Abstract
Objectives
A malignant pleural effusion (MPE) is a common complication in non-small cell lung cancer (NSCLC) with important staging and prognostic information. Patients with MPEs are often candidates for advanced therapies, however, the current gold standard, cytological analysis of pleural fluid samples, has limited sensitivity. We aimed to demonstrate the feasibility of non-invasive enumeration and immunophenotyping of EpCAM-positive cells in pleural fluid samples for the diagnosis of a MPE in NSCLC patients.
Materials and Methods
Pleural fluid specimens were prospectively collected from patients with NSCLC and the CellSearch® technology was utilized for the enumeration of pleural EpCAM-positive cells (PECs) and determination of PD-L1 expression on PECs from pleural fluid samples. The diagnostic performance of the enumeration of single PECs and PEC clusters was assessed using receiver operating characteristic (ROC) curves. The Kaplan-Meier method and Cox proportional hazards model was used to assess the impact of PECs and PEC clusters on overall survival (OS).
Results
101 NSCLC patients were enrolled. The median number of PECs was significantly greater in the malignant (n=84) versus non-malignant group (n=17) (730 PECs/mL vs 1.0 PEC/mL, p<0.001). The area under the ROC curve was 0.91. A cutoff value of 105 PECs/mL had a sensitivity and specificity of 73% and 100% for the diagnosis of a MPE, respectively. Among 69 patients with a pathology-confirmed MPE and tissue immunohistochemistry (IHC) results, 15 (22%) had greater than 50% PD-L1+ PECs. Overall concordance between tissue and PEC PD-L1 expression was 76%. Higher numbers of pleural effusion single PECs were associated with inferior overall survival (Cox adjusted HR 1.8, 95% CI: 1.02–3.05 p=0.043).
Conclusion
Non-invasive measurement of PECs in NSCLC patients, using an automated, clinically available approach, may improve the diagnostic accuracy of a MPE, allow for immunophenotyping of PECs, and provide prognostic information.
Keywords: Non-small cell lung cancer, Malignant pleural effusion, Circulating tumor cells, Biomarker
1. Introduction
Despite significant advances in detection and therapy, lung cancer remains the leading cause of cancer-related death in both men and women worldwide. Non-small cell lung cancer (NSCLC) is the most common histotype representing 85% of new diagnoses.1 Malignant pleural effusions (MPEs) occur as a frequent complication of advanced stage lung cancer, occurring in approximately 30% of patients, and denoting a poor overall prognosis.2,3 NSCLC patients with a MPE have a median overall survival of only 4.3 months and are often candidates for advanced therapies.4 Sampling of a pleural effusion in a lung cancer patient by thoracentesis is mandatory to provide accurate diagnostic and staging information. However, cytological analysis remains the gold standard for the diagnosis of a MPE despite a mean sensitivity of ~60% (range 46–89%).5–8 The detection of tumor cells in serous effusions by cytology is often limited by the scarcity of tumor cells and difficulty in distinguishing tumor cells from reactive mesothelial cells or inflammatory cells.9 Several markers have been evaluated to distinguish malignant from benign effusions,10–12 however, these have limited clinical utility.
CellSearch® is a circulating tumor cell (CTC) enrichment platform that isolates cells based on cell surface Epithelial Cell Adhesion Molecule (EpCAM) expression using an EpCAM directed capture antibody. The CellSearch® platform is currently FDA-approved for the identification and enumeration of CTCs from the peripheral blood in a variety of malignancies.13–19 The enumeration of CTCs and multicellular aggregates of CTCs, termed CTC clusters, from blood has been associated with a poor overall prognosis.16,19–22 In addition, CTC clusters have significantly increased metastatic potential when compared to individual CTCs.23–26 While CellSearch® has primarily been employed as an assay to detect single CTCs in peripheral blood, this approach has had very low sensitivity for NSCLC.13,27 To address this, we have previously demonstrated the ability to adapt this technology to detect PECs from MPEs in patients with epithelial malignancies.28 The ability to detect rare PECs from pleural effusions (analogous to CTCs detected in blood) in patients with NSCLC could improve the diagnostic accuracy of conventional cytological analysis and provide improved prognostic information. In addition, the CellSearch® system allows one to further characterize tumor cells with user-defined markers of interest, by adding a fluorochrome-conjugated antibody to the open channel. This approach has previously been used to study cell surface markers on CTCs isolated from the peripheral blood, including IGF1R, HER2, EGFR, and PD-L1.29–33 To our knowledge, this non-invasive approach for immunophenotyping has not previously been demonstrated for PECs from MPEs. The assessment of pleural fluid samples using CellSearch® could represent a novel approach to enrich tumor cells often undetectable in the blood of NSCLC patients13 and further phenotype these cells for markers of interest that could not otherwise be detected in circulating tumor DNA.
The expression of the programmed death-ligand 1 (PD-L1) on the surface of tumor cells has emerged as a promising biomarker for the selection of NSCLC patients to receive immune targeted agents. Several studies have shown improved response rates to immunotherapy in NSCLC patients with high levels of PD-L1 expression.34,35 Moreover, additional immune checkpoints, including TIM-3 and LAG-3 have been identified as being up-regulated in tumors at the time of progression on PD-1-targeted therapy.36 Inhibitors targeting these checkpoints are currently in clinical trial. To address this ongoing need for development of non-invasive immunophenotyping approaches, we aimed to demonstrate the feasibility of using the CellSearch® CTC enrichment platform for the non-invasive enumeration and immunophenotyping of PECs in pleural fluid from NSCLC patients.
