Abstract
Background
Loss to follow-up (LTFU) is a term used to classify patients no longer being seen in a clinical care program, including HIV treatment programs. It is unclear if these patients have transferred their care services elsewhere, died, or if there are other reasons for their LTFU. To better understand the status of patients meeting the criteria of LTFU, we traced a sample of HIV-infected patients that were LTFU from the Lagos University Teaching Hospital (LUTH) antiretroviral program.
Methods
We conducted a cross-sectional study of HIV-infected adult patients who enrolled for care between 2010 and 2014 at LUTH and were considered LTFU. Patients with locator information were traced using phone calls. Face-to-face interviews were used to collect data from successfully traced and consenting participants. Predictors of LTFU from LUTH, disengagement from care and willingness to re-engage in care in LUTH were assessed.
Results
Of 6108 registered patients, 3397 (56%) were LTFU and being unmarried was a predictor of being LTFU from LUTH. Of 425 patients that were traced, 355 (84%) were alive and 70 (16%) were dead. Two hundred and sixty-eight patients consented to interviews; 96 (35.8%) of these had transferred to another clinic for care while 172 (64.2%) were disengaged from care. More than half (149/268; 55.6%) were not on antiretroviral therapy (ART). Some of the primary reasons for LTFU were; long distance to clinic (56%) and feeling healthy (6.7%). Predictor of disengagement from care within the interviewed cohort was not having started ART. The predictors of willingness to re-engage in care included, not having started ART, male sex and longer duration in HIV care prior to LTFU.
Conclusion
Most of the interviewed cohort that was LTFU were truly disengaged from care and not on ART. Interventions are required to address processes of re-engagement of patients that are LTFU.
Introduction
Of the estimated 36.7 million people living with human immunodeficiency virus (HIV), about 20.9 million were on antiretroviral therapy (ART) by the middle of 2017 [1]. Nigeria’s HIV epidemic is the second largest globally, while its new HIV infection rate is among the highest in Africa’s sub-Saharan region [2]. By 2016, about 3.2 million people were living with HIV in Nigeria with the highest prevalence in the southern states of the country [2]. It is estimated that only 31% of the adults and 21% of the children living with HIV are on ART [2].
In recognition that the nation was one of the hardest hit countries both globally and in the African region, the Nigerian government implemented one of the region’s largest ART programs and subsequently received support from the United States President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund, leading to free, HIV care services, laboratory investigations, and ART drugs [3,4]. There are now about 200 facilities providing comprehensive ART services across the nation [5].
With the growth of HIV programs, there is an increased focus on measures to sustain long-term ART benefits and reduce new HIV infection rates [5]. Sustained retention of patients in ART programs is essential for combating the HIV epidemic and for the success of the programs [6,7]. However, the retention in care among patients enrolled in ART programs in sub-Saharan Africa is generally poor, about 60% by the end of the second year, as noted by Rosen et al in their systematic review [7].
Various outcomes have been described for patients who were classified as lost to follow-up (LTFU) from ART programs: death, withdrawal from care, or transfer to other facilities [7–9]. Patient tracing has helped to further characterize the true status of patients classified as LTFU [9], sometimes with the positive effect of reengagement in care [10]. Although patient tracing has been explored widely in Africa [9], few studies in Nigeria have been documented despite the heavy burden of HIV in the country and the well-documented high rates of LTFU [3,11–13]. Questions still remain concerning the magnitude of LTFU in HIV programs and the contributory factors in the densely populated and industrialized region of southwest Nigeria.
In the AIDS Prevention Initiative in Nigeria (APIN), Public Health Initiatives (PHI) and PEPFAR HIV program at the Lagos University Teaching Hospital (LUTH), over 15,000 patients have been enrolled into care since October 2004. Of these, only 8,000 patients remain actively in care. In this study, we used routine clinic data to describe LTFU and identify patients at high-risk. To better characterize reasons for LTFU in the LUTH patient setting, we investigated a sample of HIV-infected patients that were lost to follow-up from the program and determined their current status.
Methods
Study site, design and population
The study was carried out in Lagos, southwest Nigeria, between January and November 2017. We conducted a cross-sectional study on patients that had been identified as LTFU using electronic medical record data from the HIV treatment program at LUTH.
This program was officially established with the help of a Harvard-PEPFAR grant (U51HA025522) in October 2004. Patients are enrolled into care following confirmation of HIV infection. The total enrollment into HIV care services at LUTH has grown from 759 patients in 2004 to over 20,000 patients as at the end of December 2018. The clinic has a dedicated laboratory that offers viral load and CD4+ cell count (for free) as well as hematology and chemistry assays (at a cost to patients) that are done at baseline and every 6 months thereafter. The majority of patients receive their HIV prescriptions monthly, while those with stable undetectable viral loads are switched to receiving 3 month prescriptions. Prior to 2016, when the test and treat strategy commenced [14], patients not yet on ART were seen in the clinic every 6 months.
The study population was laboratory-confirmed HIV-infected adult patients who enrolled into care between January 1, 2010 and December 31, 2014 and were LTFU from the clinic. The inclusion criterion for tracing was complete locator information (11-digit phone number) in the electronic medical records system [15]. Patients who were known to have died or known to have been transferred out while still in active care were excluded from the study. During the tracing process, patients who had moved out of state were also excluded from the study due to logistical reasons.
Definition of variables
We defined LTFU as patients who were lost to care that did not receive any clinical, laboratory or pharmacy services for at least 6 months and did not later return to the clinic by December 31, 2016. Our consideration for this definition was to minimize the false-positive rate (i.e., the proportion of individuals who were considered LTFU who later returned to care) [16]. Retention in care was defined as receiving continued HIV care at any HIV clinic while disengagement from care was defined as not receiving continued HIV care services at an HIV clinic [6].
