Abstract
Lung cancer mortality can be reduced by 20% via low dose CT lung cancer screening (LCS) and treatment of early-stage disease. Providing tobacco use treatment to high risk cigarette smokers in the LCS setting may result in health benefits beyond the impact of LCS. As one of the nine trials in the National Cancer Institute’s Smoking Cessation at Lung Examination (SCALE) collaboration, the goal of the Lung Screening, Tobacco, and Health (LSTH) trial is to develop a scalable and cost-effective cessation intervention for subsequent implementation by LCS programs. Guided by the RE-AIM Framework, the LSTH trial is a two-arm RCT (N = 1330) enrolling English- and Spanish-speaking smokers registered for LCS at one of seven collaborating sites. Participants are randomly assigned to Usual Care (UC; three proactive telephone counseling sessions/two weeks of nicotine patches) vs. Intensive Telephone Counseling (ITC; eight proactive sessions/eight weeks of nicotine patches, plus discussion of the LCS results to increase motivation to quit). Telephone counseling is provided by tobacco treatment specialists. To increase continuity of care, referring physicians are notified of participant enrollment and smoking status following the intervention. Outcomes include: 1) self-reported 7-day, 30-day, and sustained abstinence, and biochemically-verified at 3-, 6-, and 12-months post-randomization, 2) reach and engagement of the interventions, and 3) cost-effectiveness of the interventions. The Cancer Intervention and Surveillance Modeling Network (CISNET) will model long-term impacts of six SCALE trials on the cost per life year saved, quality-adjusted life years saved, lung cancer mortality reduction, and population mortality.
1. INTRODUCTION
Lung cancer mortality can be reduced by 20% via low dose CT screening and treatment of early stage disease [1]. If widely adopted, it is estimated that lung cancer screening (LCS) can prevent up to 12,000 lung cancer deaths annually in the U.S. [2]. The U.S. Preventive Services Task Force (USPSTF) [3] began recommending LCS in 2013 for the estimated 8 million individuals at high-risk for lung cancer, approximately one-half of whom are current cigarette smokers [2]. Maximum health impact from screening will only be achieved if high-risk smokers are able to stop smoking [4,5]. Consequently, the Centers for Medicare and Medicaid Services (CMS) mandate that cessation assistance be provided to all smokers undergoing screening [6]. While there are several known effective smoking cessation interventions, many of these are not feasible within diverse community-based LCS programs or with smokers who are not ready to stop smoking. The goal of the Lung Screening, Tobacco, and Health (LSTH) trial is to develop a scalable and cost-effective cessation intervention for implementation that will yield the greatest benefits with the lowest costs to both individuals and society. The LSTH study is part of the NCI’s Smoking Cessation at Lung Examination (SCALE) collaboration, a group of nine trials that aims to expand the evidence base for effective cessation interventions in the context of LCS [7].
Guided by the RE-AIM Framework [8], we have designed a randomized controlled trial (RCT) using the evidence-base of telephone counseling (TC) [9-11]. Our approach is designed to be at the intersection of scalability and intensity for future implementation within the national tobacco quitline. This trial builds on our prior studies in the lung screening setting [12-16] and tests the effectiveness and cost-effectiveness of intensive treatment (eight TC sessions with eight weeks of nicotine replacement therapy (NRT) and motivational discussions of the lung screening result) vs. usual care (three TC sessions with two weeks of NRT). To increase treatment continuity, we notify the referring physician at trial enrollment and again at the six-month follow-up with the participant’s reported smoking status.
Proactive TC provides the opportunity to leverage the increased motivation provided by the teachable moment [17] of the LCS setting. Importantly, TC also serves to counteract reduced motivation to quit that can occur following a normal result, accomplished by providing education about LCS as well as cessation strategies through a framework of motivational interviewing [18]. By including all smokers, regardless of readiness to quit, personalizing tobacco-related health risks, and providing an evidence-based cessation intervention that includes NRT, we expect that the intensive intervention will yield significantly higher quit rates than the usual care intervention, while also maintaining potential for widespread implementation. Further, two important aspects of our approach include cost-effectiveness analyses and microsimulation modeling. We will use the well-established University of Michigan Lung Cancer Intervention and Surveillance Modeling Network (CISNET) model to evaluate the impact of the interventions on short- and long-term smoking-related outcomes [3,19-21]. We are collaborating with six of the other SCALE trials in order to project the impact of different cessation interventions conducted within LCS programs on long-term costs, quality-adjusted life years, and lung cancer deaths averted.
2. METHODS
2.1. Study Aims
1) To compare the smoking outcomes between the Intensive Telephone Counseling (ITC) and Usual Care (UC) telephone counseling arms at 3-, 6- and 12-months. We hypothesize that the ITC arm will have significantly improved 7-day and 30-day point prevalence abstinence, as well as 6-month sustained abstinence compared to UC. We will measure potential mediators, including teachable moment factors (e.g., perceived risk based on screening results) and process measures (e.g., TC and nicotine replacement therapy (NRT) adherence, discussion of smoking habits with primary care physician). We will also assess potential moderators, including the lung screening result, readiness to quit, and nicotine dependence.
2) To evaluate the representativeness of the smokers enrolled in the trial, we will calculate reach (percentage of current smokers enrolled in the trial, out of those current smokers who were screened for lung cancer) and engagement (percentage who receive the interventions out of those screened for lung cancer), both overall and among subgroups (site, gender, age, nicotine dependence), as well as feasibility for implementation in both community-based and academically-based screening centers.
3) To conduct an economic evaluation to test the hypothesis that while costlier, ITC will be more effective than UC in the LCS setting, making it more cost-effective in terms of costs per quit and quit attempt at 3-, 6- and 12-months. We will use these results as inputs to the Lung CISNET model to project the long-term impact of the interventions on costs per life year saved, quality-adjusted life years saved, lung cancer deaths averted, lung cancer mortality reduction, and overall population mortality.
2.2. Conceptual Framework
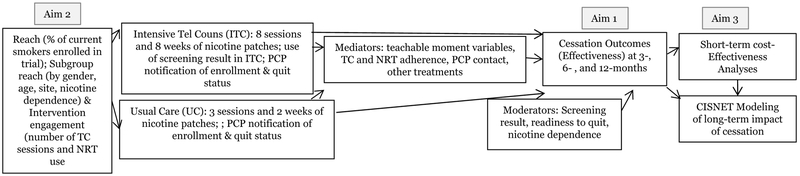
To guide our study design, we used the RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance) [8] framework, which was developed to increase the utilization of health promotion interventions in real-world settings. Figure 1 depicts the three study aims as they relate to the RE-AIM dimensions.
Figure 1.
Study Aims
Notes. Abbreviations include telephone counseling (TC), nicotine replacement therapy (NRT), intensive telephone counseling (ITC), usual care (UC), primary care physician (PCP)
The primary goals of the LSTH trial are to test the effectiveness and cost-effectiveness of interventions that can be adopted and implemented in new LCS settings through collaborations with state and national quitlines. We are maximizing reach by including all smokers, with a particular focus on those who are not ready to quit, as well as providing the intervention in both English and Spanish. We are measuring participant engagement with the interventions and the costs associated with intervention delivery to facilitate future implementation. Although we are not measuring the RE-AIM dimension of maintenance (i.e., the long-term collaboration between a LCS program and this cessation program) in the current trial, we are facilitating this aim via the cost-effectiveness analysis. Finally, we are conducting an analysis using the University of Michigan CISNET model [21] in order to project the costs and long-term morbidity and mortality of what can be expected if the interventions are broadly implemented in the US population.
2.3. Study Setting and Participants
This multisite RCT includes seven geographically-diverse LCS sites that serve a broad population of patients (see Table 1), and includes both community-based hospitals and academic medical centers. Each site has an ongoing screening program and is part of a major medical center, which includes a thoracic tumor board, radiation oncologists, medical oncologists, surgeons, pulmonologists, and palliative care clinicians, who provide diagnostic work-ups and treatment as needed. The Georgetown University Medical Center Oncology Institutional Review Board (IRB) oversees six of the seven LCS sites, with one site (Lahey Hospital and Medical Center) providing their own IRB review.
