Abstract
In the Mediterranean Sea, brown algae belonging to the Cystoseira genus play a valuable role as foundation species. Due to evidences of regression/loss of the habitats of these species caused by the interplay of human and climatic disturbances, active restoration measures have been encouraged by EU regulations. In particular, nondestructive restoration techniques, which avoid the depletion of threatened species in donor populations, are strongly recommended. In the framework of the EU project ROCPOP-Life, the first ex situ outplanting experience of Cystoseira amentacea var. stricta has been implemented in the Cinque Terre Marine Protected Area (northwestern Mediterranean). A total of 400 clay tiles, hosting approximately three mm-long germlings of C. amentacea, were fixed to the rocky shore with screws: the tiles were monitored for the next 2 months by photographic sampling, and survival (presence/absence of juveniles on the tiles), cover and growth were assessed. Additional sampling was performed 6 months after tile deployment, after which an unprecedented storm surge severely affected the restoration performance. After 2 months, over 40% of the tiles were covered with Cystoseira juveniles, which reached approximately eight mm in total length. The tiles that survived the storm hosted three to six cm-long juveniles. The high cover (≥25%), assuring moisture and shading, and the appropriate size of the juveniles, to avert micro-grazing, at time of deployment were key to the survival and growth of the outplanted juveniles, increasing the potential for restoration success. Our findings show that outplanting of midlittoral canopy-forming species is a feasible approach for restoration efforts, with particular attention given to the early phases: (i) laboratory culture, (ii) transport, and (iii) juvenile densities. These results are strongly encouraging for the implementation of restoration actions for C. amentacea on a large scale, in light of EU guidelines.
Keywords: Restoration, Cystoseira amentacea var. stricta, Marine forests, Ex situ outplanting, Canopy-forming brown algae
Introduction
Approximately 35% of brown algae species (Laminariales and Fucales; Guiry & Guiry, 2019) play a key role as ecosystem engineers, building kelp forests, and fucoid canopies, which enhance habitat complexity, biodiversity, ecosystem functioning, and the natural capital of littoral ecosystems (Thompson et al., 1996; Christie, Jørgensen & Norderhaug, 2007; Airoldi et al., 2014). Kelp forests (i.e., Macrocystis, Lessonia, and Laminaria) constitute one of the most diverse and productive ecosystems distributed worldwide (Steneck et al., 2002). In the Mediterranean Sea, some Laminariales (e.g., Laminaria and Sacchoriza) and Fucales (e.g., Sargassum and Cystoseira) also play a role as foundation species in some specific locations, but the canopy-forming brown algae of the Cystoseira genus are the most important because they are widespread in this biogeographic region (Rodríguez-Prieto et al., 2013). However, they are exposed to multiple disturbances that cause a decline in their abundance in many coastal areas (Airoldi, Balata & Beck, 2008; Mineur et al., 2015). The main pressures affecting the valuable ecosystems formed by Cystoseira are sedimentation (Perkol-Finkel & Airoldi, 2010), low water quality (Arévalo, Pinedo & Ballesteros, 2007; Sales et al., 2011), anthropization (Mangialajo, Chiantore & Cattaneo-Vietti, 2008), and overgrazing (Sala, Boudouresque & Harmelin-Vivien, 1998; Hereu, 2004; Verges et al., 2014).
Several studies conducted in the Mediterranean have reported on the past and the present distribution and abundance of Cystoseria canopies (Thibaut et al., 2014; Mancuso et al., 2018), detecting regressions or losses caused by the above mentioned factors (Cormaci & Furnari, 1999; Thibaut et al., 2005; Falace et al., 2010; De La Fuente et al., 2018). Their natural recovery, in the absence of adults, is hampered by the very limited dispersal of Cystoseira species due to the rapid fertilization of their large eggs and zygote sinking (Falace et al., 2018).
In this framework, active marine restoration is strongly encouraged to prevent the loss of the valuable habitats formed by Cystoseira species that enhance biodiversity and preserve ecosystem functions and services, according to the Biodiversity Strategy to 2020 (Target 2; European Commission, 2011).
Three different restoration techniques have been implemented in the Mediterranean Sea for Cystoseira species: (i) transplanting juveniles or adults (Falace, Zanelli & Bressan, 2006; Susini et al., 2007), (ii) positioning fertile receptacles in the target area (in situ; Verdura et al., 2018), or (iii) outplanting juveniles cultured in the laboratory along the shore (ex situ; Sales et al., 2011; Verdura et al., 2018). The latter two techniques are strongly recommended for the restoration of threatened species to avoid the depletion of natural donor populations (Falace et al., 2018). Active restoration actions must be implemented depending on the biological traits of the target species (i.e., reproductive strategy) and the environmental characteristics (i.e., depth and hydrodynamics). The in situ technique seems to be especially suitable for species with high dispersal capacity (e.g., kelps; Reed, Laur & Ebeling, 1988; Gaylord et al., 2002), while the ex situ technique is more appropriate for species with a low dispersal capacity (e.g., Cystoseira amentacea; Mangialajo et al., 2012). Both techniques have been recently validated for a shallow species (C. barbata; Verdura et al., 2018) living in low hydrodynamic conditions, but at present, such approaches have never been tested for the midlittoral habitat.
