 Fentanyl has been found to only weakly bind the σ1-receptor, and its close derivatives have submicromolar affinities.
Fentanyl has been found to only weakly bind the σ1-receptor, and its close derivatives have submicromolar affinities.
Abstract
Fentanyl and its 11 commercially available derivatives were investigated as to their affinity for the σ1 receptor. The parent compound is a rather poor binder (IC50 = 4973 nM), but its close derivatives (benzylfentanyl or p-fluorofentanyl) have submicromolar affinities. Modelling provides a structural basis for the observed trends in activity.
The σ1 receptor (σ1R) belongs to the most intriguing proteins from the point of view of medicinal chemistry. In the past, it had been mistakenly taken as one of the opioid receptor subtypes. Nowadays, it is known that the σ1R is unique and shares no homology to other receptor classes.§ ,1 Over the years, much evidence has been gathered on the involvement of the σ1R in physiological, pathological and pharmacological phenomena as diverse as addiction, pain and analgesia, depression, psychotic effects, schizophrenia, neurodegenerative diseases, motor disorders, memory formation, etc. (reviewed in several papers2–5). This is why the receptor has been of great interest as a molecular target for the development of novel drugs.2,4–8 Currently, no σ1R selective ligand is approved for medical use but several are under clinical trials for the treatment of neuropathic pain,9,10 Alzheimer's disease11 or ischemic stroke.12,13
What is particularly interesting about the σ1R is that it can bind with a notable diversity of ligands with moderate to high affinity. Among those ligands one can find butyrophenones, phenothiazines, thioxanthenes, tricyclic antidepressants, steroid hormones, alkylamines, benzomorphans, 4-N-substituted piperidines and others.14 The known binders include also many drugs in use whose main targets are other receptors (e.g. dextromethorphan, haloperidol, fluoxetine, quetiapine, and clemastine).14,15 There are also a few substances of abuse (e.g. cocaine and methamphetamine) which are shown to interact with the σ1R.14 This raises questions as to the possible involvement of the receptor in the pharmacological action or side-effects of these drugs.4,5,16 On the other hand, this fact can also pave the way for interesting repurposing programmes. Novel σ1R ligands could be developed by structural optimization of known drugs (with some σ1R binding) aimed at increasing the σ1R affinity while decreasing the affinity for the original major target.
Motivated by the known σ1R affinity of various CNS drugs, as well as by the issue of potential repurposing, we have decided to examine the σ1R affinity of fentanyl (1) and its 11 commercially available derivatives (2–12, cf.Table 1 for their list and Fig. ESI-CHEM-1 in the ESI‡ for their structures). Fentanyl (N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]propanamide, Chart 1) is one of the most important analgesics in medicinal practice.17 At the same time, it is a very popular substance of abuse.18 Both actions are mediated via the μ-opioid receptor (μOR) for which the compound has a nanomolar affinity.19,20 Regarding the σ1R binding, two reports describe fentanyl as a weak binder (IC50 = 354 ± 52 nM, displacement of (+)-[3H]3-PPP;21Ki > 1 μM, displacement of [3H]-SKF10047).22 To the best of our knowledge, no information on the σ1R affinity was published for fentanyl derivatives.
Table 1. Affinities of fentanyl derivatives for the σ1R and μOR19.