2. Materials and Methods
2.1. Patients
This was a single-center, prospective, observational study conducted at the Hospital of the University of Pennsylvania from February 2016 to June 2017. Enrolled subjects had a history of NSCLC and underwent sampling of a pleural effusion at our institution. Patients with less than 200 mL of pleural fluid obtained from a diagnostic thoracentesis were excluded from the study to ensure sufficient volume of fluid for routine clinical tests. A total of 101 subjects were included in the study. Baseline demographics and clinical variables, such as tissue mutation status, pathology results, treatment regimens and outcome data were obtained from periodic chart review of the electronic medical record (patient characteristics in Table 1). Study data were collected and managed using the REDCap electronic data capture tools hosted at the University of Pennsylvania.37 A malignant pleural effusion was defined as having at least one of the following features 1) positive cytological analysis 2) positive pleural biopsy or 3) determined to be malignant by the treating physician based on clinical characteristics. Clinical characteristics to determine a MPE in the absence of pathologic confirmation included evidence of tumor involvement of the pleural surface by cross-sectional imaging or patients with metastatic NSCLC with no alterative explanation for a pleural effusion. An effusion that had a definitive benign etiology or did not meet criteria for a MPE was defined as a non-malignant effusion. Mutations in the EGFR gene that confer sensitivity to an EGFR-targeted therapy were defined as targetable EGFR mutations. Specific EGFR mutations detected in our cohort included: Exon 19 deletions, L858R, L861K, G719S, and E709A. This study was approved by the University of Pennsylvania Institutional Review Board.
Table 1.
Patient characteristics
| All Patients (n = 101) |
||
|---|---|---|
| Number | % | |
| Median age (range) | 66 (37–91) | -- |
| Sex | ||
| Male | 46 | 46 |
| Female | 55 | 54 |
| Race | ||
| Asian | 11 | 11 |
| Black | 14 | 14 |
| White | 73 | 72 |
| Other | 3 | 3 |
| Smoking status* | ||
| Never smoker | 33 | 33 |
| Former smoker | 60 | 59 |
| Current smoker | 7 | 7 |
| ECOG status | ||
| 0–1 | 78 | 77 |
| 2 | 18 | 18 |
| 3 | 5 | 5 |
| Stage | ||
| I | 8 | 8 |
| II | 4 | 4 |
| III | 13 | 13 |
| IV | 76 | 75 |
| Histology | ||
| Adenocarcinoma | 82 | 81 |
| Squamous cell carcinoma | 11 | 11 |
| Poorly differentiated carcinoma | 8 | 8 |
| Therapeutic regimen at time of effusion | ||
| Chemotherapy | 23 | 23 |
| Tyrosine Kinase Inhibitor | 14 | 14 |
| Immunotherapy | 12 | 12 |
| None | 52 | 51 |
Smoking status unknown for 1 patient
2.2. Pleural effusion sample collection and preparation
10 mL of unprocessed pleural fluid was collected in a CellSave preservative tube (Menarini Silicon Biosystems) following thoracentesis. Samples were maintained at ambient temperature and 1 mL of pleural fluid was processed within 96 hours of collection in accordance with manufacturer guidelines.
2.3. Enumeration of PECs and PEC Clusters from pleural effusions
CTC enumeration from blood using the CellSearch® technology has been previously described.13 We adapted the CellSearch® AutoPrep (Menarini Silicon Biosystems) platform to isolate pleural EpCAM-positive cells (PECs) present in the pleural fluid of NSCLC patients for our study. Briefly, the CellSearch® CXC kit (Menarini Silicon Biosystems) utilizes a ferromagnetic particle reagent solution incubated with anti-EpCAM antibodies to capture and enrich EpCAM+ cells present in the pleural fluid. Enriched cells were stained with the following fluorescent antibodies: anti-cytokeratin (CK) to identify epithelial cells, anti-CD45-Allophycocyanin (APC) to identify white blood cells, and 4’,6-diamidino-2-phenylindole (DAPI) to identify nucleated cells. Identification, enumeration, and marker expression of PECs were performed using the CellTracks Analyzer II (Menarini Silicon Biosystems), a semi-automated fluorescent microscope system. EpCAM+/CK+/CD45−/DAPI+ cells were enumerated and counted as individual PECs. Results are expressed as the number of PECs/mL of pleural fluid. A PEC cluster was defined as two or more EpCAM+/CK+/CD45−/DAPI+ cells with surface membrane contact within a single image frame on the CellTracks Analyzer II (representative images shown in Supplemental Figure 1). PEC clusters were enumerated and results expressed as the number of PEC Clusters/mL of pleural fluid.
2.3. PEC PD-L1 expression validation and evaluation
The anti-human CD274 (PD-L1) antibody (BioLegend) was added to the open 4th channel of the CellSearch® AutoPrep allowing for marker staining during sample processing. To validate PEC PD-L1 expression using the anti-human CD274 (PD-L1) antibody, peripheral blood spiking experiments with PD-L1 positive (PD-L1 transfected EM-Meso) and PD-L1 negative (BT-474) control cell lines were conducted using normal donor blood and analyzed using the CellSearch® platform with results compared to flow cytometry analysis (Supplemental Figure 2). The percentage of PD-L1 positive PECs was determined by dividing the number of PD-L1+ PECs by the total number of PECs detected in the pleural effusion sample. Patients with a cytologically positive MPE had the pleural effusion cellblock stained for PD-L1 expression using the E1J2J PD-L1 antibody (Cell Signaling Technology). This was the standard antibody for PD-L1 testing of formalin-fixed paraffin-embedded tissue during the study period and binds the same PD-L1 domain as the anti-human CD274 (PD-L1) antibody (Biolegend). Human tonsillar tissue was used as a positive staining control. Each slide was reviewed by an independent cytopathologist and scored for membranous PD-L1 staining on the tumor cells present in less than or greater than 50% of the cells. 50 of the 69 patients with a pathologically confirmed MPE had sufficient cytological tissue for IHC analysis.
