Abstract
The active phase of conventional static composting systems varies dramatically, ranging from several weeks to several months. Therefore, this study was to examine the effect of a combined continuous aeration-rotation process on shortening the active phase of composted material. A mixture of tomato plant residues with 20%-chicken manure (v/v) was composted in two identical pilot-scale bioreactors. One of them was static, and the other was continuously rotated at 3 rpm; each was supplied with continuous aeration. Compost temperatures (Tc) were measured throughout the composting process; the moisture content (MC) and carbon/nitrogen ratio (C/N) were measured at the beginning and end of the experiment. The quality and stage of compost were evaluated at the end of the experiment using Dewar, Solvita, and visual tests. Continuous aeration-rotation significantly reduced the active phase period to 4.5 days, increased the compost temperature (Tc) to 60°C after 3 days of operation, and remained at 50–65°C for approximately 3 consecutive days (thermophilic stage). In contrast, compost in the static bioreactor remained in the mesophilic stage (Tc < 45°C). During the composting process, the C/N ratio was reduced from 30/1 to 23/1 in the rotating bioreactor, while it remained at 30/1 in the static bioreactor, indicating that the nitrogen content was not a limiting factor affecting the composting process. The MC was within the optimum range for microorganisms (58–61%) for both bioreactors. After the active phase had ended in the rotating bioreactor, the compost was inactive and ready for further maturation, while compost from the static bioreactor was still immature and active. These results show that the proposed method can be done on a commercial scale to significantly reduce the composting period and to enhance the compost stability and productivity.
Introduction
Tomato (Solanum lycopersicum Mill.) production represents approximately 50% of the total greenhouse vegetable production (8000 hectares) in Saudi Arabia [1]. Greenhouse tomatoes produce approximately 15 tons of fresh plant residues per hectare per year, which makes plant residues among the most plentiful biomasses suitable for use as a compost material [2]. The large amount of plant residues that are produced by pruning and post-crop harvesting can be considered a sustainable source of organic matter to be composted and returned back to the soil as horticultural growth media [3, 4, 5]. Adding compost to soil improves soil structure by increasing organic matter, thereby improving soil fertility [6, 7].
A common composting method is open field composting, in which residues are left in windrows or static piles. However, these methods cannot be used in the Arabian Peninsula due to the high daily water evaporation rate (15 mm day-1 in July with a yearly increasing trend) and water shortage [8]. A more efficient and promising technique is composting in enclosed (in-vessel) systems using bioreactors. Bioreactors can process large amounts of waste in a limited space and can accommodate any type of organic waste (e.g., meat, animal manure, bio-solids and food scraps). This allows good control of the environmental factors, i.e., temperature, moisture content and airflow rate [9]. Moreover, bioreactor systems produce compost in a relatively short time, and are more efficient at breaking down materials, decreasing unpleasant odors and preventing disease transmission [10]. It is well known that aeration and rotation are the most critical operational parameters affecting the aerobic composting process. Mechanically forced aeriation is often used in both open and enclosed composting systems. Because excessive or insufficient aeration can adversely affect decomposition [11]; therefore, it is necessary to adjust aeration to the appropriate level for successful composting. In windrows and static piles, oxygen was found to be consumed within two hours after a pile was manually or mechanically turned; making the reaction anaerobic and inefficient [12, 13, 14, 15]. On the other hand, rotating bioreactors provide good mixing and uniform temperature distribution and produce a quick, consistent and uniform end product without any odor or leachate-related problems [11, 16, 17, 18, 19, 20, 21]. The combined rotation-aeration techniques may provide a favorable environment with a sufficient amount of oxygen and bioavailability of organic material that allows aerobic microbes to decompose the waste quickly. However, few studies have examined intermittent manual rotation with natural aeration for enclosed bioreactors. For example, a pilot-scale rotary drum bioreactor was manually turned three revolutions at fixed (6-, 12-, 18-, and 24-hr) intervals [16]. It was found that turning at 24-hr intervals provided the best composting performance. Other studies experimented with various types of materials using different numbers of rotations at 24-hr intervals, including one [17], two [19], three [20], four [18] and six [21] rotations. The previous studies maintained aerobic conditions by opening the side doors on the upper half of the drums for a period of time after the turning process. Successful composting requires sufficient oxygen distribution (via aeration-rotation) and adjustment of control parameters such as composting temperature (Tc), moisture content (MC) and the C/N ratio [7]. In addition, specific biological and chemical tests are required to evaluate the compost quality and quantity [5, 22].
Little is known about the impact of continuous aeration-rotation on bioreactor performance, the composting process and the duration of the active phase. However, a continuous aerating-rotating pilot-scale bioreactor was recently used to evaluate the heat generation and losses during the composting process [23]. The active phase duration can be defined as “the period that starts at the end of the lag period and ends when the combined aeration-rotation no longer reheats a compost having C/N ratio below 25/1 and MC between 40% and 60%”.
Accordingly, the objective of this study was to evaluate the ability of a combination of continuous aeration and rotation to accelerate composting by reducing the active phase period for composting plants residues in a rotary bioreactor. To this end, we used two identical pilot-scale bioreactors, one static and one rotating at 3 rpm. We evaluated (i) the uniformity of compost temperature, (ii) changes in compost temperature with time and the length of the active phase, and (iii) MC, C/N ratio and compost stability and maturity indexes. We also developed a visual test that farmers could use to evaluate compost quality.
