Abstract
This paper describes how the ligand shell containing immunostimulatory oligonucleotides surrounding gold nanoparticles affects the in vitro activation of macrophages. Nanoconstructs with similar ligand densities but different oligonucleotide compositions (from 0 to 100% immune-active cytosine-phosphate-guanine, CpG) were compared. Maximum immunostimulation was achieved with CpG content as low as 5% (with total oligonucleotide surface coverage remaining constant), correlating to high levels of anti-tumor cytokine release and low levels of cancer-promoting ones. Independent of CpG content, gold nanoparticles with low oligonucleotide densities exhibit poor cellular uptake, leading to insignificant immunostimulation and cytokine release. By identifying effects of ligand shell composition on macrophage activation, we can inform the design rules of therapeutic nanoconstructs to achieve specific immune responses.
Keywords: Gold nanoparticles, CpG, ligand composition, immunostimulatory activity, immunotherapy, spherical nucleic acids, SNAs
INTRODUCTION
Activation of the immune system has received increased interest in the development of cancer vaccines,1 where antibodies, proteins and oligodeoxynucleotides (ODNs) are used as immunomodulatory agents.2,3 ODNs are attractive because of their sequence-dependent immunostimulatory (IS) activity.4 For instance, toll-like receptor 9 (TLR9) recognizes ODNs with unmethylated cytosine-phosphate-guanine (CpG) moieties naturally found in microorganisms.5 Therefore, the binding between CpG and TLR9 activates macrophages that then release cytokines that recruit and regulate other leukocytes.5 Some cytokines, such as tumor necrosis factor α (TNF-α), have anti-tumor activities, and hence controlling their release is necessary for immunotherapy.6 ODNs in free form, however, exhibit limited IS performance because of their poor cellular uptake7 and low stability in the bloodstream caused by nuclease degradation.8
When ODNs are densely packed onto the surface of nanoparticles and liposomes, these nanostructures, which are often referred to as spherical nucleic acids (SNAs), show higher cellular uptake and stability against degradation compared to free nucleic acids.9–12 Gold nanoparticles (AuNPs) can be used as ODN carriers because they are easily functionalized and biocompatible.13 Certain Au nanoconstructs presenting ODNs with CpG motifs at high surface densities exhibit stronger IS activity compared to free nucleic acids because of enhanced cellular uptake and stability.14 The ligand shell plays the key role in the interactions between cell and nanoconstructs, where ODN sequences, backbones (phosphodiester or phosphorothioate), and spatial orientations (chemisorption of the 3’ or 5’-terminus of ODNs) can modulate immune activation.15 Structural aspects of the Au core, such as size and shape, can control the specificity and intensity of TLR9-mediated IS but not types of cytokines released16 that define macrophage biological responses.17 Although ODN-AuNPs containing CpG can induce strong immune activation,14 the role of polyvalency on TLR9-mediated IS remains unclear. Previous work demonstrated the importance of polyvalency of oligonucleotide presentation to cellular uptake and to overall nanoconstruct function,18–20 but the contribution of polyvalency to interactions with TLR9 (or other targeted receptors) is not known. To differentiate between the effects of polyvalency on IS response and uptake, the CpG content of ODN-AuNPs needs to be varied while preserving the overall ODN density.
Here we show that the immune activation responses of macrophages to ODN-AuNPs are influenced by both the ODN composition (% of immune active CpG) and density of total ODN. For nanoconstructs prepared with high ligand densities (~55 ODNs per particle), a CpG content of only 5% was sufficient to achieve maximal IS activity. ODN-AuNPs with low CpG content shells (1 to 10 %) resulted in release of high levels of anti-tumor cytokines and low levels of cancer-promoting ones. AuNPs with low ligand densities (~3 ODNs per particle) did not show significant IS activity and cytokine production due to poor cellular uptake. These results indicate that the combination of both ODN composition and density determine IS intensity and type of cytokines released, and that lower CpG content may be a feature useful in the design of nanoconstructs for targeting macrophage activation for cancer immunotherapy.
RESULTS AND DISCUSSION
Synthesis of Au Nanoconstructs with Different ODN Sequences and Densities.
