Abstract
Hypoxic-ischemic encephalopathy (HIE) is a serious disease for neonates. However, present therapeutic strategies are not effective enough for treating HIE. Previous study showed that mesenchymal stem cells (MSCs) can exert neuroprotective effects for brain damage, but its mechanism remains elusive. Using in vitro coculture of rat cortical primary neurons and MSCs in HI conditions, we demonstrated that MSCs help increase brain derived neurotrophic factor (BDNF) and autophagy markers (LC3II and Beclin1) in the cultures and decrease cells death (lactate dehydrogenase levels). We demonstrated a similar mechanism using an in vivo rat model of HI in combination with MSCs transplantation. Using a behavioral study, we further showed that MSCs transplantation into the rat brain after HI injury can attenuate behavioral deficits. Finally, we found that the increase in BDNF and autophagy related factors after HI injury combined with MSCs transplantation can be reversed by anti-BDNF treatment and strengthen the point that the protective effects of BDNF work through inhibition of the mammalin target of rapamycin (mTOR) pathway. Collectively, we proposed that coculture/transplantation of MSCs after HI injury leads to increased BDNF expression and a subsequent reduction in mTOR pathway activation that results in increased autophagy and neuroprotection. This finding gives a hint to explore new strategies for treating neonates with HIE.
Keywords: Mesenchymal stem cells, Autophagy, Hypoxia-ischemia, Brain damage, Brain derived neurotrophic factor, Mammalian target of rapamycin
Introduction
Hypoxic-ischemic encephalopathy (HIE) is a common and serious disease with high mortality and long-term disabilities such as mental retardation, cerebral palsy, seizures, and learning disabilities [1]. The incidence of HIE is 1–8 per 1,000 live births in the developed countries and is as high as 26 per 1,000 live births in the underdeveloped countries [2]. However, therapeutic strategies for HIE remain scarce. Hence, developing new treatment options for neonates that effectively prevent or diminish the development of HIE is important. To research the pathogenesis and prevention measures of HIE, scientists established hypoxia-ischemia brain damage (HIBD) animal model to simulate HIE in clinics.
Autophagy has been reported to participate in the pathogenesis of HIE. Autophagy, a major intracellular degradation process, is a main pathway for the degradation of abnormal and aggregated proteins. Autophagy was also acting as a cytoprotective response, especially under stress or injury conditions such as hypoxia-ischemia [3, 4]. There are many lines of evidence suggesting that the autophagy pathway is involved in HIBD [5–8]. The induction of autophagy has been found in both neonatal and adult rodents after HIBD [5, 7, 8]. Ginet et al. [6] showed increase autophagosomal markers such as LC3 and Beclin1 in the dying neurons of HIE human newborns, which suggests that autophagy might be closely coupled with HIE pathogenesis. However, whether cellular autophagy could protect neurons and what are the mechanisms after HIE are not clear.
Previous studies showed that mesenchymal stem cells (MSCs) exert neuroprotective effects through complex mechanisms, such as secreting neurotrophic factor, angiogenesis, inhibiting apoptosis, and modulating the immune system [9–12]. MSCs are multipotent stem cells present in adult bone marrow that are capable of differentiating into various cell types under the appropriate conditions. Recently, Shin et al. [13] reported that MSCs could exert a neuroprotective effect by enhancing autophagy in an Alzheimer’s disease model. However, whether autophagy is regulated in HIBD after MSCs transplantation and the underlying mechanisms are not clear. Thus, we investigated whether autophagy is involved in the protective mechanism of MSCs.
Recent studies have reported that one of the protective mechanisms of MSCs was the secretion of various neurotrophic factors including brain derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) [14–16]. Furthermore, BDNF triggers the phosphatidylinositol 3-kinase (PI3K)/mammalin target of rapamycin (mTOR) signaling pathway, which is important in the induction of autophagy [17, 18]. Furthermore, the mTOR signaling pathway is also involved in the regulation of cell death and survival [18]. Our previous study showed that mTOR induced neuronal apoptosis at 24 hours after HIBD in developing rat brain, suggesting mTOR pathway plays a role in the pathogenesis of HIBD [19]. However, whether this pathway exerted its function in HIBD after MSCs transplantation is not clear. In the present study, we try to evaluate this problem.
Materials and Methods
Culture of MSCs
Bone marrow derived MSCs derived from Sprague-Dawley (SD) rats were purchased from ScienCell Research Laboratories (Catalog Number: 0133). Fluorescence-activated cell sorting was performed for the identification of MSCs. MSCs were negative for CD11b, CD34, CD45 and positive for CD29, CD44, CD90. The MSCs were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (ScienCell, CA, USA) and an antibiotic mixture of penicillin (100 U/ml) and streptomycin (100 μg/ml). When these cells reached 70%–80% confluence, the cells were trypsinized and subcultured. Cells were used at passages three to five in the following experiments.
Culture of Primary Neurons
All animal protocols were approved by the Sichuan University Committee on Animal Research and complied with the ARRIVE guidelines. Primary neurons were prepared from the cortex of postnatal day 1 SD rats. The whole cerebral cortex was isolated from the fetuses and cells were dissociated in a trypsin solution (1.25 mg/ml in Hank’s buffered salt solution) for 10 minutes at 37°C. The cell suspension was centrifuged and resuspended, then seeded into six-well plates precoated with poly-d-lysine (Sigma, MO, USA, P1149), and grown in neurobasal medium (Life, CA, USA, 21103–049) with 2% B27 supplement (Life, 17504–044) and 500 μM glutamine (Life, 25030081) in a humidified incubator with 5% CO2 at 37°C.
Cultured cells at day in vitro 7 were exposed to oxygen glucose deprivation (OGD) for 3 hours followed by reoxygenation. To induce OGD, cultured cells were gently washed twice with phosphate-buffered saline (PBS) and were then placed in DMEM without glucose (Life, 11966–025). Cells were exposed to hypoxia (95% N2/5% CO2) at 37°C in an airtight chamber for 3 hours. After 3 hours of OGD treatment, the cell culture medium was changed back to normal neurobasal medium and the cells were returned to the 5% CO2, 37°C incubator.
Coculture of Primary Neurons and MSCs
To test the effects of coculturing of primary neurons and MSCs without cell contact, primary neurons were plated on transwell bottoms at a density of 4.0 × 104 cells/cm2 (Corning, 3412) and MSCs were plated in transwell inserts at a density of 1.0 × 104 cells/cm2 24 hours before OGD. Primary neurons cocultured with MSCs were collected for assays 6, 12, and 24 hours after OGD. To investigate the protective effects of MSC-secreted BDNF on neurons, 20 μg/ml anti-BDNF neutralizing antibody (Millipore, MA, USA, AB1513P) was added to transwell bottoms 24 hours before OGD. The primary neurons were then collected for assays 24 hours after OGD. 3-methyladenine (3-MA) (M9281, Sigma) is an autophagy specific inhibitor. To test whether MSCs induces autophagy, 10 nm 3-MA was added to transwell bottoms 24 hours before OGD. Bafilomycin A1 (B1793, Sigma) is an autophagic flux inhibitor blocking the autophagosome-lysosome fusion, which is commonly used in research of autophagy flux. To test whether MSCs induces autophagic flux, 10 nm bafilomycin A1 was added to transwell bottoms 24 hours before OGD. The primary neurons were then collected for assays 24 hours after OGD.