Data collection
Three research assistants with postgraduate public health degrees were recruited and trained on the study implementation. As a training and tool refinement opportunity, we conducted a pilot study on a cohort of 25 patients that were LTFU after which we refined the tracing process and the data collection tool; data from the pilot study cohort are not included in this evaluation. Eligible LTFU patients were first contacted by phone to explain the study and arrange appointments for face-to-face interviews. Tracing was deemed successful if the patient could be reached by phone or if an informant familiar with patient could be reached to confirm if the patient was alive or dead. At least three phone call attempts were made on different days and at different times of the day without success before tracing was considered unsuccessful.
Patient tracing and data collection was conducted between June and November 2017. Following collection of written informed consent, research assistants used a structured questionnaire [17–19] (questionnaire in S1 Appendix) in face-to-face interviews to collect information on: socio-demographic characteristics (age, sex, marital status, religion, ethnicity, education and occupation); reasons for discontinuation of care; and, use of antiretroviral medication, including if they were placed on ART at the clinic, if they were still on ART, source of medication, reasons for non-use of ART and adherence to ART. Patients were also asked about registration for care at another clinic and their willingness to return to care in LUTH.
The reasons for discontinuation in care were divided into 5 main categories: access to care; clinic quality; work and family; medical; and alternative treatment and advice [16]. In addition, patients ranked their top 3 reasons for discontinuation.
Data analysis
Data were analyzed using Stata version 15.1 (StataCorp, USA). Continuous variables were tested for the assumption of normality. Categorical variables were presented in frequencies and percentages while non-normal continuous variables were presented as median and interquartile range (IQR). For the clinic population, Chi-square and Wilcoxon rank sum tests were used to determine differences in patient characteristics within loss to follow-up groups (LTFU vs. not LTFU, traced vs. not traced, traced and interviewed vs. traced and not interviewed). Time to LTFU was modelled using survival analysis methods. All patients that remained in care were right censored. Univariable and multivariable Cox proportional hazards (PH) regressions were conducted to examine associations between baseline patient characteristics and LTFU. Backward elimination was used to derive a parsimonious model. However, some variables such as age and enrollment year were chosen a priori. Adjusted and unadjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were computed for each predictor variable. Schoenfeld’s global test of residuals was used to assess for violation of the PH assumption; variables that violated the PH assumption were excluded from the final model.
For the LTFU patients that were successfully traced and interviewed, we used Chi-square and Wilcoxon rank sum tests to evaluate bivariate associations between independent variables (socio-demographic characteristics, months in care prior to LTFU and ART initiation in LUTH) and dependent variables (disengagement from care and willingness to re-engage in the LUTH ART program). Univariable and multivariable binary logistic regression were conducted to examine associations between respondents’ characteristics and disengagement from care and willingness to re-engage in the ART program from which they were LTFU. All independent variables in bivariate analyses were considered for inclusion in the logistic regression analyses irrespective of statistical significance. We used the Box-Tidwell test to test the assumption of linearity between continuous predictor variables (age and months in care prior to LTFU) and the dependent variables, which generated power transformed predictor variables that were included in the logistic regression model. Crude and adjusted odds ratios (OR) and 95% CI were computed for each predictor variable. The Hosmer-Lemeshow’s goodness of fit test was performed. Two-tailed test of hypothesis was assumed. Level of significance was set at 0.05.
Ethics
The study protocol was approved by the Health Research Ethics Committee (HREC) of the College of Medicine of the University of Lagos (CM/HREC/03/16/006) and the Harvard T. H. Chan School of Public Health Institutional Review Board. This study only included patients that provided voluntary consent for use of their clinic data for future research purposes. Written informed consent was collected from traced patients prior to interviews and those that indicated willingness to return to LUTH were linked back to care.
Results
Outcome of tracing
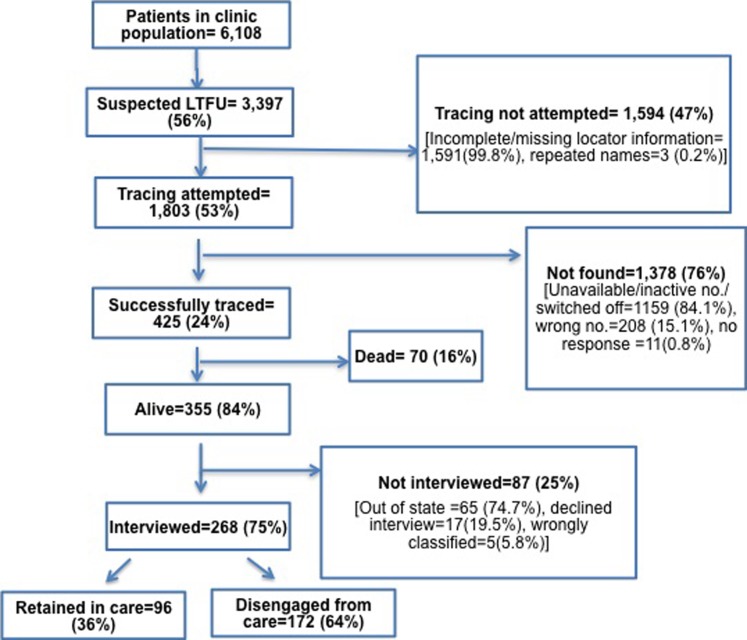
A total of 6,108 patients enrolled into the program between 2010 and 2014, out of which 3,397 (56%) were LTFU. Tracing was attempted among 1,803 patients who had enough information for tracing and, of those, 425 (24%) were successfully traced. Two hundred and sixty-eight of the successfully traced patients consented to interviews. In total, 172 (64%) of the traced and interviewed patients reported being disengaged from HIV care while the remaining 96 (36%) reported having transferred to another treatment site to receive HIV care services (Fig 1).
Fig 1. Flow chart depicting LTFU patients from LUTH HIV treatment program who enrolled into care from January 2010-December 2014.