AuNPs were functionalized with two different alkylthiolated ODNs in sodium citrate buffer following our published protocol16 (Experimental Section). We selected 15-nm spherical AuNPs as the core size (Figure S1) because larger NPs and cores with anisotropic morphologies, such as Au nanostars, induce higher but non-specific immune activation.16 ODNs had either immune-active CpG or non-active GpC motifs (Figure 1a). Nanoconstructs with different CpG to GpC ratios and ODN densities were selected to evaluate independently the effect of ODN composition and overall ODN density on immune activation (Figure 1b and Table S1). For ODN composition studies, six types of ODN-AuNPs with different CpG content (from 0 to 100%) were compared. For ligand density effects, nanoconstructs with ~55 and ~3 ODNs / AuNP (two significantly different ligand loadings previously used in nanomedicine21,22) were tested. All nanoconstructs were backfilled with mercaptoundecyl hexa(ethylene glycol) (MUHEG) to prevent NP aggregation by steric repulsion.23 The localized surface plasmon resonances of all nanoconstructs in both phosphate-buffered saline and cell media were between 523 and 527 nm, which indicates that the particles did not aggregate and that the NPs were well dispersed in solution (Figure S2).
Figure 1. Composition of the Au nanoconstructs.
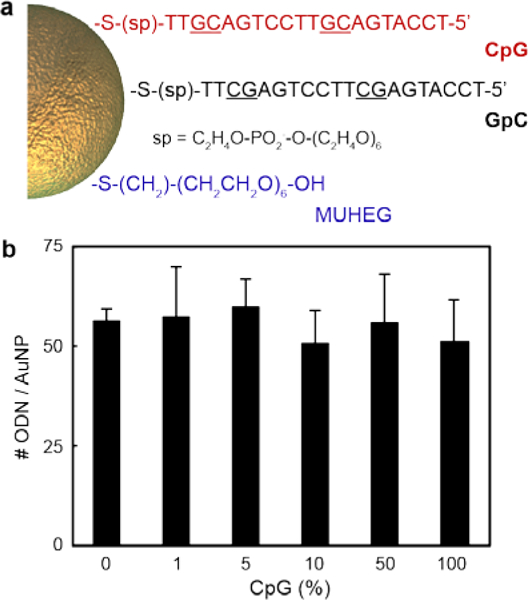
(a) ODN sequences and MUHEG used to functionalize the AuNPs. (b) Number of ODN per particle under high oligonucleotide density. The differences of ODN loading between samples with different CpG content were not significant (p < 0.05, one-way ANOVA). ODN quantification experiments were performed in quadruplicate; error bars represent one standard deviation of the measurements.
Effect of CpG Content on Immune Activation.
Murine Raw-blue macrophages were used as the model cell line because several TLRs are expressed, including TLR9.15 The macrophages were transfected with the secreted embryonic alkaline phosphatase (SEAP) reporter gene, which upon activation, triggers the release of SEAP molecules into the extracellular surroundings.24 Immune activation was quantified by SEAP levels in cell media. ODN-AuNPs with high ligand densities (~55 ODNs / AuNP) exhibited enhanced IS activity as the CpG (%) increased (Figure 2a). AuNPs with higher CpG content reached half maximal activity (EC50) at lower particle concentrations (Table S1), which indicated that IS depends on the amount of CpG delivered by the AuNPs to macrophages. At higher particle concentrations (10 AuNPs), all nanoconstructs with at least an average of 3 CpG per particle (ODN-AuNPs with ≥5% CpG content) achieved the same maximal IS (Figure S3). These results indicate that above a (relatively low) critical CpG percentage but fixed high ODN density, maximum TLR9 activation can be realized; note the major shift in IS activity occurs between 0 and 3 CpG strands per particle and that increasing the number above these values had a much smaller effect. In free form, CpG without a NP core required ca. 1000 higher concentration than CpG-AuNP to induce similar IS activation levels (Figure S4). Importantly, IS behavior was not caused by cell death, as all samples showed >90% cell viability (Figure S5).
Figure 2. Small fraction of CpG is necessary to immune activate macrophages.