Detection of Lactate Dehydrogenase
Lactate dehydrogenase (LDH) release from primary neurons cocultured with MSCs 0, 24, 48 hours before OGD was determined with an LDH detection kit (Roche, NJ, USA, 04744926001) as an index of cytotoxicity. The primary neurons were then collected for assay 24 hours after OGD. The assay procedure was performed according to the instructions included in the kit.
Enzyme-Linked Immunosorbent Assay
To determine the secretion of the BDNF protein by MSCs, BDNF levels in the culture supernatants of the bottom chamber of the transwell were measured by using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Millipore, CYT306) according to the manuscript’s instructions 24 hours after OGD. The absorbance at 450 nm was measured with a microplate reader (Bio-Rad, CA, USA).
Animal Model of HIBD
Seven-day-old SD rats were purchased from the Medical Animal Center of Sichuan Province. Neonatal HIBD model was established as described before [20]. Briefly, the rats were anesthetized by ether and subjected to ischemia with their right common carotid artery permanently double ligated and sliced in the middle. After recovery for 1 hour, the rats were exposed to hypoxia (8% O2, 92% N2) at 37°C for 2 hours and then returned to their cage. The sham rats were only subjected to isolation and stringing of the vessels without occlusion and subsequent ischemia.
MSCs Administration in Animals
Twenty-four hours after HIBD, 2 × 105 MSCs in 5 μl PBS or PBS alone were transplanted into the ipsilateral hemisphere at 2.0 mm anterior and 2.0 mm lateral to bregma, and 2.0 mm deep to the dural surface, using a 5 μl Hamilton syringe [21]. The rats were sacrificed 12, 24, 48 hours after MSCs transplantation, and their ipsilateral cortexes were collected for the following experiments.
MSCs Tracking
Twenty-four hours after HIBD, 2 × 105 MSCs were labeled with PKH-26 red fluorescent cell linker kit (Sigma-Aldrich) and were transplanted into the ipsilateral hemisphere. The rats were sacrificed 2, 7 days after MSCs transplantation and perfused intracardially with PBS followed by 4% paraformaldehyde in 0.1 mol/l PBS (4% paraformaldehyde). The brains were cut consecutively into 60-μm coronal sections. Then, one of every six sections were collected and stained with 4′,6-diamidino-2-phenylindole (DAPI, 1:500, Sigma, D9542) for nuclei staining. Images were taken using a confocal laser scanning microscope (Olympus FV1000, Tokyo, Japan). The number of transplanted MSCs was quantified using average fluorescence intensity by confocal laser scanning microscope.
Anti-BDNF Neutralizing Antibody Administration in Animals
To investigate the protective effects of MSC-secreted BDNF on neurons, 1 μg anti-BDNF neutralizing antibody (Millipore, AB1513P) or PBS was administered 1 hour after HIBD. The anti-BDNF neutralizing antibody or PBS alone was transplanted into the ipsilateral hemisphere at 2.0 mm anterior and 2.0 mm lateral to bregma, and 2.0 mm deep to the dural surface, using a 5 μl Hamilton syringe. Twenty-four hours after HIBD, 2 × 105 MSCs in 5 μl PBS or PBS alone were transplanted into the ipsilateral hemisphere. Forty-eight hours after HIBD, the rats were sacrificed, and their ipsilateral cortexes were collected.
Recombinant BDNF Administration in Animals
To investigate whether BDNF enhance autophagy, recombinant BDNF (0.05 μg, 0.25 μg) (Peprotech, NJ, USA, AF-450–02) was administered into the ipsilateral hemisphere 24 hours after HIBD [22]. Forty-eight hours after HIBD, the rats were sacrificed and their ipsilateral cortexes were collected.
Morris Water Maze
The morris water maze was carried out 4 weeks after MSCs transplantation [23]. The morris water maze was undertaken to investigate the impact of transplantation on HIBD-induced spatial memory impairment as described previously [24]. Briefly, the spaced training protocol was performed for five successive days. On each day, the rats received four consecutive training trials during which the hidden platform was kept in a constant location. A different starting location was used for each trial, which consisted of a swim followed by a 30 seconds platform sit. Any rat that could not find the platform within 60 seconds was guided to it by the experimenter. Memory retention was evaluated in a 60 seconds probe trial performed in the absence of the escape platform 24 hours after the last training session.
Tissue Preparation
The pups were deeply anesthetized at the indicated times. For the Western blot analysis, their brains were rapidly removed and stored at −70°C. For H&E staining and immunofluorescence, the brains of the pups were perfusion-fixed with 4% PFA, postfixed with 4% PFA at 4°C for 24 hours, and then embedded in 3% agarose in ddH2O. The brains were cut into coronal sections and mounted on poly-L-lysine-coated slides.
H&E Staining
Forty-one days after HIBD, the brains were rapidly removed and used for H&E staining. After routine deparaffinization, the sections were stained with hematoxylin for 5 minutes and dyed with eosin for 5 minutes. Alcohol, dimethylbenzene, and neutral gum were separately used for dehydration, hyalinization, and sealing in sequence. A Leica inverted optical microscope was used to capture images (Leica, Germany).
Western Blot
For Western blot, cocultured primary neurons or brains were harvested and extracted using lysis buffer containing protease inhibitors. Equal amounts of protein (50 μg) were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Roche, 03010040001). The membranes were blocked and incubated overnight with primary antibodies against BDNF (1:1,000, Sigma, AV41970), p62 (1:1,000, CST, 5114), LC3 (1:1,500, Novus Biological, NB100–2220), Beclin1 (1:1,000, CST, 3738S), mTOR (1:1,000, CST, 2983S), phosphorylated-mTOR (1:1,000, CST, 2971S), cleaved caspase-3 (CC3, 1:1,000, CST, 9661), or β-actin (1:1,000, CST, 4967), washed and incubated with secondary antibodies (1:3,000, Zymed Laboratories, sc11427). Immunoreactive bands were visualized using enhanced chemiluminescence (Millipore, WBKLS0100). The band intensity was analyzed using Photoshop software.
Immunofluorescence Staining
Primary neurons on slides or in brain sections were treated with 0.3% (vol/vol) Triton X-100, blocked with 10% (vol/vol) serum, incubated with antibodies against LC3 (1:200, Novus Biological, NB100–2220) and NeuN (1:500, Millipore, MAB377), and then incubated with secondary antibodies conjugated to DyLight 488 or Cy3 (1:500, Millipore, AP124JD, AP132C). After DAPI staining, the slides were observed using a confocal laser scanning microscope (Olympus FV1000, Tokyo, Japan). Digital images were captured with FV10-ASW-3.1 software. The purity of neurons was measured by staining with NeuN using immunofluorescence staining.