2.4. Statistical Analysis
Descriptive statistics such as mean, median, and proportions were used to summarize patient demographics and tumor characteristics. Non-parametric Wilcoxon rank-sum test was utilized to compare the number of PECs and PEC clusters between malignant and non-malignant MPEs. The performance of the number of PECs and PEC clusters for distinguishing malignant and non-malignant MPEs was assessed using receiver operating characteristic (ROC) curves and the area under the curve (AUC). Optimal cutoff points were selected as the point on the ROC curve with the minimum distance from the upper-left corner of the unit graph. Sensitivity and specificity and the associated 95% confidence intervals (CI) were computed. Among 50 patients with a pathologically confirmed MPE and sufficient cytological tissue for IHC analysis, PD-L1 expression between tissue and PECs was considered concordant if both PD-L1 expressions were above or below a threshold value of 50%. To determine whether the number of PECs and PEC Clusters were associated with overall survival (OS), Kaplan-Meier curves were plotted according to high and low PECs and PEC Clusters and compared by a log-rank test. High and low groups were defined by whether the number of PECs and PEC clusters were above or below the median values detected in the clinically confirmed MPEs. To control for possible confounding effects, a multivariable Cox proportional hazards model was fitted while adjusting for age, sex, smoking history (yes/no), ECOG status (≥ 2), and EGFR mutation status (mutated/not-mutated). Stata v. 14 (StataCorp) was used for all analyses, and a two-sided p-value of <0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
Pleural fluid specimens were collected from 101 NSCLC patients at the Hospital of the University of Pennsylvania between February 2016 and June 2017 (see Table 1 for patient characteristics). The majority of patients, 76 (75%), had stage IV disease at the time of enrollment. A MPE was present at the initial diagnosis of NSCLC in 27 patients (27%), and the confirmation of a MPE resulted in the diagnosis of stage IV disease in an additional 10 patients previously diagnosed with stage I-III NSCLC. Adenocarcinoma was the most common histology (81%), with only 11% of subjects having squamous cell carcinoma (SqCC). The lower incidence of SqCC in this study compared to the general population (20–50%)38 may be attributable to the fact that one-third of the patients were never smokers. Approximately half of the patients (49%) were receiving systemic therapy at the time of their pleural fluid sampling. 86 patients had evaluable tissue sequencing results for clinically relevant mutations in EGFR, EML4-ALK, and KRAS. A targetable EGFR mutation was found in 20 patients (20%), 5 patients (5%) had an EML4-ALK fusion, and 17 patients (17%) were identified to have a KRAS variant. The increased frequency of EGFR mutations in NSCLC patients with a MPE compared to the general NSCLC population in the US (~10–14%)39,40 is consistent with previous reports.41–44
3.2. PEC Enumeration in Malignant vs. Non-Malignant MPE
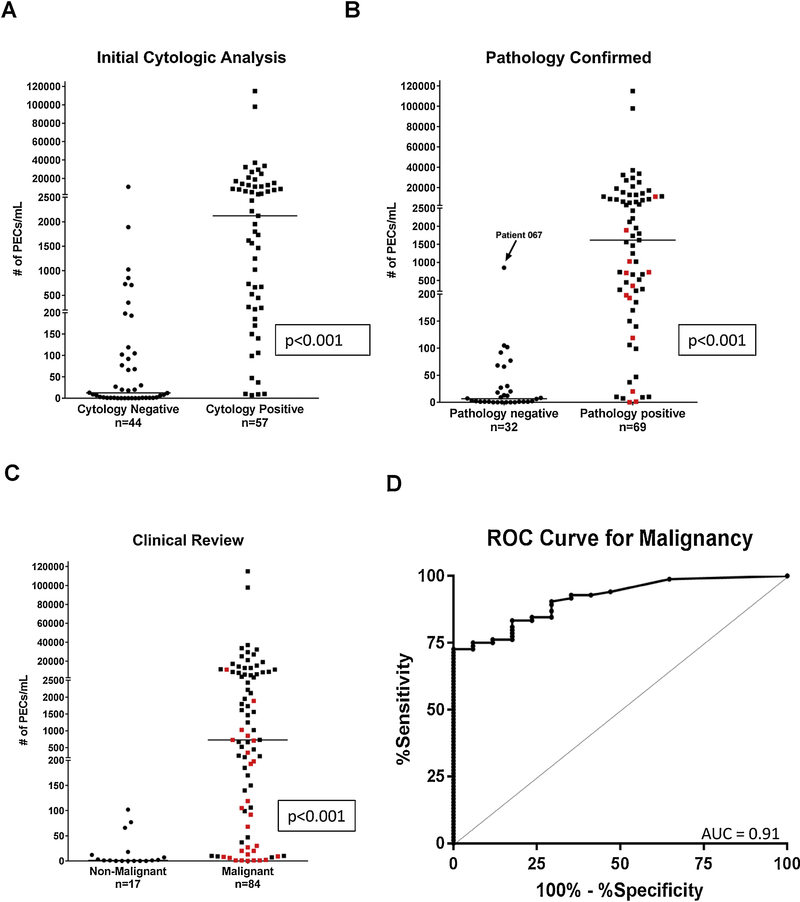
A malignant or non-malignant etiology for the pleural effusion was first established by cytology prior to or concurrently with pleural fluid PEC enumeration. We used the CellSearch® CTC enumeration platform to quantify the number of PECs present per mL of pleural fluid, and PEC counts were compared between the malignant and non-malignant cohorts. Initial cytological examination determined that 57 patients (56%) had a MPE, while 44 patients (44%) were determined to have a non-malignant effusion. The median number of PECs in the cytology positive and cytology negative groups was 2,121 PECs/mL (range:0–114,920 PECs/mL) and 12.5 PECs/mL (range:0–11,007 PECs/ml) (p<0.001, Figure 1A), respectively. In the cytology negative group, 36 (82%) patients underwent repeat pleural fluid sampling or pleural biopsy to further evaluate the etiology of the effusion, and 12 of these patients were ultimately diagnosed with a pathologically confirmed MPE. The median number of PECs detected in subjects with a pathologically confirmed MPE was significantly higher than the pathology negative group (1,616 PECs/mL vs 6.5 PECs/mL, p<0.001) (Figure 1B). An additional 15 patients were determined to have a MPE based on clinical characteristics but were not pathologically confirmed. In total, 84 patients were determined by cytology, pathology, or clinical characteristics to have a MPE, with 17 patients identified to have a benign effusion after clinical review. The median number of PECs detected in subjects with a MPE after clinical review was significantly higher than the nonmalignant group (730 PECs/mL vs 1.0 PEC/mL, p<0.001) (Figure 1C). There was no significant difference in the number of PECs detected in pleural effusions between the 49 patients receiving therapy at the time of effusion sampling and the 52 not receiving therapy (p=0.442; data not shown). The performance of PEC enumeration for the diagnosis of a MPE was assessed using ROC curves. The area under the ROC curve was 0.91 (95% CI: 0.86–0.97) demonstrating excellent discriminatory ability (Figure 1D). The optimal cutoff point of 105 PECs/mL was selected and had a sensitivity of 73% and specificity of 100% for the diagnosis of a MPE in NSCLC (Supplemental Table 1). Using this threshold value of 105 PECs/mL to diagnose a MPE would have reclassified 11 (11%) patients with an initial cytologically negative effusion that were ultimately identified as a MPE (Figure 1C). Three of the 11 patients required a thoracoscopic pleural biopsy to diagnose a MPE.