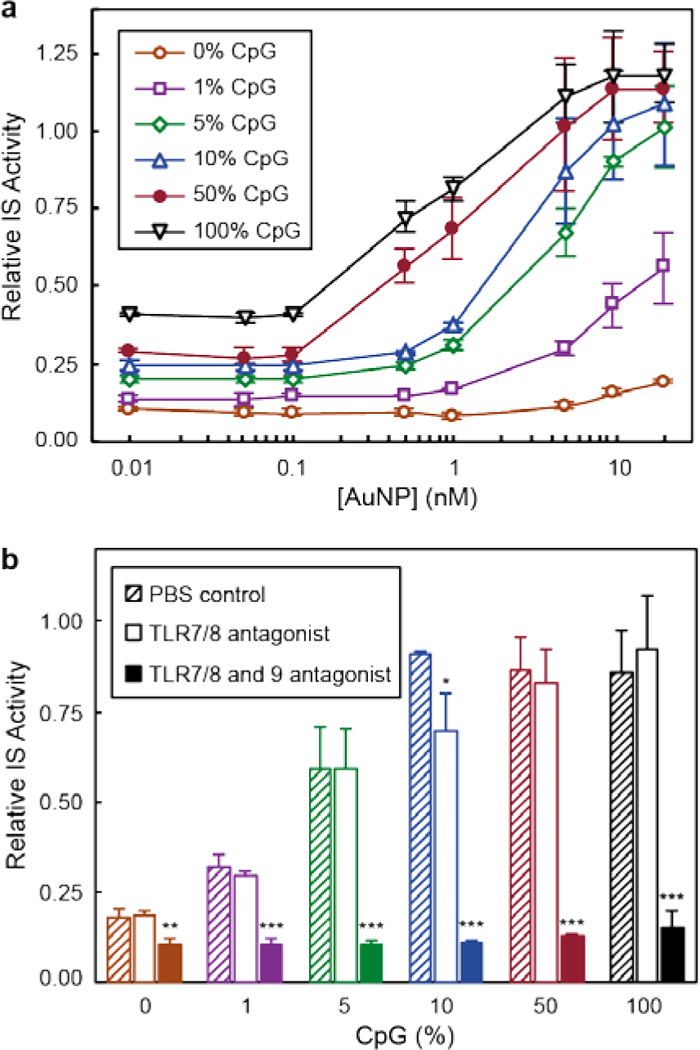
(a) Relative IS activity of Raw-blue cells after 24-h treatment with AuNPs with different CpG content. Calibration curves have been offset for clarity. (b) Relative IS activity of different nanoconstructs (5 nM ODN-AuNPs) in the presence of TLR antagonists. (*), (**) and (***) indicate groups that are significantly different from PBS with p < 0.001, p < 0.005 and p < 0.01, respectively (one-way ANOVA with post hoc Tukey HSD test). All experiments were performed in triplicate; error bars represent one standard deviation of the measurements.
CpG-functionalized Au Nanoconstructs Specifically Activated TLR9.
Since previous work showed that the NP core affects CpG targeting specificity,16 we studied whether changes in the ligand shell also influenced nanoconstruct selectivity towards TLR9 (Figure 2b). The targeting specificity of nanoconstructs on IS was studied by pre-incubating Raw-blue cells with TLR 7/8 (ODN 2087) or TLR7/8 and 9 (ODN 2088) antagonists before the addition of 5-nM ODN-AuNPs (Experimental Section). The immune activity of all CpG-containing AuNPs decreased only when TLR9 was blocked, indicating that all nanoconstructs targeted TLR9. Cell viability experiments showed that neither TLR antagonists nor nanoconstructs caused significant cytotoxicity (Figure S6).
Dependence of Immune Activation on ODN Density.
To determine how ligand density influences the IS activity of ODN-AuNPs, we compared nanoconstructs with high (~55 ODNs / AuNP) and low (~3 ODNs / AuNP) ligand loading at fixed 5-nM particle concentration. At high ligand density, nanoconstructs with higher CpG content induced stronger macrophage immune activation (Figure 3a). Nanoconstructs with low ODN loading, however, did not show significant immune activity. The difference of IS between high and low ligand densities was caused by the different levels of endocytosis of the nanoconstructs (Figure 3b), which were quantified by inductively coupled plasma mass spectrometry (Experimental Section). Larger amounts of ODNs on the AuNP surface increased the nanoconstruct endocytosis and the delivery of CpG into the cells. The particles with same ODN density and different CpG content had similar uptake levels (Figure 3b). These results are in agreement with literature that showed higher ODN loading enhances nanoconstruct cellular uptake.25 Although treatment with 5-nM ODN-AuNPs with 5% CpG (at high loading) or 100% CpG (at low loading) delivered the same total amount of CpG into the solution (ca. 15 nM), they induced high and no IS activity, respectively. These results confirm that ligand density affects IS activity of AuNPs by improving the cellular uptake of the nanoconstructs.
Figure 3. ODN density of AuNPs strongly affects their IS performance and endocytosis.
