have previously reported plasma oxLDL is significantly increased in HF+MVE C57Bl/6 mice (Suwannasual et al., 2018); thus, we investigated the role of Ang II-AT1 signaling in alterations of BBB microvascular integrity using both an in vivo exposure and in vitro BBB coculture model. Importantly, the tissue used from the in vivo inhalation study in C57Bl/6 mice for this study are the same tissues that we previously reported to have increased cerebral microvascular permeability, associated with decreased TJ protein expression, resulting from MVE-exposure (Suwannasual et al., 2018).
In this study, we observed that MVE-exposure mediated increased circulating plasma Ang II levels, regardless of the fat content in the diet, but was statistically increased in the HF+MVE compared with the LF+MVE, suggesting the interaction of diet and exposure mediated the exacerbated outcomes. In addition, there is a statistical increase in AT1 receptor expression in the cerebral microvasculature of these same mice. Such results, coupled with our previously reported results of altered BBB permeability and integrity in these same animals, suggests that Ang II is associated with BBB disruption, likely through an Ang II-AT1 signaling mechanism; however, this has not yet been confirmed in in vivo exposure studies. Using our in vitro BBB coculture system, which comprised a murine primary brain EC (apical) layer grown on a transwell membrane placed in a well with primary murine astrocytes (basal layer), we assessed the potential role of “circulating factors” in the plasma, such as Ang II, in mediating alterations in BBB integrity. When plasma from HF+MVE-exposed animals, which have significantly increased Ang II levels, were placed on the EC (apical) layer of the coculture, we observed a significant decrease in TEER measurements, compared with LF+FA, LF+MVE, and HF+FA groups. In addition, we measured a concurrent decrease in EC TJ proteins, claudin-5 and occludin, indicating altered barrier integrity in the HF+MVE group, compared with LF+FA (and HF+FA in the claudin-5) group. Such findings are in agreement with previous reports that show Ang II-induced AT1 expression in brain microvessel ECs (MECs) is associated with decreased TEER and increased permeability (Fleegal-DeMotta et al., 2009). Interestingly, we did not observe any significant alteration in BBB permeability or TJ protein expression in the LF+MVE group from our previously reported in vivo studies (Suwannasual et al., 2018); however, we did observe an increase in plasma Ang II, cerebral microvascular AT1 expression in the brains from these same animals. In addition, we only observed changes in TEER measurements and TJ protein expression in ECs treated with plasma from the LF+MVE mice at the 48 h post-plasma treatment time point (vs no change at the 24 h time point), even though plasma Ang II levels were elevated above control. Such findings suggest there may likely be a time-dependent signaling effects of Ang II-AT1 signaling. Alternatively, it could also be a dose-related signaling event, via the AT1 receptor, as circulating Ang II levels were only 1.5-fold higher in the LF+MVE than LF+FA controls, compared with 2.5-fold higher in the HF+MVE group. Previous in vivo studies have reported an association between Ang II and decreased cerebral microvascular integrity, as C57Bl/6 mice injected with Ang II are reported to develop an intracerebral hemorrhage during acute hypertension through activation of MMPs and oxidative stress (Wakisaka et al., 2010).
To investigate the role of Ang II-AT1 signaling mediating alterations in EC integrity in our in vitro model, we utilized the AT1 antagonist Losartan. We found that pretreatment of the EC cells with Losartan, prior to adding plasma from the MVE-exposed animals, attenuated alterations in TEER and TJ protein expression (occludin and claudin-5) in our BBB coculture. Taken together, these results suggest that exposure to MVE promotes altered BBB integrity and TJ protein expression through activation of AT1. Future studies will investigate the time course of Ang II signaling in the brain that may account for differences seen in our MVE exposures/diet groups at 24 versus 48 h post-plasma treatment.
