Abstract
Purpose:
In 2016, non-invasive encapsulated follicular variant of papillary thyroid carcinoma (NI-EFVPTC) was renamed as noninvasive thyroid follicular neoplasm with papillary-like nuclear features (NIFTP). However, as the study cohort did not mention tumors with oncocytic features, such lesions are still labeled by some as FVPTC. It is therefore crucial to evaluate the outcome and molecular profile of oncocytic NI-EFVPTC.
Methods:
A multi-institutional clinico-pathologic review was conducted to select 61 patients having oncocytic NI-EFVPTC. A detailed molecular profile was carried out in 15 patients.
Results:
Oncocytic NI-EFVPTCs predominantly affected women in their 50s. There was no distant metastasis, lymph node metastases, or structural recurrence in the entire cohort. Among patients with ≥ 5 years of FU, all 33 individuals did not recur with a median FU of 10.2 years. Oncocytic NI-EFVPTC commonly had RAS (33%) mutations, a high frequency of mitochondrial DNA mutations (67%) and multiple chromosomal gains/losses (53%). No fusion genes were detected.
Conclusions:
Oncocytic NI-EFVPTC, when stringently selected for, lacks metastasis at presentation and follows an extremely indolent clinical course, even when treated conservatively with lobectomy alone without RAI therapy. These tumors share a similar mutational profile as NIFTP, FVPTC and follicular neoplasm and are predominantly RAS-related. Like Hurthle cell neoplasms, they harbor a high frequency of mitochondrial DNA mutations, which contribute to the oncocytic cytomorphology. However, they lack the widespread chromosomal alterations observed in Hurthle cell carcinoma. Consideration should be given to include oncocytic NI-EFVPTCs as NIFTP in order to avoid overtreatment of these highly indolent tumors.
Keywords: Encapsulated follicular variant, papillary thyroid carcinoma, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), RAS, oncocytic
Introduction
The incidence of thyroid carcinoma has increased more than any other cancer in the United States, with an annual increase of 3.6% per year and 56,430 new cases diagnosed annually [1,2]. The increase is in part attributed to a rise in the prevalence of the follicular variant of papillary thyroid carcinoma (FVPTC), a diagnosis that is rendered with a certain degree of inter-observer subjectivity [3]. For example, the percentage of FVPTC among all papillary thyroid carcinomas (PTC) regardless of tumor size has nearly tripled from 18% to 57% over the past four decades, becoming the most common architectural patterns encountered in PTC [3].
Histologically, PTC can be subtyped using architectural patterns or cytologic features. FVPTC is a variant of PTC that is characterized by an exclusively follicular growth pattern, while the oncocytic variant is used to describe PTC with prominent oncocytic cytomorphology characterized by abundant eosinophilic granular cytoplasm [4,5]. Recently, compelling clinical outcome data and molecular evidence, including The Cancer Genomic Atlas (TCGA) of PTC, have demonstrated that noninvasive FVPTC follows a highly indolent clinical course and has a molecular signature resembling follicular adenoma/follicular carcinoma with activating RAS mutations as the most frequently encountered genetic alteration [6–9,5]. In 2016, a group of 28 endocrine experts critically reexamined this entity and advocated for a nomenclature revision to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), in an effort to reduce overtreatment of this highly indolent tumor by eliminating the term “carcinoma” [5]. Such nomenclature revision was subsequently recognized and adopted by the World Health Organization (WHO) [4].
As the cohort of 109 patients with NIFTP studied by the consensus conference [5] and in a previous report[8] did not explicitly address tumors with oncocytic features, i.e. oncocytic noninvasive encapsulated follicular variant of papillary thyroid carcinoma (O-NI-EFVPTC), there is currently no study with long-term follow up specifically designated to investigate the clinical behavior and outcome of O-NI-EFVPTC. As a consequence, such lesions are labeled and staged by many pathologists as O-NI-EFVPTC rather than NIFTP. Indeed, the United Kingdom endocrine pathology society currently considers the presence of oncocytic features as an exclusion criterion for the use of the NIFTP diagnostic term [10]. In 2013, Ganly et al. studied a group of Hurthle cell carcinomas (HCC), i.e. carcinoma with invasion, oncocytic cytoplasm, absence of nuclear features of PTC and solid and/or follicular growth patterns [11]. In this study, HCC showed a distinct mutational, transcriptional and copy number profile different from those of PTC with a low frequency (being 11%) of RAS mutations and no BRAF mutations [11]. However, the molecular profile of oncocytic FVPTC has not yet been studied to date.
In the current retrospective multi-institutional study, we gathered and analyzed the clinical outcome of 61 patients with unifocal O-NI-EFVPTC from three tertiary hospitals who were not treated with post-operative RAI. A subgroup of 15 O-NI-EFVPTC were also subjected to targeted next generation sequencing to explore the molecular profile of these lesions and compare it to that of Hurthle cell adenoma (HCA), PTC, FVPTC and HCC.
Methods
Study cohort and histopathologic review:
After obtaining approval from the various institutional review boards, the pathology database of three tertiary hospitals, namely Memorial Sloan Kettering Cancer center, (MSKCC), New York, NY, USA, Sunnybrook Health Sciences Centre (SHSC), Toronto, ON, Canada, and Ospedale Maggiore, Bologna, Italy, were searched for candidate cases of unifocal O-NI-EFVPTC. All cases were reviewed independently by three endocrine pathologists (BX, GT, and RG) to confirm the diagnosis using the criteria proposed by Nikiforov et al. [5]. In brief, PTC NI-EFVPTC was diagnosed when a PTC fulfilled all of the following criteria: 1) encapsulation or clear demarcation; 2) exclusive/predominant follicular growth pattern lacking psammoma bodies and with <1% papillae and < 30% solid growth pattern; 3) nuclear atypia in the form of nuclear enlargement, nuclear membrane irregularity and/or chromatin clearing with a nuclear score of 2–3; 4) absence of invasion (vascular or capsular); 5) no tumor necrosis; and 6) mitotic index < 3 per 10 high power fields (400X). A tumor was considered as oncocytic only when at least 75% of the lesional cells exhibited unequivocal Hurthle cell/oncocytic phenotype as defined by the Armed Forces Institute of Pathology (AFIP) fascicle [12]. The tumor cells must show abundant eosinophilic granular cytoplasm (i.e. oncocytic/Hurthle cell phenotype, Figure 1). As Hurthle cell lesions commonly have large nuclei with slight nuclear membrane irregularity, convincing diagnostic nuclear features of papillary thyroid carcinoma, e.g. marked nuclear enlargement, membrane irregularity and/or chromatin clearing, had to be present for a lesion to be considered as O-NI-EFPTC. Additionally, the tumor cells typically lacked prominent central nucleoli as those seen in Hurthle cell neoplasm, but rather had peripherally located nucleoli. Patients whose tumor was less than 1 cm, who had separate foci of carcinoma, who had no follow up, or who received post-operative RAI were excluded from the current study. A total of 61 cases operated between 1984 and 2017 fulfilled the above inclusion criteria.