Table 1.
Descriptive Information of LCS Sites During Six Month Period Prior to Beginning LSTH Enrollment
| GUMCi | BHSFii | LHMCiii | HHiv | HUMCv | UPHTvi | MSMGvii | ||
|---|---|---|---|---|---|---|---|---|
| Inclusive Dates | 12/1/16-05/1/17 | 12/1/16-6/1/17 | 01/01/17-6/01/17 | 11/1/16-4/30/17 | 11/1/16-4/30/17 | 4/1/17-9/30/17 | 4/3/17-10/4/17 | |
| Current Smokers Screening Status n (%) | ||||||||
| New | 8 (66) | . | 212 (36) | 82(100) | 66 (80) | 119 (59) | 115 (100) | |
| Repeat | 4 (33) | . | 371 (64) | 0 | 17 (20) | 84 (41) | 0 | |
| Total | 12(100) | 96(100) | 583(100) | 82(100) | 83(100) | 203(100) | 115(100) | |
| Female Current Smokers n (%) | 5 (42) | 33 (34) | 263 (45) | 44 (54) | 33 (40) | 65 (55) | 42(37) | |
| Current Smokers Race n (%) | ||||||||
| Caucasian | 3(25) | . | . | 71(87) | . | 116 (97) | 101(88) | |
| Asian | 0 | . | . | 1(1) | . | . | . | |
| African American | 6(50) | . | . | 4(5) | . | 3(3) | 11(10) | |
| Other race | 3(25) | . | . | 6(7) | . | . | . | |
| Unknown/Not Reported | . | 96(100) | 583(100) | . | 83(100) | . | 3(.02) | |
| Total | 12(100) | 96(100) | 583(100) | 82(100) | 83(100) | 119(100) | 115(100) | |
| Current Smokers Ethnicity n (%) | ||||||||
| Hispanic Origin | 1(8) | 47(49) | . | 9(11) | . | 0 | 0 | |
| Unknown/Not Reported | . | . | 583(100) | . | 83(100) | . | 10(11.5) | |
| Current Smokers: Average Pack Years | ||||||||
| Pack Yrs | 30 | 43 | 46.6 | 42 | 48 | 53 | 35 | |
| Payer Mix of Screening Participants (%) | ||||||||
| Medicare | 27 | 50 | 33 | 35 | 41 | 50 | 50 | |
| Medicaid | 6 | 0 | 10 | 13 | 1 | 20 | 15 | |
| Private Insurance | 55 | 45 | 57 | 52 | 58 | 30 | 35 | |
| No Insurance | 12 | 5 | 0 | 0 | 0 | 0 | 0 | |
Notes.
GUMC: Georgetown University Medical Center, Washington, DC
BHSF: Baptist Health South Florida, Miami, FL
LHMC: Lahey Hospital and Medical Center, Burlington, MA
HH: Hartford HealthCare, Hartford, CT
HUMC: Hackensack University Medical Center, Hackensack, NJ
UPHT: UnityPoint Health Trinity, Moline, IL
MSMG: MedStar Shah Medical Group, Hollywood, MD
We expect that the enrollment duration will extend from May 2017 to June 2020, and that the final follow-up assessment will be completed in July 2021. In an effort to enroll all participants at our collaborating LCS sites, we used the broadest inclusion criteria available, which are the National Comprehensive Cancer Network (NCCN) lung cancer screening guidelines [22]. Both NCCN Group 1 and Group 2 patients are included: (a) 50–80 years of age and (b) 20+ pack-year smoking history. Further, eligible participants must be (c) registered for LCS at one of our seven LCS sites, (d) smoking cigarettes (or cigarillos or little cigars) within the past seven days regardless of number smoked per day; (e) English- or Spanish-speaking; and (f) accrued prior to LCS. The exclusion criteria are (a) prior or current diagnosis of lung cancer and (b) non-English or non-Spanish speaker. Individuals are not excluded due to prior LCS, prior or current cessation treatment, psychiatric conditions, and do not need to be ready to quit.
Based on our prior studies in the LCS setting, we project a 45% participation rate, 95% completion of the CT screening exam, and a 5%−20% loss at each follow-up assessment [16]. Also, based on the current trends in the demographic characteristics of individuals undergoing LCS [23], as well as what is typically found in new cancer screening modalities, we expect that the majority of participants will be insured, white, and non-Hispanic. This will be a limitation of all studies conducted within LCS settings until screening becomes more widely integrated and promoted in clinical settings.
2.4. Assessment Procedures
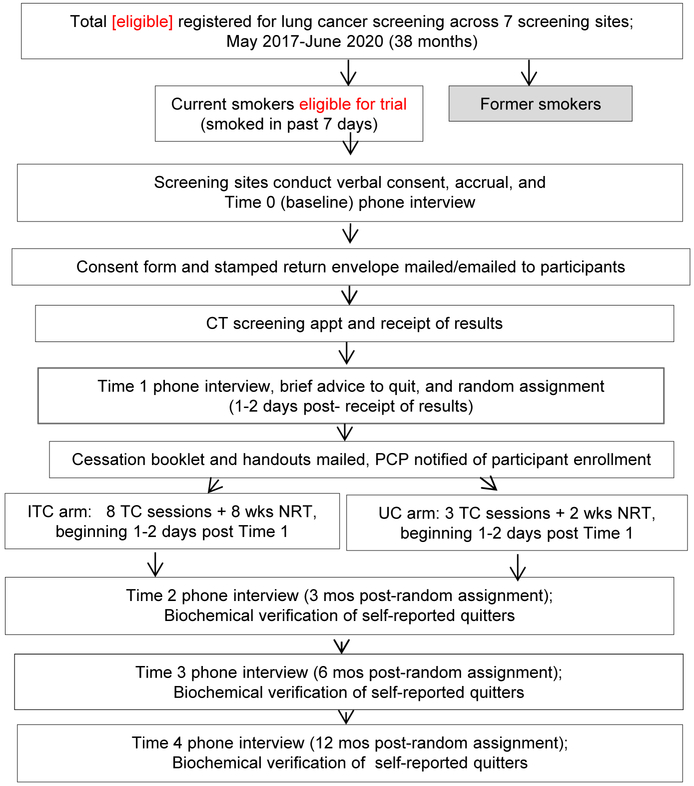
2.4.1. Accrual, consent, and the baseline (T0) interview (Figure 2).
Figure 2.
Study Flow Chart
Following a physician referral, individuals call the LCS site to schedule their screening appointment. At four of the LCS sites, the scheduler then describes the study, seeks verbal consent, and conducts the baseline interview with those interested in participating. At the other three LCS sites, this is done by the site coordinator during a subsequent call with those willing to hear more about the study. As these procedures are conducted by the staff from each LCS site, the goal is for all eligible individuals to be approached for the study. We conduct enrollment by phone so that the baseline assessment of current smoking status and readiness to quit can be completed prior to undergoing screening.
The LCS site coordinators make up to ten call attempts to reach potential participants to describe the study, obtain verbal consent, and complete the 15-minute baseline (T0) interview. The goal is to reach potential participants within one to three days of the screening registration, although in many cases, the screening appointment is not scheduled for several weeks, providing ample time for study enrollment. Responses to all interviews are entered directly into the web-based Research Electronic Data Capture (REDCap) [24] database by the coordinator while speaking to the participant. As part of the consent process, participants understand that follow-up assessments and TC are conducted by Georgetown staff.
Following completion of a baseline interview, Georgetown staff access new participants in the REDCap database each weekday and mail a thank-you letter with the study description, the consent and HIPAA forms, and a stamped return envelope. For regular email users, we send these documents via secure email for return with an electronic signature. Individuals who do not return a consent form after ten reminder calls are ineligible to participate in the intervention. However, based on their verbal consent, these participants are contacted for the follow-up interviews and biochemical verification.