In addition to ECs and pericytes, astrocytes also assist with maintaining the integrity of brain microvessels in mammals (Abbott, 2002; Gotow and Hashimoto, 1984). In addition, increased TGF-β expression is also associated with increased BBB permeability in animal models, by mediating increased MMP-9 enzyme activity, which promotes degradation of TJ proteins in cerebral microvascular ECs (McMillin et al., 2015). Such findings have been confirmed through IL-6 inhibitor studies which were reported to prevent BBB disruption after brain ischemia in the ovine fetus (Zhang et al., 2015). Increased TNF-α expression/signaling has also been shown to mediate BBB disruption and increased permeability, via NF-κβ pathway, in primary cultured bovine brain MECs (Trickler et al., 2005). Our results show that when plasma from our study animals is applied to ECs in BBB coculture, there is an alteration in expression of inflammatory and/or protective factors in the astrocytes, or astrocyte media in the basal compartment. For example, treatment of ECs with plasma from MVE-exposed animals resulted in increased expression in the astrocyte media of TGF-β from BBB coculture, regardless of the diet consumed; however IL-6 was only significantly increased in the astrocyte media of the HF+MVE group, compared with all other exposure/diet groups. Although our experiments cannot distinguish whether the increased inflammatory factors in the media were secreted from the ECs, astrocytes, or both cell types, such findings indicate that exposure-mediated alteration in BBB integrity may promote the expression of inflammatory factors in the brain. It has previously been reported that increased IL-6 secretion is associated with reduced TEER in rat cerebral ECs (de Vries et al., 1996). We have previously reported that MVE-exposure results in increased MMP-9 activity in the cerebral microvascular of C57Bl/6 mice (Suwannasual et al., 2018); thus, taken together with this study findings suggests TGF-β secreted from astrocytes may contribute to MMP-9 activity in ECs and subsequent altered TJ protein expression.
Astrocytes are the major source angiotensinogen production in the brain (Stornetta et al., 1988) and they can also produce ACE (Alvarez et al., 2013). The angiotensinogen precursor is converted to Ang I by renin, then ACE converts Ang I to Ang II (O’Connor and Clark, 2018), which mediate responses in the CNS via ligand interactions with either AT1 or AT2. Our results show that AT1 expression was upregulated with MVE-exposure, both in vivo in the cerebral microvasculature (AT1 expression) and in the astrocytes (AT1 mRNA) of our BBB coculture. A previous study reported that increased Ang II directly mediates the upregulation of AT1 expression in astrocytes (Füchtbauer et al., 2011). This suggests that Ang II is elevated in the CNS either from crossing the disrupted BBB from the blood or via increased Ang II production directly in the CNS, which is in line with the increased ACE we observed in the astrocyte media of BBB cocultures treated with plasma (on the apical EC layer) from MVE-exposed mice. Importantly, astrogliosis and cell death are reported to be associated with activation of astrocytic AT1-mediated oxidative stress (Liu et al., 2011), which may contribute to the CNS pathologies associated with traffic-generated air pollution exposure in our exposure model.
The AhR is known as a ligand-dependent transcription factor that regulates expression of several molecules, including CYP1A1 and 1B1, which are involved in biotransformation of xenobiotics and pharmaceuticals (Chang and Puga, 1998; Quintana and Sherr, 2013; Stockinger et al., 2014). The deletion of AhR is associated with increased production of pro-inflammatory mediators; AhR activation is associated with decreased expression of pro-inflammatory molecules such as iNOS, TNFα, and IL-1β (Wheeler et al., 2017). We observed that expression of AhR mRNA was suppressed in astrocytes of BBB cocultures treated with plasma from HF+ MVE animals, compared with the LF+MVE and HF+FA groups. In a similar manner, GPx mRNA expression was also decreased in astrocytes from BBB cocultures treated with HF+MVE plasma samples. GPx is an antioxidant enzyme, which is known to reduce lipid and hydrogen peroxidases (Arthur, 2001). Thus, we observed a decrease antioxidant signaling molecules in the astrocytes from BBB cocultures treated with plasma from HF+MVE animals, as well as an increase in expression of pro-inflammatory mediators and components of the RAS system, which collectively are associated with alterations in BBB integrity in our coculture model. Further mechanistic studies are necessary to determine whether astrocytes are contributing to MVE-mediated alterations in BBB integrity and/or permeability in vivo.