Figure 1.
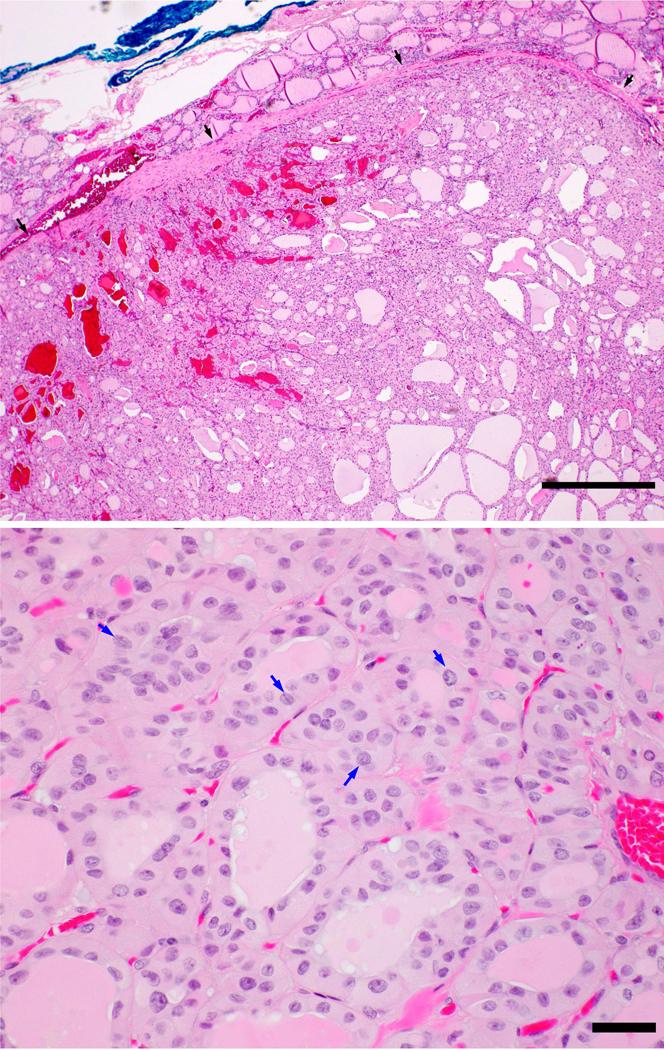
Upper panel: Oncocytic noninvasive encapsulated follicular variant of papillary thyroid carcinoma (O-NI-EFVPTC) is completely encapsulated (black arrows) without evidence of capsular or vascular invasion. Lower panel: The lesional cells show oncocytic features with abundant eosinophilic cytoplasm and nuclear features of papillary thyroid carcinoma with chromatin clearing and frequent nuclear grooves (blue arrows). Scale bars: 200 microns in upper panel, and 30 microns in lower panel.
Clinical review:
The patients’ charts were reviewed to record the following clinical parameters: age at diagnosis, sex, surgery type (total thyroidectomy vs. lobectomy/hemithyroidectomy), duration of clinical follow up (FU) and clinical outcome. Recurrence was defined as structural recurrence confirmed by imaging and/or histopathologic examination.
DNA extraction and targeted next generation sequencing
Identification of Somatic DNA mutations:
Fifteen O-NI-EFVPTC from the MSKCC cohort with tissue available for DNA extraction were subjected to the MSK-IMPACT™ (Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets) platform, an FDA (Food and Drug Administration)-approved deep-coverage, targeted next-generation sequencing assay as previously described [13,14]. In brief, the MSK-IMPACT assay detects single nucleotide variants (SNVs), small insertions/deletion (indels), copy number variants (CNVs) and fusion/structural variants in 468 oncogenes, using custom DNA probes designed for targeted sequencing of all exons and selected introns, including canonical and selected non-canonical transcripts. Genomic DNA from tumor and patient matched normal samples were extracted from formalin-fixed paraffin-embedded (FFPE) tissue and subjected to sequence library preparation (Kapa Biosystems) and exon capture (NimbleGen). Pooled libraries containing captured DNA fragments were subsequently sequenced on the Illumina HiSeq 2500 system. Paired-sample variant calling was performed on tumor samples and their respective matched normals to identify point mutations, SNVs and indels. MuTect (version 1.1.4) was used for SNV calling and SomaticIndelDetector, a tool in GATKv3.3.0, was used for detecting indel events. Variants were subsequently annotated using Annovar, and annotations relative to the canonical transcript for each gene (derived from a list of known canonical transcripts obtained from the UCSC genome browser) were reported. Annotated SNV and indel calls were subjected to a series of filtering steps to ensure only high-confidence calls were admitted to the final step of manual review [15].
Identification of Copy number alterations
The FACETS analysis [16] was performed for copy number alteration. The FACETS algorithm is an open-source allele-specific copy number analysis tool, which can enhance the sensitivity to identify amplifications or deletions in tumors by joint modeling of total and allele-specific patterns, allowing reliable determination of total and allele-specific copy number call [16]. A minimum of 1.5-fold change was required to be considered as amplification or deletion.
Identification of mitochondrial DNA (mtDNA) mutations
Somatic mtDNA mutation analysis was performed using a custom informatics pipeline for mtDNA as previously described [17]. Briefly, a pileup file for paired tumor and normal tissue sample was generated using samtools mpileup with minimal mapping quality 10 and base alignment quality 13 [18]. Mutation annotation format files for mtDNA variants were then generated using vcf2maf, and further annotated with calls from MitImpact (including the APOGEE score and MitoTIP). Putative variants were filtered if they fell in regions 302–315, 514–525, or 3106–3110 of the mitochondrial rCRS. Variants were retained if they contained at least 5 reads in support of the variant, with at least 2 reads in both the forward and reverse direction. Variants were prioritized as putatively loss-of-function if they were (1) associated with disease in MITOMAP or (2) either a nonsense or frameshift variant.