Materials and methods
Compost materials
Residues of greenhouse tomato plants (leaves, stems and some green and damaged fruits) were collected from various projects in the Riyadh area of Saudi Arabia. Prior to the composting process, the collected tomato residues had average moisture content (MC) of approximately 90%. Then, they were spread out on the ground to dry for three days, where their MC was reduced to 60%. To promote better aeration and MC distribution, the residues were chopped using a shredder (model FYS-76 Shredder, Mainland, Zhejiang, China). Furthermore, grinding was performed to decrease the particle size to approximately 1–2 cm to promote microbial degradation. The ground residues were left on the floor to dry out for two more consecutive days (MC was reduced to 15%), then tightly bagged and transported to the educational farm of the Agricultural Engineering Department, King Saud University, Riyadh, Saudi Arabia (Waste Management Lab). Thermally treated chicken manure (20% MC, 2% N) was obtained from a broiler project in the Riyadh area. Before starting the experiment, a mixture of tomato plant residues with 20% chicken manure was prepared. The mixing ratio was determined according to the recommended optimum C/N ratio of 30/1, and the MC of the final mixture was also adjusted to 60% as recommended [24]. The two bioreactors were each loaded with 50 kg of the final mixture.
Description of the bioreactors
Two identical pilot-scale bioreactors, each with a volume of 0.2 m3, were constructed at the educational farm of the Agricultural Engineering Department, King Saud University. Each bioreactor was a steel barrel with an inner diameter of 585.0 mm, a length of 914.4 mm and a wall thickness of 3.0 mm. Each bioreactor was designed to provide a space for 50 kg (wet weight) of compost mixture, leaving 25% of the volume as a headspace. In each bioreactor, a steel tube with a 50 mm outer diameter was installed horizontally at the centerline of the barrel for aeration and temperature measurement. In each bioreactor, a 50.0 × 40.5 cm door was constructed for loading, unloading, sampling and cleaning. A rubber gasket lining was fixed on the inner side of each door to prevent leakage. The outer surfaces of each bioreactor were insulated with a 25 mm thick glass wool blanket layer. For comparison, one bioreactor was static and the other was rotated horizontally around a fixed axis (i.e., a steel tube with a 50 mm outer diameter) at 3.0 rpm using a 0.25 hp electric motor (Model No. 220-380-3, Zhejiang, China). For aeration purposes, the perimeter of the tube included holes distributed longitudinally along the upper surface of the tube in the rotating bioreactor and in the lower surface in the static bioreactor. Layout dimensions for the rotary and fixed bioreactors, installed on steel frames with a rotating motor (for the rotary one), are illustrated (not to scale) in Fig 1A and 1B.
Fig 1.
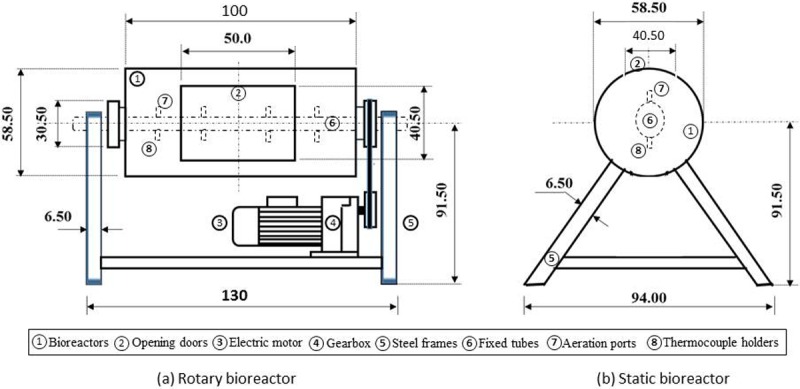
Schematic diagram showing the constructed (a) rotating bioreactor and (b) static bioreactor systems; dimensions in cm, not to scale.
Experiments and measurements
Compressed air was continuously supplied to each bioreactor at a flow rate of 0.005 m3 min−1 from a reservoir (10 bar, 0.2 m3 volume) connected to an air compressor (Model: Airmac, CRM203, 2.2 kW, Parkinson, Australia). The compressed air was supplied to the horizontal tubes around which the bioreactor rotated. The compressed air passed through flow meters (one for each bioreactor) to adjust the proper air flow rate and then to the compost via holes that were made in the horizontal tube (Fig 2A and 2B). The temperature of the compost (Tc) was measured using three copper-constantan thermocouples (type-T, Cole Parmer, Chicago, IL, USA) fixed longitudinally at three locations above and below the horizontal tube. In the tube, aeration holes were on the opposite side of the thermocouple sensors to be far enough from the inlet air to reduce the negative impact of the air on the temperature measurements. Aeration ports were located downward in the rotating bioreactor (Fig 2A) and downward and upward in the static bioreactor (Fig 2B). The thermocouple wires were placed inside the tube to the outside and connected to a portable data logger (Model: Testo 177-T4 V01-02). Ambient temperature (Tam) was measured with a Thermo-Hygrometer DMA033 (LSI-Lastem, Milano, Italy). The measured parameters were recorded every 10 seconds, averaged for every 10 min period and saved in the data logger.
Fig 2.
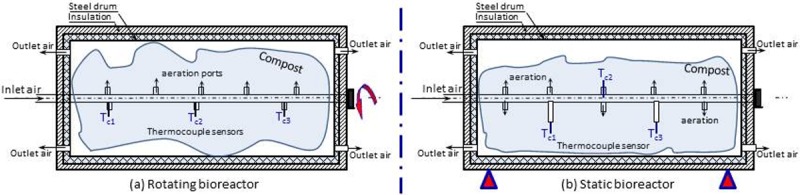
Cross-sectional views of the (a) rotating bioreactor and (b) static bioreactor Showing the inlet and outlet aeration ports and the locations of thermocouple sensors.