CONCLUSION
These results suggest that inhalation exposure of C57Bl/6 wild-type mice to the ubiquitous environmental air pollutant, MVE, results in increased plasma Ang II and expression of cerebral microvascular AT1 that is associated with decreased TJ protein expression, which is exacerbated by concurrent consumption of an HF diet. AT1 inhibition in our in vitro BBB coculture model suggests that Ang II-AT1 signaling may mediate the observed alterations in membrane integrity, as measured by TEER, and TJ protein expression, in brain microvascular ECs. Furthermore, exposure of MVE also mediates increased expression of pro-inflammatory markers in BBB coculture, associated with suppressed expression of antioxidant pathways. Collectively, these findings suggest the traffic-generated pollutant exposure induces RAS signaling in the cerebral microvasculature, which may contribute to BBB disruption via AT1 signaling, in addition to inflammatory signaling in the CNS.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
Funding from grants received from the National Institute of Environmental Health Sciences at the National Institute of Health and the Environmental Protection Agency were used to conduct some of the exposures and studies described, herein; however, the authors declare no conflict of interest or financial gains to these entities associated with this publication.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the Chemistry and Inhalation Exposure group, in the Environmental Respiratory Health Program, at Lovelace Biomedical and Environmental Research Institute for the characterization and monitoring of the animal exposures.
FUNDING
This work was supported by National Institute of Environmental Health Sciences at the National Institute of Health (R15ES026795 to A.K.L.); University of North Texas Research Initiation Grant (RIG) (GA93601 to A.K.L); and Environmental Protection Agency Center Grant RD-83479601 (Project 3 to A.K.L., coPI and Project 2 to J.D.M., coPI) for the animal exposures.
REFERENCES
- Abbott N. J. (2002). Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 200, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimohammadi H., Fakhri S., Derakhshanfar H., Hosseini-Zijoud S. M., Safari S., Hatamabadi H. R. (2016). The effects of air pollution on ischemic stroke admission rate. Chonnam Med. J. 52, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J. I., Katayama T., Prat A. (2013). Glial influence on the blood brain barrier. Glia 61, 1939–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J. R. (2001). The glutathione peroxidases. Cell. Mol. Life Sci. 57, 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G. E., White R. E., Xu L., Palm C. J., Giffard R. G. (2012). Effects of heat shock protein 72 (hsp72) on evolution of astrocyte activation following stroke in the mouse. Exp. Neurol. 238, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. L., Calderon G. L. (2009). Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 32, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-Y., Puga A. (1998). Constitutive activation of the aromatic hydrocarbon receptor. Mol. Cell. Biol. 18, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X-P, Zhang T-T, Du G-H. (2007). Lectin-like oxidized low-density lipoprotein receptor-1, a new promising target for the therapy of atherosclerosis? Cardiovasc. Drug Rev. 25, 146–161. [DOI] [PubMed] [Google Scholar]
- Chi G. C., Hajat A., Bird C. E., Cullen M. R., Griffin B. A., Miller K. A., Shih R. A., Stefanick M. L., Vedal S., Whitsel E. A., et al. (2016). Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ. Health Perspect. 124, 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Prat A. (2015). The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H. E., Blom-Roosemalen M. C., van Oosten M., de Boer A. G., van Berkel T. J., Breimer D. D., Kuiper J. (1996). The influence of cytokines on the integrity of the blood-brain barrier in vitro. J. Neuroimmunol. 64, 37–43. [DOI] [PubMed] [Google Scholar]
- Dietrich C. (2016). Antioxidant functions of the aryl hydrocarbon receptor. Stem Cells Int. 2016, 7943495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R. (2000). Metabolism and functions of glutathione in brain. Prog. Neurobiol. 62, 649–671. [DOI] [PubMed] [Google Scholar]
- Du Y., Xu X., Chu M., Guo Y., Wang J. (2016). Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 8, E8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C. M. (2006). Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J. Renin Angiotensin Aldosterone Syst. 7, 3–14. [DOI] [PubMed] [Google Scholar]
- Fleegal-DeMotta M. A., Doghu S., Banks W. A. (2009). Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J. Cereb. Blood Flow Metab. 29, 640–647. [DOI] [PubMed] [Google Scholar]
- Füchtbauer L., Groth-Rasmussen M., Holm T. H., Løbner M., Toft-Hansen H., Khorooshi R., Owens T. (2011). Angiotensin II type 1 receptor (AT1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav. Immun. 25, 897–904. [DOI] [PubMed] [Google Scholar]