Results
Clinico-pathologic findings in O-NI-EFVPTC:
Sixty-one patients with unifocal O-NI-EFVPTC who did not receive RAI were included in this study. The number of included cases according to the institutions was as follows: MSKCC n = 34; Bologna-Ospedale Maggiore n = 22; and SHSC n = 5. All cases from MSKCC and SHSC (39 of 61, 64%) had an initial diagnosis of encapsulated FVPTC while the cases from Ospedale Maggiore were diagnosed as Hurthle cell adenomas 22 (36%) of 61.
The clinico-pathologic characteristics are summarized in Table 1. O-NI-EFVPTC predominantly affected female patients with a female to male ratio of 2.2:1. The median age of diagnosis was 56 (range: 8 – 82). The median tumor size was 2.5 cm (range 1.0 – 5.0 cm). Twenty-eight patients (46%) underwent lobectomy, while the remaining 33 (54%) had total thyroidectomy. In 37 (61%) cases, the entire tumor or tumor capsule was submitted for histologic examination, while in the remaining 24 patients (39%), the tumors were sampled representatively. In cases where the tumor and its capsule were representatively sampled, an average of 5 tumor sections (median=4, range: 2–12) and 2 tumor sections per centimeter of tumor (median =2, range: 1–4) were examined per case. All tumors were confined to the thyroid and were resected completely with negative surgical margins. Lymph node(s) were sampled in 12 (20%) patients. No lymph node metastases were detected at diagnosis clinically and/or pathologically. Benign conditions such as chronic lymphocytic thyroiditis and nodular hyperplasia, were observed in 9 (15%) and 28 (46%) patients respectively.
Table 1.
Clinico-pathologic characteristics of patients with unifocal oncocytic noninvasive encapsulated follicular variant of papillary thyroid carcinoma (O-NI-EFVPTC) who did not receive post-operative radioactive iodine treatment.
| All patients (n = 61) | |||
|---|---|---|---|
| Site | MSKCC, US | 34 | 56% |
| Bologna, Italy | 22 | 36% | |
| SHSC, Canada | 5 | 8% | |
| Sex | Female | 42 | 69% |
| Male | 19 | 31% | |
| Age: mean, median (range) | 52, 56 (8–82) | ||
| Surgical procedure | Lobectomy | 28 | 46% |
| Total/subtotal thyroidectomy | 33 | 54% | |
| Tumor sampling | Entirely or entire capsule | 37 | 61% |
| Representative | 24 | 39% | |
| Number of sections sampled per tumor in cases that were representatively sampled: mean, median (range) | 5, 4 (2–12) | ||
| Number of sections per cm of tumor in cases that were representatively sampled: mean, median (range) | 2,2 (1–4) | ||
| Tumor Size(cm): mean, median (range) | 2.5, 2.5 (1.0 −5.0) | ||
| Background Thyroid | Chronic lymphocytic thyroiditis | 9 | 15% |
| Nodular hyperplasia | 28 | 46% | |
| Other/none | 25 | 41% | |
| Sampling and status of lymph nodes | Not sampled | 49 | 80% |
| Benign lymph node(s) | 12 | 20% | |
| Post-operative radioactive iodine | None | 61 | 100% |
| Follow up (FU) duration (years): mean, median (range) | 7.2, 5.3 (0.1–20.5) | ||
| Disease status at last FU | No evidence of disease (NED) | 61 | 100% |
| Patients with at least 1-year FU (n = 55) | |||
| Surgical procedure | Lobectomy | 26 | 47% |
| Total/subtotal thyroidectomy | 29 | 53% | |
| FU duration (years): mean, median (range) | 7.9, 6.7 (1–20.5) | ||
| Disease status at last FU | NED | 55 | 100% |
| Patients with at least 2-year FU (n = 52) | |||
| Surgical procedure | Lobectomy | 23 | 44% |
| Total/subtotal thyroidectomy | 29 | 56% | |
| FU duration (years): mean, median (range) | 8.3, 6.9 (2.7 −20.5) | ||
| Disease status at last FU | NED | 52 | 100% |
| Patients with at least 5-year FU (n = 33) | |||
| Surgical procedure | Lobectomy | 17 | 52% |
| Total/subtotal thyroidectomy | 16 | 48% | |
| FU duration(years):mean, median (range) | 10.9, 10.2 (5.1–20.5) | ||
| Disease status at last FU | NED | 33 | 100% |
Clinical outcome of O-NI-EFVPTC:
The median follow up in our cohort was 5.3 years (range:0.1 – 20.5). Among them, 55 (87%) had at least 1-year follow up with a median follow up of 6.7 years, 52 (85%) had at least 2-year follow up with a median follow up of 6.9 years, 33 (54%) had at least 5-year follow up with a median follow up of 10.2 years, and 17 (28%) had at least 10-year follow up. No structural recurrence or disease specific death was observed in the entire cohort.
Molecular profile of O-NI-EFVPTC:
The molecular profile of 15 cases of O-NI-EFVPTC is listed in Table 2.
Table 2.