Characteristics of patients enrolled at LUTH
Table 1 presents baseline characteristics of 6,108 patients that received care between 2010 and 2014. The median age was 41 years (IQR: 35–47). They were mostly female (67%), married (58%), had secondary education or higher (71%) and median baseline CD4+ cell count of 229 cells/mm3 (IQR: 97–402).
Table 1. Baseline demographic and clinical characteristics of 6,108 patients enrolled into care in LUTH HIV treatment program from January 2010-December 2014.
| Variables | All patients N = 6108 | Patients not LTFU N = 2711 | Patients LTFU N = 3397 | p-value | Patients LTFU and not traced N = 2972 | Patients LTFU and traced N = 425 | p-value | Patients traced and not interviewed N = 157 | Patients traced and interviewed N = 268 | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Median age, in years (IQR) | 41(35–47)a | 40(35–47) | 41(35–47) | 0.458 | 41(35–47) | 40(34–47) | 0.053 | 41(35–49) | 39(34–45) | 0.037 |
| Female, n (%) | 4065 (66.6) | 1881(69.4) | 2184 (64.3) | <0.001 | 1915(64.4) | 269(63.3) | 0.646 | 103(65.6) | 168(62.7) | 0.546 |
| Marital status, n (%) | ||||||||||
| Married Single Other* Missing |
3032(58.2) 1457(28.0) 723(13.9) 896 (14.7) |
1546(61.2) 648 (25.7) 331 (13.1) |
1486 (55.3) 809 (30.1) 392 (14.6) |
<0.001 | 1267 (54.3) 720 (30.6) 356 (15.1) |
210(62.7) 89 (26.6) 36 (10.8) |
0.010 | 69(58.5) 36(30.5) 13(11.0) |
141(65.0) 53(24.4) 23(10.6) |
0.451 |
| Education, n (%) | ||||||||||
| None Primary Secondary Tertiary Missing |
507(9.7) 1004 (19.2) 2318 (44.4) 1397 (26.7) 882 (14.4) |
211 (8.5) 419 (16.8) 1147 (45.9) 720 (28.8) |
296 (10.9) 585 (21.4) 1171 (42.9) 677 (24.8) |
<0.001 | 264(11.1) 529(22.2) 1017(42.7) 573(24.1) |
32(9.3) 56(16.2) 154(44.5) 104(30.1) |
0.014 | 16(12.6) 22(17.3) 51(56.5) 38(29.9) |
16(7.3) 34(15.5) 103(47.0) 66(30.1) |
0.327 |
| WHO Stage, n (%) | ||||||||||
| 1 2 3 4 Missing |
1210 (34.9) 685 (19.8) 1150 (33.2) 423 (12.2) 2640 (43.2) |
747 (36.5) 415 (20.3) 716 (35.0) 169 (8.3) |
462 (32.5) 270 (19.0) 434 (30.6) 245 (17.9) |
<0.001 | 382(31.3) 234(19.2) 367(30.0) 239(19.6) |
80(40.4) 36(18.2) 67(33.8) 15(7.6) |
<0.001 | 21(28.0) 8(10.7) 36(48.0) 10(13.3) |
59(48.0) 28(22.8) 31(25.2) 5(4.1) |
<0.001 |
| Median CD4, cells/mm3 (IQR) | 229 (97–402)b | 242 (123–392) | 211 (78–411) | <0.001 | 199(73–398) | 292(130–501) | <0.001 | 201(68–434) | 334(199–529) | <0.001 |
| Median viral load, copies/mL (IQR) | 52951(3911–287143)c | 47200(2686–236606) | 57487(4934–337358) | <0.001 | 59856(4962–354400) | 45231(4805–191000) | 0.112 | 119593(16522–454038) | 27800(2397–114032) | <0.001 |
*Divorced/Separated/Widowed
aMissing: 809 (13.2%)
bMissing: 522 (8.6%)
cMissing: 1650 (27%)
In this larger patient cohort overall, lower proportions of patients LTFU were female, married, had tertiary-level education; a higher proportion were in WHO stage 4 of disease and they had lower median CD4+ cell count and higher median viral load compared to patients that were not LTFU.
Among the cohort of LTFU patients, higher proportions of patients that were successfully traced were married, had tertiary education, were in WHO stage 1 and had higher median CD4+ cell count than those that could not be traced. The patients that were successfully traced and interviewed were slightly younger, had higher proportions of patients in WHO stage 1 and had higher median CD4+ cell count and lower median viral load compared to those that were not interviewed.
Predictors of LTFU
Table 2 provides the adjusted and unadjusted HRs for the effect of patient characteristics on LTFU from the ART program. A total of 3,603 (59%) patients were included in the final model. Compared with married patients, single (never married) patients were 1.2 times more likely to become LTFU (aHR: 1.21, 95% CI: 1.08–1.36) and divorced, separated or widowed patients were 1.3 times more likely to become LTFU (aHR: 1.30, 95% CI: 1.12–1.50).
Table 2. Unadjusted and adjusted hazard ratios of being lost to follow-up among 6,108 patients enrolled into care in LUTH HIV treatment program from January 2010-December 2014.