- Fuks K. B., Weinmayr G., Basagaña X., Gruzieva O., Hampel R., Oftedal B., Sørensen M., Wolf K., Aamodt G., Aasvang G. M., et al. (2017). Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (escape). Eur. Heart J. 38, 983–990. [DOI] [PubMed] [Google Scholar]
- Gillespie J. L., Anyah A., Taylor J. M., Marlin J. W., Taylor T. (2016). A versatile method for immunofluorescent staining of cells cultured on permeable membrane inserts. Med. Sci. Monit. Basic Res. 22, 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow T., Hashimoto P. H. (1984). Plasma membrane organization of astrocytes in elasmobranchs with special reference to the brain barrier system. J. Neurocytol. 13, 727–742. [DOI] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. (1989). Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 119, 203–210. [DOI] [PubMed] [Google Scholar]
- Hawkins B. T., Davis T. P. (2005). The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57, 173–185. [DOI] [PubMed] [Google Scholar]
- Johnson J. Y., Rowe B. H., Villeneuve P. J. (2010). Ecological analysis of long-term exposure to ambient air pollution and the incidence of stroke in Edmonton, Alberta, Canada. Stroke 41, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Levesque S., Surace M. J., McDonald J., Block M. L. (2011). Air pollution and the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates markers of neurodegenerative disease. J. Neuroinflamm. 8, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S., Taetzsch T., Lull M. E., Johnson J. A., McGraw C., Block M. L. (2013). The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. J. Neurochem. 125, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Saldeen T., Romeo F., Mehta J. L. (2000). Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: The potential role of transcription factor NF-Κβ. Circulation 102, 1970–1976. [DOI] [PubMed] [Google Scholar]
- Li D. Y., Zhang Y. C., Philips M. I., Sawamura T., Mehta J. L. (1999). Upregulation of endothelial receptor for oxidized low-density lipoprotein (LOX-1) in cultured human coronary artery endothelial cells by angiotensin II type 1 receptor activation. Circ. Res. 84, 1043–1049. [DOI] [PubMed] [Google Scholar]
- Liu G., Hosomi N., Hitomi H., Pelisch N., Fu H., Masugata H., Murao K., Ueno M., Matsumoto M., Nishiyama A. (2011). Angiotensin II induces human astrocyte senescence through reactive oxygen species production. Hypertens. Res. 34, 479.. [DOI] [PubMed] [Google Scholar]
- Lucero J., Suwannasual U., Herbert L. M., McDonald J. D., Lund A. K. (2017). The role of the lectin-like oxldl receptor (LOX-1) in traffic-generated air pollution exposure-mediated alteration of the brain microvasculature in apolipoprotein (Apo) E knockout mice. Inhal. Toxicol. 29, 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A. K., Lucero J., Harman M., Madden M. C., McDonald J. D., Seagrave J. C., Campen M. J. (2011). The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am. J. Respir. Crit. Care Med. 184, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. D., Barr E. B., White R. K., Chow J. C., Schauer J. J., Zielinska B., Grosjean E. (2004). Generation and characterization of four dilutions of diesel engine exhaust for a subchronic inhalation study. Environ. Sci. Technol. 38, 2513–2522. [DOI] [PubMed] [Google Scholar]
- McMillin M., Frampton G., Seiwell A., Patel N., Jacobs A., DeMorrow S. (2015). TGFβ1 exacerbates blood-brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab. Invest. 95, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Horiuchi M. (2013). Effect of angiotensin II type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr. Gerontol. Int. 13, 13–18. [DOI] [PubMed] [Google Scholar]
- Morawietz H., Catar R. A., Goettsch C., Taye A., Muller G., Lehmann S., Ziegler C. G., Bornstein S. R., Krug A. W., Walther T. (2009). Abstract 5645: Novel cross-talk between oxidized LDL, angiotensin II and oxidative stress after AT1 blockade and AT1a/AT1b double knockout. Circulation 120, S1133. [Google Scholar]
- Morawietz H., Rueckschloss U., Niemann B., Duerrschmidt N., Galle J., Hakim K., Zerkowski H.-R., Sawamura T., Holtz J. (1999). Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation 100, 899–902. [DOI] [PubMed] [Google Scholar]
- Nickenig G., Harrison D. G. (2002). The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part I: Oxidative stress and atherogenesis. Circulation 105, 393–396. [DOI] [PubMed] [Google Scholar]
- O’Connor A. T., Clark M. A. (2018). Astrocytes and the renin angiotensin system: Relevance in disease pathogenesis. Neurochem. Res. 43, 1297–1307. [DOI] [PubMed] [Google Scholar]
- Oppenheim H. A., Lucero J., Guyot A.-C., Herbert L. M., McDonald J. D., Mabondzo A., Lund A. K. (2013). Exposure to vehicle emissions results in altered blood brain barrier permeability and expression of matrix metalloproteinases and tight junction proteins in mice. Part. Fibre Toxicol. 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen O. 2010. Role of the blood-brain barrier in disposition of endobiotics and xenobiotics. Front. Pharmacol. 1. 10.3389/conf.fphar.2010.02.00005.