Molecular profile of O-NI-EFVPTC.
| Case genotyped | RAS and BRAF | Other mutations | Copy number alteration | Mitochondrial DNA mutation |
|---|---|---|---|---|
| 1 | NRAS p.Q61K | GLI p.E316Q | - | MT-CO1 nonsense mutation G6930A MT-ND5 missense mutation T12569C |
| 2 | NRAS p.Q61R | - | - | - |
| 3 | KRAS p.G12D | AURKA p.L159fs | Loss in part of 9 | MT-3’flank T12228C TS2 |
| 4 | KRAS p.Q61R | - | Loss in 21 | MT-TL1 5’ flank A3243G |
| 5 | HRAS p.Q61R | CDKN2C p.D67N | Loss of 22 | MY-CYB missense mutation T15729C |
| 6 | BRAF p.K601E | TET2 p.V1718L MCL1 p.E41A | - | MT-ND1 missense mutation c.G3901A |
| 7 | - | NFE2L2 p.29_32del | Gain of 9p | MT-RNR1 5’flank G709A |
| 8 | - | EZH1 p.Q571R | - | MT-ND4 frame shift deletion A11038- |
| 9 | - | TSHR p.M453T | Gain of 5, 10, 12, 17, 18, 20, 21, X; WCD of 7; Copy neutral LOH in 9 | - |
| 10 | - | - | Gain of 5, 9, 12, 16, 20; WCD of 7; Copy neutral LOH in X | - |
| 11 | - | - | Loss of 2p; balanced gain in 4 | - |
| 12 | - | - | Gain of 4, 12, 18, 21; WCD of 7 | MT-ND2 missense mutation T5412C |
| 13 | - | - | - | MT-ND4 nonsense mutation G11390A |
| 14 | - | - | - | MT-ND4 frame shift insertion −10952C |
| 15 | - | - | - | - |
WCD: whole chromosome duplication, LOH: loss of heterozygosity. Frameshift or nonsense mutations in bold characters.
Somatic mutations:
The nuclear DNA mutation profile of O-NI-EFVPTC is illustrated in Figure 2. Mutations in the RAS gene family appear to be the main driver with alterations detected in 5 (33%) cases, with a particular preference for mutations to the homologous Q61 position. This included two mutations to the Q61 hotspot in NRAS (Q61R and Q61K, 13% of samples), two mutations to KRAS (13% of samples), including the hotspot mutation G12D, as well as a less common Q61R variant homologous to the NRAS Q61 variant. An additional mutation to HRAS at position Q61 was also observed and one tumor harbored a relatively rare BRAF K601E mutation. Additional mutations were detected in other cancer-associated genes including TSHR (thyroid stimulating hormone receptor), EZH1 (enhancer of zest 1 polycomb repressive Complex 2 Subunit), NFE2L2 (nuclear factor erythroid 2 like 2), TET2 (TET methylcytosine dioxygenase 2), MCL1, CDKN2C (cyclin dependent kinase inhibitor 2C), AURKA (aurora kinase A) and GLI (glioma-associated oncogene family zinc finger 1): each was detected in one case (7%). Aggressive molecular signatures, e.g. TP53 and TERT promoter mutations, were not seen in our cohort. No mutations were detected in 6 (40%) cases.
Figure 2.
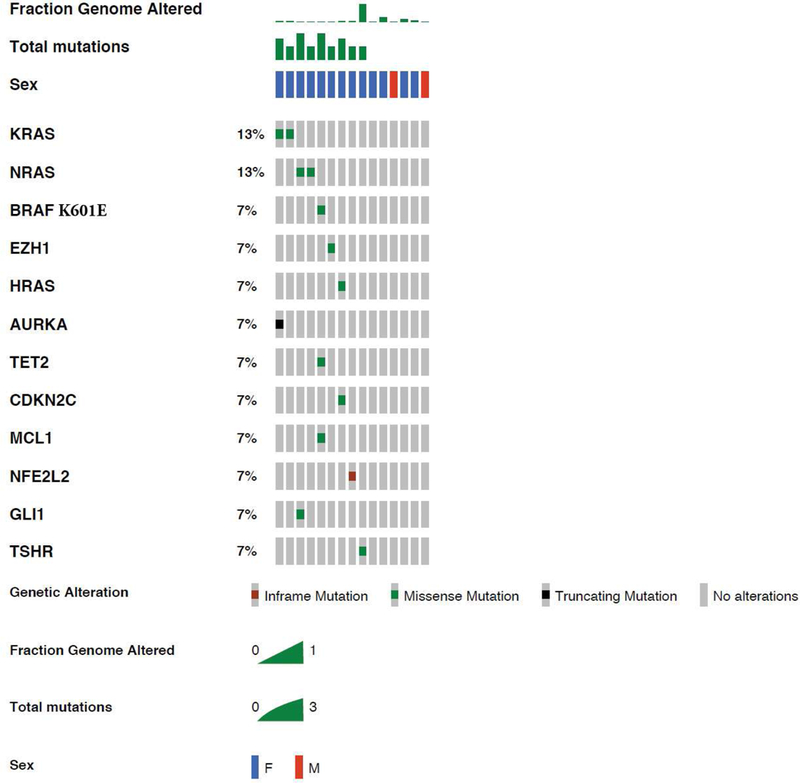
Somatic mutations in O-NI-EFVPTC. Oncoplot shows that O-NI-EFVPTC is enriched with RAS mutations. The only BRAF mutation found is BRAF K601E.
Fusion genes:
None of the 15 tested O-NI-EFVPTC contained fusions that were reported in thyroid carcinoma [6], e.g. PAX8-PPARγ as well as those involving RET, BRAF, NTRK3, ALK, THADA, FGFR1, MET and LTK loci. In addition, none of the fusion genes recently reported in HCC [17] were identified.
Copy number alterations
The FACETS plots for all 15 cases are shown in Supplementary Figure 1. Seven cases (47%) had a copy number quiet profile with no detectable copy number gains or losses. Five cases (cases 3, 4, 5, 7, and 11) had focal chromosomal alterations in chromosomes 2, 4, 9, 21, and 22. Three cases showed quite different copy number alterations (cases 9, 10, and12) in which there was whole chromosome duplication of chromosome 7 (Figure 3). These tumors did not show the widespread uniparental disomy typical of HCC [17].
Figure 3.
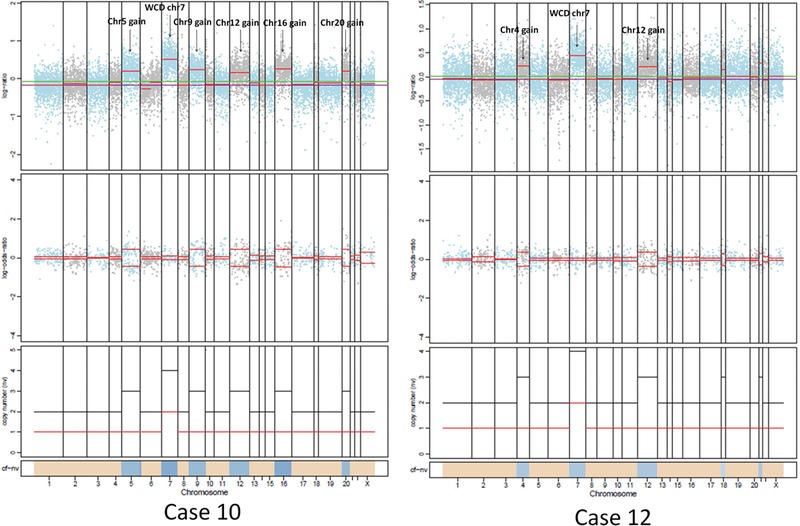
Allele specific copy number alterations in case 10 and 12. In case 10, the tumor has whole chromosome duplication of chromosome 7 in addition to gain of Chromosomes 5, 9, 12, 16 and 20. In case 12, the tumor has whole chromosome duplication of chromosome 7 in addition to gain of chromosome 4 and 12.