The aim of this study was to apply the ex situ technique (outplanting) to midlittoral C. amentacea var. stricta Montagne (hereafter C. amentacea), in the Ligurian Sea (northwestern Mediterranean), assessing its survival, cover and growth in the first, most critical, months following implementation. The outplanting was performed in the Cinque Terre Marine Protected Area (Cinque Terre MPA), where, at present, only the more tolerant congener C. compressa is found in continuous and discontinuous belts (Asnaghi et al., 2009; De La Fuente et al., 2018). Here, C. amentacea is presently missing as well as across the whole easternmost side of the Ligurian coastline starting from Punta Manara, which is approximately 20 km northwest of the Cinque Terre MPA (author’s personal observation). This species was recorded in the area until the end of the 19th century (authors’ personal observation through herbarium records, De La Fuente et al., 2018) and its disappearance can be explained by habitat fragmentation due to coastal developments, water pollution and high sediment loads. Such stressors were related to the intense excavating activities that were carried out to extract construction material (mostly for building railways and highways in the area), which occurred in the first half of the 20th century. Even though such disruptive activities have largely been reduced in recent decades, with significant changes in the riverine basin and sediment load to the sea, and a MPA was established (1997), this sensitive species is still absent along these stretches of coastline. This is likely due to its above mentioned low dispersal capacity (<1 m, Johnson & Brawley, 1998; Mangialajo et al., 2012) which hampers the natural recovery of this species, in addition to other local factors, such as mussel farming (as a result of competition for space with the settled mussel juveniles).
The effectiveness of the ex situ approach over the first 6 months following its implementation, was assessed in terms of the presence, cover and growth of the outplanted C. amentacea juveniles in the framework of the EU project ROCPOP-Life.
Materials and Methods
Study sites
The ex situ outplanting of the midlittoral species C. amentacea was performed in summer 2018 following a nondestructive strategy, with apical fronds (ca. three cm in length) holding mature receptacles collected in June from a healthy population located in the Portofino MPA (donor site) along a 200-m stretch of coastline. After a laboratory culturing period, the juveniles were transplanted into the Cinque Terre MPA (receiving site; approximately 80 km from the donor site). Both MPAs are located in the Ligurian Sea, northwestern Mediterranean (Fig. 1). The sites are characterized by a tide in the range of 30 cm (under these conditions, barometric pressure effects may have a dominant effect on the water level) and an average spring temperature of 20 °C. After sampling, the apices were gently cleaned with tweezers and rinsed with filtered seawater to remove adherent biofouling and detritus. Then, the apices, which were wrapped in seawater-wetted towels, were delivered within 24 h to the laboratory in Trieste (northeastern Italy; Fig. 1) under dark, cold, and humid conditions for culturing in environmentally controlled rooms.
Figure 1. Map showing the location of the culture laboratory facilities and the donor (DS) and receiving (RS) sites.
Laboratory ex situ cultivation
Three apices with mature receptacles (additionally cleaned with a brush and rinsed with autoclaved seawater) were placed on each substrate constituted of a rough round tiles composed of clay (4.5 cm in diameter) with a 0.6 cm hole at the center to fix the tile onto the rocks with a screw. On the next day, the fertile apices were removed, and the zygotes attached to the tiles were cultured over a 3-week period (≈450 tiles).
Temperature (+20 °C) and photoperiod (15:9 h light:dark cycle) and light intensity (125 μmoL photons m−2 s−1) were selected according to the protocol provided in Falace et al. (2018). Von Stosch’s enriched filtered seawater was used as the culture medium to accelerate the culturing time for the C. amentacea individuals to reach a greater size at the time of outplanting (Falace et al., 2018). The medium was enriched with antibiotic (two μL of amikacin sulfate and 500 μg of ampicillin sodium L−1 of culture medium) and GeO2 (Falace, Zanelli & Bressan, 2006) to prevent bacterial and diatom growth. The culture medium in the aquaria was renewed every 3 days to minimize any possible effects of nutrient limitation and was continuously aerated by bubbling and water multifunction pumps (≈300 L h−1 flow) to increase oxygenation and hydrodynamics.
After 24 days of controlled growth in the Trieste laboratory, photographs were taken (17th July; 160 random tiles) in order to assess the percent cover and juvenile length.
Outplanting and monitoring in the field
On 19th July 2018 the tiles were transported to Cinque Terre MPA. All the tiles were carefully placed in small boxes filled with filtered seawater, which were placed in a large insulated container that was maintained at a cool temperature with icepacks. The container was transported by car with an air conditioner (≈7-h trip, temperature in the range of 20–22 °C) to the receiving site, where the boxes were stored in an air-conditioned room overnight (at 22 °C).
On the next day (20th July 2018, Time 0), the tiles were carefully transported to the field, using a rubber boat. Eight patches (Fig. S1) were established in the previous weeks: 50 holes were drilled in each patch and screws placed within them in advance. On the day of implementation, the tiles were quickly screwed to the rocks. Overall, the deployment of 400 tiles was performed in approximately 5 h. On the days of transport and deployment, the air temperature was in the range of 23–27 °C and the sea temperature was in the range of 25–26 °C.
Monitoring of the clay tiles started on the same day (Time 0) and continued over the following 2 months: July 27th (Time 1), August 6th (Time 2), August 29th (Time 3), and September 27th (Time 4). During each sampling time, photos were taken of a randomly selected 20 of the 50 clay tiles in each patch. The photos were analyzed in the lab using Image J to assess percent cover and survival (i.e., overall presence/absence of juveniles). Thallus length was measured for 40 randomly selected individual specimens with ImageJ at each sampling time except Time 5 (measured in the field).
Additional sampling was performed 4 months later (Time 5). This sampling occurred after an unprecedented storm surge that affected the Ligurian Sea at the end of October 2018 (ANSA, 2018; The MediTelegraph, 2018), which caused great destruction along the entire Ligurian coastline, with estimated damages of hundreds of millions of euros. This storm caused the destruction of entire harbors and ships in marinas, particularly along the eastern side of the Ligurian coast. Huge damage was reported among the shallow benthic communities, particularly the Posidonia meadows (IL Secolo XIX, 2018). Because most of the tiles were detached by this unprecedented event (180 km h−1 winds and waves greater than 10 m high), it was not possible to quantitatively assess restoration performance in terms of percent cover but only in terms of juvenile growth (length) on the 16 tiles with C. amentacea juveniles of the approximatively 69 remaining tiles.