| No | Compound | Structural variation (cf.Chart 1) |
Affinity for |
|||||||
| N-Sub | Rβ | R3 | R4-ax | Rpara | Rω-1 | Rω | σ1R | μOR from ref. 19 | ||
| IC50 ± S.D. a | IC50 ± S.D. b | |||||||||
| 1 | Fentanyl | PhEth c | H | H | H | H | H | H | 4973 ± 2.3 | 1.23 ± 0.14 |
| 2 | Benzylfentanyl | Benz d | n/a e | H | H | H | H | H | 322.1 ± 1.9 | 489.7 ± 28.6 |
| 3 | Thenylfentanyl | ThMet f | n/a e | H | H | H | H | H | 1185 ± 2.0 | 245.5 ± 12.9 |
| 4 | β-Hydroxyfentanyl g | PhEth | OH | H | H | H | H | H | >10 000 h | 2.81 ± 0.13 |
| 5 | ω-Hydroxyfentanyl | PhEth | H | H | H | H | H | OH | >10 000 h | 97.7 ± 5.8 |
| 6 | ω-1-Hydroxyfentanyl g | PhEth | H | H | H | H | OH | H | >10 000 h | 489.0 ± 40.6 |
| 7 | p-Fluorofentanyl | PhEth | H | H | H | F | H | H | 495.3 ± 2.0 | 0.48 ± 0.03 |
| 8 | 3-Methylthiofentanyl g | ThEth i | H | Me | H | H | H | H | 465.0 ± 1.2 | 1.10 ± 0.10 |
| 9 | Sufentanil | ThEth | H | H | CH2OMe | H | H | H | 2077 ± 2.1 | 0.40 ± 0.03 |
| 10 | Norcarfentanil | H | n/a e | H | C(O)OMe | H | H | H | >10 000 h | 295.1 ± 1.3 |
| 11 | Remifentanil | C2H4C(O)OMe | n/a e | H | C(O)OMe | H | H | H | >10 000 h | 0.60 ± 0.08 |
| 12 | Alfentanil | TetrEth j | H | H | CH2OMe | H | H | H | >10 000 h | 38.9 ± 2.8 |
| 13 | SKF10047 (reference) | n/a e | n/a e | n/a e | n/a e | n/a e | n/a e | n/a e | 69.4 ± 2.1 | n/d k |
aHalf maximal inhibitory concentration in nM (± standard deviation) of [3H]-(+)-pentazocine specific binding; the results given are means from at least three experiments performed in duplicate; values recalculated to Ki are given in Table ESI-BIND-1.
bHalf maximal inhibitory concentration in nM ± standard deviation, taken from ref. 19.
c2-Phenyleth-1-yl.
dBenzyl.
eNot applicable.
f2-Thienylmethyl.
gTested as an enantiomeric or diastereoisomeric (8) mixture.
hThe values of [3H]-(+)-pentazocine displacement at 10 μM are given in Table ESI-BIND-1.
i2-Thien-2-ylethyl.
j2-(4-Ethyl-5-oxo-4,5-dihydro-1H-1,2,3,4-tetrazol-1-yl)ethyl.
kn/d – not determined.
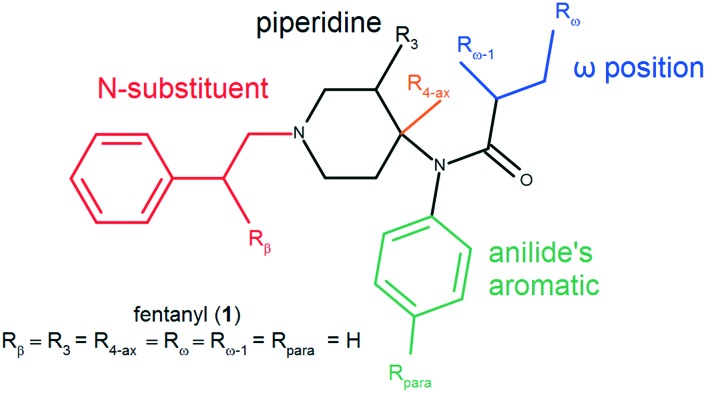
Chart 1. Structure of fentanyl (1) and its logical disassembly.