Calibration test
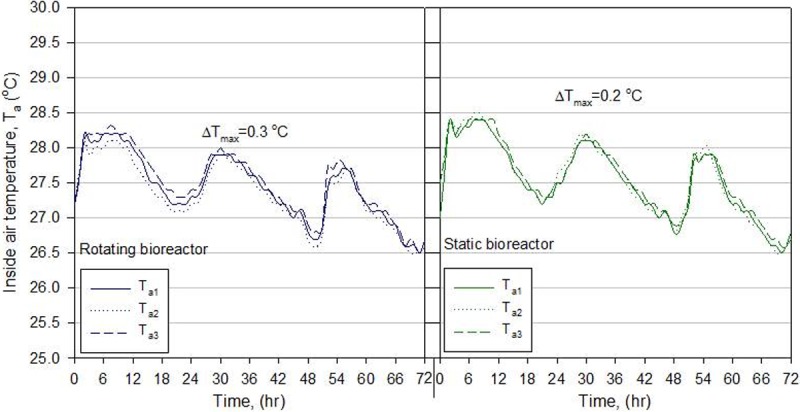
To ensure that the temperature sensors were working properly and for accurate comparisons between the rotating and static bioreactors, before starting the experiment, the two bioreactors were operated empty and the air temperatures were measured by the three sensors in each bioreactor (Ta1, Ta2, and Ta3) and recorded over 72 hours and illustrated in Fig 3. During 72 hours, the maximum temperature difference among the three sensors ΔTmax was 0.2°C (in the static bioreactor) and 0.3°C (in the rotating bioreactor). The temperature levels in the rotating bioreactor were somewhat lower than those in the static bioreactor because the rotation and air mixing in the rotating bioreactor may have increased the heat loss to the air outside the bioreactor. Based on the average air temperature [(Ta1+Ta2+Ta3)/3] in each bioreactor, the maximum temperature difference during 72 hours was estimated to be 0.33°C, which is considered the maximum expected error in the comparison of temperature.
Fig 3. Time course of air temperatures recorded by the three sensors (Ta1, Ta2, and Ta3) fixed at three different locations inside the rotating and static bioreactors.
Determining the compost parameters
Moisture content (MC, %) was measured by the oven-drying method (ASTM procedure D3173-73). A representative compost sample was placed in an air oven at 105°C for 24 hours, until a constant weight was achieved. The total organic carbon (TOC) content was calculated assuming that it was equal to 55% of the organic matter (OM), according to Haug [24] as:
| (1) |
Total nitrogen (TN, %) was analyzed based on the Kjeldahl method using Foss-Kjeltec (Model: 8100, Denmark). Consequently, the C/N ratio was calculated using the values of TOC and TN. The moisture content (MC, %) and carbon to nitrogen (C/N) ratio were determined at the beginning and end of the experiment (day 0 and day 8). To determine the maturity level of the composted material, three samples were taken randomly from each bioreactor just after taking the compost material out of the bioreactors (at day 8) for testing and evaluation.
For the Solvita test, the moisture content (MC) of the tested samples (100 mL of composted materials) was adjusted to 60%, and the samples were incubated in 200 mL containers with a Solvita reactor for 4 hours following the manufacturer’s instructions [25]. Then, the extent of color change was measured using the new DCR (Digital CO2 & NH3 Color Reader, Solvita).
For the Dewar self-heating test [26], the six samples were stored at 4°C until use. Each sample was kept in a flask for six days, then the maximum temperature (Tmax) and the corresponding room temperature (Troom) were recorded, and consequently, the temperature difference ΔT (ΔT = Tmax−Troom) was estimated.
For visual testing, the color components of each compost sample were determined on days 0 and 8 using a ColorFlex spectrophotometer (Hunter Lab-ColorFlex, Hunter Associates Laboratory, Inc.-Reston, US). Each sample was placed in a sample cup, and then the cup was covered and inserted into the measuring chamber. Consequently, color components were measured where L* is the lightness or darkness (black: L* = 0; white: L* = 100), +a* is redness, -a* is greenness, +b* is yellowness and–b* is blueness. The average value of each component was obtained for the three samples from each bioreactor. The measuring device was calibrated with standard calibration plates provided by the manufacturer. The compost color change (ΔE) during the composting process (8 days) was determined for each bioreactor using the color difference equation [27]:
| (2) |
where are the color components measured at the initial stage (day-0) and L*, a*, b* are the color components measured at the final stage (day-8).
Results and discussion
The main parameters affecting the composting process of agricultural residues are oxygen, moisture content (MC), carbon to nitrogen ratio (C/N) and compost temperature (Tc). In the present study, we monitored MC, C/N ratio and Tc but not oxygen because both bioreactors had a continuous supply of oxygen that makes oxygen isn’t a limiting factor controlling the process.
Moisture content (MC)
The optimum moisture content (MC) of compost is a vital factor for the microbial decomposition of organic waste. The initial MC of approximately 60% is proper for an acceptable composting [28]. However, excessive moisture content leads to a lower rate of oxygen resulting in anaerobic conditions that decrease the organic matter degradation rate. In the present study, however, the MC of the composted material was estimated on the 1st and 8th days for each bioreactor. During the experiment, MC remained in the range 57.8% - 60.0% in the rotating bioreactor and in the range 60.0% - 61.4% in the static bioreactor, which was in the optimum range for microbial activity [29]. Therefore, it can be concluded that the MC was not a limiting factor during the composting process in either bioreactor.
Carbon to nitrogen ratio (C/N)
The C/N ratio is used to describe organic waste decomposition and compost quality with respect to organic matter and N cycling. Several studies have indicated that for high-quality final compost, the C/N ratio should be in the range from 12/1 to 25/1, for example [30, 31]. In compost with a high C/N ratio (> 40/1), microorganisms take considerable time to break down waste because a deficiency of N reduces composting performance [32]. However, compost with a low C/N ratio causes ammonium toxicity [33]. In the present study, the C/N ratio decreased from 30/1 to 23/1 (in the rotating bioreactor) and remained at 30/1 (in the static bioreactor) during the composting process. Bazrafshan et al. (2016) [33] attribute the reduction in the C/N ratio during the composting process to the transformation of carbon to CO2 followed by a reduction in the organic acid concentration and an increase in the N content per unit material. Therefore, N was not a limiting factor during the composting process in either bioreactor.