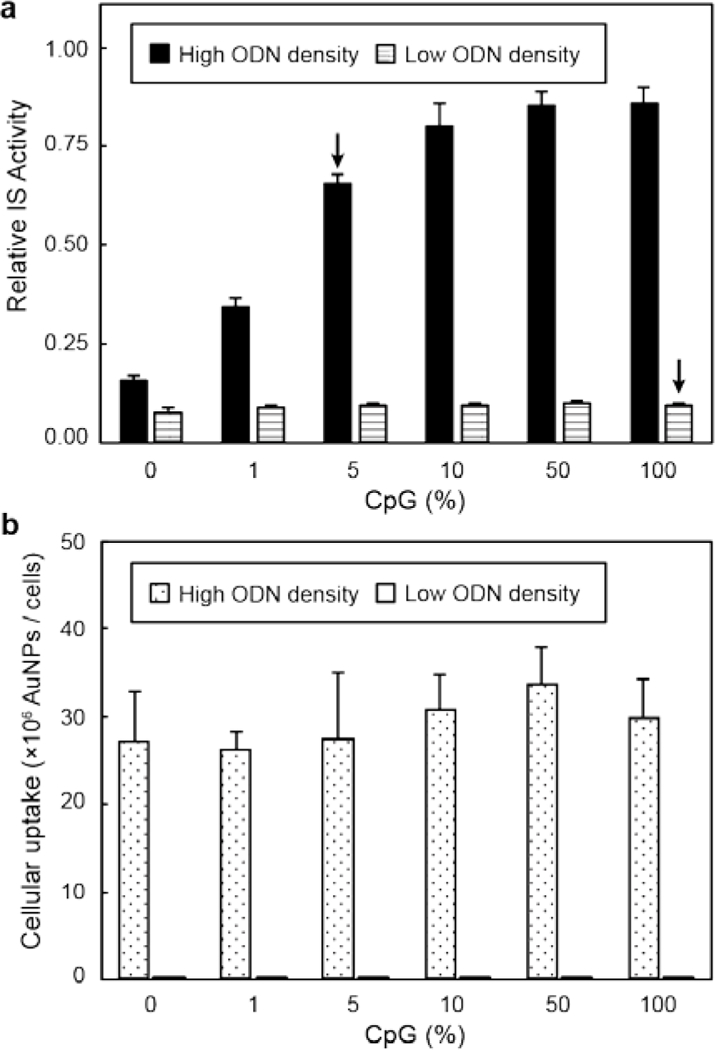
Raw-blue cells were treated with 5 nM AuNPs with high or low oligonucleotide density and different CpG content for 24 h and (a) relative IS activity and (b) cellular uptake were measured. Arrows highlight nanoconstructs with 5% (high ODN density) and 100 % CpG (low ODN density), which contained the same amount of total CpG but showed different IS performance. The differences of endocytosis between samples with same ODN density were not significant (p < 0.05, one-way ANOVA). All experiments were performed in triplicate; error bars represent one standard deviation of the measurements.
Structural parameters, such as the size and shape of Au core, have been reported to affect the sub-cellular localization of nanoconstructs, which also influence down-stream biological effects.16,26 Hence, we studied the intracellular distribution of AuNPs with different ODN compositions by transmission electron microscopy (Figure 4 and Figure S7). Macrophages were treated with 5-nM ODN-AuNPs for 24 h and double-stained after fixation and sectioning to increase the contrast of the organelles (Experimental section). Only high-ligand density AuNPs were studied since they are the only constructs that showed significant endocytosis. Independent of ODN composition, the majority of all AuNPs appeared to be located inside well-defined vesicles. These results are in agreement with previously reported transmission electron microscopy and confocal laser scanning microscopy images that show small AuNPs functionalized with oligonucleotides accumulated within endosomes and lysosomes.16,26,27 The subcellular location of the particles is important, because TLR9 receptors are expressed in intracellular membrane compartments5 such as endosomes, and their activation depends on endosomal delivery of the Au nanoconstructs.
Figure 4. AuNPs are located inside vesicles independently of their CpG content.
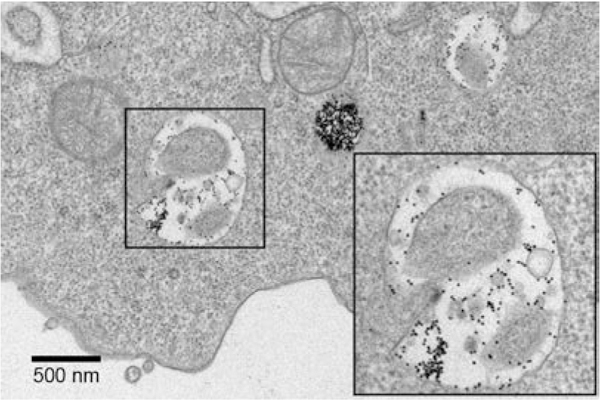
Representative TEM image of Raw-blue cell after treatment with ODN-AuNPs (100% CpG). The box dimensions are 1 μm × 1 μm.
Profile of Released Cytokines Induced by Au Nanoconstructs.