Data analysis
One-way ANOVA was applied to assess possible differences in the percent cover of the juveniles on the tiles when leaving the laboratory versus at the time of the deployment (Time 0) after arcsin transformation of the data and verification of assumptions (normality using the Shapiro–Wilk test and homoscedasticity using Bartlett’s test).
The effect of percent cover at the time of deployment was assessed using a generalized linear model (GLM) for both the percent cover (family = quasibinomial) and the presence/absence (family=binomial) of juveniles on the tiles at Time 4, using the “cover class” of each patch at the start of the experiment as the predictor variable. Patches were classified according to 3 “cover classes” (based on the percent cover at the start of the experiment): Low (18.2 ± 1.6, avg ± SE; patches 2, 3, and 5), Medium (25.0 ± 2.1; patches 1, 7, and 8), and High (32.2 ± 2.5; patches 4 and 6). Statistical analyses were performed with the free software R (R Core Team, 2015) using the stats package.
Results
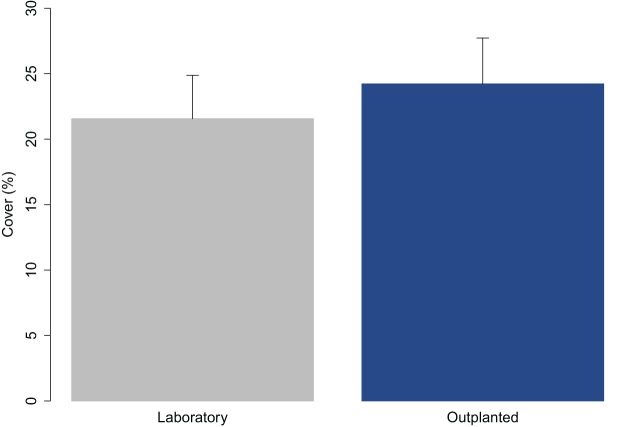
The juveniles were 2.65 ± 0.05 mm (avg ± SE) in length when they were transported to the receiving site. The percent cover on the tiles measured at Time 0 was compared with that at the time they left the laboratory (3 days before) to assess the effect of transport (Fig. 2). The percent cover in the laboratory and in outplants at Time 0 did not significantly differ (one-way ANOVA; p = 0.327).
Figure 2. Percent cover of C. amentacea (average + SE) on tiles when leaving the controlled growth conditions (laboratory) and at the time of positioning (outplanting) at the receiving site (72 h later).
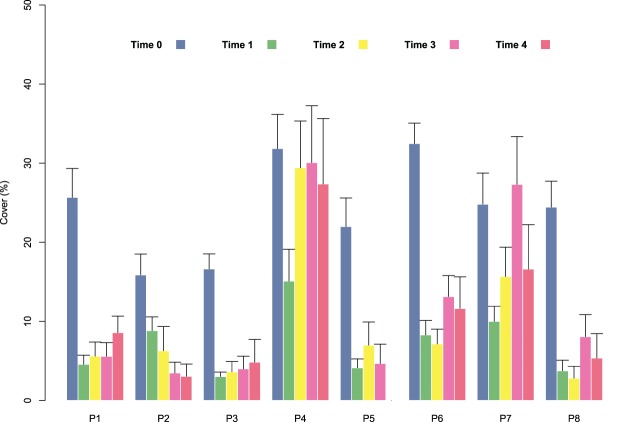
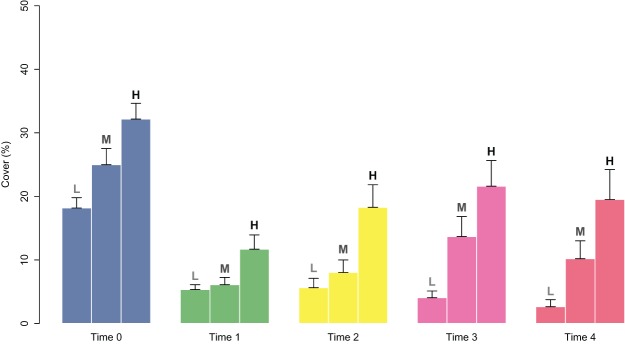
At the start of tile deployment, the average percent cover of the juveniles was 24.22 (±1.24%). The percent cover data of the juveniles on the tiles in the eight patches over time (Time 0–Time 4) are reported in Fig. 3. All patches showed a sharp decline in percent cover from Time 0 to Time 1 due to the predictable loss of some juveniles in the field. Some patches clearly showed lower average values of cover at Time 0 (ranging from 15.88 ± 2.63 in patch 2 to 32.48 ± 2.59 in patch 6; avg ± SE), and this difference in cover at the start of the experiment affected the survival and growth of the juveniles over time (Fig. 3). In the patches characterized by higher cover of juveniles at the beginning of the experiment (patches 4 and 6), after the first decline, cover increased on the following sampling dates (reaching 27.37 ± 2.63 and 11.65 ± 3.99 at Time 4 in patches 4 and 6, respectively) because of the growth of the juveniles (Figs. 3 and 4), while the patches characterized by lower cover showed a general decrease in percent cover (reaching 3.06 ± 1.55 and 4.85 ± 2.88 at Time 4 in patches 2 and 3, respectively; juveniles lost in patch 5). The GLM for the percent cover on the tiles at Time 4 showed significant differences among the classes (p = 0.0143). The Low class was significantly differed from the others, while the Medium class did not differ from the High class (Table 1).
Figure 3. Percent cover of C. amentacea (average + SE) on the clay tiles over time (Time 0–Time 4) in the eight patches at the receiving site.
Figure 4. Percent cover of C. amentacea juveniles (average + SE) in each cover class (Low-L, Medium-M, High-H) on the clay tiles over time (Time 0–Time 4) at the start of the experiment.