- Pirillo A., Norata G. D., Catapano A. L. (2013). LOX-1, oxLDL, and atherosclerosis. Mediat. Inflamm. 2013, 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana F. J., Sherr D. H. (2013). Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 65, 1148–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M., Lorenzo O., Ruperez M., Esteban V., Suzuki Y., Mezzano S., Plaza J. J., Egido J. (2001). Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension 38, 1382–1387. [DOI] [PubMed] [Google Scholar]
- Sandhu J. K., Gardaneh M., Iwasiow R., Lanthier P., Gangaraju S., Ribecco-Lutkiewicz M., Tremblay R., Kiuchi K., Sikorska M. (2009). Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol. Dis. 33, 405–414. [DOI] [PubMed] [Google Scholar]
- Schroeter M. L., Mertsch K., Giese H., Müller S., Sporbert A., Hickel B., Blasig I. E. (1999). Astrocytes enhance radical defence in capillary endothelial cells constituting the blood-brain barrier. FEBS Lett. 449, 241–244. [DOI] [PubMed] [Google Scholar]
- Skultetyova D., Filipova S., Riecansky I., Skultety J. (2007). The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Patents Cardiovasc. Drug Discov. 2, 23–27. [DOI] [PubMed] [Google Scholar]
- Stockinger B., Meglio P. D., Gialitakis M., Duarte J. H. (2014). The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432. [DOI] [PubMed] [Google Scholar]
- Stornetta R., Hawelu-Johnson C., Guyenet P., Lynch K. (1988). Astrocytes synthesize angiotensinogen in brain. Science 242, 1444–1446. [DOI] [PubMed] [Google Scholar]
- Suwannasual U., Lucero J., McDonald J. D., Lund A. K. (2018). Exposure to traffic-generated air pollutants mediates alterations in brain microvascular integrity in wildtype mice on a high-fat diet. Environ. Res. 160, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickler W. J., Mayhan W. G., Miller D. W. (2005). Brain microvessel endothelial cell responses to tumor necrosis factor-alpha involve a nuclear factor kappa b (NF-κβ) signal transduction pathway. Brain Res. 1048, 24–31. [DOI] [PubMed] [Google Scholar]
- Villeneuve P., Chen L., Stieb D., Rowe B. (2006). Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur. J. Epidemiol. 21, 689–700. [DOI] [PubMed] [Google Scholar]
- Volpe M., Tocci G., Giovannelli F. (2006). Cerebrovascular protection by new antihypertensive drugs—Focus on RAS blocking agents in stroke prevention. Eur. Cardiovasc. Dis. 2, 14–16. [Google Scholar]
- Wakisaka Y., Chu Y., Miller J. D., Rosenberg G. A., Heistad D. D. (2010). Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J. Cereb. Blood Flow Metab. 30, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. A., Rothhammer V., Quintana F. J. (2017). Control of immune-mediated pathology via the aryl hydrocarbon receptor. J. Biol. Chem. 292, 12383–12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosik K., Cayrol R., Dodelet-Devillers A., Berthelet F., Bernard M., Moumdjian R., Bouthillier A., Reudelhuber T. L., Prat A. (2007). Angiotensin II controls occludin function and is required for blood-brain barrier maintenance: Relevance to multiple sclerosis. J. Neurosci. 27, 9032–9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Kakino A, Takeshita H, Hayashi N, Li L, Nakano A, Hanasaki-Yamamoto H, Fujita Y, Imaizumi Y, Toyama-Yokoyama S, et al. (2015). Oxidized LDL (oxLDL) activates the angiotensin II type 1 receptor by binding to the lectin-like oxLDL receptor. FASEB J. 29, 3342–3356. [DOI] [PubMed] [Google Scholar]
- Zhang J., Sadowska G. B., Chen X., Park S. Y., Kim J.-E., Bodge C. A., Cummings E., Lim Y.-P., Makeyev O., Besio W. G., et al. (2015). Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J. 29, 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.