In order to revisit those older data regarding fentanyl (1) and to complement them with affinities of fentanyl derivatives, we determined the binding affinity for compounds 1–12 and a reference selective σ1R agonist SKF10047 (13).¶ ,23 The assay was carried out with membrane preparations of guinea pig brains,‖ ,** ,24 following a typical procedure of competitive radioligand binding assays.† The results of the assays are presented in Table 1 as well as in Table ESI-BIND-1 and Fig. ESI-BIND-1.‡
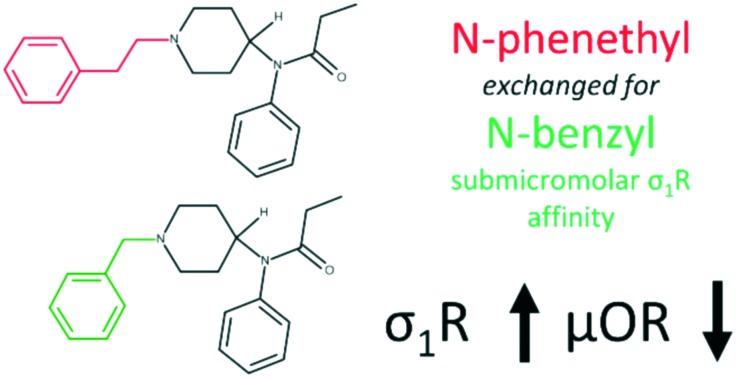
Consistent with previous reports, we found that fentanyl (1) exhibits a rather low σ1R affinity with an IC50 of 4973 nM. Its affinity is approximately 72 times worse than that of reference SKF10047 (13). Strikingly, removal of just one –CH2– unit from the N-phenethyl chain (benzylfentanyl, 2) results in over 15 times improvement of affinity yielding a submicromolar IC50 of 322.1 nM. Still, isosteric exchange of N-benzyl (2) to the N-2-thienylmethyl chain (thenylfentanyl, 3) gives an almost 4-fold drop to IC50 = 1185 nM. Introduction of the hydroxyl group to the fentanyl structure is highly unfavourable for binding. β- (4), ω- (5) or ω-1-hydroxy (6) substituted fentanyls exhibit no more than 50% displacement of [3H]-(+)-pentazocine at a concentration as high as 10 μM (Table ESI-BIND-1‡). On the other hand, a conservative H/F exchange at the para-position of anilide's aromatic (p-fluorofentanyl, 7) yields a 10-fold affinity improvement (IC50 = 495.3 nM) compared to that of the parent (1). A derivative of a similar binding strength is 3-methylthiofentanyl (8, IC50 = 465.0 nM), in which 3-methyl substitution is accompanied by replacement of phenyl to 2-thienyl in the N-chain. Out of 4-axially substituted fentanyl derivatives tested here, only sufentanil (9, IC50 = 2077 nM) shows some improvement when compared to the parent fentanyl (1). The remaining norcarfentanil (10), remifentanil (11) and alfentanil (12) inhibit no more than 35% of [3H]-(+)-pentazocine specific binding at 10 μM (Table ESI-BIND-1‡).
What deserves notice is that some σ1R SAR trends observed here are opposite to those reported for fentanyl derivatives with regard to the μOR binding.19 Firstly, benzylfentanyl (2) binds the μOR almost 400-times worse than fentanyl (1), while in the case of the σ1R, the shorter derivative is a significantly better binder. Secondly, 4-axially substituted derivatives which are very potent μOR binders (11 and 12) have only a negligible affinity for the σ1R. On the other hand, another congener of this group, sufentanil (9), exhibits slightly improved σ1R binding compared to the parent (1), so the 4-axial substitution does not seem to be entirely adverse. Similar to the fentanyls' SAR at the μOR, p-F and 3-Me substitutions are clearly beneficial, and in the case of the σ1R, the improvement of affinity is even more pronounced.
In order to find a probable structural basis for the reported affinity trends, we set out to model‡ the complexes of the σ1R with the parent fentanyl (1), as well as two submicromolar derivatives (2 and 7). The modelling protocol included docking (in AutoDock Vina)25 and molecular dynamics (MD) simulations (5 replicas of 300 ns in GROMACS26).