The compost temperature (Tc)
Composting is an exothermic process that generates heat due to the aerobic metabolic reactions of the composting materials. This, in turn, increases the temperature of the compost and the bioreactor components. Mixing of compost materials in the rotating bioreactor created uniform temperature distribution of compost. Therefore, no significant differences were observed among the three temperatures of compost (Tc1, Tc2 and Tc3) measured in rotating bioreactor (Fig 4A). While, in the static bioreactor, a maximum temperature difference of 5°C was observed among the three compost temperatures (Fig 4B). In the upcoming discussion, the average of the three compost temperatures was used to represent the compost temperature (Tc) in each bioreactor.
Fig 4. Time course of compost temperatures recorded by the three sensors (Tc1, Tc2, and Tc3) fixed at three different locations inside the rotating and static bioreactors.
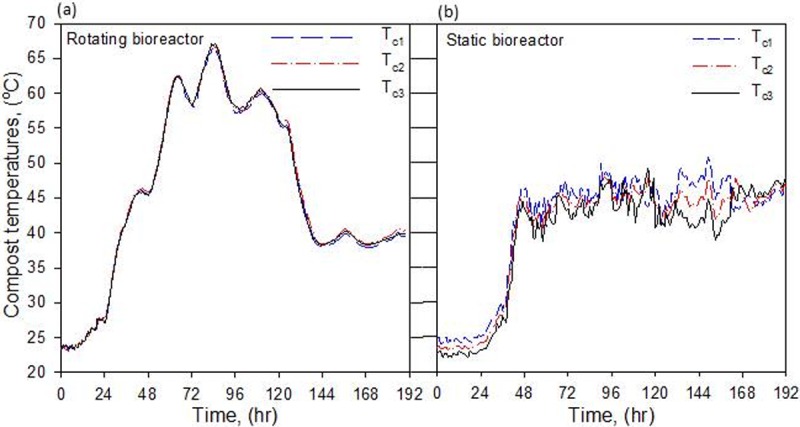
The time course of the average compost temperatures (Tc) and ambient air temperature, (Tam) in the static and rotating bioreactors are illustrated in Fig 5. Rotation provides optimal conditions for the composting process to proceed through the three standard phases, enhanced the metabolic exothermic reactions, and increased the internal energy of the compost. Thus, rotation created optimal composting conditions and increased the breakdown of the available organic matter and nitrogenous compounds through microbial activity. This rapidly increased Tc to approximately 55°C after 3 days, where it remained for approximately three days in the thermophilic stage (Fig 5). In the static bioreactor, microbial activity was depressed, lowering Tc to the mesophilic stage during the entire experiment (Fig 5). Moreover, Tc was generally below 50°C, which increases the risk of weed seed survival and plant pathogens in the end product. Mixing the compost materials in the rotary bioreactor significantly reduced the active phase period (high-temperature phase) to less than 3 days compared to several weeks, or possibly several months, for the static composting systems. In addition, keeping Tc in the range of 50–65°C for 2–3 consecutive days is sufficient to meet the optimum requirements for the destruction of pathogens and weed seed viability [24]. Temperatures above 65°C may inactivate most of the beneficial decomposing microorganisms (fungi, actinomycete and some bacteria), limiting further decomposition to thermophilic spore forming bacteria [34]. However, in the rotating bioreactor, high temperature (> 65°C) occurred for only a few hours and quickly declined (Fig 5), which was not enough to kill the microorganisms. On the fifth day, Tc decreased to below 55°C indicating that bioavailable carbon was starting to be depleted.
Fig 5. Time course of compost temperatures measured in the rotating and static bioreactors (Tc) and for the ambient air (Tam) during the composting process.
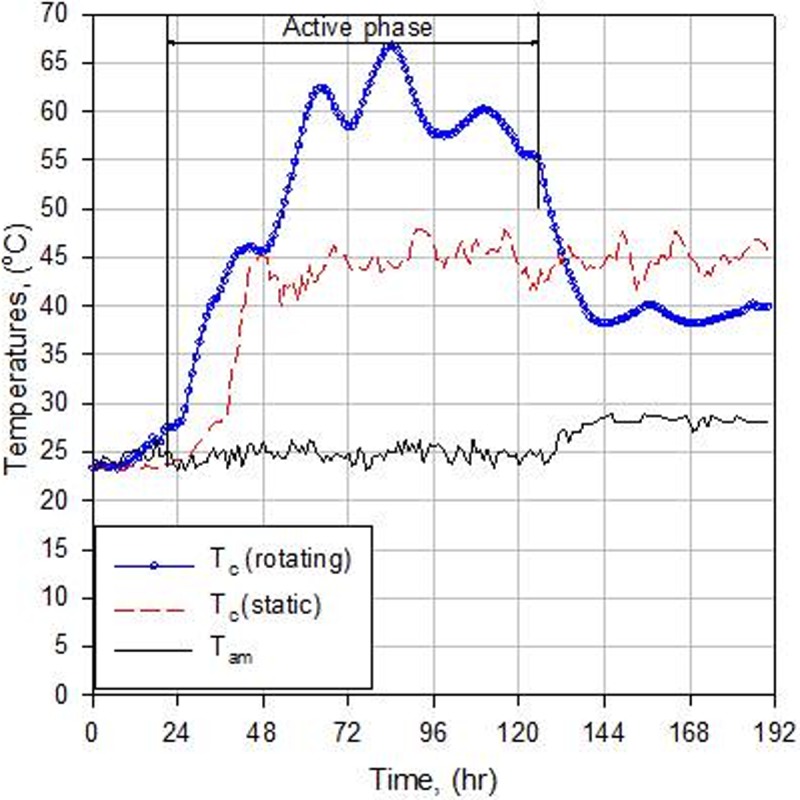
Based on the previous discussion, Oxygen, MC and C/N ratio were not limiting factors affecting the composting process; however, the compost temperature can be used as an indirect measured of microbial activity [34]. Therefore, in the rotating bioreactor, the level of microbial heat generation due to composted material degradation was clearly reduced after the bioavailable carbon was utilized. Hence, microbial activity declined, resulting in a decreased compost temperature below 40°C at the end of the active phase of the composting process.