Fig. 1.
Pleural effusion PEC counts as determined by first cytological analysis, any pathological review, or clinical characteristics. (A) PEC counts per mL of fluid in the cytology negative and cytology positive groups as determined by first cytological analysis (n=101, p<0.001). The median number of PECs/mL was significantly higher in the cytology positive compared to the cytology negative groups (2,121 vs 12.5, p<0.001) (B) PEC counts per mL after any pathological review of pleural effusion. The median number of PECs/mL was significantly higher in the pathologically confirmed MPEs compared to the pathology negative groups (1,616 vs 6.5, p<0.001). Data points marked in red denote the 12 cytology negative patients who were ultimately determined to have a malignant phenotype after pathological review. (C) PEC counts per mL after any clinical review of pleural effusion. The median number of PECs/mL was significantly higher in the malignant compared to the non-malignant groups (730 vs 1, p<0.001). Data points marked in red denote 27 patients with an initial cytologically negative effusion who were ultimately determined to have a malignant phenotype after pathology or clinical review (D) Receiver Operator Characteristics curve (ROC curve) for PECs in pleural fluid does discriminate between benign and malignant effusions: cutoff 105 PECs/mL sensitivity 73%, specificity 100%, AUC 0.91(95% CI: 0.86–0.97). Solid horizontal lines indicate median values in panels A, B, and C.
3.3. Clinical Utility of PEC enumeration for the diagnosis of MPE in NSCLC
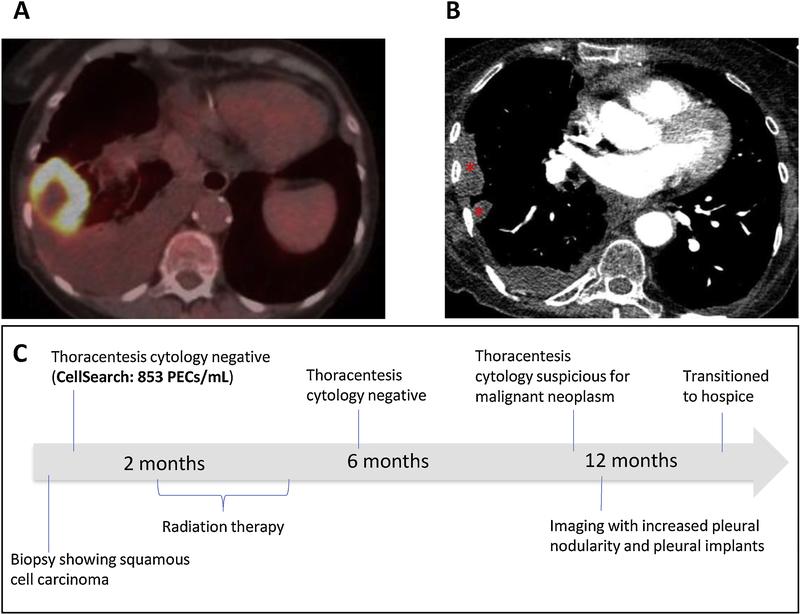
Among the 32 patients without pathologic evidence of a MPE (Figure 1B), patient 067 appeared to be an outlier with a PEC count of 853 PECs/mL, well above the median of 6.5 and the next highest patient with 102 PECs/mL. This patient was a 73-year-old female found to have a 5 cm right lower lobe mass with a moderately sized right pleural effusion (Figure 2). A biopsy of the right lower lobe mass confirmed NSCLC, squamous histology. Pleural fluid sampling at the time of diagnosis was negative for malignancy by cytological analysis. PEC enumeration of the initial pleural fluid sample revealed 853 PECs/mL. Based on cytology, the patient was diagnosed with stage IIA NSCLC and treated with definitive radiation therapy. Her pleural effusion persisted and she underwent repeat pleural fluid sampling at 6 months and 11 months after initial diagnosis. Both of these pleural fluid samples were negative by cytological analysis, however, the second sample was “suspicious” for malignancy. Neither was evaluated by CellSearch®. A CT chest performed 12 months after the initial diagnosis showed new pleural tumor implants and pleural nodularity consistent with stage IV disease. This patient was ultimately diagnosed with a MPE based on clinical characteristics.
Fig. 2.
Patient 067 diagnosed with stage IIA NSCLC based on negative cytology from thoracentesis. (A) Baseline PET/CT showing a PET avid right lower lobe mass abutting the pleura and a moderate size pleural effusion. (B) Imaging 12 months after initial diagnosis showing metastatic pleural implants (*) and pleural nodularity. (C) Timeline depicts patient history over 14 months. The initial effusion had 853 PECs/mL. Patient was treated with localized radiation with curative intent. Despite treatment, patient had a progressive decline in her functional status and was ultimately transitioned to hospice 14 months after diagnosis. Results of PEC enumeration suggest this patient likely had a malignant pleural effusion at diagnosis despite negative cytology results.