To evaluate the effect of the ODN shell composition and density on cytokine production, we quantified the six main cytokines released by macrophages upon pathogen activation at fixed ODN-AuNPs (5 nM).16 Constructs with higher CpG content and ligand loading maximized release of tumor necrosis factor α (TNF-α), RANTES, and MIP-2 (Figure 5). These three cytokines promote anti-tumor activity through acute pro-inflammatory reaction (TNF-α)28 and intra-tumor infiltration of leukocytes (RANTES and MIP-2).29 Elevated levels of G-CSF and IL-6 were also detected after macrophage incubation with AuNPs having high CpG content. G-CSF enhances the activity of neutrophils (the most abundant type of white cells that participate in the innate immune system) associated with tumor rejection.29 IL-6 promotes both pro-and anti-inflammatory responses, and high levels of this cytokine have been linked to disease progression30 and remission.29 LIF, which induces anti-inflammatory response and is associated with cancer development,31 was the cytokine with the lowest levels in cell media. LIF was minimal especially upon treatment with ODN-AuNP with low CpG loading and increased with CpG content. These results suggest that AuNPs with lower CpG content (1 to 10%) are better candidates for cancer immunotherapy, since the release of anti-tumor cytokines is maximized and lower levels of anti-inflammatory IL-6 and LIF are induced. CpG and GpC in free form (without a NP core) did not promote the release of cytokines (Figure S8), which was consistent with the low IS activity of free CpG shown in Figure S4. Finally, nanoconstructs with low ODN loading did not induce significant cytokine release, likely because of low cellular uptake.
Figure 5. Nanoconstructs with high CpG/ODN ratio promote the release of large concentrations (>5×103 pg/mL) of pro-inflammatory and chemotactic cytokines.
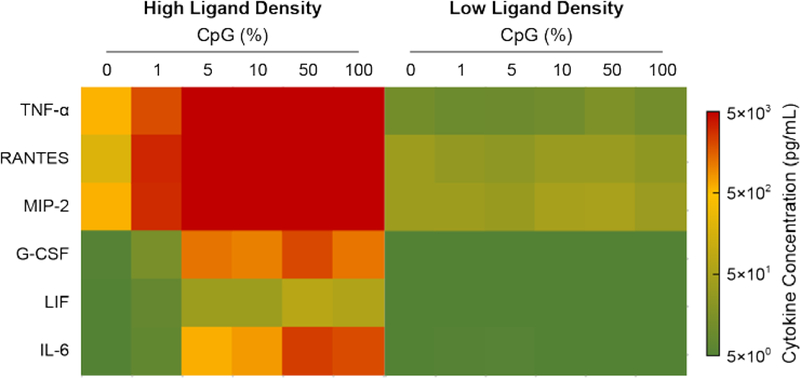
Heat map of cytokines released by Raw-blue cells after 24-h treatment with high and low ODN loading AuNPs (5 nM).
CONCLUSIONS
In summary, we found that ODN shell composition and density are key features in the response of macrophages by Au nanoconstructs. Although nanoconstructs with higher CpG percentages showed lower EC50 values, only 5% CpG was necessary to induce maximal immune activation at high particle concentrations, which maximized the release of cytokines related to cancer therapy and minimized the production of disease-promoting ones. At a fixed CpG content (total CpG amount), cellular uptake, which correlates with ODN shell density, is a primary contributor to immune stimulation. Therefore, ligand shell tunability will be critical in the development of cancer therapeutic agents to achieve specific immune responses.
EXPERIMENTAL SECTION
Materials
Chloroauric acid, tri-sodium citrate dihydrate (sodium citrate), tris(2-carboxyethyl)phosphine hydrochloride (TCEP), potassium iodide, iodine, 11-mercaptoundecyl hexa(ethylene glycol) (MUHEG), sodium borohydride, monosodium phosphate, dithiothreitol, hydrochloric acid (37%), and nitric acid (70%) were bought from Sigma Aldrich, St. Louis, MO. 15-nm AuNPs were synthesized by citrate reduction of chloroauric acid following a previous protocol.32 CpG ODN (5’-TCCATGACGTTCCTGACGTT-(sp18)-disulfide-3’, phosphodiester backbone) and GpC ODN (5’-TCCATGAGCTTCCTGAGCTT-(sp18)-disulfide-3’, phosphodiester backbone) were synthesized by solid phase. Zeocin, Primocin, and antagonists for TLR7/8 (ODN 2087) and TLR7/8 and 9 (ODN 2088) were obtained from Invivogen, San Diego, CA. Fetal bovine serum (FBS), Dulbecco’s phosphate-buffered saline (DPBS) and Dulbecco’s Modified Eagle’s medium (DMEM) were purchased from Thermo Fisher Scientific, Waltham, MA.
