Abstract
Background/Aims
Motility disorders are common and may affect the entire gastrointestinal (GI) tract but current treatment is limited. Multilocular sensing of GI electrical activity and variable electrical stimulation (ES) is a promising option. The aim of our study is to investigate the effects of adjustable ES on poststimulatory spike activities in 5 GI segments.
Methods
Six acute porcine experiments were performed with direct ES by 4 ES parameter sets (30 seconds, 25 mA, 500 microseconds or 1000 microseconds, 30 Hz or 130 Hz) applied through subserosal electrodes in the stomach, duodenum, ileum, jejunum, and colon. Multi-channel electromyography of baseline and post-stimulatory GI electrical activity were recorded for 3 minutes with hook needle and hook-wire electrodes. Spike activities were algorithmically calculated, visualized in a heat map, and tested for significance by Poisson analysis.
Results
Post-stimulatory spike activities were markedly increased in the stomach (7 of 24 test results), duodenum (8 of 24), jejunum (23 of 24), ileum (18 of 24), and colon (5 of 24). ES parameter analysis revealed that 80.0% of the GI parts (all but duodenum) required a pulse width of 1000 microseconds, and 60.0% (all but jejunum and colon) required 130 Hz frequency for maximum spike activity. Five reaction patterns were distinguished, with 30.0% earlier responses (type I), 42.5% later or mixed type responses (type II, III, and X), and 27.5% non-significant responses (type 0).
Conclusions
Multilocular ES with variable ES parameters is feasible and may significantly modulate GI electrical activity. Automated electromyography analysis revealed complex reaction patterns in the 5 examined GI segments.
Keywords: Electric stimulation, Electromyography, Gastrointestinal tract
Introduction
Complexly coordinated smooth muscle contraction and relaxation in the gastrointestinal (GI) tract is the underlying source of GI motility.1–3 From the esophagus to the anal sphincter, multifaceted motility disorders during the interdigestive and postprandial state may significantly impair patients’ quality of life.4–5 Furthermore, functional disorders in one GI segment increase the risk in others.6 GI smooth muscle electromyography (EMG) has been clinically tested for recording pacesetter slow waves or action potential spikes. Diagnosis of motility disorders according to the myoelectric activity and direct electrical stimulation (ES) appears to be reasonable.7,8
As long-term pharmacological or dietetic treatment may be limited due to side effects and lack of proof of efficacy,9,10 ES is a promising minimally invasive therapeutic option with potential application in the whole GI tract. However, only a few studies have investigated the effects of multilocular ES in multiple parts of the GI tract in animals or humans.11–13
To address a broad spectrum of GI motility disorders as well as specific requirements of individual patients, interacting active implants distributed along the GI tract are needed. Multilocular sensing of GI activity will be the basis for adaptive modulation of complex physiological functions. We recently reported successful evaluation of the safety and feasibility of ES in 5 parts of the GI tract. We used multilocular recordings of electrical activity in a porcine model14 to assess EMG spike activities, post-stimulatory reaction patterns, and optimal ES parameters.
The purpose of our study is to investigate the effects of adjustable ES on poststimulatory spike activities in 5 GI segments. Variable ES with 4 ES parameter sets were applied to the stomach, duodenum, ileum, jejunum, and colon, and electrical GI spike activities were algorithmically calculated. Experiments are intended to support the electrophysiological and technical groundwork for future device-based applications. The first experimental results are presented here.
Materials and Methods
Anesthesia and Surgery
Six acute experiments with healthy Piétrain pigs weighing 26–36 kg (median 30.5 kg) were performed under general anesthesia and continuous monitoring as described previously.11 The research complied with all of the relevant national regulations and institutional policies for the care and use of animals and was approved by the Regional Board of Animal Welfare, Koblenz, Rhineland-Palatinate, Germany under approval code G-17-1-052.