Thermal kinetics of compost
During the composting process, the heat was generated as a result of the degradation of organic matter, first raising the compost temperature (Tc) to the mesophilic stage (25–45°C) and then to the thermophilic stage (45–67°C), as shown in Fig 5. The maximum temperature in the rotating bioreactor was 66.8°C and was achieved after 84 hours. Temperature above 55°C (which is required for destruction of pathogens and weed seeds) were maintained for 68 hours. However, in the static bioreactor, the maximum temperature was 52°C, which was achieved within a short period after 114 hours, and the compost temperature during most of the composting process did not exceed that of the mesophilic stage.
Fig 5 shows three distinct phases; thee lag, active (mesophilic and thermophilic), and maturation (curing) phases. The initial mesophilic lag period (microbial adaptation) was reduced to 10 hours in the rotating bioreactor compared with 27 hours in the static (Fig 6). The thermophilic lag period (second lag) was 7 hours in the rotating bioreactor, whereas there was no second lag period in the static bioreactor because the compost temperature did not exceed 45°C (Fig 6). This indicated that rotating the compost decreased the mesophilic lag period by at least 37%. The transition from lag phase to active phase is determined by an exponential increase in temperature (an indirect measurement of microbial activity). In general, compost temperature increased more rapidly in the rotating bioreactor than in the static bioreactor. Table 1 compares thermal kinetic parameters in the two bioreactors.
Fig 6. Increase of compost temperature and the mesophilic and thermophilic lag periods estimated for the rotating and static bioreactors.
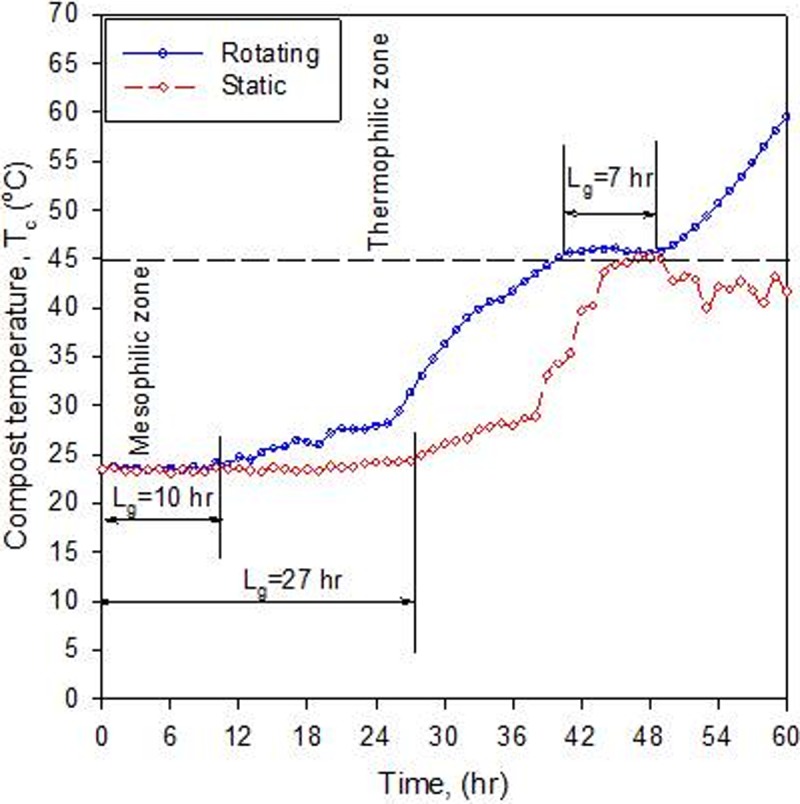
Table 1. Thermal kinetic parameters estimated for the rotating and static bioreactors.
| Parameter (unit) | Rotating bioreactor | Static bioreactor |
|---|---|---|
| Lag period (hr) | ||
| Mesophilic | 10 | 27 |
| Thermophilic | 7 | - |
| Rate of temperature increase (°C hr-1) | ||
| Mesophilic | 0.33 for 15 hours 1.25 for 13 hours | 0.42 for 12 hours 2.6 for 8 hours |
| Thermophilic | 1.7 | - |
| Maximum temperature (°C) | 66.8 | 52 |
| Peak time (hr) | 84 | 114 |
| Duration of temperature (hr) | ||
| ≥ 35°C | 160 | 150 |
| ≥ 45°C | 90 | 30 |
| ≥ 50°C | 71 | 6 |
| ≥ 55°C | 68 | - |
| ≥ 60°C | 24 | - |
Solvita maturity test
The results of Solvita test for the 6 composted samples taken from the two bioreactors on the 8th day are illustrated in Table 2. According to Solvita maturity index [25], which ranges from 1 (unstable compost) to 8 (very stable and well-matured compost). Based on the values of CO2 and NH3, the index of the rotating bioreactor's compost was estimated to be 6. This means that the compost moved beyond the active phase of decomposition and was ready for curing, reducing the need for intensive handling. In contrast, the index was estimated to be 3 for the static bioreactor’s compost, which indicated that the compost was still active, fresh and still required intensive oversight and management.