Table 1. Results of the GLMs of the differences in percent cover and presence/absence of juveniles of the cover classes (Low, Medium, High) at Time 4.
| Presence/absence | Estimate | Std. error | z-value | Pr(>|z|) | |
|---|---|---|---|---|---|
| High–Medium | 0.05084 | 0.28864 | 0.176 | 0.98288 | |
| High–Low | −0.99550 | 0.36378 | −2.373 | 0.01689 | |
| Medium–Low | 1.04634 | 0.32695 | 3.200 | 0.00387 | |
| Percent cover | |||||
| High–Medium | −0.8179 | 0.3683 | −2.220 | 0.0652 | |
| High–Low | −2.2942 | 0.5732 | −4.003 | <0.001 | |
| Medium–Low | 1.4763 | 0.5642 | 2.617 | 0.0231 |
Note:
The significant differences are highlighted in bold.
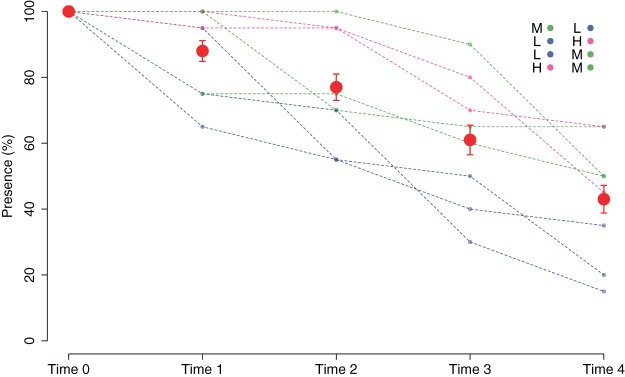
The good performance of the outplanting, at least in the early phases, is confirmed by the assessment of the percentage of tiles with the presence of juveniles (Fig. 5). In fact, from Time 0 to Time 1, this percentage changed from 100% to 88% and was still over 40% after 2 months (Time 4). Similar to cover, survival also differed in the patches characterized by higher cover of juveniles at the start of the experiment. In fact, the GLM on the presence/absence of juveniles on the tiles at Time 4 using the cover class of each patch at the start of the experiment as a predictor variable showed significant differences among the classes (p = 0.00161). The Low class significantly differed from the others, while the Medium class did not differ from the High class (Table 1): this implies that cover at the start of the experiment ≥ 25% is required for a good outplanting performance.
Figure 5. Percentage of tiles with C. amentacea juveniles over time (Time 0–Time 4) according to presence/absence data for the individual tiles in the eight patches at the receiving site.
Eight patches were allocated to the different cover classes. Red dots represent average values across the patches (average ± SE). L, low cover class; M, medium cover class; H, high cover class.
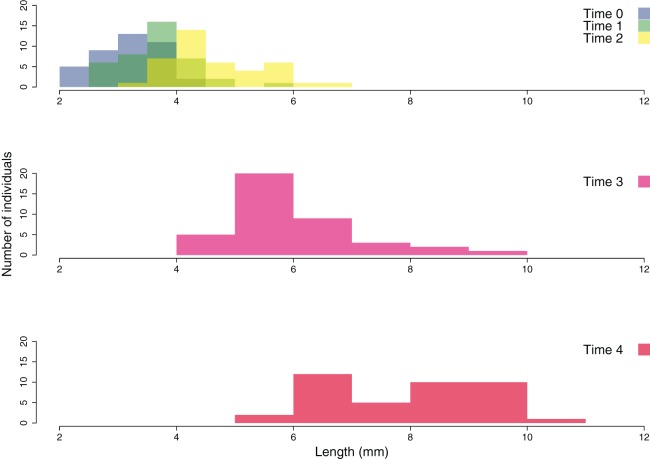
The growth of the ouplanted juveniles over time is shown in Figs. 6 and 7. The thallus length of the juveniles of C. amentacea was mostly under six mm at Time 0 (3.22 ± 0.08, avg ± SE), Time 1 (3.73 ± 0.10) and Time 2 (4.67 ± 0.13). At Time 3 (6.02 ± 0.18) and Time 4, juveniles grew to up to 11 mm, with a higher number of individuals 8–10 mm in size at Time 4 (8.03 ± 0.22). After 6 months (Time 5), the outplanted juveniles reached three to six cm in length (last record, in February 2019).
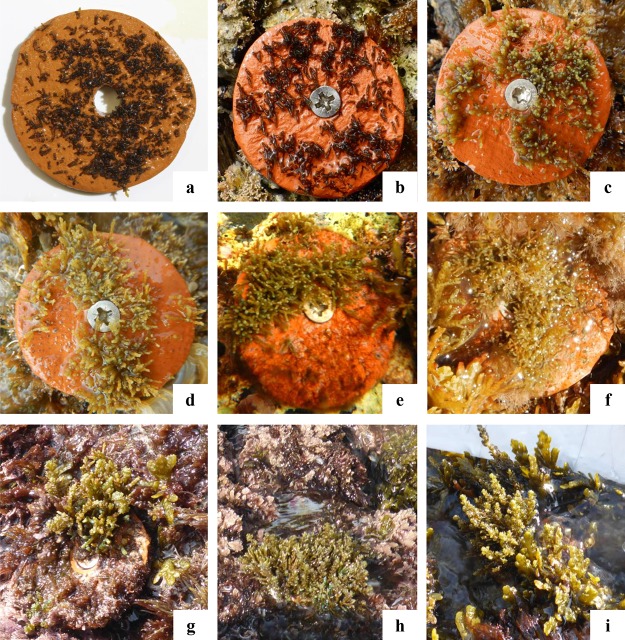
Figure 6. Development of the outplanted juveniles on tiles over time (Time 0–Time 5) at the receiving site.