| Variables | Unadjusted HR (95% CI) | p-value | Adjusted HRa (95% CI) | p-value | Adjusted HRb (95% CI) (N = 3603) | p-value |
|---|---|---|---|---|---|---|
| Age (per 10 years) | 0.99(0.94–1.03) | 0.599 | 0.96(0.90–1.02) | 0.177 | 1.00(0.95–1.05) | 0.969 |
| Sex | ||||||
| Female | Ref | Ref | ||||
| Male | 1.12(1.02–1.23) | 0.020 | 1.06(0.94–1.19) | 0.343 | - | - |
| Marital status | ||||||
| Married | Ref | Ref | Ref | |||
| Single | 1.23(1.10–1.37) | <0.001 | 1.17(1.03–1.33) | 0.014 | 1.21(1.08–1.36) | 0.001 |
| Divorced/Separated/Widowed | 1.21(1.05–1.40) | 0.007 | 1.18(1.00–1.38) | 0.046 | 1.30(1.12–1.50) | <0.001 |
| Education | ||||||
| None | Ref | Ref | ||||
| Primary | 1.07(0.89–1.29) | 0.481 | 1.20(0.97–1.47) | 0.088 | - | - |
| Secondary | 0.86(0.72–1.02) | 0.083 | 0.94(0.78–1.14) | 0.523 | ||
| Tertiary | 0.81(0.68–0.97) | 0.024 | 0.89(0.72–1.08) | 0.238 | ||
| Disease progression at baseline | ||||||
| CD4≥200 and/or WHO Stage 1–2 | Ref | Ref | ||||
| CD4<200 and/or WHO Stage 3–4 | 1.29(1.17–1.43) | <0.001 | 1.30(1.16–1.45) | <0.001 | - | - |
| Year of enrollment | ||||||
| 2010 | Ref | Ref | ||||
| 2011 | 1.09(0.96–1.23) | 0.192 | 3.15(2.74–3.61) | <0.001 | - | - |
| 2012 | 1.11(0.97–1.27) | 0.122 | 1.04(0.89–1.20) | 0.625 | ||
| 2013 | 1.24(1.07–1.43) | 0.004 | 0.88(0.71–1.08) | 0.222 | ||
| 2014 | 1.26(1.06–1.51) | 0.009 | 1.21(0.99–1.48) | 0.061 |
HR: Hazards Ratio Ref: Reference category
aFull cox’s proportional hazard model
bFinal cox’s proportional hazard model (Schoenfeld Residuals Test p = 0.062)
Characteristics of interviewed cohort
Of the 268 traced and interviewed patients, 201 (75%) had initiated ART prior to discontinuation at LUTH and the remaining 67 (25%) had only been enrolled in HIV care services. Amongst the 201 patients that had previously initiated ART, 119 (44%) were still on ARV and reported sourcing ARVs from other clinics (77%) and friends (12%). Of the 119 that were still taking ART, 91 (77%) reported still being engaged in HIV care services at another clinic and 28 (23%) reported being disengaged from care. Of these 119 patients, 19 (16%) reported gaps in their treatment. The majority (92%) of the respondents that were still on ARVs had a 100% adherence over the prior 7 days and 66% said they never skip taking their ARVs (Table 3).
Table 3. Use of antiretroviral medication among 268 traced and interviewed LTFU patients.
| Variables | N (%) |
|---|---|
| Started on ARV | |
| Yes | 201 (75.0) |
| No | 67 (25.0) |
| Still on ART (n = 201) | |
| Yes always | 100 (49.8) |
| Yes sometimes | 19 (9.5) |
| No | 82 (40.8) |
| Source of ARVs (n = 119) | |
| Other clinic | 91 (76.5) |
| Family | 4 (3.4) |
| Friend | 14 (11.8) |
| Pharmacy | 10 (8.4) |
| Doses of ARVs missed in last 7 days# (n = 119) | |
| None | 110 (92.4) |
| 1–4 | 5 (4.2) |
| All | 4 (3.4) |
| Last time ARV was missed (n = 119) | |
| Within the past week | 9 (7.6) |
| 1–4 weeks ago | 3 (2.5) |
| 1–3 months ago | 8 (6.7) |
| More than 3 months ago | 20 (16.8) |
| Never skip ARVs | 79 (66.4) |
#ARVs mentioned by all respondents were once daily prescriptions
Reasons for discontinuation in interviewed cohort
Of the 268 patients interviewed, the primary reported reason for discontinuation from LUTH was long distance to clinic (56%). In total, the most common reasons per category were: long distance to clinic (access to care, 88%), staff was not nice (clinic quality, 10%), busy at work (work and family, 3%), feeling healthy (medical, 14%) and not being permitted by religion or faith (alternate treatment and advice, 6%) [Table 4].
Table 4. Reasons for discontinuation of care from LUTH HIV treatment program among 268 traced and interviewed LTFU patients.
| Category | Reasons mentioned | Ranked 1st N (%) N = 268 |
Ranked 2nd N (%) N = 217 |
Ranked 3rd N (%) N = 129 |
Total N (%) N = 268 |
|---|---|---|---|---|---|
| Access to care | Long distance to clinic | 150 (56.0) | 65 (30.0) | 22 (17.1) | 237 (88.4) |
| Long waiting time | 12(4.5) | 43 (19.8) | 26 (20.2) | 81 (30.2) | |
| Started treatment in another clinic | 9 (3.4) | 18 (8.3) | 15 (11.6) | 42 (15.7) | |
| High cost of transportation | 11(4.1) | 15 (6.9) | 14 (10.9) | 40 (14.9) | |
| High cost of test | 5 (1.9) | 3 (2.3) | 8 (3.0) | ||
| Othersa | 3 (1.1) | 2 (0.9) | 1 (0.8) | 5 (1.9) | |
| Clinic quality | Staff was not nice | 5 (1.9) | 8 (3.7) | 13 (10.1) | 26 (9.7) |
| Afraid of scolding from clinic staff | 2 (0.8) | 5 (2.3) | 6 (4.7) | 13 (4.9) | |
| Attending clinic risked disclosure to community | 2 (0.8) | 5 (2.3) | 4 (3.1) | 12 (4.5) | |
| Othersb | 3 (1.1) | 5 (2.3) | 2 (1.6) | 10 (3.7) | |
| Work and family | Busy at work | 9 (3.4) | 17 (7.8) | 10 (7.8) | 36 (13.4) |
| Busy caring for family | 1 (0.4) | 4 (1.8) | 3 (2.3) | 8 (3.0) | |
| Spouse/baby died | 4 (1.5) | 1 (0.5) | 5 (1.9) | ||
| Medical | Feeling healthy | 18(6.7) | 12 (5.5) | 7 (5.4) | 37 (13.8) |
| Didn’t need ARV | 11(4.1) | 4 (1.8) | 2 (1.6) | 17 (6.3) | |
| Othersc | 14 (5.2) | 4 (1.8) | 0 (0.0) | 18 (6.7) | |
| Alternate treatment and advice | Not permitted by religion or faith | 9 (3.4) | 7 (3.2) | 0 (0.0) | 16 (6.0) |
| Othersd | 1 (0.4) | 2 (0.9) | 1 (0.8) | 4 (1.5) |
a No work/no money, next scheduled clinic visit was far
b Poor clinic environment, too many appointments, lack of privacy
c Was sick, had side effects with ARV, does not have HIV, tired of ARV, did not understand importance, did not want caesarian section
d Family person does not approve of clinic, started alternative medicine
Reasons for stopping ART in interviewed cohort
Of the 82 patients that reported having stopped taking ART, the most common reasons given for stopping were long distance to clinic (32%), feeling healthy (30%), having finished ARVs without restocking (23%), not being permitted by faith/religion (16%), high transportation cost to clinic (15%) and not wanting to take ARVs for life (13%). Of the 19 patients that reported gaps in their treatment, the most common reasons given were long distance to clinic (47%), finishing ARVs without restocking (37%), high transportation cost to clinic (32%) and work responsibilities (26%) [Table 5].