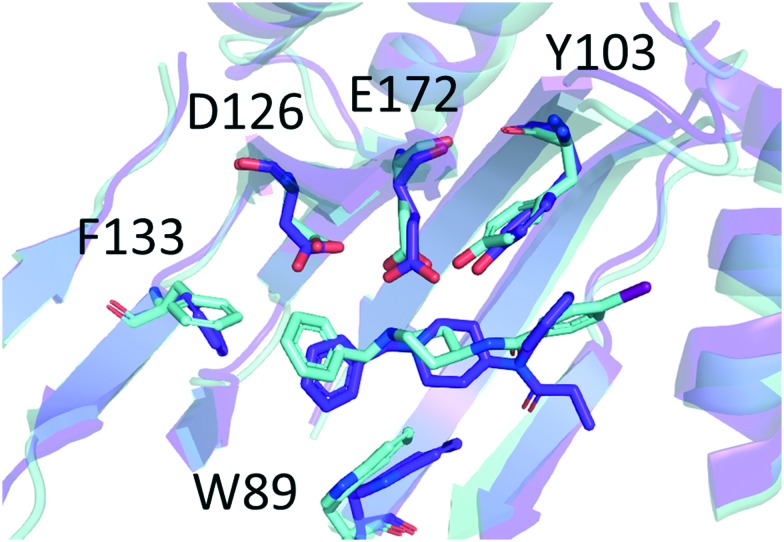
For both submicromolar derivatives (2 and 7), docking and MD predict binding in the positioning close to that of crystallographic ligand 4-IBP (N-(1-benzyl-4-piperidyl)-4-iodo-benzamide, PDB accession code: ; 5HK2;15Fig. 1 and ESI-SIM-20 and ESI-SIM-21‡). For fentanyl (1), top scored poses are also similar, but in 3 of 5 MD runs started therefrom, the ligand migrates away and assumes a binding mode perpendicular to the ‘classical’ one (Fig. ESI-SIM-23 and ESI-SIM-24‡). In the remaining two simulations, fentanyl stays close to the ‘classical’ positioning (Fig. ESI-SIM-22‡).
Fig. 1. Superimposition of benzylfentanyl (compound 2, colour: purple blue) and 4-IBP (N-(1-benzyl-4-piperidyl)-4-iodo-benzamide, colour: aquamarine). The binding mode of compound 2 is taken from simulation II, t = 300.0 ns. The binding mode of 4-IBP is taken from chain A of crystallographic structure ; 5HK2.15 Only several residues of the binding site are shown for clarity. The receptor structures are given as ribbons representing the secondary structure. Heteroatoms are coloured according to the convention: red – oxygen, dark blue – nitrogen, dark pink – iodine.
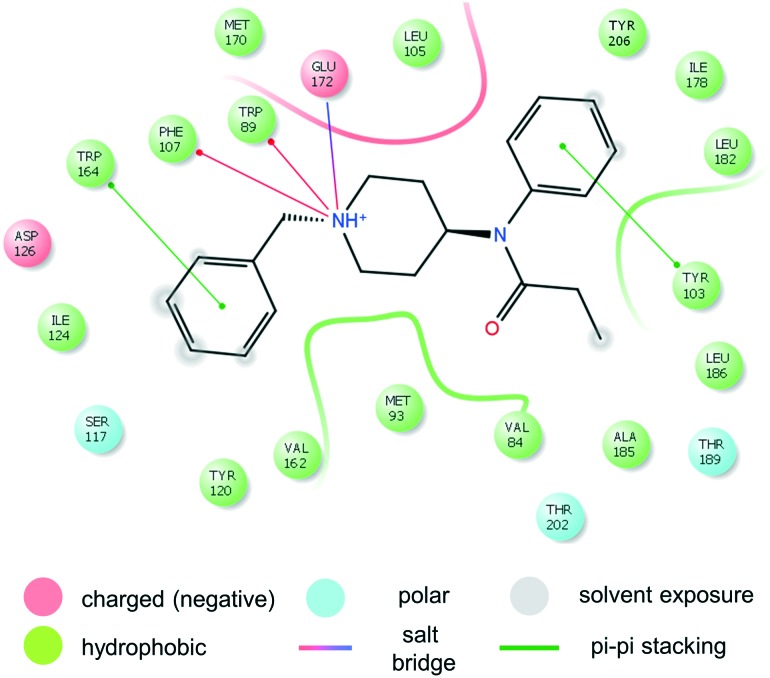
The general features of this binding mode, common to simulations with benzylfentanyl (2, Fig. 2), p-fluorofentanyl (7, Fig. ESI-SIM-27‡) and two simulations of fentanyl (1, Fig. ESI-SIM-25‡) are:
Fig. 2. Interactions of benzylfentanyl (2) with σ1R.