Electron Microscopy
The pups were intracardially perfused with 2.5% glutaraldehyde and 2% paraformaldehyde in cacodylate buffer (0.1 mol/l, pH 7.4). The brains were postfixed overnight at 4°C in the same fixative. The brains and cultured primary neurons were rinsed in 1% osmium tetroxide, dehydrated in an acetone series, infiltrated in Epon 812 for a longer period, and embedded. Ultrathin sections were stained with methylene blue, and ultrathin sections were cut with a diamond knife and stained with uranyl acetate and lead citrate. The sections were examined with a transmission electron microscope (HITACHI, H-600IV, Japan). Neuronal autophagy was quantified by the total number of autophagosomes.
Statistics
The data are presented as the mean ± SEM and were analyzed using SPSS version 17.0. Student’s t test was used to compare two groups. An analysis of variance with Fisher’s post hoc test was used to compare more than two groups. p<.05 was accepted as statistically significant.
Results
MSCs Reduced LDH and Increased BDNF in Neurons Following OGD Treatment
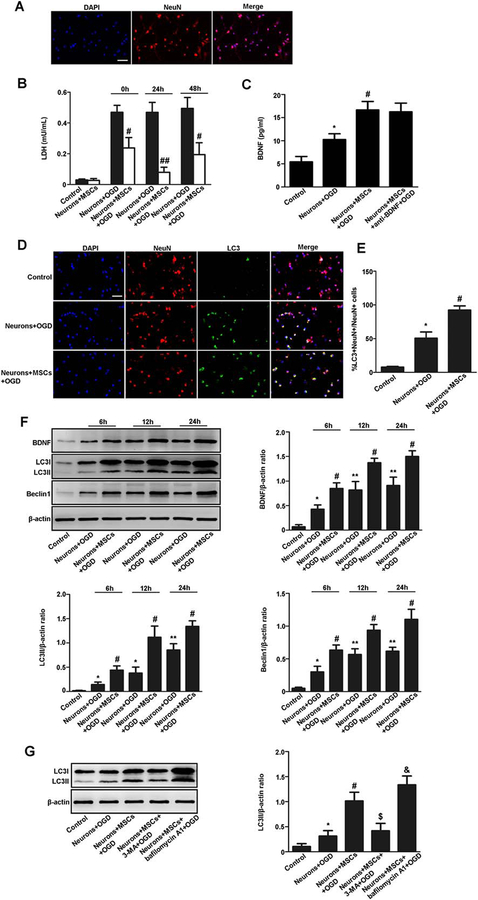
NeuN is a specific neuronal marker. We first detected NeuN-positive cells through immunofluorescent staining. We found that NeuN-positive cells were above 95% in the culture, suggesting most of the cells in the culture are neurons (Fig. 1A).
Figure 1.
MSCs have neuroprotective effects on neurons following OGD treatment by enhancing autophagy. (A): Immunofluorescent staining of NeuN (red) and quantitative analyses of NeuN positive cells. The NeuN-positive neurons were above 95% in the culture. (B): LDH was detected in the neuronal medium following OGD treatment. LDH was significantly reduced in the neuronal medium cocultured with MSCs (0, 24, 48 hours prior to OGD treatment) compared with the non-coculture group. (C): The BDNF level in the neuronal medium was detected by enzyme-linked immunosorbent assay 24 hours after OGD treatment. Neurons cultured on day in vitro 7 without OGD treatment nor cocultured with MSCs were used as control group. The anti-BDNF antibody group is a group that anti-BDNF neutralizing antibody was added to the medium of the MSCs coculture 24 hours before OGD. The BDNF level was higher in the medium of the OGD group than that in the control group. The BDNF level was also higher in the medium of the MSCs coculture group than that in the non-coculture group after OGD treatment. The BDNF level was not different between the MSCs coculture group and the anti-BDNF antibody group. (D, E): Immunofluorescent staining and quantitative analyses of NeuN (red) and LC3 (green) and quantitative analyses of LC3 positive neurons. LC3-positive neurons were increased in the MSCs coculture group compared with the non-coculture group at 24 hours after OGD treatment. Scale bar = 40 μm. (F): Representative Western blot and quantitative analyses of BDNF, LC3II, and Beclin1. Results are presented as the ratio of BDNF, LC3II, and Beclin1 normalized to β-actin. The expression of BDNF, LC3II, and Beclin1 was higher in the MSCs coculture group than that in the non-coculture group at 6, 12, and 24 hours after OGD treatment, especially at 24 hours after OGD treatment. (G): Representative Western blot and quantitative analyses of LC3II in neurons. Results are presented as the ratio of LC3II normalized to β-actin. The expression of LC3II was higher at 24 hours after OGD treatment when compared the OGD group with the control group, the MSCs coculture group with the non-coculture group, and 3-MA treatment further decreased LC3II level while bafilomycin A1 treatment increased LC3II level. Values are expressed as mean ± SEM, n = 8. *, p<.05, **, p<.01 for the non-coculture group versus the control group; #, p<.05, ##, p<.01 for the MSCs coculture group versus the non-coculture group; $, p<.05 for the MSCs coculture group with 3-MA treatment versus the MSCs coculture group; &, p<.05 for the MSCs coculture group with bafilomycin A1 treatment versus the MSCs coculture group. Abbreviations: BDNF, brain derived neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; LDH, lactate dehydrogenase; MSCs, mesenchymal stem cells; OGD, oxygen glucose deprivation.
To evaluate the effects of MSCs on primary neurons, neurons were cocultured with MSCs for 0, 24, 48 hours prior to OGD treatment. LDH is used as a marker of cell death. LDH was significantly reduced in the medium of the MSCs coculture group compared with the non-coculture group, especially at 24 hours prior to OGD (Fig. 1B). Therefore, we used neurons cocultured with MSCs 24 hours prior to OGD in the following assays.
To evaluate whether MSCs secret BDNF, BDNF level in the medium was detected by ELISA. Neurons cultured in vitro on day 7 without OGD treatment nor cocultured with MSCs were used as control group. The anti-BDNF antibody group is a group that anti-BDNF neutralizing antibody was added to the medium of the MSCs coculture group 24 hours before OGD. ELISA showed that the BDNF level was higher in the medium of the OGD group than that in the control group. The BDNF level was also higher in the medium of the MSCs coculture group than that in the non-coculture group after OGD treatment. The BDNF level was not different between the MSCs coculture group and the anti-BDNF antibody group (Fig. 1C).