Table 2. Results of the solvita test for six samples of compost taken from the static and rotating bioreactors.
| Sample No. | Rotating bioreactor | Static bioreactor | ||||||
|---|---|---|---|---|---|---|---|---|
| C/N ratio | MC (%) | Solvita index | C/N ratio | MC (%) | Solvita index | |||
| CO2 | NH3 | CO2 | NH3 | |||||
| 1 | 23.2 | 58.1 | 5.07 | 4.3 | 29.4 | 62.3 | 4.54 | 3.43 |
| 2 | 22.8 | 57.4 | 5.12 | 4.35 | 31.2 | 61.2 | 4.43 | 3.47 |
| 3 | 23.3 | 57.8 | 5.15 | 4.29 | 29.7 | 60.9 | 4.51 | 3.4 |
| Mean | 23.1 | 57.7 | 5.11 | 4.31 | 30.1 | 61.4 | 4.49 | 3.43 |
| SD | 0.22 | 0.35 | 0.04 | 0.03 | 0.96 | 0.73 | 0.05 | 0.03 |
Dewar test
Table 3 shows the results of the Dewar self-heating test results for the six samples taken from the two bioreactors on the 8th day. The maximum temperature of compost samples taken from the rotating bioreactor was very close to room temperature. An average value of ΔT of 3.4°C, which is much lower than 10°C, indicated that the mixture completed the active phase and was ready for maturation [26]. In contrast, the compost samples taken from the static bioreactor showed higher increments of ΔT exceeding 10°C and reaching 17.3°C, on average. This indicated that the materials were still decomposing (active). These results demonstrate that rotating the compost can greatly reduce the time to achieve high-quality compost.
Table 3. Dewar self-heating test results for six compost samples taken from the static and rotating bioreactors.
| Dewar self-heating test | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample No. | Rotating bioreactor | Static bioreactor | ||||||
| Tmax (°C) | Troom (°C) | ΔT (°C) | Remarks | Tmax (°C) | Troom (°C) | ΔT (°C) | Remarks | |
| 1 | 25.9 | 22.2 | 3.7 | Compost class-A (ready for maturation stage) | 38.8 | 21.3 | 17.5 | Compost class-B (Mesophilic, still active, immature) |
| 2 | 25.1 | 21.7 | 3.4 | 37.9 | 21.7 | 16.2 | ||
| 3 | 24.4 | 21.2 | 3.2 | 39.1 | 20.9 | 18.2 | ||
| Mean | 25.13 | 21.7 | 3.43 | 38.63 | 21.3 | 17.3 | ||
| SD | 0.75 | 0.5 | 0.25 | 0.62 | 0.4 | 1.01 | ||
Visual test
The texture of mature compost depends on the source materials and age. A practical and simple method that can be used by farmers to judge the maturity level of compost is the visual method, which involves observing the product with the human eye to assess its color, homogeneity and texture. Fig 6 illustrates photographs of the compost samples taken at days 0, 5 and 8 from the two bioreactors. Changes in the compost color are easily visible after 5 days of composting (Fig 7). Even though the tested samples were taken after the completion of the active phase period, the visual appearance and smell of the compost product were clearly different from those of the original sample (raw mixture). The texture of compost from the rotating bioreactor was finer, more crumby and has a strong smell of ammonia, while compost from the static bioreactor remained almost unchanged (Fig 7). The color components of the composted material can be used to describe the composting process and as an indicator of compost stability and maturity level [35]. Accordingly, to support the visual observation, as illustrated in Fig 7, the change of the color components of compost were measured at days 0 and 8; data of day 8 are summarized in Table 4 to represent the color differences. On day 8, the color index (L*) of the rotating bioreactor compost was lower than that of the static bioreactor compost (the darkness of the rotating bioreactor compost was increased). Moreover, the greenness (a*) and yellowness (b*) colors of compost were increased in the rotating bioreactor compost than those in the static bioreactor. In general, the total change in color (ΔE) was much higher in the rotating bioreactor than that in the static bioreactor. This simply indicated that rotating the bioreactor significantly improved the quality of compost and the maturity level compared with that of the static bioreactor compost.
Fig 7. Photos of compost material taken from the two bioreactors at days 0, 5 and 8 to show the changes in the color and material structure (visual testing).

Table 4. Results of the color test for six compost samples taken from the static and rotating bioreactors.
| Compost color test at day-8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. | Static bioreactor | Rotating bioreactor | ||||||||
| L* | a* | b* | ΔE | Remarks | L* | a* | b* | ΔE | Remarks | |
| 1 | 22.6 | 4.6 | 7.7 | 8.5 | Blackness, greenness and yellowness slightly increased. Texture and compost structure did not improve. | 17.6 | 2.7 | 4 | 15.1 | Blackness, greenness and yellowness increased. Texture and compost structure improved. |
| 2 | 22.8 | 4.4 | 7.8 | 8.4 | 17.5 | 2.7 | 4 | 15.2 | ||
| 3 | 22.7 | 4.5 | 7.5 | 8.4 | 17.5 | 2.7 | 4 | 15.2 | ||
| Mean | 22.7 | 4.5 | 7.7 | 8.4 | 17.5 | 2.7 | 4 | 15.2 | ||
| SD | 0.1 | 0.1 | 0.15 | 0.06 | 0.06 | 0.0 | 0.0 | 0.06 | ||
Practical implications of this study
One of the main challenges facing composting practices is the long duration of the active phase. This requires close attention and good management practice to oxygen requirements, turning frequency, temperature levels, and pathogens risk and odors control. Conventional composting systems require several weeks or even several months, in the static piles, to complete the active phase of composting and to be ready for further maturation. The obtained results from the present study showed that integrating the continuous rotation and aeration can significantly reduce the duration of the active phase to only 4.5 days, with a very short lag period (10h), and enough thermophilic stage to meet the guidelines for good compost that is free of plant pathogens. The improvement of the proposed composting protocol over other methods is illustrated in Table 5. According to the parameters reported in Table 5, the proposed method can be done on a commercial scale. Further studies are needed to examine different composting strategies for different agricultural residues. For example, composting might be improved by applying intermittent forced aeration-rotation (on-off) to the bioreactor, and applying natural aeration (via holes in the bioreactor body) combined with a continuous or intermittent rotation of the bioreactor.