1. Persistent ionic interaction of protonable piperidine's nitrogen with Glu172 (Fig. ESI-SIM-19‡),
2. Positioning of the anilide's ring towards α4 and α5 helices,
3. Location of the N-substituent towards the bottom of β-barrel (close to Asp126).
Apart from the ionic interaction, further contacts of the piperidine ring involve hydrophobic interactions with Leu105, Trp89, Phe107 and Val84. The anilide's aromatic of the ligands is able to π–π stack with Tyr103. Relatively close to the para-position of the ring located are the side chains of Ile178, Leu182 and Tyr206. It is the latter of these that interacts with the p-fluoro substituent of compound 7. Overall, the anilide's rings of our ligands are located very closely to the positioning of the phenyl ring of 4-IBP (Fig. 1 and ESI-SIM-20‡). Within 4.0 Å from the atoms of the propanamide moiety, one can find Ala185, Leu186, Thr189 and Thr202.
The greatest difference in the binding modes of the three modelled compounds lies in the interactions of the N-substituent. The shorter benzylfentanyl (2) accommodates this moiety in a manner similar to that of 4-IBP (Fig. 1). This creates dispersive interactions with hydrophobic/aromatic side chains: Trp89, Phe107, Ser117, Tyr120, Ile124, and Val162. Looking at some snapshots of the simulations, one could expect also the presence of π–π stacking with Trp164.§ On the other hand, the longer N-phenethyl substituent of fentanyl (1) and p-fluorofentanyl (7) reaches further and can additionally have contact with Phe133, Gln135, His154, and Thr160. Here however, stacking with Trp164 is impossible.
Assuming that the crystallographic binding mode of 4-IBP also shared partially by 2 and 7, is somehow optimal, one can put forward a few structural, qualitative intuitions as to the observed experimental affinity trends. Benzylfentanyl (2) is able to locate the pharmacophoric elements very closely to those of 4-IBP (RMSDs for N-benzyl's rings: 1.89 Å; the other ring pair: 1.37 Å; distance between piperidines' nitrogens: 1.20 Ŷ ). On the other hand, fentanyl (1) larger by one methylene unit in the N-substituent cannot accommodate its phenyl ring in such an advantageous manner. Moreover, it has one additional rotor the freezing whereof is associated with entropic penalty. The same applies to the p-fluoro derivative (7) but here the para substituent at the anilide's ring forms favourable interactions with Tyr206.
As to other derivatives, the hydroxyl groups at ω positions do not seem to have potential H-bonding partners reachable without at least some distortion of interaction with Glu172 and/or the stacking interactions with Tyr103. Similarly, no partner for H-bonding is apparent for the β-OH group. This points to the reason why derivatives 4, 5 and 6 might be very poor binders. Further, regarding the bulky 4-axial substituents (present in derivatives 9, 10, 11, and 12), their accommodation in the binding pocket (while preserving the binding mode similar to that of 4-IBP) would create strain, thus these compounds take another, presumably higher in energy, binding mode.