Functionalization of Gold Nanoparticles
CpG and GpC ODNs were functionalized on AuNPs following our previous published method.16 In short, the ODN disulfide bonds were reduced to thiol groups with TCEP (20 mM) at room temperature. In order to achieve different oligonucleotide compositions, the ODNs containing CpG and GpC were mixed at different ratios before being exposed to the AuNPs. The thiol terminated ODNs were added to 15-nm AuNP solutions (molar ratios of 200:1 and 25:1 ODN:AuNP for high and low ODN loading, respectively) and left reacting at room temperature under vigorous shaking for 10 min, followed by the addition of sodium citrate buffer (100 mM, pH 5.8). Lastly, MUHEG was added into the solution at a molar ratio of 2000:1 MUHEG:AuNP. The resulting solution was left shaking at room temperature for 5 h, washed three times with a centrifuge and suspended in DPBS. In order to quantify the loading of ODNs on the AuNPs, the Au cores were initially digested by sequentially adding aqueous iodine solution (160 mM iodine and 1M potassium iodide) for 10 min, monosodium phosphate for 10 min, and a mixture of 1:5 sodium borohydride:dithiothreitol for 5 min. The resulting solution was centrifuged at 21,000 ×g, the supernatant collected and the number of ODNs quantified by Quant-iT OliGreen ssDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA). The loading of ODNs per particle was calculated by dividing the number of ODNs by the AuNP concentration, which was quantified by inductively coupled plasma mass spectrometry (ICP-MS, iCAP Q, Thermo Fisher Scientific, Waltham, MA). For the preparation of ODN-AuNPs, we made the assumption that the ratios of CpG to GpC ODNs presented by the AuNPs were the same as the ratios of the solutions used to functionalize the nanoconstructs. The AuNP samples were digested prior ICP-MS analysis in a mixture of hydrochloric acid and nitric acid (1:1) for 30 min and subsequent 20-fold dilution in milli-Q water. The AuNP size was estimated with images captured by a JEOL 1230 transmission electron microscope. The zeta potential of ODN-AuNPs was measured by a ZetaPLUS (Brookhaven Instruments, Holtsville, NY).
Quantification of Macrophage Immune Activation
Murine Raw-blue macrophage (Invivogen, San Diego, CA) was used as cell line in these experiments, and DMEM supplemented with FBS (10%), Zeocin (200 μg/mL) and Primocin (100 μg/mL) was employed as growth medium. The IS activity of Au nanoconstructs was tested by Quanti-Blue assay (Invivogen, San Diego, CA). Raw-blue cells were seeded with a concentration of 2.5×104 cells per well in 96-well plates and left to adhere for 20 h at 37 ºC with 5% CO2. The supernatants were removed and replaced by fresh media containing different concentrations of AuNPs (0 to 20 nM), and the cells were further incubated for 24 h at 37 ºC with 5% CO2. The cell media were collected and centrifuged to remove the AuNPs left in solution. 20 μL of the supernatants were transferred to new wells, mixed with 200 μL of Quanti-blue assay reagent and incubated at 37 ºC with 5% CO2 for 12 h. Finally, the absorbance of the solutions at 635 nm, which correlates with the secrete embryonic alkaline phosphatase levels released by the cells, was recorded.
For the antagonist experiments Raw-blue cells were pre-incubated with 500 nM ODN 2087 or 2088 for 2 h prior the addition of the AuNPs.
Cellular Uptake Studies
Raw-blue cells were seeded with a density of 2×105 cells per well in a 12-well plate, and incubated for 20 h at 37 ºC with 5% CO2. The cell medium was replaced by fresh one containing Au nanoconstructs (5 nM). After incubating for 24 h at 37 ºC with 5% CO2, the supernatant was removed and the cells were washed with DPBS 3 times. Cells were detached from the plates with a scrapper and transferred to an Eppendorf tube for additional washing (250 g × 5 min). The cells were suspended in DPBS and counted with a hemocytometer. Lastly, the cells were digested in a solution of 2% nitric acid and 2% hydrochloric acid at 70 ºC for 12 h, and the Au quantified by ICP-MS.