Mitochondrial DNA mutations
Nonsilent mitochondrial DNA mutations were detected in ten (67%) O-NI-EFVPTC, including one case (7%) which harbored two nonsilent mutations (Figure 4, case 1 of Table 2). The 6 mtDNA encoding complex I subunits were enriched for mutations, being detected in 6 cases (40%). Of the 10 cases with mtDNA mutations, four (27% of all cases) were frameshift or nonsense mutations, which may lead to potential inactivation of mitochondrial respiration in these tumors and subsequent activation of mitochondrial biogenesis [19].
Figure 4.
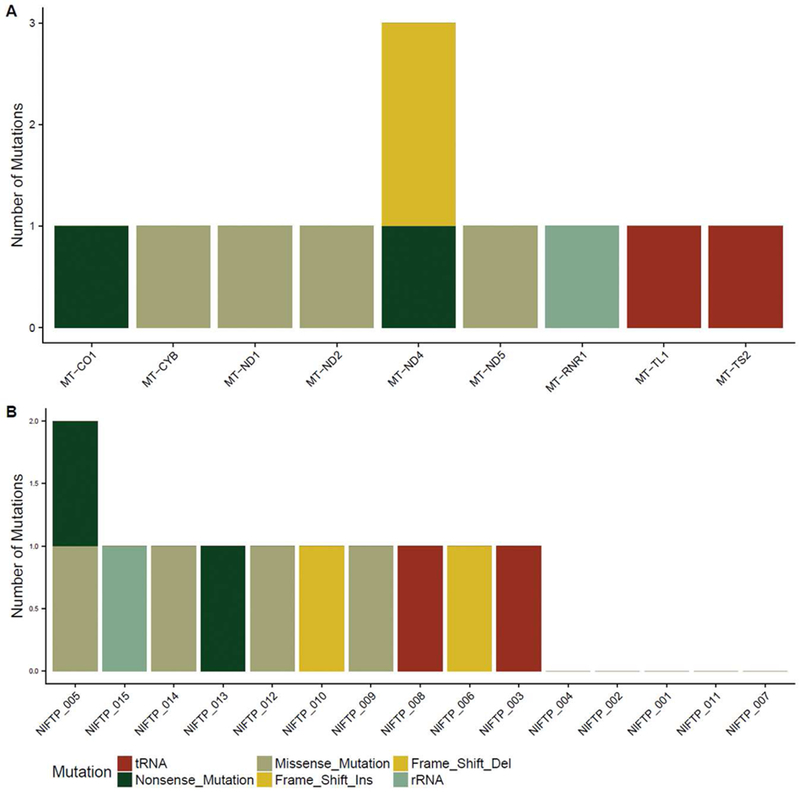
Mitochondrial DNA (mtDNA) mutations in O-NI-EFVPTC. (A) Distribution of non-synonymous, tRNA, and rRNA mutations across mtDNA-encoded genes in 15 cases of O-NI-EFVPTC. (B) mtDNA mutation(s) in each tested O-NI-EFVPTC sample.
Discussion
In the current study, we examined the clinical outcome and molecular profile of a large cohort of oncocytic NI-EFVPTC with predominant oncocytic cytomorphology but otherwise fulfilling the initial diagnostic criteria of NIFTP in order to determine the clinical behaviors and molecular signatures of these lesions. Only unifocal cases of O-NI-EFVPTC without separate carcinoma foci were included in this study to avoid the confounding effect of multifocal disease. Additionally, we only included patients treated with surgery alone (lobectomy or total thyroidectomy) without post-operative RAI, as recommended by the ATA guidelines [20,21]. This enabled us to follow the natural history of resected O-NI-EFVPTC. With regard to follow up, we relied on structural rather than biochemical recurrence to assess patients’ disease status. This was partly due to the fact that some cases were old and did not have adequate serum thyroglobulin data. Although a long follow up time was not available in all cases, this study comprised 33 patients, each followed for at least 5 years, treated by surgery without RAI therapy who did not recur with a median follow up of 10.2 years. Seventeen patients had at least 10-year follow up. As the literature has shown that most differentiated thyroid carcinomas recur during the first decade [22,23], the outcome data from the present study suggest that O-NI-EFVPTC may follow a very indolent behavior similar to their non-oncocytic counterparts [24,5]. Future studies with larger cohort size and longer follow up may be required to confirm the indolent nature of these tumors. The definition of NIFTP is in constant evolution. The most recent suggested criteria requires a total lack of true papillary formations [9], Despite the fact that we used the less stringent initial criterion of less than 1% papillae, none of the patients developed nodal metastasis. The lack of nodal disease in our cohort was also consistent with the fact that O-NI-EFVPTC behaves similarly to other encapsulated follicular neoplasms [25],in contrast to BRAFV600E-mutated tumors that are typically prone to develop local lymph nodes metastases[26,12]. Additional studies with longer follow up may be required to specifically address the long-term outcome of the patients with these tumors.