MSCs Enhanced Autophagy in Neurons Following OGD Treatment
To evaluate whether MSCs enhanced neuronal autophagy after OGD treatment, we detect a specific autophagy marker LC3-positive neurons by immunofluorescent staining. We found that LC3-positive neurons were increased in the MSCs coculture group compared with the non-coculture group at 24 hours after OGD treatment (Fig. 1D, 1E). Then, autophagy specific proteins such as LC3II and Beclin1 were detected by Western blot. It showed that the expression of LC3II and Beclin1 was higher in the MSCs coculture group than that in the non-coculture group at 6, 12, and 24 hours after OGD, especially at 24 hours after OGD (Fig. 1F). In addition, 3-MA treatment decreased LC3II level, whereas bafilomycin A1 treatment increased LC3II level (Fig. 1G).
MSCs Enhanced Autophagy by Secreting BDNF in Neurons Following OGD Treatment
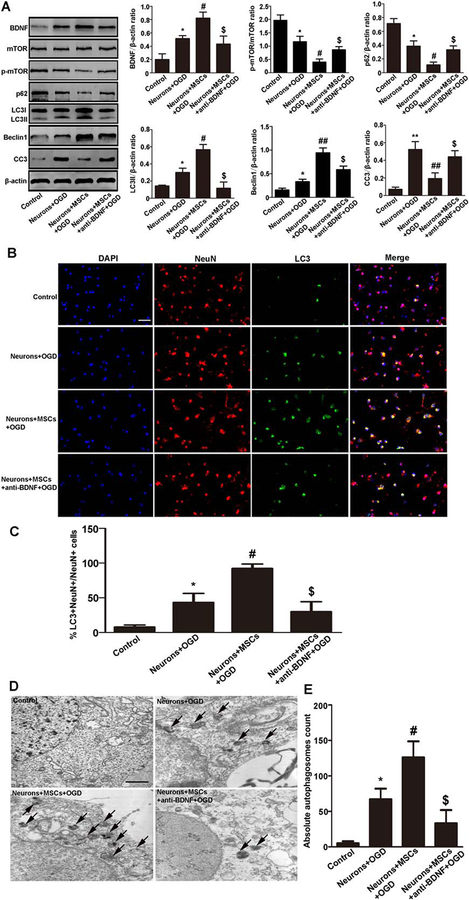
To clarify the effects of BDNF on neuronal autophagy after OGD treatment, anti-BDNF antibody was added to the coculture medium. Western blot analysis showed that anti-BDNF antibody significantly reduced the expression of LC3II and Beclin1 in neurons. To clarify the signaling pathway involved in autophagy caused by BDNF, we evaluate roles of mTOR signaling pathway. We found that p-mTOR/mTOR expression was significantly lower in the MSCs coculture group than that in the anti-BDNF antibody group (Fig. 2A). In addition, we also found that p62 and CC3 expression were significantly lower in the MSCs coculture group than that in the anti-BDNF antibody group (Fig. 2A).
Figure 2.
MSCs enhanced neuronal autophagy by secreting BDNF following OGD treatment. (A): Representative Western blot and quantitative analyses of BDNF, mTOR, p-mTOR, p62, LC3II, Beclin1, and CC3 in neurons. Neurons cultured on day 7 without OGD treatment nor cocultured with MSCs were used as control group. The expression of BDNF, LC3II, and Beclin1 was higher in the OGD group than that in the control group at 24 hours after OGD treatment. The expression of BDNF, LC3II, and Beclin1 was higher whereas p-mTOR/mTOR, p62, CC3 was lower in the MSCs coculture group than that in the non-coculture group or the anti-BDNF antibody group. (B, C): Immunofluorescent staining and quantitative analyses of NeuN (red) and LC3 (green). Neurons cultured on day 7 without OGD treatment nor cocultured with MSCs were used as control group. LC3-positive neurons were increased in the OGD group compared with the control group at 24 hours after OGD treatment. LC3-positive neurons were also increased in the MSCs coculture group compared with the non-coculture group or the anti-BDNF antibody group. Scale bar = 40 μm. (D, E): Neuronal autophagy observed by electron microscopy was quantified by counting total number of autophagosomes. Data represent 6 rats per culture and 20 fields/section. It showed that autophagosomes were increased in the OGD group compared with the control group at 24 hours after OGD treatment. Autophagosomes were also increased in the MSCs coculture group compared with the OGD group or the anti-BDNF antibody group. Scale bar = 1 μm. Values are expressed as mean ± SEM, n = 6. *, p<.05, **, p<.01 for the non-coculture group versus the control group; #, p<.05, ##, p<.01 for the MSCs coculture group versus the non-coculture group; $, p<.05 for the anti-BDNF antibody group versus the MSCs coculture group. Abbreviations: BDNF, brain derived neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; MSCs, mesenchymal stem cells; mTOR, mammalin target of rapamycin; OGD, oxygen glucose deprivation; p-mTOR, phosphorylated-mTOR.
Immunofluorescence staining showed that LC3-positive neurons were increased in the MSCs coculture group compared with the non-coculture group or the anti-BDNF antibody group (Fig. 2B, 2C).
Morphometric ultrastructural analyses also showed that autophagosomes were increased in the OGD group compared with the control group. Moreover, the autophagosomes were increased in the MSCs coculture group compared with the OGD group or the anti-BDNF antibody group (Fig. 2D, 2E).
MSCs Transplantation Has Neuroprotective Effects on Rats with HIBD
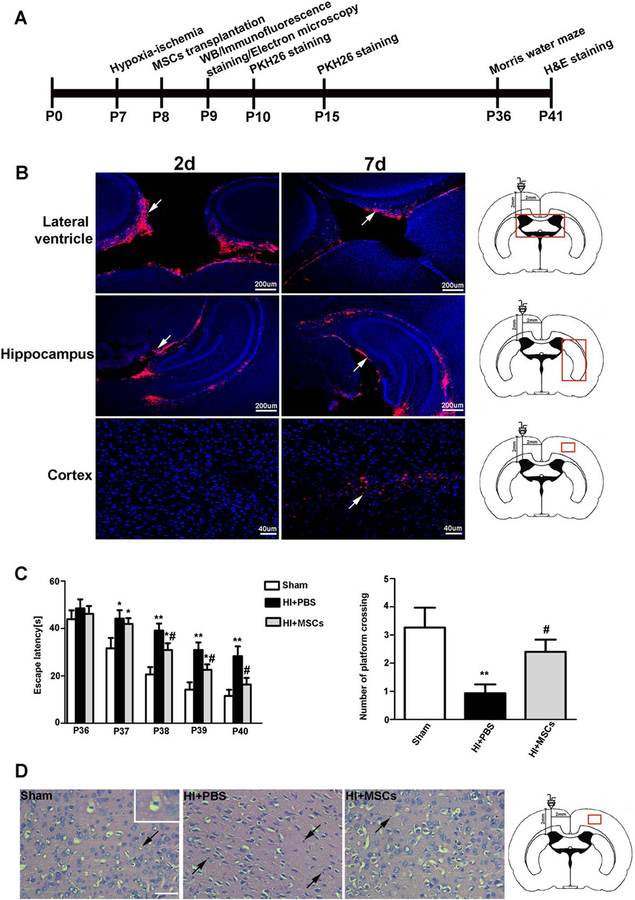
Experimental procedural timeline was shown in Figure 3A. Rats were subjected to HI insult at P7, and then they were received MSCs transplantation at P8. The rats were sacrificed, and their ipsilateral cortexes were collected for immunofluorescent staining, Western blot and electron microscopy at P9. PKH26 staining was done at P10 and P15. The morris water maze was examined at P36. H&E staining was performed at P41.