(A) Juveniles leaving the lab facilities (avg: 2.67 mm), (B) Time 0—outplanting day (avg: 3.22 mm), (C) Time 1—1 week (avg: 3.73 mm), (D) Time 2—2 weeks (avg: 4.67 mm), (E) Time 3—1 month (avg: 6.02 mm), (F) Time 4—2 months (avg: 8.03 mm), (G–I) juveniles 6 months after outplanting (three to six cm).
Figure 7. Size frequency distribution of the outplanted juveniles of Cystoseira amentacea over time.
Discussion
Outplanting represents an innovative technique for restocking of brown canopy-forming macroalgae (Falace et al., 2018), although its implementation consists of a set of delicate steps: (i) fertile material collection, (ii) culturing of juveniles in the lab, (iii) transport of juveniles to the field, and (iv) attachment of the juveniles to the rocky shore. In addition to the multiple set of phases, this technique needs to be adapted according to target species-specific requirements in the different phases of implementation.
The lab culture process involves several issues related to the wellness of the sensitive embryo stage. The thorough cleaning of the fertile apices before and after transport to the lab facilities to reduce epiphyte outbreaks and the presence of grazers helps to increase the chance of good performance. The C. amentacea embryos were cultured under their optimal temperature and light intensity conditions to maximize the yield (Falace et al., 2018), which can also unfortunately enhance spores, propagules, and bacteria proliferation. The implemented culture setting (e.g., the addition of antibacterial solutions and enhanced hydrodynamics) preserved the culture in good conditions, with healthy juveniles larger (2.65 mm) than those in previous Cystoseira restoration studies being obtained in only 3 weeks: C. amentacea (3 weeks—1.38 mm; Falace et al., 2018) and C. barbata (1 month 200–400 μm; Verdura et al., 2018). Furthermore, the length recorded 1 month after outplanting (6.02 mm; Fig. 6) is similar or even slightly larger than that recorded in a previous study on C. amentacea (4.73 mm; Falace et al., 2018) because the outplanting size was also larger in the present study.
The transport of early life stages from the nursery facility to the receiving site, which may be located a large distance away, as in this case (≈600 km), was found to not affect their fitness. The results show no significant differences in the percent cover of juveniles before and after transport. The cover of the juveniles on tiles at the time of arrival at the receiving site was even slightly greater than that when leaving the nursery facility (before transport—21.6%, after transport—24.2%; Fig. 2). This result was obtained maintaining a good temperature range (20–22 °C) during transport and during the attachment of the clay tiles in the field (outplanting action). Due to the high numbers, the clay tiles were transported, by rubber boat in cooled boxes to the rocky shore in several times over the course of the same day to prevent solar heat stress during attachment.
The attachment technique applied in this study, using pre-established screws instead of epoxy putty, which is generally used for both adults and juveniles (Susini et al., 2007; Whitaker, Smith & Murray, 2010; Perkol-Finkel et al., 2012; Verdura et al., 2018), increases the effectiveness of outplanting by reducing the time spent for attachment (the deployment of 400 tiles was performed in 5 h by six people) and the possible dislodgement by wave action on the day of deployment or the following days (Susini et al., 2007). This method additionally minimizes the esthetic and environmental impacts on the rocky shores and is reported to reinforce attachment, preventing the possible dislodgement of the clay tiles by wave action, as in the case of Lessonia nigrescens restoration (Vásquez & Tala, 1995). This is particularly important for midlittoral species living under high hydrodynamic conditions, as in the case of C. amentacea. In our study, we observed very positive results over the first 2 months (with the overall dislodgement of approximately 39 tiles). Unfortunately, the experiment was strongly affected by an unprecedented storm surge (winds over 170 km h−1 and significant wave height of six m and maximum waves of 10 m height, over approximately 12 h). This storm surge caused the destruction of entire harbors and ships in marinas and caused great damage to the shallow benthic communities. This event caused a large loss of tiles: 69 tiles survived the storm out of the 361 reported at Time 4. This means that, notwithstanding the exceptional strength of the storm, 20% survived the event, although only 16 of them hosted juveniles. Overall, the attachment method used in this study seems to be not only more environmentally friendly than the epoxy putty attachment method but also quite resistant to wave action. The screws themselves were not at all removed from the shore.
In addition to providing information for the optimization of culture and transport/outplanting techniques, our results stress the relevance of juvenile cover on the tiles. Relatively high cover (≥ 25% of the tile covered at the time of deployment) ensures the survival of the outplanted juveniles (Table 1; Fig. 4). Midlittoral species are exposed to high solar heat and desiccation stress; therefore, a higher percentage of cover allows moisture and shading to be retained, enhancing the development of early juveniles (Brawley & Johnson, 1991; Dudgeon & Petraitis, 2005).
Another key issue regulating outplanting success is grazing, which was reported in previous studies, for example, on Sargassum species, where did not obviously affect the development of the outplanted juveniles in order to establish population in the restoration site, because of the low density of major grazers (seven individuals/0.25 m2, Yu et al., 2012) and the use of cages to protect juveniles from grazing, until reaching a suitable grazing free size (Yoon, Sun & Chung, 2014). In this study, we did not formally test the effect of grazing, but we can exclude it having a relevant role. No grazers were observed crawling on the tiles at the time of sampling or in the photographs. In terms of small grazers (mostly small amphipods and isopods), their potential abundance may be estimated from the paper by Thrush et al. (2011), which reports densities of grazing crustaceans in the range of nine individuals/20 cm2 in the area, which is likely not high enough to exert a strong grazing effect. Furthermore, the tiles were located relatively high on the shore compared to the mean sea water level, and this could actually prevent grazing by fish and sea urchins, which are generally considered the most relevant herbivores (Ling et al., 2015; Gianni et al., 2017).
Conclusions
Our findings show that outplanting midlittoral canopy-forming species is a feasible approach for restoration efforts. In particular, our study addressed the early steps of restoration and provides information on best practices for the outplanting phases of laboratory culture, transport, and reintroduction in the natural environment.