Conclusions
In conclusion, herein we report σ1R affinity for fentanyl (1) and its 11 commercially available derivatives (2–12). Fentanyl (consistent with older studies) is a rather weak binder (IC50 = 4973 nM). Still, a few derivatives exhibit a submicromolar affinity, with the best of them being benzylfentanyl (2, IC50 = 322.1 nM). Strikingly, this over 15-fold difference in affinity is related to a very modest structural difference of one methylene unit in the N-substituent. By means of molecular modelling, we have provided a probable structural basis of the fentanyls' interactions with the σ1R. According to the results of modelling, the best compound benzylfentanyl (2) is able to assume a binding mode very close to that of crystallographic ligand 4-IBP. It seems that research based on fentanyl derivatives might be an interesting approach towards finding novel σ1R ligands. Given the high affinities of fentanyls for the μ-opioid receptor, it is tempting to look for ligands of mixed μOR agonist/σ1R antagonist characters within the fentanyl family. Compounds of this kind could be expected to have interesting analgesic properties.
Ethical statement
All the animal experiments were performed according to the national (1998. XXVIII; 40/2013) and European (2010/63/EU) animal ethics guidelines. The experimental protocols were approved by the Animal Experimentation and Ethics Committee of the Biological Research Centre of the Hungarian Academy of Sciences, Szeged, Hungary.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The research was funded by the National Centre of Science, Poland (Grant No. 2013/11/B/ST4/00785). Further support was provided by the Polish-Hungarian Joint Research Project between BRC HUA and MMRC PAS (2017-2019, HU identifier: PROJEKT2016-29). Simulations were performed at the Interdisciplinary Centre for Mathematical and Computational Modelling (ICM), Warsaw, Poland (Computational grant GB65-21) and at Swierk Computing Centre, National Centre for Nuclear Research, Swierk, Poland.
Footnotes
†Dedicated to the memory of Prof. Andrzej W. Lipkowski on the 5th anniversary of his death.
‡Electronic supplementary information (ESI) available: The ESI file is divided into three sections. The items in each section are independently numbered. The sections are ESI-CHEM (structures of all the studied molecules, 1 chart), ESI-BIND (binding affinity determinations, 1 figure and 1 table), and ESI-SIM (simulations, 27 figures). See DOI: 10.1039/c9md00222g
§The closest homologues are fungal sterol isomerases. On the other hand, the σ1R displays no detectable isomerase activity.
¶Chemicals. Compounds 1–12 (cf.Table 1) were obtained from Toronto Research Chemicals, North York, ON, Canada and used without further processing. Compounds 3, 4 and 12 were chlorides, compound 9 was citrate, and the remaining ones were free bases. The substances were dissolved in ethanol : water (1 : 1 v/v) and were stored in 1 mM stock solution at –20 °C. The selective σ1R agonist SKF10047 was purchased from Tocris Bioscience (Bristol, UK). [3H]-(+)-pentazocine ((+)-pentazocine, [RING-1,3-3H], specific activity: 26.9 Ci mmol–1) and the UltimaGold™ MV aqueous scintillation cocktail were obtained from PerkinElmer (Boston, USA).
‖Animals. Both male and female guinea pigs (∼700 g body weight, LAL/HA/BR strain) were used for membrane preparations. The animals were housed in LAB-ÁLL Bt. (Budapest, Hungary). All the animals were kept in a temperature controlled room (21–24 °C) under a 12 : 12 light and dark cycle and were provided with water and food ad libitum. All housing and experiments were conducted in accordance with the European Communities Council Directives (86/606/ECC) and the Hungarian Act for the Protection of Animals in Research (XXVIII.tv. 32.§). The total number of animals as well as their suffering was minimized whenever possible.
**Preparation of brain samples for binding assays. Guinea pigs were decapitated and their brains were quickly removed. The brains were prepared for membrane preparation according to Benyhe et al. as described in ref. 24. The brains were homogenized in 30 volumes (v/w) of ice-cold 50 mM Tris-HCl pH 7.4 buffer with a Teflon-glass Braun homogenizer operating at 1500 rpm. The homogenate was centrifuged at 18 000 rpm for 20 min at 4 °C, and the pellet was taken in the original volume of Tris-HCl buffer. The homogenate was incubated at 37 °C for 30 min in a shaking water bath. Then centrifugation was repeated as described before. The final pellet was suspended in 5 volumes of 50 mM Tris-HCl pH 7.4 buffer and stored at –80 °C.