Figure 3.
MSCs transplantation has neuroprotective effects on rats with HIBD. (A): Experimental procedural timeline was shown. Rats were subjected to HI insult at P7, and then they were received MSCs transplantation at P8. The rats were sacrificed, and their ipsilateral cortexes were collected for immunofluorescent staining, Western blot and electron microscopy at P9. PKH26 staining was done at P10 and P15. The morris water maze was examined at P36. H&E staining was performed at P41. (B): Immunofluorescent staining showed that the PKH-26-labeled MSCs were in the lateral ventricle and migrated out of the ventricle to the hippocampus 2 days after MSCs transplantation. The PKH-26-labeled MSCs were observed in the cortex 7 days after MSCs transplantation. (C): The morris water maze was tested in the rats. The sham rats were only subjected to isolation and stringing of the vessels without occlusion and ischemia. The sham group showed a significantly faster latency of swimming over the platform location than the HIBD group or the MSC-transplanted group from postnatal day 37 to postnatal day 39. The MSC-transplanted group showed a significantly faster latency of swimming over the platform location than the HIBD group from postnatal day 38 to postnatal day 40. Furthermore, the HIBD group showed fewer platform crossings than the sham group or MSC-transplanted group, while there was no difference between the sham group and the MSC-transplanted group. (D): H&E staining in the rats. H&E staining showed that neurons in the cortex were arranged orderly with complete cell structures in the sham group. The neurons arrangement was disordered, and a large number of neurons appeared shrunken with nuclear pyknosis in the HIBD group. MSCs transplantation markedly ameliorated these pathological changes. Scale bar = 50 μm. Values are expressed as mean ± SEM, n = 15 for morris water maze, n = 8 for H&E staining. *, p<.05, **, p<.01 for the MSC-transplanted group or the HIBD group versus the sham group; #, p<.05 for the MSC-transplanted group versus the HIBD group. Abbreviations: HIBD, hypoxia-ischemia brain damage; MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline.
To explore the neuroprotective effects of MSCs on rats with HIBD, we detect the distribution and migration of MSCs in the brain area after transplantation. PKH26 is a kind of red fluorescent general cell linker and it was used for membrane labeling for stem cells in the present study. MSCs were labeled with PKH-26 and then transplanted into the ipsilateral hemisphere. Immunofluorescent staining showed that the PKH-26-labeled MSCs (red) were in the lateral ventricle and migrated out of the ventricle to the hippocampus 2 days after MSCs transplantation (Fig. 3B). The PKH-26-labeled MSCs were observed in the cortex 7 days after MSCs transplantation. These findings suggest that MSCs have been transplanted into brain tissues and can migrate to different areas of the brain.
Next, we conducted the morris water maze to evaluate the neuroprotective effects of MSCs on rats with HIBD. The morris water maze revealed that the MSC-transplanted group showed a significantly faster latency of swimming over the platform location than the HIBD group from postnatal day 38 to postnatal day 40. Furthermore, the MSC-transplanted group showed more platform crossings than the HIBD group (Fig. 3C).
In addition, we used H&E staining to evaluate the neuroprotective effects of MSCs on rats with HIBD. H&E staining showed that the neurons arrangement was disordered in the HIBD group, with a large number of neurons appearing shrunken with nuclear pyknosis, whereas MSCs transplantation markedly ameliorated these pathological changes (Fig. 3D).
MSCs Transplantation Enhanced Neuronal Autophagy in Rats with HIBD
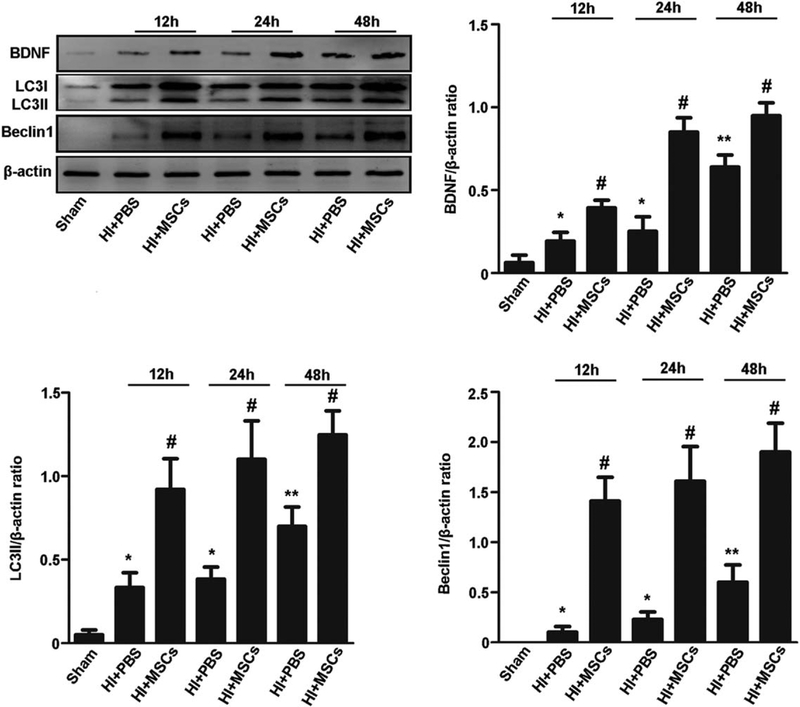
Furthermore, we evaluated whether MSCs increased the induction of autophagy in the HIBD model. The expression of LC3II and Beclin1 was significantly higher in the MSC-transplanted group than that in the HIBD group at 12, 24, 48 hours after MSCs transplantation (Fig. 4). These data indicate that MSCs enhanced neuronal autophagy induction in the HIBD model. In addition, the expression of BDNF was significantly higher in the MSC-transplanted group than that in the HIBD group at 12, 24, 48 hours after MSCs transplantation (Fig. 4).
Figure 4.
MSCs enhanced neuronal autophagy in rats with hypoxia-ischemia brain damage (HIBD). Representative Western blot and quantitative analyses of BDNF, LC3II, and Beclin1. Results are presented as the ratio of BDNF, LC3II, and Beclin1 normalized to β-actin. The expression of BDNF, LC3II, and Beclin1 protein was significantly higher in the cortex of HIBD group than that in the sham group at 12, 24, 48 hours after MSCs transplantation. The expression of BDNF, LC3II, and Beclin1 protein was significantly higher in the cortex of MSC-transplanted group than that in the HIBD group at 12, 24, 48 hours after MSCs transplantation. Values are expressed as mean ± SEM, n = 6. *, p<.05, **, p<.01 for the HIBD group versus the sham group; #, p<.05 for the MSC-transplanted group versus the HIBD group. Abbreviations: BDNF, brain derived neurotrophic factor; MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline.