Table 5. Reasons for discontinuation of ART for patients that discontinued from the LUTH HIV treatment program.
| Variables | N (%) |
|---|---|
| Reasons for stopping ARVs* (n = 82) | |
| Finished ARVs without restocking | 19 (23.2) |
| Suspected side effects of ARVs | 6 (7.3) |
| Very weak/sick | 4 (4.9) |
| Now on alternate medicine | 5 (6.1) |
| Feeling healthy | 25 (30.5) |
| High transportation cost to clinic | 12 (14.6) |
| Long distance to clinic | 30 (36.6) |
| Work responsibilities | 8 (9.8) |
| Not ready to take ARVs for life | 11 (13.4) |
| My religion/faith does not permit me | 13 (15.9) |
| Othersa | 10 (8.4) |
| Reasons for treatment gaps* (n = 19) | |
| Finished ARVs without restocking | 7 (36.8) |
| Forget to take ARVs | 4 (21.1) |
| High transportation cost to clinic | 6 (31.6) |
| Long distance to clinic | 9 (47.4) |
| Work responsibilities | 5 (26.3) |
| Othersb | 5 (26.3) |
*Multiple responses allowed
a Non-disclosure of HIV status, family person does not approve taking ARVs, high cost of tests, no longer pregnant, lack information, too much stress
b Suspected side effects of ARVs, very weak/sick, family person does not approve taking ARVs, no work/no money
Predictors of disengagement and re-engagement with care
Among the interviewed patients, bivariate analyses comparing characteristics of patients disengaged from HIV care to those still retained in HIV care outside LUTH revealed that significantly higher proportions of patients still not receiving care following LTFU from LUTH were male (73%, p = 0.020) and had not started ART in LUTH (85%, p<0.001). Additionally, a significantly higher proportions of patients that; are male (73%, p = 0.001), had not started ART in LUTH (79%, p<0.001), and had stayed longer duration in care before LTFU (82%, p = 0.017), were willing to re-engage in the LUTH antiretroviral program (Table A in S2 Appendix).
In multivariate analyses, the predictor of disengagement from care was not having started ART. The predictors of willingness to re-engage in care at LUTH were not having started ART, male sex and longer duration in HIV care prior to LTFU (Table 6).
Table 6. Multivariate analysis of characteristics associated with disengagement from care and willingness to re-engage into LUTH HIV treatment program among 268 traced and interviewed LTFU patients.
| Disengaged from carea c (N = 268) | Willingness to re-engageb d (N = 268) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | COR (95% CI) | p-value | AOR (95% CI) | p-value | COR (95% CI) | p-value | AOR (95% CI) | p-value |
| Age (per 10 years) | 0.30(0.07–1.27) | 0.103 | 0.11(0.09–1.33) | 0.083 | 0.28(0.07–1.12) | 0.073 | 0.43(0.70–2.61) | 0.357 |
| Sex | ||||||||
| Female | Ref | Ref | Ref | Ref | ||||
| Male | 1.88(1.10–3.23) | 0.021 | 1.49(0.80–2.79) | 0.208 | 2.40(1.40–4.10) | 0.001 | 1.91(1.05–3.45) | 0.033 |
| Marital status | ||||||||
| Married | Ref | Ref | Ref | Ref | ||||
| Single | 1.72(0.82–3.63) | 0.153 | 2.29(0.99–5.28) | 0.052 | 0.80(0.41–1.56) | 0.510 | 1.05(0.48–2.26) | 0.907 |
| Divorced/Separated/Widowed | 0.85(0.40–1.78) | 0.658 | 0.80(0.37–1.71) | 0.559 | 1.02(0.48–2.16) | 0.955 | 1.23(0.58–2.62) | 0.594 |
| Education | ||||||||
| None | Ref | Ref | Ref | Ref | ||||
| Primary | 0.27(0.03–2.52) | 0.253 | 0.25(0.03–2.26) | 0.217 | 0.83(0.14–4.93) | 0.841 | 0.57(0.08–4.26) | 0.583 |
| Secondary | 0.38(0.04–3.23) | 0.373 | 0.42(0.05–3.47) | 0.419 | 0.66(0.12–3.53) | 0.625 | 0.58(0.09–3.94) | 0.581 |
| Tertiary | 0.22(0.23–1.88) | 0.166 | 0.25(0.03–2.10) | 0.203 | 0.48(0.09–2.61) | 0.396 | 0.45(0.07–3.06) | 0.417 |
| ART initiation | ||||||||
| Started on ART | Ref | Ref | Ref | |||||
| Not started on ART | 4.26(2.06–8.83) | <0.001 | 4.42(2.13–9.15) | <0.001 | 3.20(1.67–6.13) | <0.001 | 3.56(1.77–7.16) | <0.001 |
| Months in care before LTFU (per 3 months) | 1.00(1.00–1.00 | 0.929 | 1.00(1.00–1.00) | 0.810 | 2.00(1.33–2.99) | 0.001 | 2.14(1.36–3.36) | 0.001 |
COR: crude odds ratio; AOR: adjusted odds ratio; Ref: Reference categories
Reference categories:
aRetained in care
bNot willing to re-engage in program
Box-Tidwell tests before transformation—age vs. disengagement from care (p = 0.494, linear relationship); months in care vs. disengagement from care (p = 0.026, non-linear relationship); age vs. willingness to re-engage (p = 0.360, linear relationship); months in care vs. willingness to re-engage (p = 0.832, linear relationship)
Hosmer-Lemeshow goodness-of-fit test:
cp = 0.625;
dp = 0.326
In subsequent follow-up, 95 of the 268 patients interviewed returned to LUTH for care services.