Intracellular location of Au Nanoconstructs
The macrophages were seeded in 12-well plates with a density of 2×105 cells per well and incubated for 20 h at 37 ºC with 5% CO2. The cell medium was replaced by fresh one containing Au nanoconstructs (5 nM). After 24 h incubation at 37 ºC with 5% CO2, the supernatant was removed and the cells were washed with DPBS 3 times. Cells were detached from the plate with a scrapper, transferred to an Eppendorf tube for additional washing (250 g × 5 min), and the pellet transferred to Karnovsky’s fixative solution to be fixed with a Pelco Biowave microwave following a previously published protocol.26 After fixation, the cells were double-stained with uranyl acetate and lead citrate, and imaged by a JEOL 1230 transmission electron microscope.
Analysis of Cytokines Released by Macrophages
To quantify the release of cytokines by macrophages upon immune activation, Raw-blue cells were seeded with a concentration of 2.5×104 cells per well in 96-well plates and incubated at 37 ºC with 5% CO2. After 20 h, the old cell media were replaced by fresh ones containing ODN-AuNPs (5 nM), and the cells were further incubated for 24 h at 37 ºC with 5% CO2. The cell media were collected and centrifuged to remove the AuNPs left in solution. 25 μL of the supernatants were used to quantify the released cytokines by a Luminex® Multiplex Kit and Luminex® 200 instrument (Invitrogen, Carlsbad, CA) following the manufacturer protocol.
Supplementary Material
ACKNOWLEDGMENTS
The scientific work reported in this article has been supported by the National Cancer Institute of the National Institutes of Health under Award Number U54CA199091 (R.M.P., C.A.M, A.L., T.W.O.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. L.E.C. acknowledges support from Northwestern University’s Cancer Nanotechnology Training Program Award T32CA186897. A.L. and C.A.M. acknowledge support from the Prostate Cancer Foundation and the Movember Foundation under award 17CHAL08. Fluorescence spectroscopy was performed at the High Throughput Analysis Laboratory. Quantification of gold was conducted at the Northwestern University Quantitative Bio-elemental Imaging Center generously supported by NASA Ames Research Center NNA06CB93G.
Footnotes
ASSOCIATED CONTENT
Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Characterization of Au cores and ODN functionalized AuNPs, stability studies, statistical analysis of IS activity of Raw-blue cells after treatment with 10 nM ODN-AuNPs, control experiment of IS with free CpG, cell viability studies, TEM of nanoconstructs inside cells, cytokine release after treatment with free CpG and GpC.
C.A.M. has financial interests in Exicure, Inc. which could potentially benefit from the outcomes of this research.
REFERENCES
- (1).Mellman I, Coukos G, and Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Khalil DN, Smith EL, Brentjens RJ, and Wolchok JD (2016) The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nature Reviews Clinical Oncology 13, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Jahrsdörfer B, and Weiner GJ (2008) CpG oligodeoxynucleotides as immunotherapy in cancer. Update on Cancer Therapeutics 3, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ishii KJ, Gursel I, Gursel M, and Klinman DM (2004) Immunotherapeutic utility of stimulatory and suppressive oligodeoxynucleotides. Curr Opin Mol Ther 6, 166–174. [PubMed] [Google Scholar]
- (5).Kumagai Y, Takeuchi O, and Akira S (2008) TLR9 as a key receptor for the recognition of DNA. Advanced Drug Delivery Reviews 60, 795–804. [DOI] [PubMed] [Google Scholar]
- (6).Arango Duque G, and Descoteaux A (2014) Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Frontiers in Immunology 5, 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Patil SD, Rhodes DG, and Burgess DJ (2005) DNA-based therapeutics and DNA delivery systems: A comprehensive review. The AAPS Journal 7, E61–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Houk BE, Hochhaus G, and Hughes JA (1999) Kinetic modeling of plasmid DNA degradation in rat plasma. AAPS PharmSci 1, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Cutler JI, Auyeung E, and Mirkin CA (2012) Spherical nucleic acids. 134, 1376–1391. [DOI] [PubMed] [Google Scholar]
- (10).Banga RJ, Chernyak N, Narayan SP, Nguyen ST, and Mirkin CA (2014) Liposomal Spherical Nucleic Acids. Journal of the American Chemical Society 136, 9866–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Luo D, and Saltzman WM (2000) Synthetic DNA delivery systems. Nature Biotechnology 18, 33. [DOI] [PubMed] [Google Scholar]