BDNF Enhanced Autophagy in Rats with HIBD
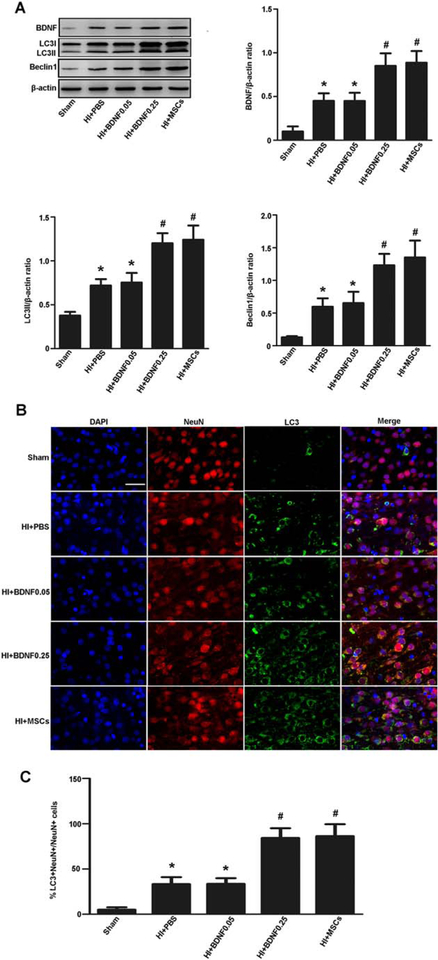
To evaluate whether BNDF enhanced autophagy, we administered recombinant BDNF in the HIBD model. Western blot showed that BDNF, LC3II, and Beclin1 were significantly higher in the BDNF-treated group (0.25 μg BDNF) than that in the HIBD group (Fig. 5A). Immunofluorescent staining showed that LC3-positive neurons were increased in the BDNF-treated group (0.25 μg BDNF) compared with the HIBD group (Fig. 5B, 5C). These data showed that BDNF enhanced autophagy in rats with HIBD.
Figure 5.
BDNF enhanced autophagy in rats with hypoxia-ischemia brain damage (HIBD). (A): Representative Western blot and quantitative analyses of BDNF, LC3II, and Beclin1. The expression of BDNF, LC3II, and Beclin1 protein was significantly higher in the cortex when compared the HIBD group with the sham group, BDNF-treated (0.25 μg BDNF) group with the HIBD group, and the MSC-transplanted group with the HIBD group. (B, C): Immunofluorescent staining and quantitative analyses of NeuN (red) and LC3 (green). LC3-positive neurons were increased in the cortex when compared the HIBD group with the sham group, the BDNF-treated (0.25 μg BDNF) group with the HIBD group, and the MSC-transplanted group with the HIBD group. Scale bar = 50 μm. Values are expressed as mean ± SEM, n = 6. *, p<.05 for HIBD group or BDNF-treated group (0.05 μg) versus the sham group; #, p<.05 for BDNF-treated group (0.25 μg) or the MSC-transplanted group versus the HIBD group. Abbreviations: BDNF, brain derived neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; MSC, mesenchymal stem cell; PBS, phosphate-buffered saline.
MSCs Enhanced Autophagy by Increasing BDNF in Rats with HIBD
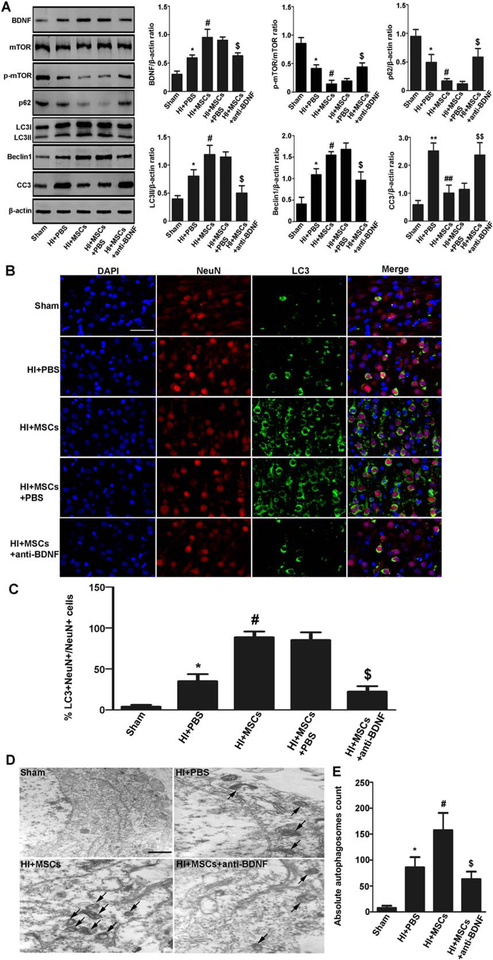
To further prove the neuroprotective mechanism of MSCs is related to BDNF production, we pretreated rats with anti-BDNF antibody to block BDNF. The expression of LC3II and Beclin1 was lower whereas p-mTOR/mTOR was significantly higher in the MSCs with anti-BDNF antibody pretreatment group than that in the MSCs with PBS pretreatment group (Fig. 6A). Immunofluorescence staining confirmed that LC3-positive neurons were reduced in the MSCs with anti-BDNF antibody pretreatment group compared with the MSCs with PBS pretreatment group (Fig. 6B, 6C). Electron microscopy also revealed that autophagosomes were reduced in the MSCs with anti-BDNF antibody pretreatment group compared with the MSCs with PBS pretreatment group (Fig. 6D). These data indicate that MSCs enhance autophagy induction by increasing BDNF in the HIBD model.
Figure 6.
MSCs enhanced autophagy by increasing BDNF in rats with hypoxia-ischemia brain damage (HIBD). (A): Representative Western blot and quantitative analyses of BDNF, mTOR, p-mTOR, p62, LC3II, Beclin1, and CC3. The expression of BDNF, LC3II, and Beclin1 was significantly higher whereas p-mTOR/mTOR, p62, and CC3 was lower in the cortex when compared the HIBD group with the sham group, the MSC-transplanted group with the HIBD group, the MSCs plus PBS pretreatment group with the MSCs plus anti-BDNF antibody pretreatment group. (B, C): Immunofluorescence staining and quantitative analyses of for NeuN (red) and LC3 (green). LC3-positive neurons were increased in the cortex when compared the HIBD group with the sham group, the MSC-transplanted group with the HIBD group, the MSCs plus PBS pretreatment group with the MSCs plus anti-BDNF antibody pretreatment group. Scale bar = 50 μm. (D, E): Neuronal autophagy observed by electron microscopy was quantified by counting total number of autophagosomes. Data represent 4 rats per group, 3 section per rats; 20 fields/section. It showed that autophagosomes were increased in the cortex when compared the HIBD group with the sham group, the MSC-transplanted group with the HIBD group, the MSC-transplanted group with the MSCs plus anti-BDNF antibody pretreatment group. Scale bar = 1 μm. Values are expressed as mean ± SEM, n = 6. *, p<.05 for HIBD group versus the sham group; #, p<.05 for the MSC-transplanted group versus the HIBD group; $, p<.05 the MSCs with PBS pretreatment group versus the MSCs with anti-BDNF antibody pretreatment group. Abbreviations: BDNF, brain derived neurotrophic factor; DAPI, 4′,6-diamidino-2-phenylindole; MSCs, mesenchymal stem cells; mTOR, mammalin target of rapamycin; p-mTOR, phosphorylated-mTOR; PBS, phosphate-buffered saline.