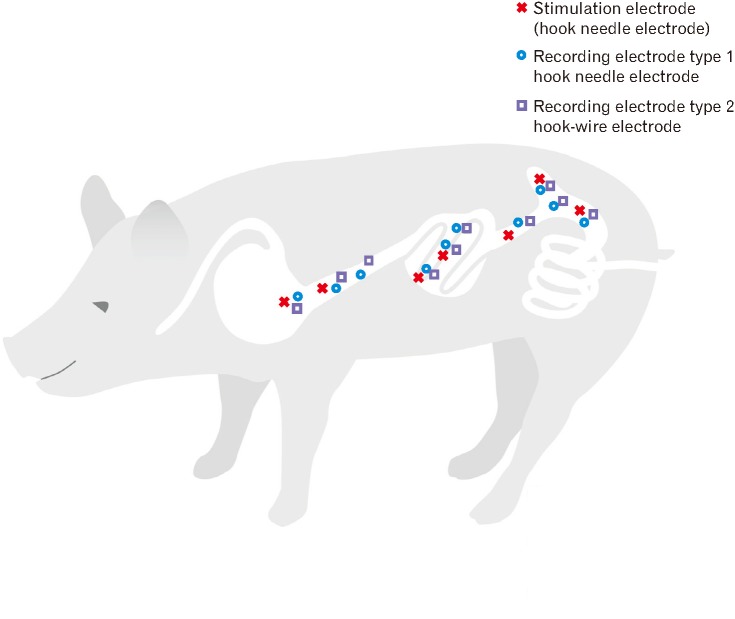
After midline laparotomy, multiple hook needle, and hook-wire electrodes (Product No. 532730 and 530603; inomed Medizintechnik GmbH, Emmendingen, Germany) were applied in the prepyloric segment of the stomach, postpyloric duodenum, distal duodenum, middle part of the jejunum, terminal ileum, cecum, and ascending colon in a fully standardized manner. Smooth muscle EMG recording was performed with hook needle and hook-wire electrodes, but separate hook needle electrodes were used for ES (Fig. 1). Needle placement was performed at the beginning and electrodes were kept in place for the whole experiment and only repositioned if impedance got high (> 5 kΩ for hook needle and > 100 kΩ for hook-wire electrodes) indicating a dislocation of the electrode. Between needle placement and the recording of the baseline EMG signals there was a break of at least 15 minutes to exclude effects due to electrode insertion.
Figure 1.
Placement of hook needle and hook-wire electrodes for electrical stimulation and electromyography in the porcine gastrointestinal tract. After laparotomy, hook needle and hook-wire electrodes were applied in the prepyloric segment of the stomach, postpyloric duodenum, distal duodenum, middle parts of the jejunum, terminal ileum, cecum, and ascending colon in a fully standardized manner. For electrical stimulation only hook needle electrodes were used. Electromyography was recorded with both hook needle and hook-wire electrodes.
Electromyography Recording and Electrical Stimulation
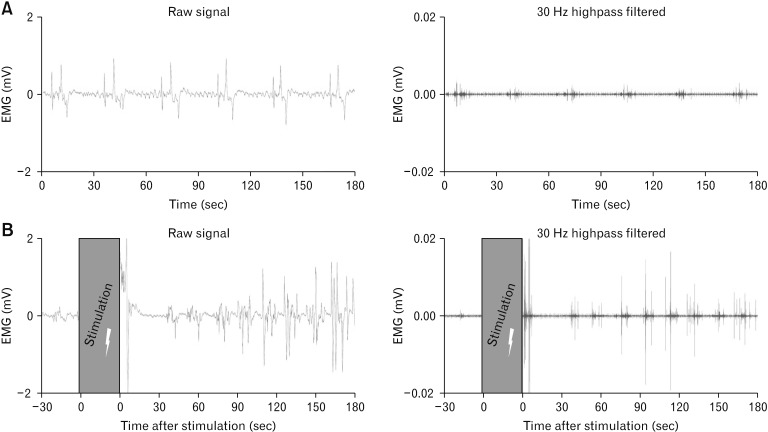
After impedance measurements, differential multi-channel baseline EMG was performed with both electrode types using the ISIS Xpress (inomed Medizintechnik GmbH) at 20 kHz within the range of 10 mVpp. The hardware and software high pass filter was set to 0.5 Hz and the low pass filter to 2 kHz. Baseline was recorded for 3 minutes before stepwise gastric (GES), duodenal (DES), jejunal (JES), ileal (IES), or colonic (CES) ES. Each stimulation period was a 30-second train of 25 mA rectangular monophasic pulses subsequently applied with 4 different parameter sets: 30 Hz and 500 microseconds pulse width, 130 Hz and 500 microseconds, 30 Hz and 1000 microseconds, or 130 Hz and 1000 microseconds. Post-stimulatory EMG was then recorded an additional 3 minutes after each ES parameter set. An example of gastric pre- and poststimulatory EMG signals is shown (Fig. 2).