3.4. PD-L1 Expression in Malignant PECs and clusters from pleural fluid
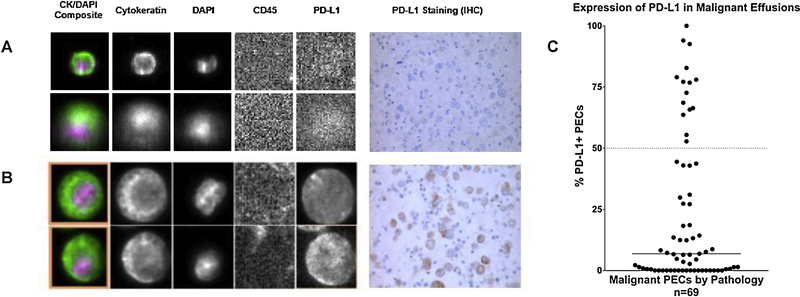
To assess the feasibility of immunophenotyping PECs present in MPEs of NSCLC patients, the anti-human CD274 (PD-L1) antibody was added to the open 4th channel of the CellSearch® platform. For the 69 patients with a pathologically confirmed MPE (see Figure 1B), the percentage of PECs expressing PD-L1 was calculated and compared to immunohistochemical PD-L1 staining from the corresponding pleural effusion cellblock. Patients with a pathologically confirmed MPE were evaluated so that a comparison between PEC PD-L1 expression and the corresponding tissue cellblock could be made. Representative PEC images demonstrating PD-L1 positive and negative PECs with associated IHC results are shown in Figure 3A and 3B. In the 69 patients with a pathologically confirmed MPE, the median percent of PD-L1+ PECs was 6.9% (range 0–100%) (Figure 3C). 15 patients (22%) had greater than 50% PEC PD-L1 expression. 50 patients with a pathologically confirmed MPE had sufficient cytological tissue from the pleural effusion cellbock for IHC analysis. The concordance between the percent PD-L1 expression as determined by CellSearch® and tissue IHC was 76%.
Fig. 3.
Representative images of low and high PD-L1 expression as detected on PECs by CellSearch and IHC staining of pleural effusions. (A) Patient 022: CellSearch detected 11,404 PECs/mL, 1.4% of which expressed PD-L1. IHC staining determined the PD-L1 expression to be 0%. (B) Patient 017: CellSearch detected 1,762 PECs/mL, 94% of which were PD-L1 positive. >95% of cells stained PD-L1 positive by IHC. Concordance between CellSearch and IHC staining for PD-L1 expression was 76%. (C) PD-L1 expression in malignant effusions for the 69 patients confirmed malignant by pathology. The median value in the malignant group was 6.9%. Dotted horizontal line indicates that 15 out of 69 patients (22%) had 50% or more PD-L1+ PECs. Solid horizontal line indicates median value in panel.
3.5. Analysis of PEC Clusters
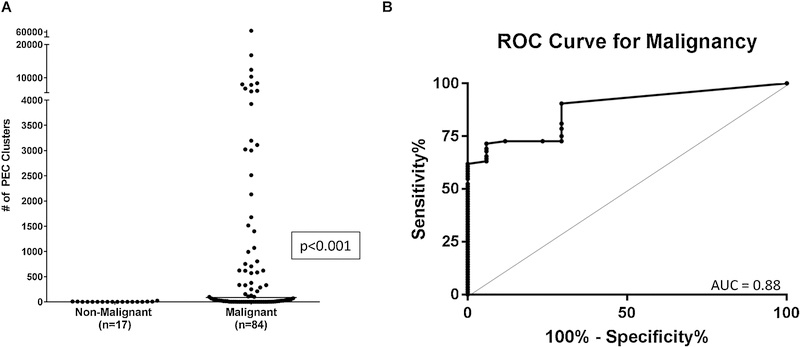
We next sought to determine the feasibility of measuring PEC clusters from pleural effusions. The number of PEC clusters was significantly higher in the MPE group (median 86 PEC Clusters/mL, range:0–63,935), when compared to the non-malignant group (median 0 PEC Clusters/mL, range:0–24) (p<0.001) (Figure 4A). The use of PEC clusters to discriminate a benign effusion from a MPE had an AUC of 0.88 (95% CI: 0.81–0.95) (Figure 4B).
Fig. 4.
Pleural effusion PEC cluster counts and Receiver Operator Characteristics Curve. (A) Total PEC cluster count/mL for malignant and non-malignant patients after clinical review. The median number of PEC clusters/mL in the malignant group was significantly higher than in the non-malignant group: 86 PEC clusters/mL (range: 0–63,935) and 0 PEC clusters/mL (range: 0–24) p<0.001. (B) Receiver Operator Characteristics curve (ROC curve) for PEC clusters in pleural fluid does discriminate between benign and malignant effusions: cutoff 28 PEC clusters/mL; sensitivity 62%, specificity 100%, AUC 0.88 (95% CI 0.81–0.95).
3.6. Survival Analysis
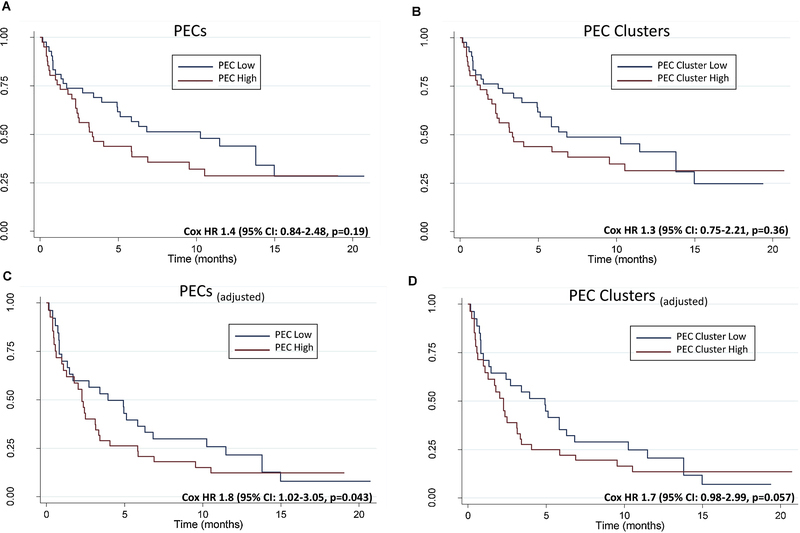
Given that the diagnosis of a MPE in patients with NSCLC confers a poor prognosis with a median survival of 4–6 months,4,45 we next sought to determine the association of the presence of PECs and PEC clusters with overall survival. For the 84 patients identified to have a MPE, the median overall survival from the diagnosis of a MPE was 6.3 months (95% CI: 3.4–11.5). In the univariate analysis, there was no significant difference in overall survival (OS) in the high PEC group (range: 731–114,920 PECs/mL) vs the low PEC group (range: 0–728 PECs/mL) (Cox HR 1.4, 95% CI: 0.84–2.48, p=0.19) (Figure 5A). High and low PECs were defined as those patients above or below the median PEC count of 730 PECs/ml. The median OS in the high PEC group was 3.3 months (95% CI: 2.26–9.54) vs 10.3 months (95% CI: 4.92–14.98) in the low PEC group. There was also no significant difference in OS in the high PEC cluster group (range: 100–63,935 PEC Clusters/mL) vs the low PEC cluster group (range: 0–71 PEC clusters/mL) (Cox HR 1.3, 95% CI: 0.75–2.21, p=0.36) (Figure 5B). We next performed a multivariate analysis including age, sex, smoking status, ECOG performance status, EGFR mutation status, high PECs, and high PEC clusters. Age, smoking status, and ECOG performance status were not significantly associated with OS and were removed from the final multivariate model (Supplemental Table 2). In the multivariate analysis, a higher number of PECs was associated with inferior OS (Cox adjusted HR 1.8, 95% CI: 1.02–3.05 p=0.043) (Figure 5C). The high PEC cluster group was not associated with inferior OS (Cox adjusted HR 1.7, 95% CI: 0.98–2.99 p=0.057) (Figure 5D).