†Receptor binding assays. Aliquots of frozen guinea pig brain membrane homogenate were suspended in 50 mM Tris-HCl buffer (pH 8.0). The membrane was incubated in the presence of the unlabeled SKF10047, fentanyl and fentanyl analogues in increasing concentrations (10–10–10–5 M) at 37 °C for 90 min with [3H]-(+)-pentazocine. The non-specific and total binding was determined in the presence and absence of 10 μM unlabelled SKF10047. The reaction was terminated by rapid filtration under vacuum (Brandel M24R Cell Harvester), and washed three times with 5 ml ice-cold 50 mM Tris-HCl (pH 7.4) buffer through Whatman GF/C glass fibers. The radioactivity of the dried filters was detected in the UltimaGold™ MV aqueous scintillation cocktail with a Packard Tricarb 2300TR liquid scintillation counter. The competitive binding assays were performed in duplicate and repeated at least three times. Experimental data were presented as means ± standard deviation (S.D.). Points were fitted with a professional curve fitting program, GraphPad Prism 5.0 (GraphPad Prism Software Inc., San Diego, CA), using non-linear regression. In the competition binding assays, the ‘one site competition’ fitting was used to establish the half maximal inhibitory concentration, ligand potency (IC50). Inhibition was given as percent of the specific binding observed.
‡Molecular modelling. Structures of compounds 1, 2 and 7 were docked into the 5HK2 PDB structure using AutoDock Vina.25 Only the chain A was used for docking. The box was set so as to encompass the crystallographic ligand and a large margin. The top scored poses were visually inspected, and upon finding no counterintuitive elements, they were chosen as starting points for molecular dynamics simulations. MD was performed on a simplified receptor without the membrane and without the α1 helix. The shortened protein models with bound ligands were solvated (TIP3 water model) by using the CHARMM-GUI service. Also Na+ and Cl– ions were added to obtain 0.154 M concentration. The protonation states were set as expected at pH = 7, with the exception of Asp126 which is believed to be protonated in the presence of the ligand, and so it was set. The protein, water and ions were typed with the CHARMM 36 force-field, while for ligands, CHARMM CGenFF was used. The complexes were minimized and equilibrated. The production step (NPT ensemble, 303.15 K, integration step = 2 fs, cut-off scheme Verlet, Nose–Hoover thermostat, Parrinello–Rahman barostat, LINCS H-bond constraints) was run to obtain at least 300 ns of equilibrated trajectory. There were 5 independent production runs for each derivative. For the analysis of simulation data, we used GROMACS utilities,26 our in-house Python scripts. Molecular graphics were prepared using PyMol 2.1.1 (ref. 27) and using Maestro of Schrödinger suite.28
§One should remember that classical MD force fields do not take into account interactions of this kind (π–π stacking) therefore geometries where these would be present may be somehow undersampled in the trajectories.