Discussion
In this study conducted at a high-volume HIV clinic in southern Nigeria, we found a high proportion of LTFU. Of the patients that were traced and interviewed, majority were truly disengaged from care and not receiving ART from any source. We discovered that the most common reason for discontinuation from HIV care from the large clinic, stopping ART, or having treatment gaps (for those still on ART) was long distance to the clinic.
The LTFU rate found in this evaluation was consistent with that reported in other studies of LTFU for patients receiving HIV care services in sub-Saharan Africa (2.6–57.4%) [20]. Our rate was similar to that reported from programs in north central Nigeria with rates of 56.4% and 58% in hub and spoke sites respectively [13], but higher than a study from the southeast with rates of 32.8% and 11% in public and private programs, respectively [3].
A possible explanation for the regional differences is that the private southeastern program takes efforts to trace lost patients [3], along with the increased mobility of people in metropolitan areas of the southwest region [21]. Our value was higher than the LTFU rate of 28% from an earlier study involving part of this patient cohort, as that earlier study focused only on patients on ART [12]. Being unmarried was a predictor of being LTFU in our study, similar to findings from other studies [12,22], implying that having a committed spousal support system could be a motivation for patients to overcome existing barriers to continuing care.
The rate of true disengagement from care and ART among interviewed patients in this study was higher than in similar studies in sub-Saharan Africa [6,8,17,19]. This is particularly worrisome as patients not receiving care are at risk of progressive disease and death when they do not have access to life-saving medication and continual care, possibly evidenced by the outcome of death among 16% of the traced patients in our study; a rate of mortality lower than pooled estimates of 39% in sub-Saharan Africa [23]. In addition, those that were previously on ART are at an increased risk of developing and transmitting drug resistant strains of HIV [3].
Similar to other studies, we found patients in our study cohort reported that long distance to clinic was a major reason for discontinuation of HIV care and stopping ART [19,24]. Prior studies have shown that patients are more likely to remain in HIV care when ART services are decentralized to centers closer to patients’ homes [25–27]. The cohort evaluated in this study originally enrolled at LUTH when ART services were not decentralized. Several patients also reported having discontinued care and stopping ART because they were feeling healthy. This could occur among patients when they increase in weight, have high CD4+ cell count or undetectable viral load [28,29]; the dangers of discontinuing ART even when feeling healthy should be stressed during health education sessions.
An encouraging finding for the LUTH clinic was that only few of the interviewed patients discontinued care secondary to clinic quality such as poor clinic environment and lack of privacy. This is interesting because the clinic environment was compromised by a fire incident in 2012 without being adequately rehabilitated [30] and may be due to the subjective sense of connection patients have to each other, the clinic’s site, providers and processes [31].
Similar to other studies, we found that patients that were not yet on ART were more likely than those on ART to be disengaged from care [32]. Our finding underscores the importance of the test and treat strategy to aid retention in care, which was adopted in Nigeria in 2016 [14]. Further research could examine any difference in LTFU and disengagement rates in the current test and treat strategy era as well as the re-engagement to care kinetics within the ART program.
Most of the patients interviewed were willing to return to the LUTH clinic and 95 of them were linked back to care. This positive attitude despite identified barriers to continued care is encouraging and could potentially translate to high re-engagement in care once barriers are addressed. It is not immediately clear why female patients were less willing to return to care and this would require exploratory research. Those patients with long duration in HIV care prior to LTFU might be challenged by long duration of physical symptoms, psychosocial distress, co-morbidities, social stigma, treatment fatigue, as well as the adverse effects that may be associated with ART [33–35], which could result in their unwillingness to return to care.
Some limitations must be considered in our study. First, our findings cannot be generalized to all the LTFU patients within the cohort because a significant number of them could not be traced as a result of missing and inaccurate locator information in the secondary data or as a result of having moved out of state resulting in a potential bias. A salient recommendation would be updating and verification of patient information at each follow-up visit. Our results thus pertain to only those patients that were traced and interviewed as they differed in some regard to those that were not interviewed. Secondly, our definition of retention in care subsequent to LTFU from LUTH was based on self-report of attendance in other facilities other than LUTH. This may be considered simplistic as it does not account for frequency or regularity of visits or use of ART [36].