Fig. 5.
Overall survival in patients with a MPE (n=84) between (A) the high and low PEC groups and (B) high and low PEC cluster groups, univariate analysis. High and low groups were defined by whether the number of PECs and PEC clusters were above or below the median values detected in the clinically confirmed MPEs. Median values in each group were: PECs =730 PECs/ml, PEC clusters= 86 PEC clusters/ml. Multivariate analysis of overall survival between (C) the high and low PEC groups and (D) high and low PEC clusters.
4. Discussion
Here we present evidence for the enumeration and characterization of pleural EpCAM-positive cells (PECs) from pleural effusions for the management of NSCLC patients. Malignant pleural effusions occur as a common complication of advanced malignancies, with lung cancer being the leading cause of a MPE.46 The accurate evaluation of a pleural effusion in lung cancer is critical because the presence of a MPE is diagnostic of stage IV disease, informs therapy selection, and denotes a poor overall prognosis.47 Cytological analysis of the pleural fluid remains the gold standard to diagnose a MPE. However, the sensitivity is often limited due to the scarcity of tumor cells, inherent difficulty in distinguishing cancer cells from reactive mesothelial cells or inflammatory cells, and the experience of the cytopathologist.9 Lung cancer patients with a pleural effusion are often subjected to repeated invasive procedures to ultimately diagnose a MPE. Several studies have evaluated various molecular markers in pleural fluid to improve the diagnostic accuracy of a MPE,10–12,28 however, none of these markers are currently routinely used in clinical practice.
We have previously reported on the ability of the CellSearch® platform to detect PECs in pleural effusions. In this previous study, we identified that the number of PECs recovered from pleural fluid in patients with epithelial malignancies was significantly greater than detected in blood with 90% of NSCLC patients having greater than 100 PECs detected.28 Here, we expand upon our previous work and demonstrate the clinical utility of the CellSearch® platform for evaluating pleural effusions of NSCLC patients. In this prospective study of 101 NSCLC patients, 84 patients were ultimately found to have a MPE. The median number of PECs detected was significantly greater in the malignant vs non-malignant group (730 PECs/mL vs 1 PEC/mL, p<0.001). The use of PECs to diagnose a MPE showed excellent discriminatory ability with an AUC of 0.91. The low level of PECs detected in the non-malignant group is likely secondary to the presence of contaminating benign epithelial cells and reactive mesothelial cells that may coexpress epithelial markers.48,49 A cutoff value of 105 PECs/mL had a sensitivity and specificity of 73% and 100%, respectively. Thus, a high number of PECs detected in a pleural fluid sample could alert the cytopathologist to perform further immunohistochemical stains or suggest that the patient undergo further invasive testing to ensure adequate staging of disease. A clinician may weigh the sensitivity and specificity of different threshold values of PECs/ml to help guide management decisions in NSCLC patients with a pleural effusion (Supplemental Table 1). Given the AUC of 0.91, this technology could be deployed in a clinical lab and utilized clinically in patients with a high number of PECs/ml to make the diagnosis of a MPE more definitive in the setting of diagnostic uncertainty. The potential clinical utility of this technology is highlighted by our case example in which Patient 067 had 853 PECs/mL detected from their initial diagnostic thoracentesis. The high number of PECs detected in the pleural fluid would suggest that this patient had a MPE and stage IV disease. In addition, the high number of PECs detected in only 1 mL of pleural fluid could be particularly useful in patients with a limited volume of pleural fluid available for analysis, both for the diagnosis of a MPE and for downstream genetic profiling to determine the presence of actionable genetic alterations. The CellSearch® platform has been used to identify CTCs in the cerebral spinal fluid of NSCLC patients with leptomeningeal disease with nextgeneration sequencing of CTCs demonstrating high concordance (89.5%) with the primary tumor.50
An additional advantage of our approach is the ability to non-invasively quantify biomarkers of interest such as checkpoint ligands and receptors. In our cohort, 15 of 69 patients (22%) with a pathologically confirmed MPE had greater than 50% PD-L1+ PECs. This rate of PD-L1 positivity is consistent with previous reports for NSCLC patients with greater than 50% PD-L1 expression by tissue IHC.34,51 The concordance between tissue IHC and CellSearch® for PD-L1 expression was 76%. The open 4th channel on the CellSearch® platform could also be utilized to detect other immune checkpoint receptors expressed on the tumor cell surface such as Galectin 9 and TIGIT ligand.52 The ability to rapidly immunophenotype the tumor cells from a MPE could assess candidacy for an immune checkpoint inhibitor or newer combination therapies or detect the emergence of resistance to an immune targeted agent. This technology may also be applied to other epithelial malignancies that frequently cause MPEs, such as breast cancer, to identify ER and HER2 positivity. This application has already been validated in peripheral blood samples of breast cancer patients.53
Our analysis demonstrates that higher numbers of PECs were independently associated with inferior overall survival. Similar to previous reports, the presence of an activating EGFR mutation was associated with improved overall survival.54–57 Higher numbers of PECs and PEC clusters detected in peripheral circulation have been associated with worse overall survival in a variety of malignancies.16,19–22 However, to our knowledge, this is the first study demonstrating the prognostic significance of the enumeration of PECs in MPEs. The impact on survival from higher numbers of PECs may be related to increased burden of disease, however this would need to be further validated. PEC clusters have been detected by CellSearch® and other rare cell enrichment approaches and are associated with inferior survival,21,25,58 However, while there was a trend toward worse survival for patients with high PEC clusters (Figure 5D) in our study, this relationship was not significant (p=0.057). This is perhaps due to differences in PEC clusters detected within a site of metastasis such as a pleural effusion compared to circulating in blood, along with the advanced level of disease exhibited by our patients and the low overall survival.