Discussion
In the present study, we found for the first time that MSCs transplantation significantly attenuates neuronal damage by enhancing neuronal autophagy through BDNF/mTOR signaling pathway after hypoxia-ischemia.
Autophagy has been found to play roles in many fundamental biological processes including aging, development, immunity, tumorigenesis, and cell death [25–28]. Autophagy maintains cellular homeostasis under basal conditions. It also can be activated under conditions such as hypoxia, excitotoxicity, starvation, stress, and neurodegeneration [29–33]. It has been suggested that autophagy is an adaptive mechanism that helps maintain cellular homeostasis during the early stage of disease in response to cellular stress. However, in some cases, paradoxically, autophagy may be deleterious [34–36]. Therefore, autophagy can function as both a prosurvival and a pro-death mechanism.
Recent studies suggest that there may be a link between autophagy and brain damage [5–8]. Autophagosomes have been found in HIBD model in the adult and developing rats [5, 7, 8]. LC3, an important autophagy marker, has also been found to be increased in the HIBD model [5–8]. Consistently, we found that autophagosomes and LC3II were increased in the HIBD model, which suggests that autophagy play an important role in HIBD. Recent studies have shown that induction of autophagy can produce neuroprotective effects [37–40]. Therefore, a therapeutic strategy to enhance autolysosome induction may be an important pharmacological target in HIBD.
Currently, MSCs treatment is thought to be a potent therapeutic strategy for some diseases such as adult stroke, traumatic brain injury, and Alzheimer’s disease [41–43]. The possible mechanisms involved including secreting neurotrophic factor, promoting angiogenesis, inhibiting apoptosis, modulating the immune system and chemoattraction [9–12, 44]. Moreover, MSCs could exert neuroprotective effects by enhancing autophagy in an Alzheimer’s disease model [13]. However, the mechanism is unclear. Interestingly, our study showed that LDH release was reduced in neurons cocultured with MSCs after hypoxia-ischemia in vitro. Consistently, we found that MSCs transplantation significantly reduced brain damage and improved motor impairment in vivo. These findings indicate that MSCs can protect neurons from HIBD. In addition, we found that MSCs can migrate out of the lateral ventricle to other areas of the brain, which is consistent with previous study that the chemoattraction of MSCs may guide MSCs home to the ischemic lesion site [44]. Further study showed that MSCs transplantation induced autophagosomes both in vitro and in vivo after hypoxia-ischemia stimulation. This increasing of autophagosomes is in parallel with the decreasing of LDH and brain damage. In addition, we found autophagy inhibitor 3-MA treatment decreased LC3II level while autophagosome-lysosome inhibitor bafilomycin A1 treatment increased LC3II level. These findings suggest that MSCs play a neuroprotective role by increasing autophagy after HIBD. However, the protective mechanisms need to be clarified.
Previous reports have demonstrated that one of the protective mechanisms of MSCs is the secretion of various factors, including neurotrophins such as BDNF and GDNF [14–16]. BDNF is a member of the mammalian neurotrophin family, which has been demonstrated to be a potent growth factor that is beneficial to adult stroke [45]. In this study, we found that BDNF levels were higher in the neuronal medium cocultured with MSCs than those without MSCs. These are in line with previous studies that MSCs could secret BDNF [14, 15]. Moreover, BDNF was also found to be higher in the neurons cocultured with MSCs. Thus, we think that MSCs could secret BDNF, which may be benefit for neuroprotection in HIBD model.
It was reported that BDNF can trigger three enzymes, mitogen-activated protein kinase, PI3K, and phospholipase C-γ [17]. The PI3K signaling pathway is upstream of mTOR, which is important in the induction of autophagy. It has been shown that the activation of the mTOR signaling pathway may promote necrotic cell death via regulation of apoptosis and autophagy [18, 19]. Increased mTOR activity is seen in an Alzheimer’s disease model and related with the abnormal proteins accumulated caused cognitive impairments. Inhibit mTOR activity could reverse cognitive impairments in the Alzheimer’s disease model and decrease abnormal proteins accumulated by inducing autophagy [46]. However, whether the mTOR signaling pathway is involved in the HIBD model is not clear. In the present study, we found that BDNF, LC3II, and Beclin1 levels were increased with MSCs treatment and decreased with anti-BDNF treatment. In addition, recombinant BDNF injection in vivo showed that recombinant BDNF can enhance autophagy. These findings suggest that MSCs could enhance autophagy in neurons by increasing BDNF level. We also found that p-mTOR/mTOR expression was significantly decreased with MSCs treatment. Furthermore, this decreasing could be reversed by anti-BDNF treatment. These findings suggest that the mTOR signaling pathway is involved in BDNF mediated neuronal autophagy after HIBD. In addition, we found that CC3 expression was significantly decreased with MSCs treatment in vitro and in vivo, suggesting that the BDNF/mTOR signaling pathway might be also involved in regulating neuronal apoptosis after HIBD, the exact mechanism need to be further examined.
Conclusion
The present study found that MSCs exert neuroprotective effects by enhancing autophagy through BDNF/mTOR signaling pathway in the rat model of HIBD. This finding gives a hint to explore new strategies for treating neonates with HIE in clinic.
Significance Statement.