Interestingly, the molecular profile of O-NI-EFVPTC straddles between FVPTC/NIFTP and HCC, a thyroid carcinoma that shares the eosinophilic cytoplasmic features of O-NI-EFVPTC but lacks the nuclear features of PTC (Table 3) [6,5,27,11]. The mutation landscape of O-NI-EFVPTC largely resembles those of FVPTC and NIFTP and differs significantly from Hurthle cell neoplasms. NRAS, KRAS, and HRAS hotspot mutations are detected in a significant proportion (33%) of O-NI-EFVPTC, comparable with the 37% frequency found in the FVPTC of the TCGA PTC study [6,28], 30% in the NIFTP consensus cohort [5] and 63% of NIFTP reported by Johnson et al. [7]. However, the NRAS, KRAS, and HRAS mutations in O-NI-EFVPTC are significantly higher than in Hurthle cell neoplasms, being 9–11% in HCC and 0% in Hurthle cell adenoma [11,29,17]. BRAF V600E, a driver mutation commonly seen in classical variant and tall cell variant of PTC, is absent in O-NI-EFPTC (current study), as well as in NIFTP, Hurthle cell adenoma, and HCC as shown in previous studies [6,5,27,11,17]. A single case of O-NI-EFVPTC harbored BRAF K601E, which was also described in 1 to 3% of FVPTC and NIFTP [5,6,27].
Table 3.
Molecular profile in follicular variant of papillary thyroid carcinoma (FVPTC, The Cancer Genomic Atlas TCGA cohort, data retrieved from cBioPortal(1)), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), Hurthle cell carcinoma (HCC), Hurthle cell adenoma (HCA) and O-NI-EFVPTC.
| FVPTC (n=99, TCGA) (1, 2) | NIFTP (n=27, Nikiforov) (3) | NIFTP (n=32, Johnson) (4) | HCC (n=27, Ganly) (5) | HCC (n=56, Ganly) (6) | HCA (n=8) (5) | O-NI-EFVPTC Current study (n=15) | |
|---|---|---|---|---|---|---|---|
| Mutations | |||||||
| NRAS | 25% | 19% | 34% | 11% | 9% | 0 | 13% |
| HRAS | 10% | 7% | 19% | 0 | 0 | 0 | 7% |
| KRAS | 2% | 4% | 9% | 0 | 0 | 0 | 13% |
| BRAF K601E | 2% | 3% | 3% | 0 | 0 | 0 | 7% |
| Total RAS/BRAF K601E | 39% | 33% | 66% | 11% | 9% | 0 | 40% |
| Fusions | |||||||
|
RET (4%) THADA (3%) BRAF (3%) PPARγ (1%) MET (1%) NTRK3 (1%) |
PPARγ (22%) THADA (22%) |
NA | PPARγ (0) |
PPARγ (0) THADA (0) CHCHD10-VPREB3 (13%) HEPHL1-PANX1 (9%) TMEM233-PRKAB1 (9%) |
None | None | |
| Copy number alterations | |||||||
| NA for FVPTC PTC: 1q gain (15%) 22q loss (10%) High frequency of focal gains/losses (2.4%) Copy number quiet (73%) |
NA | NA | Gain: 4p, 5p, 6p, 7p, 8p, 10p, 12p, 16q Loss: 4q, 6p, 7p, 9q, 12q, 16q |
Gain: 1q, 2q, 3q, 5p, 7p, 10q, 12q, 14q, 20p, Xp. WCD of chr 5, 7, 12 in widely invasive HCC Loss: 1q, 2q, 3q, 4q, 7q, 9q, 10q, 11p, 15q, 16q, 17q. Global UPD in widely invasive HCC. |
NA | Gain: 4, 5, 9, 10, 12, 16, 17, 18, 20, 21. WCD chr 7. Loss: 2p, 9, 21, 22 |
|
| Mitochondria DNA | |||||||
| NA | NA | NA | NA | 71% with nonsilent mutations, including 37% of frameshift or nonsense mutations | NA | 67% with nonsilent mutations, including 27% of frameshift or nonsense mutations | |
NA: not available, UPD: uniparental disomy, WCD: whole chromosome duplication.
Additional molecular alterations observed in O-NI-EFVPTC include GLI, AURKA, CDKN2C, TET2, MCL1, NFE2L2, EZH1, and TSHR mutations. CDKN2C, EZH1 and TSHR mutations were also detected in four PTCs in the TCGA cohort: including three cases each with CDKN2C, EZH1 or TSHR mutation and one case with both TSHR and EZH1 mutation. Interestingly, all four cases are follicular variant, including two O-NI-EFVPTC, as shown in cBioPortal [30,31]. CDKN2C encodes a protein that belongs to the INK4 family of cyclin-dependent kinase inhibitors, which functions as a cell growth regulator by controlling cell cycle G1 progression. EZH1 is the catalytic subunit of PRC2 complex which mediates methylation of histone H3 lys27 (H3K27), leading to transcription repression of the targeted genes. TSHR encodes thyroid stimulating hormone receptor. The contribution of these gene mutations to the pathogenesis of O-NI-EFVPTC remains to be investigated.
No gene fusions were detected in this cohort of O-NI-FVPTC. In comparison, PPARγ and THADA rearrangement has been reported in 22% of NIFTP [5] and 1 to 3% of FVPTC in the TCGA cohort [6,28]. We do not know the reason behind this lack of gene fusions in O-NI-EFVPTC.
Oncocytic change in thyroid neoplasms is the result of an aberrant increase in mitochondrial mass [32], and has been described in a spectrum of thyroid follicular-cell derived neoplasms, including Hurthle cell adenoma, HCC, and various variants of papillary thyroid carcinoma [4]. This accumulation of mitochondria has been linked to mutations in the genes coding for some of the subunits of the five multimeric complexes of the oxidative phosphorylation (OXPHOS) system localized to the inner mitochondrial membrane [33,34]. If mutations of these subunits render them missing/defective, the entire multimeric complex does not assemble properly, OXPHOS is impaired, and there is a compensatory accumulation of mitochondria, with phenotypic (i.e., accumulation of mitochondria), biochemical and metabolic defects [34,33]. In the past decade a high frequency of somatic mitochondrial DNA variants has been reported in oncocytic thyroid neoplasms [35,32,17,34], and much less frequently in nuclear coded mitochondrial genes [35,32,17]. As high as 71% of HCC harbors mtDNA mutations, with 37% of tumors demonstrating detrimental frameshift or nonsense variants [17].