2.4.2. Lung cancer screening procedures.
All LCS sites use the LungRADS system [25]. Approximately one-week post-screening, participants receive the screening results via a phone call, in-person consultation with a nurse or physician, or letter. The referring physician receives the results via e-mail or fax. Referring physicians call those with results suspicious for lung cancer to discuss recommended follow-up procedures. Based on the experience of the LCS sites, we expect approximately 10–15% of results to require follow-up of some form, but only ~1% to have a lung cancer diagnosis. We offer TC to those diagnosed with lung cancer, but we do not conduct follow-up assessments with them as they are no longer eligible for the trial.
2.4.3. Post-screening (T1) interview.
One to two days after participants receive their screening results, Georgetown tobacco treatment specialists (TTS) call to complete the 20-minute T1 phone interview, using the same 10 call protocol. The interviewer asks all participants to self-report their screening results. Once participants return the signed HIPAA form, the LCS sites release the LungRADS scores to Georgetown via the REDCap database.
At the conclusion of the T1 interview, the TTS provides brief advice to quit, including presentation of the health risks of continued smoking, as well as the health benefits of quitting. The TTS provides both groups with encouragement to engage in the TC and to use the NRT patches, and to try to quit if they become ready. For continuity, the T1 interview and the counseling sessions (described below) are conducted by the same TTS.
2.4.4. Randomization.
At the end of the T1 interview, subjects are randomized in blocks of size four and stratified by LCS site, readiness to quit (next 30 days vs. next six months vs. not considering quitting), LCS result (LungRADS 1/2 vs. 3/4), and language (English vs. Spanish). Georgetown coordinators conduct randomization using a password-protected Microsoft Access program, inform participants of their study arm, and describe the next steps of the protocol. Participants who report having quit smoking for ≥ 8 days at the T1 interview are not randomized and excluded from the trial. However, we conduct the follow-up interviews to track the long-term quit rates in the absence of the cessation intervention.
2.4.5. Overview of follow-up interviews.
Georgetown staff members conduct the 20-minute telephone interviews at 3-, 6-, and 12-months post-randomization. In accordance with the Society for Research on Nicotine and Tobacco guidelines for interventions that include smokers not ready to quit, the timing of the follow-up interviews is based on randomization date [26]. Participants receive a $15 gift card following completion of each follow-up interview. The telephone interviewers are blinded to study arm. Counselors do not conduct assessments with participants for whom they were the telephone counselor. To keep interviewers blinded to study arm, questions about satisfaction with the intervention occur at the end of the interview, following the assessment of smoking status.
2.4.6. Biochemical Verification Procedures.
Within two weeks of self-reported abstinence at the 3-, 6-, and 12-month follow-up assessments, participants are asked to complete biochemical verification using either 1) a NicAlert saliva strip, mailed to their home with instructions to complete and return by mail, or 2) a carbon monoxide (CO) test for persons using NRT or an electronic nicotine delivery device. Participants provide a breath sample using a CO monitor at the LCS site. The standard abstinence cutoffs are: <1 for NicAlert and ≤ 6ppm for CO. The equivalence between NicAlert and CO has been demonstrated [27-28]. We cover parking costs as needed and provide a $25 gift card for completing verification at each assessment.
2.5. Intervention Procedures
2.5.1. Overview of the telephone counseling interventions.
Proactive TC, defined as the counselor calling the participant, is a well-suited cessation intervention in the LCS setting. Its effectiveness has been demonstrated with older smokers [9, 29-31], with smokers who are not ready to quit [32-34] and with non-treatment seeking smokers [11,34]. It is also intensive enough to fully assist those who are ready to quit [10,11,35-37] by providing tailored support for cessation. TC is a relatively low-cost method of reaching all LCS participants [38].
We considered four key issues when selecting the intervention components. We sought interventions with data supporting: 1) the effectiveness among older, chronic, and addicted smokers, 2) the feasibility of implementation in LCS settings, 3) the ability to reach large numbers of screening participants, and 4) the likelihood that LCS participants would engage in the intervention.
The ITC protocol is based on the Project Ascend protocol [39]. We modified it for use with older, chronic smokers, many of whom are not seeking tobacco treatment. A more intensive intervention than some other TC interventions [11] is justified by the dose-response relationship between quitting and number of sessions in non-treatment seeking smokers [34]. Further, our pilot data showed that a more intensive intervention was useful for those who were not ready to quit at baseline [13, 16]. Thus, readiness to quit is one of the potential moderating factors we will assess.
The UC arm also utilizes the content of the Project Ascend protocol, but with regard to level of intensity, the UC arm was designed to represent what is currently offered in state quitlines. For example, in FY 17, an average of two calls per person was provided and 44% of quitlines offered 2-weeks of the nicotine patch (and 56% offered 4 weeks or more) [40].
We considered beginning TC prior to LCS, to leverage the health concerns that led to choosing to undergo lung screening. However, this would introduce a varying number of calls pre- and post-screening, depending on each site’s scheduling practices. As we expect that the LCS result may impact motivation to quit, we determined that the intervention should start following the LCS exam, in order to capitalize on the receipt of the screening result.
2.5.2. Procedures common to both intervention arms.
Following randomization, we mail a packet (in English or Spanish) containing materials used during the counseling sessions: 1) the NCI’s “Clear Horizons” booklet for older smokers (for English speakers) or the American Lung Association’s “Freedom From Smoking en Español: La guía para ayudarle a dejar de fumar” (for Spanish speakers), 2) a cigarette tracker, 3) a decision-making activity sheet [41], and 4) a worksheet we developed to assist participants in thinking through physical, behavioral, and motivational changes needed for quitting or reducing. For regular email users, resources are sent via email. All materials are also available on the LSTH website (https://lunghealth.georgetown.edu) in both English and Spanish.
TC is comprised of empirically-validated cessation techniques as identified in the Public Health Service guidelines [9] and recent meta-analyses [11,42]. A central component is motivational interviewing (MI) [43], a strategy that emphasizes individual responsibility for change, enhances self-efficacy, minimizes resistance by building on natural ambivalence, and provides assistance for setting goals for change. MI is most appropriate for non-treatment seeking smokers who may not be ready to quit. There is strong empirical support for the utility of MI within brief interventions and TC for both English and Spanish speakers [44-48].
Prior to the first TC session, the TTS reviews the participant’s smoking history and goals for cessation collected at the T0 and T1 assessments. Core intervention components implemented during each TC call include an assessment of readiness to quit, confidence in and importance of quitting, a discussion of smoking-related goals, discussion of the nicotine patch, ways of dealing with triggers and urges associated with smoking, and encouragement to use the mailed cessation materials. These elements are approached using MI techniques with a focus on open-ended questions and reflections, while cultivating a non-judgmental, supportive, and collaborative atmosphere. Participants are not asked to set a quit date until they indicate being ready. Those who quit during the intervention are encouraged to continue engaging in the calls which focus on relapse prevention.
2.5.2.1. NRT procedures.
Participants in both arms are offered free NRT (NicoDerm CQ 21mg, 14mg, and 7mg patches). NRT is express mailed in four two-week batches (ITC) or one two-week batch (UC) after the first TC session is completed. For ITC participants, subsequent batches are mailed only after additional TC calls are completed to encourage continued engagement with TC and to reduce costs for non-use. The TTS confirms that the first two-week batch is being used, assesses dosage, and ships the second two-week batch so that it arrives prior to completion of the first batch. This is a standard approach used by quitlines [49].
For participants planning to use the nicotine patch, the TTS emphasizes the importance of using a patch beginning on the predetermined quit day. This encourages setting a quit date or a 24-hour ‘practice’ quit date to learn where difficulties may lie and what additional behavioral changes may be needed to prepare for an actual quit date. If slips occur during patch use, participants are encouraged to remove the patch if more than five cigarettes are smoked.
For participants not interested in using the nicotine patches, the TTS discusses additional pharmacological aids for cessation and encourages participants to discuss these options with their primary care physician.
2.5.2.2. Letters to Referring Physicians.
Prior to starting recruitment at each LCS site, we mail a letter describing the study to the physicians who have previously referred patients to that LCS program. The letter is intended to raise awareness about the study and to promote additional referrals to the LCS program. We also send copies of a study recruitment flyer that physicians can give to eligible patients.