Limitations to our study include it being a single-center study with 101 NSCLC patients; other prospective, multi-center studies would be needed to validate our results. Given that CellSearch® is already FDA-approved for enumeration of CTCs from blood, such a large-scale validation study could lead to rapid clinical deployment of a pleural effusion-based test. A second limitation is that 49% of patients were receiving systemic therapy at the time of pleural effusion sampling, and this may impact levels of PD-L1 expression and PEC enumeration in our study. A third inherent limitation is the use of EpCAM as the capture antibody to detect PECs. EpCAM expression in NSCLC may range from 82–94% depending on the histologic subtype.59 Low level EpCAM expression may explain the 23 out of 84 patients with a MPE as determined by clinical review who, nevertheless, had a PEC count below the threshold of 105 PECs/ml (Figure 1C) To address this, a future direction would be to employ combinations of capture antibodies to epithelial cell markers such as MUC1 in addition to EpCAM to improve the yield of PEC detection.60 In addition, although EpCAM has been demonstrated to be similarly expressed in both adenocarcinoma and squamous cell carcinoma of the lung,61,62 given the limited number of patients with squamous cell lung cancer in our cohort, future studies would need to be performed to further validate our results in patients with this histology. Nevertheless, to our knowledge, this prospective study is the first of its kind to demonstrate the feasibility of using the CellSearch® CTC enrichment platform for the non-invasive enumeration and immunophenotyping of PECs in pleural fluid from NSCLC patients. The ability to identify rare tumor cells in a small volume of pleural fluid using an automated technology has the potential to be utilized as an adjunct to conventional cytological analysis to improve the diagnostic yield of a MPE. We demonstrate the feasibility of using this approach to determine PD-L1 expression on PECs from MPE, and a future application would determine whether PEC PD-L1 expression from MPE predicts response to immunotherapy. The high number of PECs detected in the MPE group with a median value of 730 PECs/mL of pleural fluid could also be used for downstream genetic profiling to detect the presence of actionable mutations, suggesting a high-throughput, automated platform for enumeration, immunophenotyping, and eventual molecular analysis of non-invasively obtained PECs.
5. Conclusion
In summary, we have utilized PEC enumeration to demonstrate the ability to discriminate benign from MPEs in patients with NSCLC. We demonstrate the feasibility to detect PD-L1 expression on PECs from MPE. Such immunophenotyping could potentially be utilized to determine candidacy for immunotherapy or assess markers of acquired resistance. The enumeration of PECs was independently associated with poor overall survival. Further studies are needed to validate the detection of PECs in MPE and explore their use as a diagnostic and prognostic marker.
Supplementary Material
Highlights.
First study to evaluate PEC enumeration and immunophenotyping in pleural fluid
Detection of PECs in pleural effusions may be utilized to diagnose a MPE
CellSearch® enables the non-invasive immunophenotyping of PECs from MPEs
First study to demonstrate the prognostic value of the enumeration of PECs in MPEs
Acknowledgments
Funding:
Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001879. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Supported, in part, by the Abramson Cancer Center Lung Cancer Translational Center of Excellence.
Footnotes
Conflicts of interest disclosure
In the interest of full transparency, the CellSearch® platform was provided to our laboratory through an agreement with Menarini Silicon Biosystems. Aside from that agreement, and on behalf of my co-authors and myself we would like to disclose that there are no potential conflicts of interest and all authors have read and approved the manuscript.