References
- Hanner M., Moebius F. F., Flandorfer A., Knaus H. G., Striessnig J., Kempner E., Glossmann H. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Lucke-Wold B. P., Mookerjee S. A., Cavendish J. Z., Robson M. J., Scandinaro A. L., Matsumoto R. R. J. Pharmacol. Sci. 2015;127:17–29. doi: 10.1016/j.jphs.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Su T.-P., Su T.-C., Nakamura Y., Tsai S.-Y. Trends Pharmacol. Sci. 2016;37:262–278. doi: 10.1016/j.tips.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T., Su T.-P. Pharmacol. Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos E. J., Entrena J. M., Nieto F. R., Cendán C. M., Del Pozo E. Curr. Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishback J. A., Robson M. J., Xu Y.-T., Matsumoto R. R. Pharmacol. Ther. 2010;127:271–282. doi: 10.1016/j.pharmthera.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata K., Hashimoto K. Curr. Pharm. Des. 2006;12:3857–3876. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- Hayashi T. J. Pharmacol. Sci. 2015;127:2–5. doi: 10.1016/j.jphs.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Bruna J., Videla S., Argyriou A. A., Velasco R., Villoria J., Santos C., Nadal C., Cavaletti G., Alberti P., Briani C., Kalofonos H. P., Cortinovis D., Sust M., Vaqué A., Klein T., Plata-Salamán C. Neurotherapeutics. 2018;15:178–189. doi: 10.1007/s13311-017-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castany S., Codony X., Zamanillo D., Merlos M., Verdú E., Boadas-Vaello P. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard V., Espallergues J., Keller E., Vamvakides A., Maurice T. J. Psychopharmacol. 2011;25:1101–1117. doi: 10.1177/0269881110379286. [DOI] [PubMed] [Google Scholar]
- Xu R., Lord S. A., Peterson R. M., Fergason-Cantrell E. A., Lever J. R., Lever S. Z. Bioorg. Med. Chem. 2015;23:222–230. doi: 10.1016/j.bmc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer R., Moebius H. J., Skoloudik D., Santamarina E., Sato W., Mita S., Muir K. W. Stroke. 2014;45:3304–3310. doi: 10.1161/STROKEAHA.114.005835. [DOI] [PubMed] [Google Scholar]
- Narayanan S., Bhat R., Mesangeau C., Poupaert J. H., McCurdy C. R. Future Med. Chem. 2011;3:79–94. doi: 10.4155/fmc.10.279. [DOI] [PubMed] [Google Scholar]
- Schmidt H. R., Zheng S., Gurpinar E., Koehl A., Manglik A., Kruse A. C. Nature. 2016;532:527–530. doi: 10.1038/nature17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurciński M., Jarończyk M., Lipiński P. F. J., Dobrowolski J. C., Sadlej J. Molecules. 2018;23:456. doi: 10.3390/molecules23020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortazzo M. H., Copenhaver D. and Fishman S. M., in Practical Management of Pain, Elsevier, 2014, pp. 495–507.e3. [Google Scholar]
- Burns S. M., Cunningham C. W., Mercer S. L. ACS Chem. Neurosci. 2018;9:2428–2437. doi: 10.1021/acschemneuro.8b00174. [DOI] [PubMed] [Google Scholar]
- Lipiński P. F. J., Kosson P., Matalińska J., Roszkowski P., Czarnocki Z., Jarończyk M., Misicka A., Dobrowolski J. C., Sadlej J. Molecules. 2019;24:740. doi: 10.3390/molecules24040740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe D. A., McMahon Tobin G. A., Mellon R. D., Katki A. G., Parker R. J., Colatsky T., Kropp T. J., Verbois S. L. Regul. Toxicol. Pharmacol. 2011;59:385–390. doi: 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Largent B. L., Wikström H., Gundlach A. L., Snyder S. H. Mol. Pharmacol. 1987;32:772–784. [PubMed] [Google Scholar]
- Chen J. C., Smith E. R., Cahill M., Cohen R., Fishman J. B. Life Sci. 1993;52:389–396. doi: 10.1016/0024-3205(93)90152-s. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M., Cortés-Montero E., Pozo-Rodrigálvarez A., Sánchez-Blázquez P., Garzón-Niño J. Oncotarget. 2015;6:35458–35477. doi: 10.18632/oncotarget.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyhe S., Farkas J., Tóth G., Wollemann M. J. Neurosci. Res. 1997;48:249–258. doi: 10.1002/(sici)1097-4547(19970501)48:3<249::aid-jnr7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Trott O., Olson A. J. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., Lindahl E. SoftwareX. 2015;1:19–25. [Google Scholar]
- The PyMOL Molecular Graphics System. Available online: www.pymol.org (accessed on 9 April 2018).
- Schrödinger Release 2019-1: Maestro, Schrödinger, LLC, New York, NY, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.