When a new participant enrolls in the LSTH trial, we send a personalized letter to inform the referring physician that the patient has enrolled. After the six-month follow-up (T3), we send a second letter to inform the physician of the participant’s smoking status (or if the participant has been lost to follow-up), whether the participant engaged in TC, as well as his/her use of study-provided NRT. The letter also reminds physicians to use the 5 As [9], to discuss cessation at each visit, to consider Chantix or Zyban when clinically appropriate, and to provide support for relapse prevention.
2.5.3. Procedures specific to each arm
2.5.3.1. UC arm.
The UC arm includes three 20-minute counseling sessions and one 2-week supply of NRT patches. Calls are scheduled according to participant preference, but must be completed within three months post-randomization. Counselors make a maximum of three follow-up attempts (with voicemail messages) for each of the three sessions. Participants are considered unreachable if they do not return any calls. However, sessions are resumed if a participant calls back prior to three months post-randomization. Further, TTS do not facilitate discussion of screening results in the UC arm.
2.5.3.2. ITC arm.
The ITC arm includes eight 20-minute counseling sessions and up to four 2-week boxes of NRT patches. We included eight sessions based on evidence from our pilot study, which suggested that for those who began TC not ready to quit and subsequently became ready to quit during the course of the calls, six sessions was not sufficient to discuss relapse-prevention strategies [16].
A maximum of six follow-up attempts (with voicemail messages) are made for each session. As noted above, participants who do not return calls are considered unreachable unless they attempt to reach the counselor. Calls are scheduled at the participant’s convenience, but all calls must be completed within three months post-randomization. Unlike the UC arm, the TTS initiates discussion of the LCS results and, if applicable, of subsequent follow-up procedures, as motivators to quit.
The rationale for reaching participants shortly following the T1 interview is to allow the TTS to capitalize on the impact of the screening result as a potential “teachable moment” [17]. The counselor addresses the potential impact of undergoing screening and the screening result during each of the first three sessions, and only after that if it is particularly relevant to a participant or if the participant brings it up. Discussion of the screening result is framed as a primary motivator to quit, including 1) emphasis on the importance of quitting following any abnormal finding, and 2) encouragement to use the lung screening event as an opportunity to quit, to reduce future health risks, including lung cancer and other tobacco-related diseases, and 3) to maximize quality of life. For those with a normal result or whose abnormal result is ultimately considered normal after a work-up, the TTS assesses thoughts that reflect minimization of the need to quit (e.g., ‘this result means I can continue smoking’ or ‘this result is a license to smoke’ [15]), discusses converting the original motivation for undergoing screening into motivation to quit, and discusses the importance of using the screening as an opportunity to quit for disease prevention. Providing education that a normal result is not a permanent ‘clean bill of health,’ that quitting can increase the possibility of having a normal result again next year, that older adults who quit can add years to their life [50], each serve to challenge the potential for minimization of the consequences of smoking. As guided by the teachable moment model [17] we expect that discussion of the screening result as a motivator will increase risk perceptions and emotional reactions to the result, and challenge one’s self-concept as a smoker, which may ultimately translate to behavior change.
2.6. Collaboration with LCS Sites.
The LCS site coordinators are trained by Georgetown project coordinators using ZOOM, a HIPAA-compliant web and teleconferencing platform [51]. The training focuses on the methods used to recruit patients, including data entry in REDCap, roleplays to conduct baseline interviews, and data tracking in Microsoft Excel to manage information about eligible participants. A detailed site protocol manual, which includes telephone scripts, a comprehensive study description, the verbal consent process, and the baseline interview protocol, was developed and shared with all sites to provide consistent information and training for site coordinators. The information is available on a secure website that is accessible to all of the LCS sites and is updated as needed. Additionally, the use of these online resources facilitates consistent training in the case of staff turnover.
The Georgetown staff and PI have monthly phone meetings with each LCS site to ensure adherence to the study protocol, discuss participant accrual progress, and brainstorm methods to maximize enrollment and minimize dropout. We also e-mail weekly accrual reports to communicate recruitment rates. Georgetown project coordinators and the LCS sites use e-mail to clarify data entered into the REDCap system. Finally, approximately every four months, the Georgetown team hosts a teleconference with all of the LCS sites to communicate information that applies to the entire study. This also allows the sites to share information with each other and provide updates on participant accrual.
2.7. Training and Supervision of the Tobacco Treatment Specialists
All telephone counselors received training at one of the Association for the Treatment of Tobacco Use and Dependence (ATTUD) accredited week-long programs for TTS. Prior to counseling trial participants, each counselor performs roleplays with experienced staff members, our patient advocate (a former smoker who quit while participating in our LCS pilot study), and doctoral-level investigators (clinical psychologists). The TTS also counsels two pilot participants, completing the full UC and ITC protocols. These calls with pilot participants are recorded and feedback is provided on protocol adherence and MI skills.
Twice per year, our study consultant, a member of the Motivational Interviewing Network of Trainers (MINT), provides a full-day training for the TTSs and supervisors. Both didactics and roleplaying are used to demonstrate central constructs of MI, including the use of open-ended questions and reflections, cultivating change talk, softening sustain talk, providing a collaborative atmosphere, and expressing a deep understanding of the participant’s perspective. TTSs receive monthly individual supervision from our MINT consultants (both English- and Spanish-speaking), which includes detailed feedback using the Motivational Interviewing Treatment Integrity (MITI) protocol on a recorded counseling call to ensure compliance with MI techniques. Finally, the team has a monthly meeting with the MINT trainer to cover general MI techniques that are applicable to recent sessions conducted by the TTSs, using roleplays and discussion to improve the counselors’ MI skills.
All of the counseling sessions are recorded for quality assurance. Weekly supervision (individual and group) is provided by two clinical psychologists for each counselor. Supervision of the Spanish-language TTS is conducted in Spanish by both the project director and a MINT trainer. Each supervision meeting involves listening to an audio recording of one session per counselor. Supervisors (and other counselors during the group meetings) provide feedback on protocol adherence and MI techniques. We use fidelity coding forms (described below) to assess protocol adherence and to discuss areas requiring improvement.
Assessment of Intervention Fidelity.
We will assess intervention fidelity by recording and coding a random selection of 10% of the counseling calls [52] selected from each six month period of data collection, to insure inclusion of calls conducted in the early and later periods of the trial. We will select 10% of the calls from each of the following three groups: sessions 1–3 in the UC arm, sessions 1–3 in the ITC arm, and sessions 4–8 in the ITC arm. All sessions are audio-recorded using ZOOM.
Using a coding manual, two investigators will train three coders to conduct the fidelity ratings, using audio recordings from pilot participants. Inter-rater reliability will be calculated for 20% of the coded calls. We will hold regular meetings and come to consensus on disagreements. The coders will periodically code with one of the investigators to assess for drift from the fidelity coding protocol. Coders will be blinded to study arm and session number.
Fidelity coding will include an assessment of the core topics to be covered during each call, including an assessment of current smoking status and readiness to quit (three items); a discussion of behavioral strategies for reducing or quitting smoking (five items), assessment of NRT use or NRT adherence for those using the patch (one item), use of MI techniques (five items), discussion of the print materials, and limiting the session to 20 minutes. In the ITC arm, a discussion of the LCS result and its impact on readiness to quit is required in the first three sessions (two items). Relapse prevention items are coded for sessions in which the participant has stopped smoking (four items).
2.8. Measures (Table 2).
Table 2.