Table 5. Results of the proposed method compared to results of other composting methods.
| Composted material | Size (Liter) | Rotation protocol | Aeration protocol | Lag phase duration (hr) |
Active phase duration+ (day) |
Tc, max (°C) |
Ref. |
|---|---|---|---|---|---|---|---|
| Cattle manure & green vegetables & sawdust | 250 | 3 rotations/ | |||||
| 6hr | Intermittent | Not reported | 9 | 53 | [16] | ||
| 12hr | 11 | 53 | |||||
| 18hr | 9 | 55 | |||||
| 24hr | 8 | 58 | |||||
| Different mixtures of vegetables wastes & cow manure & sawdust | 550 | 1 rotation/24 hr | Intermittent | Not reported | 8–12 | 61–66 | [17] |
| Pig & poultry carcasses | 360 | 24-min rotations/ | |||||
| 1hr | Intermittent | Not reported | 11 | 60 | [21] | ||
| 2hr | 16 | 62 | |||||
| 3hr | 17 | 70 | |||||
| 4hr | 18 | 70 | |||||
| Penicillin mycelial dreg & sewage sludge & sawdust & rice straw | 390 | static | Continuous | Not reported | 28 | 65 | [14] |
| Tomato residues & chicken manure | 200 | Continuous | Continuous | 10 | 4.5 | 66.8 | Present study |
+ The active periods were determined, based on the definition in this study, from the Tc evolution figures reported in the corresponding references.
Conclusion
Continuous aeration-rotation of compost significantly reduced the active phase of composting to only 4.5 days; whereas several weeks or even several months may be required to finalize the active phase of the static compost. Unlike the static bioreactor, the rotating bioreactor with continues aeration increased and maintained the compost temperature uniformly distributed and in the range between 50–65°C for three consecutive days, achieving a successful active phase of the composting process. However, the compost in the static bioreactor remained in the mesophilic stage, and the compost temperature remained below 45°C during the experiment. Based on the Solvita, Dewar-self heating, visual and color tests, the compost in the rotating bioreactor completed the active phase and became inactive and ready for further maturation, while that of the static bioreactor was still active.
Nomenclature
- a*
compost color index (+ ve is redness,—ve is greenness) (-)
- b*
compost color index (+ ve is yellowness,—ve is blueness) (-)
- C/N
carbon to nitrogen ratio (-)
- d
day
- L*
compost color index (0 black to 100 white) (-)
- L
liter
- hr
hour
- MC
moisture content (%)
- NC
nitrogen content (%)
- OM
organic matter content (%)
- Ta
air temperature measured in the empty bioreactor (°C)
- Tam
ambient air temperature (°C)
- Tc
temperature of compost (°C)
- Tc, max
maximum compost temperature (°C)
- Tmax
maximum temperature recorded by Dewar's flask (°C)
- Troom
room air temperature (°C)
- TN
total nitrogen content (%)
- TOC
total organic carbon (%)
- ΔE
index of the color difference (-)
- ΔT
temperature difference (Tmax-Troom) (°C)
- v
volume
Data Availability
All relevant data are within the manuscript.
Funding Statement
This research was funded by the National Plan for Science, Technology and Innovation (NSTIP), 12-ENV-2814-02 to FNA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Agriculture Statistical Annual Book (ASAB-2016). Ministry of Environment, Water and Agriculture, General Authority for Statistics, Saudi Arabia, 29, 298 pp. Available online at: https://www.stats.gov.sa/en (accessed: July 4, 2018).
- 2.Pane C, Celano G, Piccolo A, Villecco D, Spaccini R, Palese A M, et al. Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chem. Biolog. Technolo. Agric.2015; 2(4): 2–13. [Google Scholar]
- 3.Perez-Murcia MD, Moreno-Caselles J, Moral R, Perez-Espinosa A, Paredes C, Rufete B. Use of composted sewage sludge as horticultural growth media: effects on germination and trace element extraction. Comm Soil Sci Plant Anal. 2005; 36: 571–582. 10.1081/CSS-200043286 [DOI] [Google Scholar]
- 4.Jindo K, Suto K, Matsumoto K, Garcia C, Sonoki T, Sancchez-Monedero MA. Chemical and biochemical characterization of biochar-blended composts prepared from poultry manure. Bioresour. Technol. 2012; 110: 396–404. 10.1016/j.biortech.2012.01.120 [DOI] [PubMed] [Google Scholar]
- 5.Alkoaik FN, Khalil AI, Al-Mahasneh MA, Fulleros RB, El-Waziry AM. Changes in color and germination index as indicators for compost maturity. J. Pure & App. Microbiol. 2014; 8(2): 409–417. [Google Scholar]
- 6.Togun A, Akanbi W, Adediran JA. Growth, nutrient uptake and yield of tomato in response to different plant residue composts. Food, Agric. & Environ. 2004; 2(1); 310–316. [Google Scholar]
- 7.Gao M, Liang F, Yub A, Li B, Yang L. Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 2010; 78: 614–619. 10.1016/j.chemosphere.2009.10.056 [DOI] [PubMed] [Google Scholar]
- 8.El-Nesr M, Alazba A, Abu-Zreig M. Analysis of evapotranspiration variability and trends in the Arabian Peninsula. American J. Environ. Sci. 2010; 6: 535–547. [Google Scholar]
- 9.Vuorinen AH, Saharinen MH. Evolution of microbiological and chemical parameters during manure and straw co-composting in a drum composting system. Agriculture, Ecosystem and Environment 1997;66, 19–29. [Google Scholar]