The laboratory culture phase requires appropriate species-specific protocols to reduce outbreaks of epiphytes, obtain high cover and a large size of the juveniles for field deployment, and increase the potential for restoration success. The use of an appropriate culture medium, the addition of an antibacterial solution and thorough fertile material cleaning are relevant elements likely to guarantee good culture performance and obtain high densities of healthy embryos.
The feasibility of large distance transport from the laboratory to the field has been remarkably shown, providing a potential option for replication also on a large scale.
The applied screw attachment technique was found to be effective in terms of increasing the efficiency of tile deployment and resistance to dislodgement, particularly in the early stages of restoration, when the traditional epoxy putty technique may be strongly affected by wave action, which is particularly relevant for midlittoral species.
These results are strongly encouraging for the implementation of restoration actions of canopy-forming species on a large scale, in light of EU guidelines.
Supplemental Information
Map data ©2018 Google using QGIS v 3.4.4.
Acknowledgments
We would like to thank the contributions of all colleagues and students who helped in the field and during samples processing: Saul Ciriaco (Shoreline Soc. COOP), Sara Menon (Shoreline Soc. COOP), Marco Segarich (Shoreline Soc. COOP), Massimo Andreoli (Cinque Terre MPA), Enrico Agostini (Nemo-Italia), Davide Monteggia (University of Genoa), Maria Paola Ferranti (University of Genoa), Lorenzo Meroni (University of Genoa), Greta Fallanca (University of Genoa), Francesca Piga (University of Genoa). We are sincerely grateful to the referees Enric Ballesteros, Kiran Liversage and an anonymous third referee, as well as to the editor Anastazia Banaszak for improving this manuscript with their valuable comments.
Funding Statement
This study was supported by the LIFE financial instrument of the European Community, project ROC-POP-LIFE (LIFE16 NAT/IT/000816). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Gina De La Fuente conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Mariachiara Chiantore conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Valentina Asnaghi conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Sara Kaleb conceived and designed the experiments, performed the experiments, approved the final draft.
Annalisa Falace conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field experiments were performed under permission of the Cinque Terre Marine Protected Area.
Data Availability
References
- Airoldi, Balata & Beck (2008).Airoldi L, Balata D, Beck MW. The gray zone: relationships between habitat loss and marine diversity and their applications in conservation. Journal of Experimental Marine Biology and Ecology. 2008;366(1–2):8–15. doi: 10.1016/j.jembe.2008.07.034. [DOI] [Google Scholar]
- Airoldi et al. (2014).Airoldi L, Ballesteros E, Buonuomo R, Van Belzen J, Bouma TJ, Cebrian E, De Clerk O, Engelen AH, Ferrario F, Fraschetti S, Gianni F, Guidetti P, Ivesa L, Mancuso FP, Micheli F, Perkol-Finkel S, Serrao EA, Strain EM, Mangialajo L. Marine forests at risk: solutions to halt the loss and promote the recovery of Mediterranean canopy-forming seaweeds. 5th Mediterranean Symposium on Marine Vegetation; Portoroz, Slovenia: 2014. [Google Scholar]
- ANSA (2018).ANSA Storm death toll up to 11, Liguria on its knees. 2018. http://www.ansa.it/english/newswire/english_service/2018/10/30/ansa-storm-death-toll-up-to-11-liguria-on-its-knees_0973b7e8-492d-4802-8e16-9820abe65783.html. [30 October 2018]. http://www.ansa.it/english/newswire/english_service/2018/10/30/ansa-storm-death-toll-up-to-11-liguria-on-its-knees_0973b7e8-492d-4802-8e16-9820abe65783.html
- Arévalo, Pinedo & Ballesteros (2007).Arévalo R, Pinedo S, Ballesteros E. Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: descriptive study and test of proposed methods to assess water quality regarding macroalgae. Marine Pollution Bulletin. 2007;55(1–6):104–113. doi: 10.1016/j.marpolbul.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Asnaghi et al. (2009).Asnaghi V, Chiantore M, Bertolotto R-M, Parravicini V, Cattaneo-Vietti R, Gaino F, Moretto P, Privitera D, Mangialajo L. Implementation of the European water framework directive: natural variability associated with the CARLIT method on the rocky shores of the Ligurian Sea (Italy) Marine Ecology. 2009;30(4):505–513. doi: 10.1111/j.1439-0485.2009.00346.x. [DOI] [Google Scholar]
- Brawley & Johnson (1991).Brawley SH, Johnson LE. Survival of fucoid embryos in the intertidal zone depends upon development stage and microhabitat. Journal of Phycology. 1991;27(2):179–186. doi: 10.1111/j.0022-3646.1991.00179.x. [DOI] [Google Scholar]
- Christie, Jørgensen & Norderhaug (2007).Christie H, Jørgensen NM, Norderhaug KM. Bushy or smooth, high or low; importance of habitat architecture and vertical position for distribution of fauna on kelp. Journal of Sea Research. 2007;58(3):198–208. doi: 10.1016/j.seares.2007.03.006. [DOI] [Google Scholar]
- Cormaci & Furnari (1999).Cormaci M, Furnari G. Changes of the benthic algal flora of the Tremiti Islands (southern Adriatic) Italy. Sixteenth International Seaweed Symposium; Dordrecht: Springer; 1999. pp. 75–79. [Google Scholar]