Among the 15 O-NI-EFVPTC tested, a very similar mtDNA mutation profile (compared to HCC) was identified with 10 cases (67%) showing nonsilent mtDNA mutations, including three cases with frameshift or nonsense mutations. This high rate of mtDNA mutations is consistent with the oncocytic phenotype of O-NI-EFVPTC and further confirms the importance of mtDNA in the development of oncocytic thyroid neoplasms. The similar rate of mtDNA mutations in the extremely indolent O-NI-EFVPTC and the much more aggressive HCC suggests that mitochondrial DNA mutations are probably not sufficient to cause an aggressive behavior in oncocytic thyroid tumors.
Multiple previous studies, including our own, have reported a plethora of chromosomal aberrations in Hurthle cell neoplasms using array comparative genomic hybridization and next generation sequencing techniques [11,29,32,36,35,17]. Ganly et al. recently reported the unique chromosomal alterations seen in 56 cases of HCC (18). In this study, the more aggressive widely invasive HCC had whole chromosome duplication of chromosome 7 and 5 with global uniparental disomy (UPD) of the remaining chromosomes. This global UPD results in widespread loss of heterozygosity (Table 3). Numerous chromosomal gains and losses involving chromosome 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 16, 17, 19, 20 and 22 have been described previously in HCC (Table 3). Among O-NI-FVPTC, several tumors lacked any chromosome alteration and 5 showed single chromosome alterations. Three tumors showed whole chromosome duplication of chromosome 7 as seen in HCC. However, these tumors did not show the widespread UPD that is seen in HCC [17].
In conclusion, the above data strongly suggest that O-NI-EFVPTC are similar to NIFTP at the molecular level in regard to their MAPK alterations (enrichment in RAS-like mutations) and at the outcome level having an indolent clinical behavior with negligible risk of metastasis and recurrence, even when treated conservatively without post-operative RAI. These findings further reinforce the fact that invasion rather than nuclear or cytoplasmic features drives outcome in encapsulated follicular patterned tumors [37,38].The presence of mitochondrial DNA mutations confirms that O-NI-EFVPTC are part of the spectrum of oncocytic neoplasms, and further supports the role of mitochondrial DNA mutations in the acquisition of the oncocytic phenotype. Therefore, given the molecular and behavioral similarity, it is worth considering rebranding oncocytic noninvasive encapsulated follicular variant of papillary thyroid carcinoma as NIFTP just as its non-oncocytic counterpart. A note in the pathology report should mention the presence of oncocytic features. Including O-NI-EFVPTC in the NIFTP category will help reduce unnecessary treatments with its side effects and will prevent the adverse psychosocial and financial consequences of a cancer diagnosis.
Supplementary Material
Acknowledgments
Funding: Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarilyrepresent the official views of the National Institutes of Health. Research reported in this publication was also supported in part by an Italian Government-Ministero della Salute Grant No. RF-2011–02350857 (to G.T.)
Footnotes
Compliance with ethical standards:
Disclosure of potential conflicts of interest: All authors declares that he/she has no conflict of interests.
Research involving human participants and/or animals: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: not applicable.
References
- 1.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM: Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. Jama 317(13), 1338–1348 (2017). doi: 10.1001/jama.2017.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA: a cancer journal for clinicians 68(1), 7–30 (2018). doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr., Sigurdson AJ, Nikiforov YE: The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. The Journal of clinical endocrinology and metabolism 99(2), E276–285 (2014). doi: 10.1210/jc.2013-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd RV, Osamura RY, Kloppel G, Rosai J: WHO classification of tumours of endocrine organs International Agency for Research on Cancer (IARC), Lyon: (2017) [Google Scholar]
- 5.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA: Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA oncology 2(8), 1023–1029 (2016). doi: 10.1001/jamaoncol.2016.0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research, N.: Integrated genomic characterization of papillary thyroid carcinoma. Cell 159(3), 676–690 (2014). doi: 10.1016/j.cell.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DN, Furtado LV, Long BC, Zhen CJ, Wurst M, Mujacic I, Kadri S, Segal JP, Antic T, Cipriani NA: Noninvasive Follicular Thyroid Neoplasms With Papillary-like Nuclear Features Are Genetically and Biologically Similar to Adenomatous Nodules and Distinct From Papillary Thyroid Carcinomas With Extensive Follicular Growth. Archives of pathology & laboratory medicine 142(7), 838–850 (2018). doi: 10.5858/arpa.2017-0118-OA [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Singh B, Tallini G, Carlson DL, Katabi N, Shaha A, Tuttle RM, Ghossein RA: Follicular variant of papillary thyroid carcinoma: a clinicopathologic study of a problematic entity. Cancer 107(6), 1255–1264 (2006). doi: 10.1002/cncr.22138 [DOI] [PubMed] [Google Scholar]
- 9.Lloyd RV, Asa SL, LiVolsi VA, Sadow PM, Tischler AS, Ghossein RA, Tuttle RM, Nikiforov YE: The evolving diagnosis of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Human pathology 74, 1–4 (2018). doi: 10.1016/j.humpath.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 10.Johnson SJ, Stephenson TJ, Poller DN: NIFTP addendum to the RCPath Dataset for thyroid cancer histopathology reports http://www.ukeps.com/docs/niftp.pdf (2016).