- (12).Zhu S, Xing H, Gordiichuk P, Park J, and Mirkin CA (2018) PLGA Spherical Nucleic Acids. Advanced Materials 30, 1707113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Dykman L, and Khlebtsov N (2012) Gold nanoparticles in biomedical applications: recent advances and perspectives. Chemical Society reviews 41, 2256–2282. [DOI] [PubMed] [Google Scholar]
- (14).Wei M, Chen N, Li J, Yin M, Liang L, He Y, Song H, Fan C, and Huang Q (2012) Polyvalent Immunostimulatory Nanoagents with Self-Assembled CpG Oligonucleotide-Conjugated Gold Nanoparticles. Angewandte Chemie International Edition 51, 1202–1206. [DOI] [PubMed] [Google Scholar]
- (15).Radovic-Moreno AF, Chernyak N, Mader CC, Nallagatla S, Kang RS, Hao L, Walker DA, Halo TL, Merkel TJ, Rische CH, et al. (2015) Immunomodulatory spherical nucleic acids. Proceedings of the National Academy of Sciences 112, 3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yue J, Pallares RM, Cole LE, Coughlin EE, Mirkin CA, Lee A, and Odom TW (2018) Smaller CpG-Conjugated Gold Nanoconstructs Achieve Higher Targeting Specificity of Immune Activation. ACS Applied Materials & Interfaces 10, 21920–21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cavaillon JM (1994) Cytokines and macrophages. Biomedicine & Pharmacotherapy 48, 445–453. [DOI] [PubMed] [Google Scholar]
- (18).Shiang Y-C, Ou C-M, Chen S-J, Ou T-Y, Lin H-J, Huang C-C, and Chang H-T (2013) Highly efficient inhibition of human immunodeficiency virus type 1 reverse transcriptase by aptamers functionalized gold nanoparticles. Nanoscale 5, 2756–2764. [DOI] [PubMed] [Google Scholar]
- (19).Cutler JI, Zhang K, Zheng D, Auyeung E, Prigodich AE, and Mirkin CA (2011) Polyvalent Nucleic Acid Nanostructures. Journal of the American Chemical Society 133, 9254–9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, and Mirkin CA (2007) Oligonucleotide Loading Determines Cellular Uptake of DNA-Modified Gold Nanoparticles. Nano Letters 7, 3818–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pei H, Li F, Wan Y, Wei M, Liu H, Su Y, Chen N, Huang Q, and Fan C (2012) Designed Diblock Oligonucleotide for the Synthesis of Spatially Isolated and Highly Hybridizable Functionalization of DNA–Gold Nanoparticle Nanoconjugates. Journal of the American Chemical Society 134, 11876–11879. [DOI] [PubMed] [Google Scholar]
- (22).Bouyer, F., Gérardin, C., Fajula, F., Putaux, J. L., and Chopin, T. pp 179–184. [DOI] [PubMed]
- (23).Gentili D, Ori G, Ortolani L, Morandi V, and Cavallini M (2017) Cooperative and Reversible Anisotropic Assembly of Gold Nanoparticles by Modulation of Noncovalent Interparticle Interactions. ChemNanoMat 3, 874–878. [Google Scholar]
- (24).Lewis RE, Liao G, Young K, Douglas C, and Kontoyiannis DP (2014) Macrophage Reporter Cell Assay for Screening Immunopharmacological Activity of Cell Wall-Active Antifungals. Antimicrobial Agents and Chemotherapy 58, 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Choi CHJ, Hao L, Narayan SP, Auyeung E, and Mirkin CA (2013) Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Yue J, Feliciano TJ, Li W, Lee A, and Odom TW (2017) Gold Nanoparticle Size and Shape Effects on Cellular Uptake and Intracellular Distribution of siRNA Nanoconstructs. Bioconjugate Chemistry 28, 1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wu XA, Choi CHJ, Zhang C, Hao L, and Mirkin CA (2014) Intracellular Fate of Spherical Nucleic Acid Nanoparticle Conjugates. Journal of the American Chemical Society 136, 7726–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, and Mantovani A (2008) Macrophage polarization in tumour progression. Seminars in Cancer Biology 18, 349–355. [DOI] [PubMed] [Google Scholar]
- (29).Zhu Eric F., Gai Shuning A., Opel Cary F., Kwan Byron H, Surana R, Mihm Martin C., Kauke Monique J., Moynihan Kelly D., Angelini A, Williams Robert T., et al. (2015) Synergistic Innate and Adaptive Immune Response to Combination Immunotherapy with Anti-Tumor Antigen Antibodies and Extended Serum Half-Life IL-2. Cancer Cell 27, 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, and Mantovani A (2003) The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunological Reviews 177, 141–149. [DOI] [PubMed] [Google Scholar]
- (31).Yue X, Wu L, and Hu W (2015) The regulation of leukemia inhibitory factor. Cancer cell & microenvironment 2, e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, and Letsinger RL (1998) One-Pot Colorimetric Differentiation of Polynucleotides with Single Base Imperfections Using Gold Nanoparticle Probes. Journal of the American Chemical Society 120, 1959–1964. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