- De La Fuente et al. (2018).De La Fuente G, Chiantore M, Gaino F, Asnaghi V. Ecological status improvement over a decade along the Ligurian coast according to a macroalgae based index (CARLIT) PLOS ONE. 2018;13(12):e0206826. doi: 10.1371/journal.pone.0206826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudgeon & Petraitis (2005).Dudgeon S, Petraitis PS. First year demography of the foundation species, Ascophyllum nodosum, and its community implications. Oikos. 2005;109(2):405–415. doi: 10.1111/j.0030-1299.2005.13782.x. [DOI] [Google Scholar]
- European Commission (2011).European Commission . Brussels: 2011. Communication from the Commission to the European Parliament, the Council, the Economic and Social Committee of the Region. Our Life Insurance, Our Natural Capital: An EU Biodiversity Strategy to 2020. [Google Scholar]
- Falace et al. (2010).Falace A, Alongi G, Cormaci M, Furnari G, Curiel D, Cecere E, Petrocelli A. Changes in the benthic algae along the Adriatic Sea in the last three decades. Chemistry and Ecology. 2010;26(supp 1):77–90. doi: 10.1080/02757541003689837. [DOI] [Google Scholar]
- Falace et al. (2018).Falace A, Kaleb S, De La Fuente G, Asnaghi V, Chiantore M. Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLOS ONE. 2018;13(2):e0193011. doi: 10.1371/journal.pone.0193011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falace, Zanelli & Bressan (2006).Falace A, Zanelli E, Bressan G. Algal transplantation as a potential tool for artificial reef management and environmental mitigation. Bulletin of Marine Science. 2006;8:161–166. [Google Scholar]
- Gaylord et al. (2002).Gaylord B, Reed DC, Raimondi PT, Washburn L, McLean SR. A physically based model of macroalgal spore dispersal in the wave and current-dominated nearshore. Ecology. 2002;83(5):1239–1251. doi: 10.1890/0012-9658(2002)083[1239:APBMOM]2.0.CO;2. [DOI] [Google Scholar]
- Gianni et al. (2017).Gianni F, Bartolini F, Pey A, Laurent M, Martins GM, Airoldi L, Mangialajo L. Threats to large brown algal forests in temperate seas: the overlooked role of native herbivorous fish. Scientific Reports. 2017;7(1):6012. doi: 10.1038/s41598-017-06394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiry & Guiry (2019).Guiry MD, Guiry GM. AlgaeBase. Galway: National University of Ireland, World-wide electronic publication; 2019. [4 February 2019]. [Google Scholar]
- Hereu (2004).Hereu B. The role of trophic interactions between fishes, sea urchins and algae in the northwestern Mediterranean rocky infralittoral. 2004. PhD thesis. University of Barcelona.
- IL Secolo XIX (2018).IL Secolo XIX Mareggiata, una catastrofe che ha sconvolto i fondali di Portofino. 2018. https://www.ilsecoloxix.it/p/italia/2018/11/20/ADqanphC-mareggiata_sconvolto_catastrofe.shtml. [20 November 2018]. https://www.ilsecoloxix.it/p/italia/2018/11/20/ADqanphC-mareggiata_sconvolto_catastrofe.shtml
- Johnson & Brawley (1998).Johnson LE, Brawley SH. Dispersal and recruitment of a canopy-forming intertidal alga: the relative roles of propagule availability and post-settlement processes. Oecologia. 1998;117(4):517–526. doi: 10.1007/s004420050688. [DOI] [PubMed] [Google Scholar]
- Ling et al. (2015).Ling SD, Scheibling RE, Rassweiler A, Johnson CR, Shears N, Connell SD, Salomon AK, Norderhaug KM, Pérez-Matus A, Hernández JC, Clemente S, Blamey LK, Hereu B, Ballesteros E, Sala E, Garrabou J, Cebrian E, Zabala M, Fujita D, Johnson LE. Global regime shift dynamics of catastrophic sea urchin overgrazing. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1659):20130269. doi: 10.1098/rstb.2013.0269. [DOI] [Google Scholar]
- Mancuso et al. (2018).Mancuso FP, Strain EMA, Piccioni E, De Clerck O, Sarà G, Airoldi L. Status of vulnerable Cystoseira populations along the Italian infralittoral fringe, and relationships with environmental and anthropogenic variables. Marine Pollution Bulletin. 2018;129(2):762–771. doi: 10.1016/j.marpolbul.2017.10.068. [DOI] [PubMed] [Google Scholar]
- Mangialajo, Chiantore & Cattaneo-Vietti (2008).Mangialajo L, Chiantore M, Cattaneo-Vietti R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Marine Ecology Progress Series. 2008;358:63–74. doi: 10.3354/meps07400. [DOI] [Google Scholar]
- Mangialajo et al. (2012).Mangialajo L, Chiantore M, Susini M-L, Meinesz A, Cattaneo-Vietti R, Thibaut T. Zonation patterns and interspecific relationships of fucoids in microtidal environments. Journal of Experimental Marine Biology and Ecology. 2012;412:72–80. doi: 10.1016/j.jembe.2011.10.031. [DOI] [Google Scholar]
- Mineur et al. (2015).Mineur F, Arenas F, Assis J, Davies AJ, Engelen AH, Fernandes F, Malta E-J, Thibaut T, Van Nguyen T, Váz-Pinto F, Vranken S, Serrao EA, De Clerk O. European seaweeds under pressure: consequences for communities and ecosystem functioning. Journal of Sea Research. 2015;98:91–108. doi: 10.1016/j.seares.2014.11.004. [DOI] [Google Scholar]
- Perkol-Finkel & Airoldi (2010).Perkol-Finkel S, Airoldi L. Loss and recovery potential of marine habitats: an experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic Sea. PLOS ONE. 2010;5(5):e10791. doi: 10.1371/journal.pone.0010791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkol-Finkel et al. (2012).Perkol-Finkel S, Ferrario F, Nicotera V, Airoldi L. Conservation challenges in urban seascapes: promoting the growth of threatened species on coastal infrastructures. Journal of Applied Ecology. 2012;49(6):1457–1466. doi: 10.1111/j.1365-2664.2012.02204.x. [DOI] [Google Scholar]