Appendix A. Supplementary data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin 68:7–30, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Massarelli E, Onn A, Marom EM, et al. : Vandetanib and indwelling pleural catheter for non-small-cell lung cancer with recurrent malignant pleural effusion. Clin Lung Cancer 15:379–86, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Panadero F, Borderas Naranjo F, Lopez Mejias J: Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 2:366–9, 1989 [PubMed] [Google Scholar]
- 4.Thomas JM, Musani AI: Malignant pleural effusions: a review. Clin Chest Med 34:459–71, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Nance KV, Shermer RW, Askin FB: Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol 4:320–4, 1991 [PubMed] [Google Scholar]
- 6.Garcia LW, Ducatman BS, Wang HH: The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol 7:665–8, 1994 [PubMed] [Google Scholar]
- 7.Arnold DT, De Fonseka D, Perry S, et al. : Investigating Unilateral Pleural Effusions: The role of cytology. Eur Respir J, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Hooper C, Lee YC, Maskell N: Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65 Suppl 2:ii4–17, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bedrossian CW: Diagnostic problems in serous effusions. Diagn Cytopathol 19:131–7, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Sriram KB, Relan V, Clarke BE, et al. : Pleural fluid cell-free DNA integrity index to identify cytologically negative malignant pleural effusions including mesotheliomas. BMC Cancer 12:428, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tse HT, Gossett DR, Moon YS, et al. : Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci Transl Med 5:212ra163, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Chang JH: Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, neuron-specific enolase, and cytokeratin 19 fragments in patients with effusions from primary lung cancer. Chest 128:2298–303, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Allard WJ, Matera J, Miller MC, et al. : Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10:6897–904, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Budd GT, Ellis MJ, et al. : Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781–91, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Cristofanilli M, Broglio KR, Guarneri V, et al. : Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer 7:471–9, 2007 [PubMed] [Google Scholar]
- 16.Hayes DF, Cristofanilli M, Budd GT, et al. : Circulating tumor cells at each followup time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12:4218–24, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Budd GT, Cristofanilli M, Ellis MJ, et al. : Circulating tumor cells versus imaging-predicting overall survival in metastatic breast cancer. Clin Cancer Res 12:6403–9, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Shaffer DR, Leversha MA, Danila DC, et al. : Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res 13:2023–9, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Cohen SJ, Punt CJ, Iannotti N, et al. : Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26:3213–21, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Danila DC, Heller G, Gignac GA, et al. : Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13:7053–8, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hou JM, Krebs MG, Lancashire L, et al. : Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 30:525–32, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Au SH, Edd J, Stoddard AE, et al. : Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci Rep 7:2433, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddipati R, Stanger BZ: Pancreatic Cancer Metastases Harbor Evidence of Polyclonality. Cancer Discov 5:1086–97, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung KJ, Padmanaban V, Silvestri V, et al. : Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A 113:E854–63, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aceto N, Bardia A, Miyamoto DT, et al. : Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158:1110–1122, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Au SH, Storey BD, Moore JC, et al. : Clusters of circulating tumor cells traverse capillary-sized vessels. Proc Natl Acad Sci U S A 113:4947–52, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascalchi M, Falchini M, Maddau C, et al. : Prevalence and number of circulating tumour cells and microemboli at diagnosis of advanced NSCLC. J Cancer Res Clin Oncol 142:195–200, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Schwed Lustgarten DE, Thompson J, Yu G, et al. : Use of circulating tumor cell technology (CELLSEARCH) for the diagnosis of malignant pleural effusions. Ann Am Thorac Soc 10:582–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Liu Q, Wang T, et al. : Circulating tumor cells in HER2-positive metastatic breast cancer patients: a valuable prognostic and predictive biomarker. BMC Cancer 13:202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punnoose EA, Atwal S, Liu W, et al. : Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 18:2391–401, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Ligthart ST, Bidard FC, Decraene C, et al. : Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann Oncol 24:1231–8, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Nicolazzo C, Raimondi C, Mancini M, et al. : Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci Rep 6:31726, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375:1823–1833, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–50, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Cyriac G, Gandhi L: Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langer CJ, Besse B, Gualberto A, et al. : The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol 28:5311–20, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Shigematsu H, Lin L, Takahashi T, et al. : Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339–46, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Lynch TJ, Bell DW, Sordella R, et al. : Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–39, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Tsai MF, Chang TH, Wu SG, et al. : EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci Rep 5:13574, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu SG, Gow CH, Yu CJ, et al. : Frequent epidermal growth factor receptor gene mutations in malignant pleural effusion of lung adenocarcinoma. Eur Respir J 32:924–30, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Soh J, Toyooka S, Aoe K, et al. : Usefulness of EGFR mutation screening in pleural fluid to predict the clinical outcome of gefitinib treated patients with lung cancer. Int J Cancer 119:2353–8, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Smits AJ, Kummer JA, Hinrichs JW, et al. : EGFR and KRAS mutations in lung carcinomas in the Dutch population: increased EGFR mutation frequency in malignant pleural effusion of lung adenocarcinoma. Cell Oncol (Dordr) 35:189–96, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porcel JM, Gasol A, Bielsa S, et al. : Clinical features and survival of lung cancer patients with pleural effusions. Respirology 20:654–9, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Roberts ME, Neville E, Berrisford RG, et al. : Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 65 Suppl 2:ii32–40, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Goldstraw P, Chansky K, Crowley J, et al. : The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 11:39–51, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Saleh HA, El-Fakharany M, Makki H, et al. : Differentiating reactive mesothelial cells from metastatic adenocarcinoma in serous effusions: the utility of immunocytochemical panel in the differential diagnosis. Diagn Cytopathol 37:324–32, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Davidson B, Nielsen S, Christensen J, et al. : The role of desmin and N-cadherin in effusion cytology: a comparative study using established markers of mesothelial and epithelial cells. Am J Surg Pathol 25:1405–12, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Jiang BY, Li YS, Guo WB, et al. : Detection of Driver and Resistance Mutations in Leptomeningeal Metastases of NSCLC by Next-Generation Sequencing of Cerebrospinal Fluid Circulating Tumor Cells. Clin Cancer Res 23:5480–5488, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Garon EB, Rizvi NA, Hui R, et al. : Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–28, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Burugu S, Dancsok AR, Nielsen TO: Emerging targets in cancer immunotherapy. Semin Cancer Biol, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Paoletti C, Muniz MC, Thomas DG, et al. : Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer. Clin Cancer Res 21:2487–98, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mok TS, Wu YL, Thongprasert S, et al. : Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–57, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Maemondo M, Inoue A, Kobayashi K, et al. : Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–8, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Kris MG, Johnson BE, Berry LD, et al. : Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311:1998–2006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin JJ, Cardarella S, Lydon CA, et al. : Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol 11:556–65, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mu Z, Wang C, Ye Z, et al. : Prospective assessment of the prognostic value of circulating tumor cells and their clusters in patients with advanced-stage breast cancer. Breast Cancer Res Treat 154:563–71, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Patriarca C, Macchi RM, Marschner AK, et al. : Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev 38:68–75, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Kubisch I, de Albuquerque A, Schuppan D, et al. : Prognostic Role of a Multimarker Analysis of Circulating Tumor Cells in Advanced Gastric and Gastroesophageal Adenocarcinomas. Oncology 89:294–303, 2015 [DOI] [PubMed] [Google Scholar]
- 61.Piyathilake CJ, Frost AR, Weiss H, et al. : The expression of Ep-CAM (17–1A) in squamous cell cancers of the lung. Hum Pathol 31:482–7, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Went P, Vasei M, Bubendorf L, et al. : Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer 94:128–35, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.