Summary of Measures and Assessment Points
| LCS Site and Electronic Medical Record |
Baseline Interview (T0) |
Post- Screening Interview (T1) |
Counseling Intervention (ITC and UC) |
3,6,&12 Month Assessments (T2, T3, T4) |
|
|---|---|---|---|---|---|
| Demographic & Clinical Information | |||||
| Age, gender, language, past history of cancer, height, weight, date of birth, health insurance [53,54] | x | ||||
| Race and ethnicity [53,54] | x | x | |||
| Marital status and education [53,54] | x | ||||
| Tobacco Related Comorbid Illnesses [1,53,54] | x | x | |||
| Family History of Lung Cancer [1,53,54] | x | ||||
| Lung Cancer Screening | |||||
| CT scan results (self-reported & EMR) [53,54,25] | x | x | x | ||
| Impact of screening results on attitudes/feelings about smoking | x | x | |||
| Recommended follow-up procedures (self-reported & EMR) [53,54] | x | x | x | ||
| Diagnostic work-up procedures [53,54] | x | ||||
| Final diagnosis: lung cancer, other cancer [53,54] | x | ||||
| Smoking & Cessation History | |||||
| Current tobacco use: cigarettes per day [53,54,55] | x | x | x | x | |
| Other tobacco use (pipes, cigars, smokeless, e-cigarettes) [53,54,55] | x | x | x | ||
| Cessation Methods (previous, current, future interest) [15] | x | x | |||
| Nicotine Dependence [53,54,56] | x | x | x | ||
| Pack years [53,54] | x | ||||
| Smoking/Tobacco Outcomes | |||||
| Readiness to quit [53,54,57] | x | x | x | x | |
| Confidence & motivation to quit [53,54,58] | x | x | x | x | |
| 24 hour quit attempts [53,54] | x | x | x | ||
| Self-reported 7-day, 30-day, and sustained abstinence [26,53,54,59] | x | x | x | ||
| Past 30 day smoking frequency (some days, every day, not at all) [53,54] | x | x | x | ||
| Biochemical verification: NicAlert saliva test, Expired CO [26,53,54,59] | x | ||||
| Mental Health & Substance Use | |||||
| Alcohol use [60] | x | ||||
| Marijuana use [61] | x | ||||
| Quality of life [53,54,62] | x | x | x | ||
| Psychological distress [53,54,63] | x | x | x | ||
| Distress thermometer [53,54,62] | x | x | x | ||
| Perceived Risk for Lung Cancer | |||||
| Perceptions of smoking and quitting on risk of being diagnosed with lung cancer [15,53,54] | x | x | x | ||
| Treatment Utilization and Satisfaction Outcomes | |||||
| Use of and satisfaction with telephone counseling and nicotine patches [53,54] | x | ||||
| Self-report of discussion of smoking/cessation methods with PCP [64] | x | ||||
| Amount of nicotine mailed and number of TC calls completed | x | ||||
Table 2 describes each of the measures included in the baseline and follow-up telephone assessments, the process data regarding the intervention delivery, and medical record data provided by the LCS sites. We have indicated the measures that are in common with the SCALE collaborators.
2.9. Economic data collection.
Trial costs include fixed costs (space and overhead) and variable costs (intervention delivery and participation). We will use our established methods [65-67] to collect data from project staff on time spent on intervention delivery and the participants’ time spent undergoing the intervention. We will use average US costs to micro-cost resource units to enhance generalizability for dissemination.
We will consider the following study outcomes at 3-, 6-, and 12-months: 1) the average cost per arm per participant for biochemically verified abstinence; and 2) the average cost per improved readiness to quit and quit attempts. Cost units will be assigned dollar values using several sources, such as the average US gender-specific wage rates from the Current Population Survey of the Bureau of Labor Statistics, national data for overhead, and Redbook costs for pharmacotherapy. Longer-term costs for the Lung CISNET modeling (e.g., lung screening, diagnostic evaluations, treatment of lung cancer) will be based on Medicare allowable charges and SEER-Medicare cost data. Medicare claims data do not provide detailed information on smoking status, therefore, to estimate costs of non-lung cancer care among smokers, we will conduct additional analyses among non-cancer controls who have received intensive cessation counseling using HCPCS/CPT codes. We will vary the costs of lung cancer and non-lung cancer care among smokers to test the net impact of different approaches to estimating costs.
3. ANALYSES
3.1. Overview.
We have identified the primary and secondary outcomes and performed power calculations based on the primary outcomes for Aims 1 and 2. We will perform the analyses based on the intent-to-treat principle. The Aim 1 analyses will focus on the primary outcome of biochemically-verified abstinence (7-day and 30-day point prevalence) at the 12-month assessment. Secondary outcomes for Aim 1 will use a similar approach to evaluate the outcomes of readiness to quit and 24-hour quit attempts at 3-, 6-, and 12-months. All statistical models that compare study arms will control for site, baseline readiness to quit, screening result, language, and baseline nicotine dependence.
3.2. Sample size and power analysis.
We anticipate accruing 1330 and randomizing 1200 participants, 600 per arm. All power calculations were performed for the primary outcome of biochemically-verified abstinence at 12-months using conservative effect size estimates.
At a significance level of 0.05, we will have about 80% power to detect abstinence differences of 21% (ITC) and 14% (UC) at 12-months. These conservative abstinence rates are based on our pilot study, related studies [68-69] and a meta-analysis involving 43 studies for higher- vs. lower-intensity counseling [9].
Aim 1:
Mediators include teachable-moment factors (e.g., perceived risk for lung cancer) and process measures (e.g., adherence to TC and NRT, contact with referring physician, use of other pharmacotherapy). At a significance level of 0.05, using the product of coefficients approach [71], we will have >80% power to detect mediation effects of small size (with coefficient parameters of 0.14) for each mediator of interest.
Aim 1:
Moderators include the screening result, readiness to quit, and nicotine dependence. At a significance level of 0.05, we will have 80% power to detect interaction ORs of 2.2–2.4, corresponding to the interaction between study arm and the hypothesized binary moderators, assuming ORs ranging between 1.5 and 2.0 for the associations between study arm and abstinence at 12 months, and the associations between each moderator and abstinence at 12 months [70]. The power calculation was performed assuming a logistic regression model that includes the study arm, a binary moderator, and their interaction, and it was based on the Wald test for the coefficient of the interaction term [70].
Aim 2:
Assuming 45% participation, at a significance level of 0.05, we will have 80% power to detect differences in reach of 6% between subgroups (gender, age, nicotine dependence, and site).
3.3. Data monitoring and missing data.
Given our randomized pilot study [16] and our data collection procedures, we expect to have a negligible amount of missing data collected by telephone interview. However, we expect to have participant dropout by the follow-up assessments and we will compare the baseline characteristics of those with and without missing data. All participants who are missing outcome data at 3-, 6-, and 12-months will be assumed to be continued smokers. In sensitivity analyses, we will handle missing data using multiple imputation methods. We will generate 10 multiple imputed datasets, analyze each separately, and then combine results across the 10 datasets.
3.4. Statistical analyses.
3.4.1. Overview of statistical analyses.
We will calculate descriptive statistics and perform bivariate analyses using t-tests (or Wilcoxon rank sum tests), chi-square tests (or Fisher’s exact tests), and Pearson (or Spearman) correlation coefficients to describe the associations of baseline factors (demographics, smoking history, LCS history, etc.) with the potential moderators, mediators and outcomes at 3-, 6-, and 12-months. In what follows, we are providing details only about the binary outcomes of interest. A similar approach, employing Poisson regression models instead of logistic regression models, will be used for the secondary outcomes of interest that are count variables, e.g., the number of quit attempts at 3-, 6-, and 12-months.
3.4.2. Aim 1 analyses.
H.1.1.
We expect that the ITC arm will result in improved cessation outcomes relative to the UC arm. We will use mixed effects logistic regression models to compare the study arms on the primary outcomes, which include biochemically verified 7-day and 30-day point prevalence abstinence, and six month sustained abstinence. Secondary outcomes include the binary outcome of change in readiness to quit and the occurrence of at least one 24 hour quit attempt. These models will include participant as a random effect and the following fixed effects: study arm, time (3-, 6-, or 12-months), study arm by time interaction, and the covariates (site, readiness to quit, screening result, language, and nicotine dependence). We will calculate adjusted ORs and 95% confidence intervals (CIs) for the associations between study arm and the binary outcomes of interest.