- R Core Team (2015).R Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- Reed, Laur & Ebeling (1988).Reed DC, Laur DR, Ebeling AW. Variation in algal dispersal and recruitment: the importance of episodic events. Ecological Monographs. 1988;58(4):321–335. doi: 10.2307/1942543. [DOI] [Google Scholar]
- Rodríguez-Prieto et al. (2013).Rodríguez-Prieto C, Ballesteros E, Boisset F, Afonso-Carrillo J. Guía de las macroalgas y fanerógamas marinas del Mediterráneo occidental. Barcelona: Ediciones Omega; 2013. [Google Scholar]
- Sala, Boudouresque & Harmelin-Vivien (1998).Sala E, Boudouresque CF, Harmelin-Vivien M. Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos. 1998;82(3):425–439. doi: 10.2307/3546364. [DOI] [Google Scholar]
- Sales et al. (2011).Sales M, Cebrian E, Tomas F, Ballesteros E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta) Estuarine, Coastal and Shelf Science. 2011;92(3):347–357. doi: 10.1016/j.ecss.2011.01.008. [DOI] [Google Scholar]
- Steneck et al. (2002).Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation. 2002;29(4):436–459. doi: 10.1017/S0376892902000322. [DOI] [Google Scholar]
- Susini et al. (2007).Susini ML, Mangialajo L, Thibaut T, Meinesz A. Development of a transplantation technique of Cystoseira amentacea var. stricta and Cystoseira compressa. Hydrobiologia. 2007;580(1):241–244. doi: 10.1007/s10750-006-0449-9. [DOI] [Google Scholar]
- The MediTelegraph (2018).The MediTelegraph Liguria hit by record-breaking storm surge. 2018. http://www.themeditelegraph.com/en/shipping/2018/11/08/liguria-hit-record-breaking-storm-surge-zs6juoPVgGkXvBWQJL9KuJ/index.html. [8 November 2018]. http://www.themeditelegraph.com/en/shipping/2018/11/08/liguria-hit-record-breaking-storm-surge-zs6juoPVgGkXvBWQJL9KuJ/index.html
- Thibaut et al. (2014).Thibaut T, Blanfuné A, Markovic L, Verlaque M, Boudouresque CF, Perret-Boudouresque M, Mácic V, Bottin L. Unexpected abundance and long-term relative stability of the brown alga Cystoseira amentacea, hitherto regarded as a threatened species, in the north-western Mediterranean Sea. Marine Pollution Bulletin. 2014;89(1–2):305–323. doi: 10.1016/j.marpolbul.2014.09.043. [DOI] [PubMed] [Google Scholar]
- Thibaut et al. (2005).Thibaut T, Pinedo S, Torras X, Ballesteros E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean) Marine Pollution Bulletin. 2005;50(12):1472–1489. doi: 10.1016/j.marpolbul.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Thompson et al. (1996).Thompson RC, Wilson BJ, Tobin ML, Hill AS, Hawkins SJ. Biologically generated habitat provision and diversity of rocky shore organisms at a hierarchy of spatial scales. Journal of Experimental Marine Biology and Ecology. 1996;202(1):73–84. doi: 10.1016/0022-0981(96)00032-9. [DOI] [Google Scholar]
- Thrush et al. (2011).Thrush SF, Chiantore M, Asnaghi V, Hewitt J, Fiorentino D, Cattaneo-Vietti R. Habitat-diversity relationships in rocky shore algal turf infaunal communities. Marine Ecology Progress Series. 2011;424:119–132. doi: 10.3354/meps08960. [DOI] [Google Scholar]
- Verdura et al. (2018).Verdura J, Sales M, Ballesteros E, Cefalì ME, Cebrian E. Restoration of a canopy-forming alga based on recruitment enhancement: methods and long-term success assessment. Frontiers in Plant Science. 2018;9:1832. doi: 10.3389/fpls.2018.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verges et al. (2014).Verges A, Steinberg PD, Hay ME, Poore AGB, Campbell AH, Ballesteros E, Heck KL, Booth DJ, Coleman MA, Feary DA, Figueira W, Langlois T, Marzinelli EM, Mizerek T, Mumby PJ, Nakamura Y, Roughan M, van Sebille E, Gupta AS, Smale DA, Tomas F, Wernberg T, Wilson SK. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proceedings of the Royal Society B: Biological Sciences. 2014;281(1789):20140846. doi: 10.1098/rspb.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez & Tala (1995).Vásquez JA, Tala F. Repopulation of intertidal areas with Lessonia nigrescens in northern Chile. Journal of Applied Phycology. 1995;7(4):347–349. doi: 10.1007/BF00003791. [DOI] [Google Scholar]
- Whitaker, Smith & Murray (2010).Whitaker SG, Smith JR, Murray SN. Reestablishment of the Southern California rocky intertidal brown alga, Silvetia compressa: an experimental investigation of techniques and abiotic and biotic factors that affect restoration success. Restoration Ecology. 2010;18:18–26. doi: 10.1111/j.1526-100X.2010.00717.x. [DOI] [Google Scholar]
- Yoon, Sun & Chung (2014).Yoon JT, Sun SM, Chung G. Sargassum bed restoration by transplantation of germlings grown under protective mesh cage. Journal of Applied Phycology. 2014;26(1):505–509. doi: 10.1007/s10811-013-0058-8. [DOI] [Google Scholar]
- Yu et al. (2012).Yu YQ, Zhang QS, Tang YZ, Zhang SB, Lu ZC, Chu SH, Tang XX. Establishment of intertidal seaweed beds of Sargassum thunbergii through habitat creation and germling seeding. Ecological Engineering. 2012;44:10–17. doi: 10.1016/j.ecoleng.2012.03.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Map data ©2018 Google using QGIS v 3.4.4.
Data Availability Statement
The following information was supplied regarding data availability:
Percent cover and presence/absence data of the Cystoseira outplanted juveniles from T0 to T4 are available, respectively, in Data S1 and S2.