In conclusion, retention in HIV care is a major challenge in HIV/AIDS management programs in sub-Saharan Africa. There might be site-based variability in reasons for discontinuation and outcomes of patients LTFU and our understanding of these in the Nigerian setting is limited. Our study fills in this gap in knowledge by describing the status as well as willingness to re-engage in care of patients fitting the criteria of LTFU from one the largest ART programs in the country. It is crucial that ART programs incorporate LTFU tracing routinely into their care [9,10,37] and further research should explore innovative ways of re-engaging patients back into care in our setting. In addition to early tracing, well-organized community support initiatives are also effective strategies [38–40]. In settings where services have been decentralized, the flexibility in the system should be properly communicated to patients so they understand their options for receiving treatment and care. Health information systems should also be strengthened and patient information linked across the antiretroviral programs (including the decentralized sites) in the nation in order to enhance the documentation of transfers and to further facilitate continuity in care. The findings of our study have implications for the strengthening of healthcare systems to address the needs of a large population of HIV-infected patients.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
We are grateful to Hameed Adelabu, data manager in LUTH antiretroviral program, for his assistance in sorting through the secondary data. We also thank the research assistants; Victoria Yesufu, Eghonghon Olawepo-Adeoti and Ikenna Molobe for their work in collecting the field data and conducting the face-to-face interviews.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW010134 to MB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Co-Funding Partners: Fogarty International Center (FIC); NIH Common Fund, Office of Strategic Coordination, Office of the Director (OD/OSC/CF/NIH); Office of AIDS Research, Office of the Director (OAR/NIH); Office of Research on Women's Health, Office of the Director (ORWH/NIH); National Institute on Minority Health and Health Disparities (NIMHD/NIH); National Institute of Neurological Disorders and Stroke (NINDS/NIH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. HIV [Online]. 2018 February [cited 2018 May 01]. Available from: URL http://www.who.int/hiv/en/
- 2.Avert. HI and AIDS in Nigeria [Online]. 2018 March 26 [cited 2018 May 02] [4 screens]. Available from: URL https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/nigeria
- 3.Onoka CA, Uzochukwu BS, Onwujekwe OE, Chukwuka C, Ilozumba J, Onyedum C, et al. Retention and loss to follow-up in antiretroviral treatment programmes in southeast Nigeria. Pathog Glob Health. 2012. March; 106(1): 46–54. 10.1179/2047773211Y.0000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idigbe EO, Odutolu O, Okonkwo P, Folayan MO, Uwakwe CBR, Audu RA, et al. Evaluation of the Nigerian antiretroviral (ARV) treatment training Programme. SAHARA J. 2006. November; 3(3): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AFRIPOL. Nigeria: HIV/AIDS Treatment Medical Centers [Online]. 2013 Jun 08. [cited 2018 October 05] [18 screens], Available at: http://www.afripol.org/afripol/item/1213-nigeria-hiv/aids-treatment-medical-centers.html (accessed: 05/10/2018)
- 6.Namusobya J, Semital FC, Amanyire G, Kabami J, Chamie G, Bogere J, et al. High Retention in Care among HIV Infected patients entering care with CD4 levels>350cells/uL under routine program conditions in Uganda. Clin Infect Dis. 2013. November 01; 57(9): 1343–50. 10.1093/cid/cit490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen S, Fox MP, Gill CJ. Patient Retention in Antiretroviral Therapy Programs in Sub-Saharan Africa: a Systematic Review. PLoS Med. 2007. October; 4(10): e298 10.1371/journal.pmed.0040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clin Infect Dis. 2016. April 1;62(7):935–944. 10.1093/cid/civ1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon JH, Elliot JH, Hong SY, Bertagnolio S, Jordan MR. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS One. 2013;8(2):e56047 10.1371/journal.pone.0056047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bershetyn A, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. The Causal Effect of Tracing by Peer Health Workers on Return to Clinic Among Patients Who Were Lost to Follow-up From Antiretroviral Therapy in Eastern Africa: A "Natural Experiment" Arising From Surveillance of Lost Patients. Clin Infect Dis. 2017. June 1;64(11):1547–54. 10.1093/cid/cix191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agolory SG, Auld AF, Odafe S, Shiraishi RW, Dokubo EK, Swaminathan M, et al. High rates of loss to follow-up during the first year of pre-antiretroviral therapy for HIV patients at sites providing pre-ART care in Nigeria, 2004–2012. PLoS One. 2017. September 1;12(9):e0183823 10.1371/journal.pone.0183823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meloni ST, Chang C, Chaplin B, Rawizza H, Jolayemi O, Banigbe B, et al. Time-Dependent Predictors of Loss to Follow-Up in a Large HIV Treatment Cohort in Nigeria. Open Forum Infect Dis. 2014. August 6;1(2):ofu055 10.1093/ofid/ofu055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agaba PA, Genberg BL, Sagay AS, Agbaji OO, Meloni ST, Dadem NY, et al. Retention in Differentiated Care: Multiple Measures Analysis for a Decentralized HIV Care and Treatment Program in North Central Nigeria. J AIDS Clin Res. 2018;9(2). pii: 756. 10.4172/2155-6113.1000756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federal Ministry of Health. National Guidelines for HIV Prevention, Treatment and Care 2016. National AIDS and STIs Control Programme, Federal Ministry of Health, Abuja, Nigeria, 2016. Available at: https://aidsfree.usaid.gov/resources/2016-nigeria-national-guidelines-hiv-prevention-treatment-and-care [accessed 04/03/2018] [Google Scholar]