We will conduct sensitivity analyses to compare the cessation rates of the site with the largest number of participants to all of the other sites combined to determine if one site is having an undue influence on the findings. Further, we will assess for the presence of site differences on demographic and tobacco-related characteristics to determine whether these variables may play a role in the differences in cessation rates across sites if those differences are present.
H.1.2
In separate analyses, we will assess whether those with a normal result, those less ready to quit, and those with higher baseline nicotine dependence will have a significantly higher quit rate in the ITC vs. the UC arm, whereas intervention arm will have less of an impact among their respective counterparts. For the moderation analyses, we will expand the previous models to include the 3-way interaction between study arm, time, and the moderator, and the two 2-way interactions between study arm and the moderator, and between time and the moderator, respectively. If interaction terms are significant (p<.05), we will report stratum-specific ORs and 95% CIs for the association between study arm and outcome at 3-, 6-, or 12-months. We will also explore the role of age and gender as moderators using similar methods.
H.1.3.
We expect the effect of the interventions to be mediated by greater perceived risk for lung cancer, greater adherence to the intervention, and subsequent discussions about cessation with the referring physician. For the mediation analysis, we will use the product of coefficients method [59] to evaluate the hypothesized mediators. We will first use separate regression models to estimate the coefficients relating the study arm to each of the hypothesized mediators at 6-months, treated as outcomes at this first step. We will use linear regression models for the perceived risk for lung cancer and NRT adherence, a Poisson regression model for TC sessions completed, and logistic regression models for having had referring physician contact re cessation, and the use of other pharmacotherapy. For the second step, we will use logistic regression models to estimate the standardized coefficients relating each one of these mediating variables at 6-months to the binary outcomes at 12-months. We will calculate each mediated effect as the corresponding product of these coefficients, with corresponding 95% CIs estimated according to the methods from [59].
3.4.3. Aim 2 analyses.
The purpose of Aim 2 is to assess the representativeness of the smokers enrolled in the trial, relative to the smokers who undergo LCS. To assess reach (the absolute number, proportion, and representativeness of individuals who enroll) and engagement (percentage who receive the interventions), we will calculate descriptive statistics and perform tests to compare reach proportions between subgroups (gender, age, nicotine dependence, and site). We will explore reach and engagement among Hispanic and non-white participants. Engagement will be defined in several ways (e.g., the percentage who receive > 2 TC sessions, percentage who receive > 4 sessions, percentage who use >2 weeks of NRT). We will also assess the implementation endpoints of TC intervention fidelity and the ease of delivery (effects on clinic workflow). Finally, we will assess the impact of the interventions (defined as the product of reach and effectiveness).
3.4.4. Aim 3 analyses.
These analyses will use micro-costing approaches and follow standard recommendations [72]. Our cost-effectiveness analyses will have two components. The first is a short-term analysis of the costs of the trial interventions per unit outcome, such as costs per study arm per 3-, 6-, or 12-month cessation. The second portion of the economic analysis will leverage the University of Michigan CISNET lung cancer model [19,21,73,74]. The model will be used to project how the changes in smoking rates and costs observed in the Georgetown trial and in five of the other SCALE trials may impact long-term costs, population lung cancer rates, and overall tobacco-related mortality. This model translates individual smoking histories into lung cancer risk, and simulates the effects of CT screening on lung cancer incidence and mortality [19,73]. The model includes competing mortality due to other causes of death by age and will include costs associated with lung cancer treatment, making it feasible to deploy it to project the long-term outcomes of the patterns observed in the proposed trial.
For the short-term analysis, we will compare the average costs and incremental differences in costs by study arm. Analyses from the screening center perspective will be limited to costs of intervention delivery only; analyses from the insurer’s perspective will consider short-term follow-up procedure costs; the societal perspective will include all of the preceding costs plus patient-related costs. For all analyses, one-way and multi-way sensitivity analysis will be conducted using the range of effects and other data observed in the RCT to assess key drivers of results such as gender, ethnicity, and tobacco history. Within the context of the trial, the time horizon for the CEA is short-term (i.e., 3-, 6-, or 12-months post-randomization), so we will not discount costs or effects; in the longer-term analysis using the CISNET model, we will discount all costs and effects.
For the long-term analysis using the CISNET model, we will use the RCT cost estimates and effects as model inputs. We will use extant parameters previously employed to model trends in lung cancer incidence and mortality and the impact of LCS, and modify these accordingly to the estimated effects [21]. In particular, baseline smoking cessation rates in the CISNET model will be adjusted according to the effects of the SCALE interventions. To accomplish this, we will first simulate a cohort of individuals and their individual smoking histories using the CISNET smoking generator [21,74,75]. Next, we will simulate their enrollment into a LCS program based on their age-specific eligibility. Third, we will simulate individual screening and lung cancer outcomes using the University of Michigan model, modifying smoking histories according to individual simulated screening outcomes in the study and the estimated effects of cessation program type. We will model various types of cessation programs, including a scenario with no cessation program and/or usual care referral. Modeled outcomes for each cessation program type will include cohort smoking prevalence, lung cancer incidence and mortality, screening eligibility and screening outcomes (numbers of screens, positive, negative, and false positive), number needed to enter the smoking cessation intervention and overall tobacco-related mortality, as well as costs per life year (and quality-adjusted life year) saved by cessation and lung screening. We will conduct sensitivity analyses to evaluate uncertainty in model parameters and in the efficacy of the smoking cessation interventions.
4. Discussion
The goal of the Lung Screening, Tobacco and Health trial is to assess the impact of intensive, proactive TC and nicotine replacement on short- and long-term cigarette smoking cessation, compared to usual care. Both the ITC and UC intervention protocols have the potential to improve cessation outcomes among older, chronic smokers who are most at risk for lung cancer and other tobacco-related diseases. Using the RE-AIM framework, we will assess the reach, effectiveness, cost-effectiveness, and the long-term impact of these interventions.
TC is a feasible approach to promote smoking cessation among older, long-term smokers who may not be ready to quit and who may not be seeking treatment [29-36]. We built our approach on the evidence base of TC partly in response to the limited staffing in LCS programs. As state and national quitlines offer multiple tailored protocols for specific populations (e.g., youth, pregnant smokers) [40], quitlines provide an ideal setting for implementing the more cost-effective intervention protocol from this trial.
For all participants, we use motivational interviewing techniques to address the behavioral and psychological aspects of quitting, particularly among persons not yet ready to quit. Furthermore, the interventions use an individualized approach to establish behavior change strategies with participants and to set a quit date once they are ready. A unique feature of our approach is the utilization of the “teachable moment” offered by LCS. By delivering the cessation intervention in conjunction with LCS, participants may be particularly amenable to receiving support for quitting. In the ITC arm only, we incorporate targeted intervention strategies based upon the LCS results, which can capitalize on heightened risk perception and challenge thoughts related to reduced risk perception. Notably, if LCS is perceived as a substitute for quitting or if receipt of a normal result contributes to reduced motivation to quit [15,76], then additional education regarding the purpose of LCS and the benefits it affords may bolster screening as a contributor to cessation. Thus, in the ITC arm, we discuss the risks associated with continued smoking, as well as benefits associated with quitting, regardless of the LCS result or readiness to quit at the time of screening.
This trial may result in a substantial public health impact by addressing the scalability of a phone-based cessation intervention for LCS participants. The cost-effectiveness analyses and CISNET modeling will provide critical data to address scalability efforts via the projection of the long-term impact of the interventions on costs per life year saved, quality-adjusted life years saved, lung cancer deaths averted, and overall population mortality. Further, the CISNET analyses will include cost and cessation data from several of the SCALE trials [7], which will allow for the comparison of the potential long-term impact of alternative cessation interventions.
In summary, the LSTH trial has multiple strengths. One of the strengths is the broad inclusion criteria that will allow the results to be generalized to the broadest possible population of smokers eligible for LCS. We do not exclude participants based on their readiness to quit, psychiatric diagnoses, other concurrent or prior cessation interventions, and we include both English and Spanish speakers. Another strength is the biochemical verification of smoking status at all three follow-up assessments, up to one-year post-randomization. Third, we will conduct a cost-effectiveness analysis and leverage a CISNET model to project the long-term population impact of effective cessation interventions when conducted in the context of LCS. Finally, this trial is testing proven cessation interventions in the LCS setting that are designed for widespread implementation.