- 11.Ganly I, Ricarte Filho J, Eng S, Ghossein R, Morris LG, Liang Y, Socci N, Kannan K, Mo Q, Fagin JA, Chan TA: Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. The Journal of clinical endocrinology and metabolism 98(5), E962–972 (2013). doi: 10.1210/jc.2012-3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, G., T.: Tumor of the thyroid and parathyroid gland (AFIP atlas of tumor pathology series 4). American Registry of Pathology Press, Silver Spring, MD: (2015) [Google Scholar]
- 13.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O’Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF: Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD 17(3), 251–264 (2015). doi: 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris LG, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, Wong RJ, Lee NY, Sherman EJ, Baxi SS, Ganly I, Singh B, Shah JP, Shaha AR, Boyle JO, Patel SG, Roman BR, Barker CA, McBride SM, Chan TA, Dogan S, Hyman DM, Berger MF, Solit DB, Riaz N, Ho AL: The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA oncology (2016). doi: 10.1001/jamaoncol.2016.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP: Integrative genomics viewer. Nature biotechnology 29(1), 24–26 (2011). doi: 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen R, Seshan VE: FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic acids research 44(16), e131 (2016). doi: 10.1093/nar/gkw520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, Nanjangud G, Eng S, Bose P, Kuo F, Morris LGT, Landa I, Carrillo Albornoz PB, Riaz N, Nikiforov YE, Patel K, Umbricht C, Zeiger M, Kebebew E, Sherman E, Ghossein R, Fagin JA, Chan TA: Integrated Genomic Analysis of Hurthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 34(2), 256–270.e255 (2018). doi: 10.1016/j.ccell.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R: The Sequence Alignment/Map format and SAMtools. Bioinformatics (Oxford, England) 25(16), 2078–2079 (2009). doi: 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reznik E, Wang Q, La K, Schultz N, Sander C: Mitochondrial respiratory gene expression is suppressed in many cancers. eLife 6 (2017). doi: 10.7554/eLife.21592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, Mandel SJ, Morris JC, Nassar A, Pacini F, Schlumberger M, Schuff K, Sherman SI, Somerset H, Sosa JA, Steward DL, Wartofsky L, Williams MD: American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid : official journal of the American Thyroid Association 27(4), 481–483 (2017). doi: 10.1089/thy.2016.0628 [DOI] [PubMed] [Google Scholar]
- 21.Haugen BRM, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RMM, Wartofsky L: 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association 26, 1–133 (2016). doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzaferri EL, Jhiang SM: Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. The American journal of medicine 97(5), 418–428 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Nwatsock JF, Taieb D, Zok FD, Mundler O: Late Recurrences of Thyroid Carcinoma 24 Years after a Complete Remission: When Monitoring Should be Stopped? World J Nucl Med 11(1), 42–43 (2012). doi: 10.4103/1450-1147.98749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson LD: Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: A name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod Pathol 29(7), 698–707 (2016). doi: 10.1038/modpathol.2016.65 [DOI] [PubMed] [Google Scholar]
- 25.Rivera M, Ricarte-Filho J, Knauf J, Shaha A, Tuttle M, Fagin JA, Ghossein RA: Molecular genotyping of papillary thyroid carcinoma follicular variant according to its histological subtypes (encapsulated vs infiltrative) reveals distinct BRAF and RAS mutation patterns. Mod Pathol 23(9), 1191–1200 (2010). doi: 10.1038/modpathol.2010.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera M, Tuttle RM, Patel S, Shaha A, Shah JP, Ghossein RA: Encapsulated papillary thyroid carcinoma: a clinico-pathologic study of 106 cases with emphasis on its morphologic subtypes (histologic growth pattern). Thyroid : official journal of the American Thyroid Association 19(2), 119–127 (2009). doi: 10.1089/thy.2008.0303 [DOI] [PubMed] [Google Scholar]
- 27.Johnson DN, Furtado LV, Long BC, Zhen CJ, Wurst M, Mujacic I, Kadri S, Segal JP, Antic T, Cipriani NA: Noninvasive Follicular Thyroid Neoplasms With Papillary-Like Nuclear Features (NIFTPs) Are Genetically and Biologically Similar to Adenomatous Nodules and Distinct From Papillary Thyroid Carcinomas With Extensive Follicular Growth. Archives of pathology & laboratory medicine (2018). doi: 10.5858/arpa.2017-0118-OA [DOI] [PubMed] [Google Scholar]
- 28.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N: The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer discovery 2(5), 401–404 (2012). doi: 10.1158/2159-8290.cd-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tallini G, Hsueh A, Liu S, Garcia-Rostan G, Speicher MR, Ward DC: Frequent chromosomal DNA unbalance in thyroid oncocytic (Hurthle cell) neoplasms detected by comparative genomic hybridization. Lab Invest 79(5), 547–555 (1999). [PubMed] [Google Scholar]
- 30.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N: Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling 6(269), pl1 (2013). doi: 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N: The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery 2(5), 401–404 (2012). doi: 10.1158/2159-8290.cd-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gasparre G, Bonora E, Tallini G, Romeo G: Molecular features of thyroid oncocytic tumors. Mol Cell Endocrinol 321(1), 67–76 (2010). doi: 10.1016/j.mce.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 33.Bonora E, Porcelli AM, Gasparre G, Biondi A, Ghelli A, Carelli V, Baracca A, Tallini G, Martinuzzi A, Lenaz G, Rugolo M, Romeo G: Defective oxidative phosphorylation in thyroid oncocytic carcinoma is associated with pathogenic mitochondrial DNA mutations affecting complexes I and III. Cancer Res 66(12), 6087–6096 (2006). doi: 10.1158/0008-5472.can-06-0171 [DOI] [PubMed] [Google Scholar]
- 34.Gasparre G, Porcelli AM, Bonora E, Pennisi LF, Toller M, Iommarini L, Ghelli A, Moretti M, Betts CM, Martinelli GN, Ceroni AR, Curcio F, Carelli V, Rugolo M, Tallini G, Romeo G: Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc Natl Acad Sci U S A 104(21), 9001–9006 (2007). doi: 10.1073/pnas.0703056104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maximo V, Botelho T, Capela J, Soares P, Lima J, Taveira A, Amaro T, Barbosa AP, Preto A, Harach HR, Williams D, Sobrinho-Simoes M: Somatic and germline mutation in GRIM-19, a dual function gene involved in mitochondrial metabolism and cell death, is linked to mitochondrion-rich (Hurthle cell) tumours of the thyroid. Br J Cancer 92(10), 1892–1898 (2005). doi: 10.1038/sj.bjc.6602547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada N, Duh QY, Miura D, Brunaud L, Wong MG, Clark OH: Chromosomal aberrations by comparative genomic hybridization in hurthle cell thyroid carcinomas are associated with tumor recurrence. The Journal of clinical endocrinology and metabolism 87(10), 4595–4601 (2002). doi: 10.1210/jc.2002-020339 [DOI] [PubMed] [Google Scholar]
- 37.Ganly I, Wang L, Tuttle RM, Katabi N, Ceballos GA, Harach HR, Ghossein R: Invasion rather than nuclear features correlates with outcome in encapsulated follicular tumors: further evidence for the reclassification of the encapsulated papillary thyroid carcinoma follicular variant. Human pathology 46(5), 657–664 (2015). doi: 10.1016/j.humpath.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu B, Tallini G, Scognamiglio T, Roman BR, Tuttle RM, Ghossein RA: Outcome of Large Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid : official journal of the American Thyroid Association 27(4), 512–517 (2017). doi: 10.1089/thy.2016.0649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