The present study found that mesenchymal stem cells (MSCs) exert neuroprotective effects by enhancing autophagy through brain-derived neurotrophic factor/mammalin target of rapamycin signaling pathway in the rat model of hypoxia-ischemia brain damage. It was built on what is known in the field of HI injury/MSC protective properties/autophagy and added novelty by filling gaps of missing knowledge about specific pathways and mechanisms of action in the brain. Result of this study gives a hint to explore new strategies for treating neonates with hypoxic-ischemic encephalopathy in clinic.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFA0104200), the National Science Foundation of China (81330016, 81630038, 81771634, 81200462), the Grants from Ministry of Education of China (IRT0935), the Grants from Science and Technology Bureau of Sichuan Province (2014SZ0149, 2016TD0002), and the Grant of clinical discipline program (neonatology) from the Ministry of Health of China (1311200003303).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol 2004; 207:3149–3154. [DOI] [PubMed] [Google Scholar]
- 2.Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: A review for the clinician. JAMA Pediatr 2015;169:397–403. [DOI] [PubMed] [Google Scholar]
- 3.Cuervo AM. Chaperone-mediated autophagy: Selectivity pays off. Trends Endocrinol Metab 2010;21:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B, Cuervo AM et al. Autophagy fights disease through cellular self-digestion. Nature 2008;451:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carloni S, Albertini MC, Galluzzi L et al. Increased autophagy reduces endoplasmic reticulum stress after neonatal hypoxia-ischemia: Role of protein synthesis and autophagic pathways. Exp Neurol 2014;255:103–112. [DOI] [PubMed] [Google Scholar]
- 6.Ginet V, Pittet MP, Rummel C et al. Dying neurons in thalamus of asphyxiated term newborns and rats are autophagic. Ann Neurol 2014;76:695–711. [DOI] [PubMed] [Google Scholar]
- 7.Lu Q, Harris VA, Kumar S et al. Autophagy in neonatal hypoxia ischemic brain is associated with oxidative stress. Redox Biol 2015;6:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu C, Wang X, Xu F et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ 2005;12:162–176. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SY, Chang YS, Sung DK et al. Pivotal role of brain derived neurotrophic factor secreted by mesenchymal stem cells in severe intraventricular hemorrhage in the newborn rats. Cell Transplant 2017;26:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng N, Hao G, Yang F et al. Transplantation of mesenchymal stem cells promotes the functional recovery of the central nervous system following cerebral ischemia by inhibiting myelin-associated inhibitor expression and neural apoptosis. Exp Ther Med 2016;11:1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitkari B, Nitzsche F, Kerkela E et al. Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav Brain Res 2014;259:50–59. [DOI] [PubMed] [Google Scholar]
- 12.Zhang R, Liu Y, Yan K et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation 2013;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin JY, Park HJ, Kim HN et al. Mesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease models. Autophagy 2014;10:32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong CH, Kim SM, Lim JY et al. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed Res Int 2014;2014:129145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Wang X, Li A et aI. Effect of mesenchymal stem cell transplantation on brain-derived neurotrophic factor expression in rats with Tourette syndrome. Exp Ther Med 2016;11:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribeiro TB, Duarte AS, Longhini AL et al. Neuroprotection and immunomodulation by xenografted human mesenchymal stem cells following spinal cord ventral root avulsion. Sci Rep 2015;5:16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng M, Zhiling W, Hui Z et al. Cellular levels of TrkB and MAPK in the neuroprotective role of BDNF for embryonic rat cortical neurons against hypoxia in vitro. Int J Dev Neurosci 2005;23:515–521. [DOI] [PubMed] [Google Scholar]
- 18.Wu YT, Tan HL, Huang Q et al. Activation of the PI3K-Akt-mTOR signaling pathway promotes necrotic cell death via suppression of autophagy. Autophagy 2009;5:824–834. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Xiong T, Qu Y et al. mTOR activates hypoxia-inducible factor-1alpha and inhibits neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia. Neurosci Lett 2012;507: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice JE III, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981;9: 131–141. [DOI] [PubMed] [Google Scholar]
- 21.Gu Y, He M, Zhou X et al. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by suppressing apoptosis in astrocyte. Sci Rep 2016;6:18587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Fang Y, Lian Y et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by abeta1–42. PLoS One 2015;10:e0122415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zhang Q, Li W et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res 2014;92:35–45. [DOI] [PubMed] [Google Scholar]
- 24.de Paula S, Greggio S, Marinowic DR et al. The dose-response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia-ischemia. Neuroscience 2012; 210:431–441. [DOI] [PubMed] [Google Scholar]
- 25.Andersen AN, Landsverk OJ, Simonsen A et al. Coupling of HIV-1 antigen to the selective autophagy receptor SQSTM1/p62 promotes T-cell-mediated immunity. Front Immunol 2016;7:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Y, Miao H, Wu S et al. ABHD5 interacts with BECN1 to regulate autophagy and tumorigenesis of colon cancer independent of PNPLA2. Autophagy 2016;12:2167–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero Y, Bueno M, Ramirez R et al. mTORC1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Won H, Rubinsztein DC. Autophagy and mammalian development. Biochem Soc Trans 2013;41:1489–1494. [DOI] [PubMed] [Google Scholar]
- 29.Au AK, Chen Y, Du L et al. Ischemia-induced autophagy contributes to neurodegeneration in cerebellar Purkinje cells in the developing rat brain and in primary cortical neurons in vitro. Biochim Biophys Acta 2015; 1852:1902–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi H, Merceron C, Mangiavini L et al. Hypoxia promotes noncanonical autophagy in nucleus pulposus cells independent of MTOR and HIF1A signaling. Autophagy 2016;12: 1631–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Li M, Qin S et al. Cytosolic PINK1 promotes the targeting of ubiquitinated proteins to the aggresome-autophagy pathway during proteasomal stress. Autophagy 2016; 12:632–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishiyama J, Yuzaki M. Excitotoxicity and autophagy: Lurcher may not be a model of “autophagic cell death”. Autophagy 2010;6: 568–570. [DOI] [PubMed] [Google Scholar]
- 33.Takagi A, Kume S, Maegawa H et al. Emerging role of mammalian autophagy in ketogenesis to overcome starvation. Autophagy 2016;12:709–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Cheng TS, Qin A et al. Glucocorticoid impairs cell-cell communication by autophagy-mediated degradation of connexin 43 in osteocytes. Oncotarget 2016;7:26966–26978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Lee JH, Jin M et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med 2014;46:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melk A, Baisantry A, Schmitt R. The yin and yang of autophagy in acute kidney injury. Autophagy 2016;12:596–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodder J, Denaes T, Chobert MN et al. Macrophage autophagy protects against liver fibrosis in mice. Autophagy 2015;11:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadakis M, Hadley G, Xilouri M et al. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat Med 2013;19:351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang P, Hou H, Zhang L et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol 2014;49:276–287. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Yuan J, Lipinski MM. Live imaging and single-cell analysis reveal differential dynamics of autophagy and apoptosis. Autophagy 2013;9:1418–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acosta SA, Tajiri N, Hoover J et al. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke 2015; 46:2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naaldijk Y, Jager C, Fabian C et al. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol 2017;43:299–314. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Wang Y, Wang Z et al. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells 2015;33:456–467. [DOI] [PubMed] [Google Scholar]
- 44.Donega V, Nijboer CH, Braccioli L et al. Intranasal administration of human MSC for ischemic brain injury in the mouse: In vitro and in vivo neuroregenerative functions. PLoS One 2014;9:e112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Cejudo J, Gutierrez-Fernandez M, Otero-Ortega L et al. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke 2015;46:221–228. [DOI] [PubMed] [Google Scholar]
- 46.Caccamo A, Majumder S, Richardson A et al. Molecular interplay between mammalian target of rapamycin (mTOR), amyloidbeta, and Tau: Effects on cognitive impairments. J Biol Chem 2010;285:13107–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]