ACKNOWLEDGEMENTS
Claudia Campos, M.A., Jennifer Frey, Ph.D., Lucia Galleno, Ph.D., Charlotte Hagerman, MA, Paula Bellini, MA, Ryan Anderson, and Susan Marx.
FUNDING
This study is funded by the National Cancer Institute, Integrating Smoking Cessation Interventions with Lung Cancer Screening Programs: A Randomized Trial. 2016–2021. 1R01CA207228.
Footnotes
DECLARATION OF INTERESTS
The authors have no conflicts of interest.
CLINICAL TRIALS REGISTRATION
The trial is registered at clinicaltrials.gov:
REFERENCES
- [1].Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365(5):395–409. PM:21714641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer 2013;119(7):1381–1385. PM:23440730. [DOI] [PubMed] [Google Scholar]
- [3].Final Update Summary: Lung Cancer: Screening. U.S. Preventive Services Task Force. July 2015. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening
- [4].McMahon PM, Kong CY, Bouzan C, Weinstein MC, Cipriano LE, Tramontano AC, Johnson BE, Weeks JC, Gazelle GS. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol 2011;6(11):1841–1848. PM:21892105. PMCID:PMC3202298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One 2013;8(8):e71379. PM:23940744. PMCID:PMC3737088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Centers for Medicare & Medicaid Services (CMS). Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). 2015. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- [7].Joseph AM, Rothman AJ, Almirall D, Begnaud A, Chiles C, Cinciripini PM, Fu SS, Graham AL, Lindgren BR, Melzer AC, Ostroff JS, Seaman EL, Taylor KL, Toll BA, Zeliadt SB, Vock DM. Lung Cancer Screening and Smoking Cessation Clinical Trials: SCALE Collaboration (2018). American Journal of Respiratory and Critical Care Medicine. January 15;197(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health 2013;103(6):e38–e46. PM:23597377. PMCID:PMC3698732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treating_tobacco_use08.pdf; 2008. [Google Scholar]
- [10].Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. Am Psychol 2010;65(4):252–261. PM:20455619. PMCID:PMC3169380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2013;8:CD002850. PM:23934971. [DOI] [PubMed] [Google Scholar]
- [12].Barry SA, Tammemagi MC, Penek S, Kassan EC, Dorfman CS, Riley TL, Commin J, Taylor KL. Predictors of adverse smoking outcomes in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst 2012;104(21):1647–1659. PM:23104210. PMCID:PMC3490843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hagerman CJ, Tomko CA, Stanton CA, Kramer JA, Abrams DB, Anderson ED, Taylor KL (2015). Incorporating a smoking cessation intervention into lung cancer screening programs: Preliminary studies. J Psychosoc Oncol. Nov-Dec; 33(6):703–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tammemagi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106(6):dju084. PM:24872540. PMCID:PMC4081623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer 2007;56(1):125–134. PM:17196298. [DOI] [PubMed] [Google Scholar]
- [16].Taylor KL, Hagerman CJ, Luta G, Bellini PG, Stanton C, Abrams DB, Kramer JA, Anderson ED, Regis S, McKee A, McKee B, Niaura R, Harper H, & Ramsaier M (2017). Preliminary evaluation of a telephone-based smoking cessation intervention in the lung cancer screening setting: A randomized clinical trial. Lung Cancer. 108:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res 2003;18(2):156–170. PM:12729175. [DOI] [PubMed] [Google Scholar]
- [18].Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. Preventive Services Task Force. Ann Am Thorac Soc 2014;11(4):619–627. PM:24701999. [DOI] [PubMed] [Google Scholar]
- [19].de Koning HJ, Meza R, Plevritis SK, ten Haaf K, Munshi VN, Jeon J, Erdogan SA, Kong CY, Han SS, van RJ, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med 2014;160(5):311–320. PM:24379002. PMCID:PMC4116741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holford TR, Meza R, Warner KE, Meernik C, Jeon J, Moolgavkar SH, Levy DT. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA 2014;311(2):164–171. PM:24399555. PMCID:PMC4056770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jeon J, Holford TR, Levy DT, Feuer EJ, Cao P, Tam J, Clarke L, Clarke J, Kong CY, & Meza R. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Annals of Internal Medicine, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].National Comprehensive Cancer Network, Inc. 2018. Lung cancer Screening Guidelines Ver. 2.2019. https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf
- [23].Schütte S, Dietrich D, Montet X, & Flahault A (2018). Participation in lung cancer screening programs: are there gender and social differences? A systematic review. Public Health Reviews. 39:23 10.1186/s40985-018-0100-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. PM:18929686. PMCID:PMC2700030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS™). http://www.acr.org/Quality-Safety/Resources/LungRADS [Google Scholar]
- [26].SRNT Subcommittee on Biochemical Verification; Biochemical verification of tobacco use and cessation, Nicotine & Tobacco Research, Volume 4, Issue 2, 1 May 2002, Pages 149–159, 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- [27].Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum Psychopharmacol 2010;25(1):80–83. PM:19998321. PMCID:PMC2805052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Murray RP, Connett JE, Istvan JA, Nides MA, Rempel-Rossum S. Relations of cotinine and carbon monoxide to self-reported smoking in a cohort of smokers and ex-smokers followed over 5 years. Nicotine Tob Res 2002;4(3):287–294. PM:12215237. [DOI] [PubMed] [Google Scholar]
- [29].Joyce GF, Niaura R, Maglione M, Mongoven J, Larson-Rotter C, Coan J, Lapin P, Morton S. The effectiveness of covering smoking cessation services for medicare beneficiaries. Health Serv Res 2008;43(6):2106–2123. PM:18783459. PMCID:PMC2614005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers: tailored interventions for routine medical care. Prev Med 1996;25(3):346–354. PM:8781013. [DOI] [PubMed] [Google Scholar]
- [31].Tait RJ, Hulse GK, Waterreus A, Flicker L, Lautenschlager NT, Jamrozik K, Almeida OP. Effectiveness of a smoking cessation intervention in older adults. Addiction 2007;102(1):148–155. PM:17207132. [DOI] [PubMed] [Google Scholar]
- [32].Tzelepis F, Paul CL, Wiggers J, Walsh RA, Knight J, Duncan SL, Lecathelinais C, Girgis A, Daly J. A randomised controlled trial of proactive telephone counselling on cold-called smokers’ cessation rates. Tob Control 2011;20(1):40–46. PM:21030529. PMCID:PMC3003878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Curry SJ, McBride C, Grothaus LC, Louie D, Wagner EH. A randomized trial of self-help materials, personalized feedback, and telephone counseling with nonvolunteer smokers. J Consult Clin Psychol 1995;63(6):1005–1014. PM:8543703 [DOI] [PubMed] [Google Scholar]
- [34].Emmons KM, Puleo E, Mertens A, Gritz ER, Diller L, Li FP. Long-term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. J Clin Oncol 2009;27(1):52–60. PM:19047296. PMCID:PMC2645097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ellerbeck EF, Mahnken JD, Cupertino AP, Cox LS, Greiner KA, Mussulman LM, Nazir N, Shireman TI, Resnicow K, Ahluwalia JS. Effect of varying levels of disease management on smoking cessation: a randomized trial. Ann Intern Med 2009;150(7):437–446. PM:19349629. PMCID:PMC2825176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tzelepis F, Paul CL, Walsh RA, McElduff P, Knight J. Proactive telephone counseling for smoking cessation: meta-analyses by recruitment channel and methodological quality. J Natl Cancer Inst 2011;103(12):922–941. PM:21666098. [DOI] [PubMed] [Google Scholar]
- [37].Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. J Consult Clin Psychol 1996;64(1):202–211. PM:8907100. [DOI] [PubMed] [Google Scholar]
- [38].Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control 2007;16 Suppl 1:i53–i59. PM:18048633. PMCID:PMC2598511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Graham AL, Papandonatos GD, DePue JD, Pinto BM, Borrelli B, Neighbors CJ, Niaura R, Buka SL, Abrams DB. Lifetime characteristics of participants and non-participants in a smoking cessation trial: implications for external validity and public health impact. Ann Behav Med 2008;35(3):295–307. PM:18414962. [DOI] [PubMed] [Google Scholar]