- 15.Chaplin B, Meloni S, Eisen G, Jolayemi T, Banigbe B, Adeola J, et al. Scale-up of networked HIV treatment in Nigeria: creation of an integrated electronic medical records system. Int J Med Inform. 2015. January;84(1):58–68. 10.1016/j.ijmedinf.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 16.Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C. Universal Definition of Loss to Follow-Up in HIV Treatment Programs: A Statistical Analysis of 111 Facilities in Africa, Asia, and Latin America. PLoS Med. 2011. October; 8(10): e1001111 10.1371/journal.pmed.1001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rachlis B, Ochieng D, Geng E, Rotich E, Ochieng V, Maritim B, et al. Evaluating outcomes of patients lost to follow-up in a large comprehensive care treatment program in western Kenya. J Acquir Immune Defic Syndr. 2015. April 1; 68(4): e46–e55. 10.1097/QAI.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The AIDS Clinical Trial Group. ACTG Adherence Follow up Questionnnaire [Online] 2001 April 05. Cited 2017 Jan 05. [3 screens] Available at: https://prevention.ucsf.edu/uploads/tools/surveys/pdf/2098.4188.pdf [accessed 05/01/2017]
- 19.Tweya H, Feldacker C, Estill J, Jahn A, Ng’ambi W, Ben-Smith A, et al. Are They Really Lost? “True” Status and Reasons for Treatment Discontinuation among HIV Infected Patients on Antiretroviral Therapy Considered Lost to Follow Up in Urban Malawi. PLoS One. 2013; 8(9): e75761 10.1371/journal.pone.0075761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zürcher K, Mooser A, Anderegg N, Tymejczyk O, Couvillon MJ, Nash D. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Trop Med Int Health. 2017; 22(4): 375–87. 10.1111/tmi.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oladele OO, William RB. Rural-urban mobility in southwestern Nigeria: implications for HIV/AIDS transmission from urban to rural communities. Health Education Research. 1994; 9(4): 507–18. 10.1093/her/9.4.507 [DOI] [Google Scholar]
- 22.Ahmed I, Gugsa S T, Lemma S, Demissie M. Predictors of loss to follow-up before HIV treatment initiation in Northwest Ethiopia: a case control study. BMC Public Health. 2013; 13(867):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. 2015;20(3):365–79. 10.1111/tmi.12434 [DOI] [PubMed] [Google Scholar]
- 24.Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programmes in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010. March; 53(3): 405–11. 10.1097/QAI.0b013e3181b843f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AK, Mateyu G, Jahn A, Schouten E, Arora P, Mlotha W, et al. Outcome assessment of decentralization of antiretroviral therapy provision in a rural district of Malawi using an integrated primary care model. Trop Med Int Health. 2010; 15: 90–7. 10.1111/j.1365-3156.2010.02503.x [DOI] [PubMed] [Google Scholar]
- 26.Auld AF, Kamiru H, Azih C, Baughman AL, Nuwagaba-Biribonwoha H, Ehrenkranz P, et al. Implementation and operational research: Evaluation of Swaziland’s hub-and-spoke model for decentralizing access to antiretroviral therapy services. J Acquir Immune Defic Syndr. 2015; 69: e1–12 10.1097/QAI.0000000000000547 [DOI] [PubMed] [Google Scholar]
- 27.Johns B, Baruwa E. The effects of decentralizing anti-retroviral services in Nigeria on costs and service utilization: two case studies. Health Policy Plan. 2016; 31(2): 182–191. 10.1093/heapol/czv040 [DOI] [PubMed] [Google Scholar]
- 28.Mukumbang FC, Mwale JC, van Wyk B. Conceptualising the factors affecting retention in care of patients on antiretroviral treatment in Kabwe District Zambia, using the ecological framework. AIDS Res Treat. 2017; 2010:7356362 10.1155/2017/7356362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muessig KE, Panter AT, Mouw MS, Amola K, Stein KE, Murphy JS, et al. Medication-taking practices of patients on antiretroviral HIV therapy: control, power, and intentionality. AIDS Patient Care STDS. 2015; 29(11): 606–616. 10.1089/apc.2015.0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezeamalu B (2012) “LUTH attends to patients, despite hospital fire”, The Premium Times. 13 August. Available at: https://www.premiumtimesng.com/news/98519-luth-attends-to-patients-despite-hospital-fire-2.html [accessed 09/04/2019]
- 31.Ware NC, Wyatt MA, Geng EH, Kaaya SF, Agbaji OO, Muyindike WR, et al. Towards an understanding of disengagement from HIV treatment and care in Sub-Saharan Africa: a qualitative study. PLoS Med. 2013; 10(1): e1001369 10.1371/journal.pmed.1001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: Systematic review and meta-analysis. Trop Med Int Health. 2012; 17(12): 1509–1520. 10.1111/j.1365-3156.2012.03089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YT. Challenges and responses in providing palliative care for people living with HIV/AIDS. Int J Palliat Nurs. 2013; 19(5): 218, 220–5. 10.12968/ijpn.2013.19.5.218 [DOI] [PubMed] [Google Scholar]
- 34.Corless IB, Nicholas PK. Long-term continuum of care for people living with HIV/AIDS. J Urban Health. 2000; 77(2):176–86 10.1007/BF02390529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claborn KR, Meier E, Miller MB, Leffingwell TR. A systematic review of treatment fatigue among HIV infected patients prescribed antiretroviral Therapy. Psychol Health Med. 2015; 20(3):255–65. 10.1080/13548506.2014.945601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rollins NC, Becquet R, Orne-Gliemann J, Phiri S, Hayashi C, Baller A et al. Defining and analyzing retention-in-care among pregnant and breastfeeding HIV-infected women: unpacking the data to interpret and improve PMTCT outcomes. J Acquir Immune Defic Syndr. 2014;67:S150–S156 10.1097/QAI.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Luis S, Fuente-Soro L, Augusto O, Bernardo E, Nhampossa T, Maculuve S, et al. Reengagement of HIV-infected children lost to follow-up after active mobile phone tracing in a rural area of Mozambique. J Trop Pediatr. 2018. August 7 10.1093/tropej/fmy041 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Wouters E, Van Damme W, van Rensburg D, Masquillier C, Meulemans H. Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Serv Res. 2012. July 9;12:194 10.1186/1472-6963-12-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. Int Health. 2013. September;5(3):169–79. 10.1093/inthealth/iht016 [DOI] [PubMed] [Google Scholar]
- 40.Decroo T, Telfer B, Dores CD, White RA, Santos ND, Mkwamba A, et al. Effect of Community ART Groups on retention-in-care among patients on ART in Tete Province, Mozambique: a cohort study. BMJ Open. 2017. August 11;7(8):e016800 10.1136/bmjopen-2017-016800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.