- 10.Ghaly AE, Alkoaik F, Snow A. Thermal balance of in-vessel composting of tomato plant residues. Canadian Biosyst. Eng. 2006; 48: 6.1–6.11. [Google Scholar]
- 11.Ajay SK, Kazmi AA. Mixed organic waste composting using rotary drum composter. Int. J. Environ. Waste Manag. 2008; 2 (1/2): 24–35. [Google Scholar]
- 12.Hwang EY, Park JS, Kim JD, Namkoong W. Effect of aeration mode on the composting of diesel-contaminated soil. J. Ind. Chem. 2006; 12(5): 694–701. [Google Scholar]
- 13.Tajima K, Yoshimura M, Kato M, Tatsuno J, Tamaki K. Enhancement of composting process by the addition of chicken manure and rice bran to tree pruning chips. Jpn. J. Farm Work Res. 2003; 38(4): 207–2013. [Google Scholar]
- 14.Chen Z, Zhang S, Wen Q, Zheng J. Effect of aeration rate on composting of penicillin mycelial dreg. J. Environ. Sci. 2015; 37: 172–178. [DOI] [PubMed] [Google Scholar]
- 15.Rasapoor M, Adl M, Pourazizi B. Comparative evaluation of aeration methods for municipal soild waste composting from the perspective of resource management: A practical case study in Tihran, Iran. J. Environ. Manag. 2016; 184: 528–534. [DOI] [PubMed] [Google Scholar]
- 16.Ajay SK, Kazmi AA. Effects of turning frequency on compost stability and some chemical characteristics in a rotary drum composter. Chemosphere 2009; 74: 1327–1334. 10.1016/j.chemosphere.2008.11.058 [DOI] [PubMed] [Google Scholar]
- 17.Varma VS, Kalamdhad AS. Evaluation of chemical and biological characterization during thermophilic composting of vegetable waste using rotary drum composter. Int. J. Environ. Sci. Technol. 2015; 12: 2015–2024. [Google Scholar]
- 18.Kalamdhad AS, Pasha M, Kazmi AA. Stability evaluation of compost by respiration techniques in a rotary drum composter. Resources, Conservation & Recycling 2008; 52: 829–834. [Google Scholar]
- 19.Kalamdhad AS, Singn YK, Ali M, Khwairakpam M, Kazmi AA. Rotary drum composting of vegetable waste and tree leaves. Bioresour. Technol. 2009; 100: 6442–6450 10.1016/j.biortech.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 20.Madan S, Bhatia A, Rajpal A, Kazmi AA. Maturity assessment of rotary drum and windrow composts in terms of germination index and enzymatic activities. Int. J. App. Sci. Eng. Res. 2012; 1(3): 415–426. [Google Scholar]
- 21.Matias MO, Arlei C, Paulo BF, Paulo AVO. Aeration frequency on accelerated composting of animal carcasses. Ciencia e Agrotecnologia 2018; 24(6): 653–665. [Google Scholar]
- 22.Said-Pullicino D, Erriquens F, Gicliotti G. Changes in the chemical characteristics of water-extractable organic matter during composting and their influence on compost stability and maturity. Bioresour. Technol. 2007; 98: 1822–1831. 10.1016/j.biortech.2006.06.018 [DOI] [PubMed] [Google Scholar]
- 23.Alkoaik FN, Abdel-Ghany AM, Rashwan MA, Fulleros RB, Ibrahim MN. Energy analysis of a rotary drum bioreactor for composting tomato plant residues. Energies 2018; 11: 449, 10.3390/en11020449 [DOI] [Google Scholar]
- 24.Haug RT. Practical Handbook of Compost Engineering. Lewis Publishers, Boca, Raton, 1993. [Google Scholar]
- 25.WERL-2018. Woods End Research Laboratory, Inc. Guide to Solvita Testing for Compost Maturity Index. Available online at: https://woodsend.org/pdf-files/manual.pdf (access April, 10, 2018).
- 26.Dewar Self-Heating Test. Test Instructions for Use Application of the Dewar self- heating test to measure completion of composting. 5th REVISED EDITION– 2009. https://woodsend.com/pdf-files/dewar-instructions-2009.pdf [Google Scholar]
- 27.Mascan M. Kinetics of color change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001; 48: 169–175. [Google Scholar]
- 28.Elcik H, Zoungrana A, Bekaraki N. Laboratory-scale investigation of aerobic compost stability of municipal solid waste in Istanbul. Sigma J. Eng. & Nat. Sci. 2016; 34(2): 211–220. [Google Scholar]
- 29.Cabanas-Vargas DD, Sánchez-Monedero MA, Urpilainen ST, Kamilaki A, Stentiford EI. Assessing the stability and maturity of compost at large-scale plants. Ingeniería 2005; 9(2): 25–30. [Google Scholar]
- 30.Tiquia SM. Reduction of compost phytotoxicity during the process of decomposition. Chemosphere 2010; 79(5): 506–512. 10.1016/j.chemosphere.2010.02.040 [DOI] [PubMed] [Google Scholar]
- 31.Estevez-Schwarz I, Seoana-Labandeira S, Nunez-Delgado A, Lopez-Mosquera E. Production and characterization of compost made from garden and other waste. Pol. J. Environ. Stud. 2012; 21(4): 855–864. [Google Scholar]
- 32.Escobar N, Solarte V. Microbial diversity associated with organic fertilizer obtained by composting of agricultural waste. Int. J. Biosci., Biochem. Bioinform. 2015; 5(2): 70–79. [Google Scholar]
- 33.Bazrafshan E, Zarei A, Mostafapour FK, Poormollae N, Mahmoodi S, Zazouli MA. Maturity and stability evaluation of composted municipal solid wastes. Health Scope. 2016; 5(1): 1–9. [Google Scholar]
- 34.Tchobanoglous G. Handbook of solid waste management. New York: McGraw-Hill. United States; 2002. [Google Scholar]
- 35.Khan MAI, Ueno K, Horimoto S, Komai F, Someya T, Inoue K, et al. CIELAB color variables as indicators of compost stability. Waste Manag. 2009; 29: 2969–2975. 10.1016/j.wasman.2009.06.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.