- [40].North American Quitline Consortium. 2017. Results from the 2017 NAQC Annual Survey of Quitlines. Rudie M, editor. Available at http://www.naquitline.org/?page=2017Survey [Google Scholar]
- [41].Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM. The Tobacco Dependence Treatment Handbook: A Guide to Best Practices. New York: Guilford Press; 2003. [Google Scholar]
- [42].Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database Syst Rev. 2015. October 12;(10):CD009670. doi: 10.1002/14651858.CD009670.pub3. Review. PMID: 26457723 [DOI] [PubMed] [Google Scholar]
- [43].Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behaviors. 3rd ed. New York, NY: Guilford Press; 2012. [Google Scholar]
- [44].Soria R, Legido A, Escolano C, Lopez YA, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. Br J Gen Pract 2006;56(531):768–774. PM:17007707. PMCID:PMC1920717 [PMC free article] [PubMed] [Google Scholar]
- [45].Pineiro B, Lopez-Duran A, Fernandez del Rio E, Martinez U, Brandon TH, & Becona E (2016). Motivation to quit as a predictor of smoking cessation and abstinence maintenance among treated Spanish smokers. Addictive Behaviors, 53, 40–45. [DOI] [PubMed] [Google Scholar]
- [46].Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low-income planned parenthood clinics. Am J Public Health 2000;90(5):786–789. PM:10800431. PMCID:PMC1446229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Velasquez MM, Hecht J, Quinn VP, Emmons KM, DiClemente CC, Dolan-Mullen P. Application of motivational interviewing to prenatal smoking cessation: training and implementation issues. Tob Control 2000;9 Suppl 3:III36–III40. PM:10982903. PMCID:PMC1766310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Martino KM, Ball S, Nich SA, Frankforter C, Anez T, Paris LM,...Farentinos C (2009). A multisite randomized effectiveness trial of motivational enhancement therapy for Spanish-speaking substance users. Journal of Consulting and Clinical Psychology, 77, 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ferguson J, Docherty G, Bauld L, Lewis S, Lorgelly P, Boyd KA, McEwen A, Coleman T. Effect of offering different levels of support and free nicotine replacement therapy via an English national telephone quitline: randomised controlled trial. BMJ 2012;344:e1696. PM:22446739. PMCID:PMC3311694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013;368(4):341–350. PM:23343063. [DOI] [PubMed] [Google Scholar]
- [51].ZOOM web and telconferencing platform. https://zoom.us/
- [52].Catley D, Goggin K, Harris KJ, Richter KP, Williams K, Patten C, Resnicow K, Ellerbeck EF, Bradley-Ewing A, Lee HS, Moreno JL, Grobe JE (2016). A Randomized Trial of Motivational Interviewing: Cessation Induction Among Smokers With Low Desire to Quit. Am J Prev Med. May;50(5):573–583. doi: 10.1016/j.amepre.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Smoking Cessation at Lung Examination: The SCALE Collaboration (2018). https://cancercontrol.cancer.gov/brp/tcrb/scale-collaboration.html
- [54].Smoking Cessation at Lung Examination (SCALE) Collaboration Special Measures Collection (NCI). https://www.gem-beta.org/Public/wsoverview.aspx?wid=33&cat=8
- [55].National Health Interview Survey (2015). Sample Adult Tobacco Document Version Date: 5/27/16.ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Survey_Questionnaires/NHIS/2015/english/qcancer.pdf
- [56].Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction 1991; 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- [57].Apodaca TR, Abrantes AM, Strong DR, Ramsey SE, Brown RA. Readiness to change smoking behavior in adolescents with psychiatric disorders. Addict Behav. 2007; 32 (6): 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Latimer-Cheung, et al. “How Do Perceptions About Cessation Outcomes Moderate the Effectiveness of a Gain-Framed Smoking Cessation Telephone Counseling Intervention?” J Health Commun. 2012. ; 17(9): 1081–1098. doi: 10.1080/10810730.2012.665420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5(1):13–25. PM:12745503. [PubMed] [Google Scholar]
- [60].Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA, for the Ambulatory Care Quality Improvement Project (ACQUIP). The AUDIT Alcohol Consumption Questions (AUDIT-C) An Effective Brief Screening Test for Problem Drinking. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- [61].Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, & Sellman JD (2010). An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug and alcohol dependence, 110(1–2), 137–143. [DOI] [PubMed] [Google Scholar]
- [62].The EuroQol Group (1990). EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 16(3):199–208. [DOI] [PubMed] [Google Scholar]
- [63].Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT.. Zaslavsky AM (2002). Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- [64].An LC, Zhu SH, Nelson DB, Arikian NJ, Nugent S, Partin MR, Joseph AM. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med 2006;166(5):536–542. PM:16534040. [DOI] [PubMed] [Google Scholar]
- [65].Graham AL, Chang Y, Fang Y, Cobb NK, Tinkelman DS, Niaura RS, Abrams DB, Mandelblatt JS. Cost-effectiveness of internet and telephone treatment for smoking cessation: an economic evaluation of The iQUITT Study. Tob Control 2013;22(6):e11. PM:23010696. PMCID:PMC3626730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mandelblatt JS, Cullen J, Lawrence WF, Stanton AL, Yi B, Kwan L, Ganz PA. Economic evaluation alongside a clinical trial of psycho-educational interventions to improve adjustment to survivorship among patients with breast cancer. J Clin Oncol 2008;26(10):1684–1690. PM:18375897. [DOI] [PubMed] [Google Scholar]
- [67].Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, Nusbaum R, Huang AT, Chang Y, Graves K, Isaacs C, Wood M, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol 2014;32(7):618–626. PM:24449235. PMCID:PMC3927731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bush TM, McAfee T, Deprey M, Mahoney L, Fellows JL, McClure J, Cushing C. The impact of a free nicotine patch starter kit on quit rates in a state quit line. Nicotine Tob Res 2008;10(9):1511–1516. PM:19023843. [DOI] [PubMed] [Google Scholar]
- [69].Jardin BF, Cropsey KL, Wahlquist AE, Gray KM, Silvestri GA, Cummings KM, Carpenter MJ. Evaluating the effect of access to free medication to quit smoking: a clinical trial testing the role of motivation. Nicotine Tob Res 2014;16(7):992–999. PM:24610399. PMCID:PMC4133568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Demidenko E Sample size and optimal design for logistic regression with binary interaction. Stat Med 2008;27(1):36–46. PM:17634969. [DOI] [PubMed] [Google Scholar]
- [71].MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods 2007;39(3):384–389. PM:17958149. PMCID:PMC2819369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- [73].Meza R, ten HK, Kong CY, Erdogan A, Black WC, Tammemagi MC, Choi SE, Jeon J, Han SS, Munshi V, et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer 2014;120(11):1713–1724. PM:24577803. PMCID:PMC4031303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Caverly TJ, Cao P, Hayward RA, Meza R. (2018). Identifying Patients for Whom Lung Cancer Screening Is Preference-Sensitive: A Microsimulation Study. Ann Intern Med. July 3;169(1):1–9. doi: 10.7326/M17-2561. PMID: 29809244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jeon J, Meza R, Krapcho M, Clarke LD, Byrne J, Levy DT. Chapter 5: Actual and counterfactual smoking prevalence rates in the U.S. population via microsimulation. Risk Anal 2012;32 Suppl 1:S51–S68. PM:22882892. PMCID:PMC3478148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zeliadt SB, Heffner JL, Sayre G, Klein DE, Simons C, Williams J, Reinke LF, Au DH. Attitudes and Perceptions About Smoking Cessation in the Context of Lung Cancer Screening. JAMA Intern Med. 2015. September;175(9):1530–7. doi: 10.1001/jamainternmed.2015.3558. [DOI] [PubMed] [Google Scholar]