Figure 2.
Pre- and post-stimulatory gastric electromyography (EMG) signals recorded with a hook needle electrode. (A) Figure exemplifies raw and 30 Hz highpass filtered signals of a gastric hook needle electrode with a recording length of 3 minutes before electrical stimulation (ES) is applied. (B) Figure shows examples of EMG raw and 30 Hz highpass filtered signals of a gastric hook needle electrode with a recording length of 3 minutes after 30 seconds ES was performed.
Computer-assisted Counting and Statistical Analysis
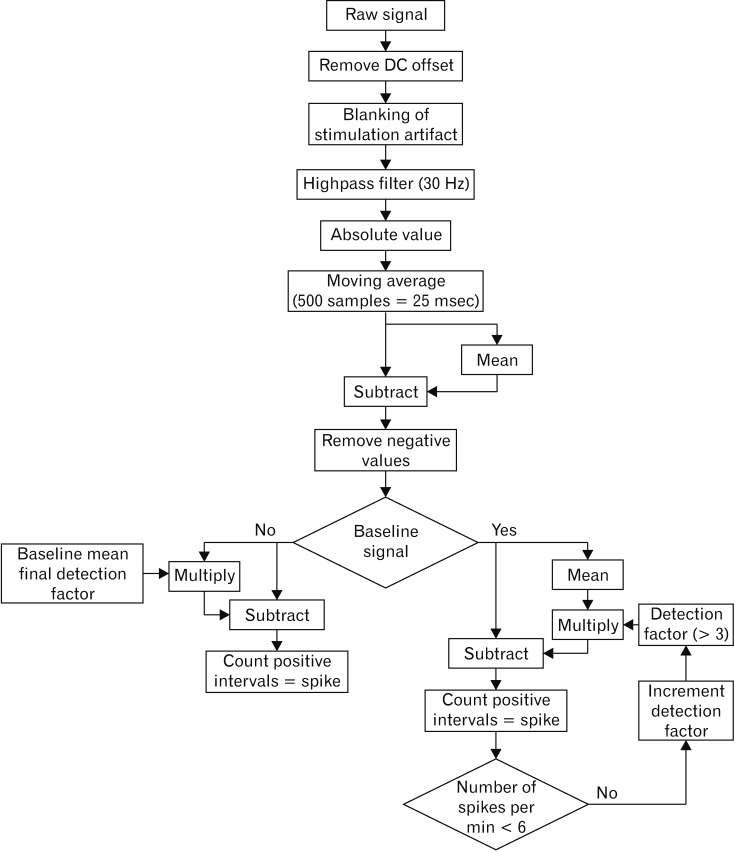
Minute by minute EMG spike activity was analyzed using pooled data for 5 locations from 3 experiments (n = 15). Automated EMG spike detection was computed based on Lammers et al.15 However modifications of the algorithm seemed appropriate as we assume Lammers et al15 recorded signals with a monopolar setup in contrast to our bipolar setting resulting in altered signal forms. Therefore we analyzed the absolute amplitude instead of the deviation (Fig. 3). An automated cutoff calculation was performed using Matlab version 2017b (The Mathworks, Inc, Natick, MA, USA). Baseline cutoff values were calculated to reach a level of less than 6 spikes per minute (spm) with application of the same cutoff values for the subsequent spike detection in the post-stimulatory periods. Pre- and post-stimulatory EMG spike activities were compared for every minute, electrode, and part of the GI tract with calculation of the absolute differences between means using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA, USA). The effects of ES on the GI spike counts were accessed in penalized mixed Poisson models with penalization for overdispersion (function glmmPQL in package MASS of R software, version 3.4.4 [R Core Team, 2018]; R Foundation for Statistical Computing, Vi-enna, Austria). These models account for correlation within animals and multiple electrodes in the same location. The number of spikes in the pre-stimulatory period is compared to the post-stimulatory periods. This type of model is estimated for each location, each ES parameter set, each electrode type and each of the 3 post-stimulatory minutes resulting in 120 models. No adjustment for multiplicity is applied, neither for the 4 tests within each model nor for the 120 models. A Poisson P-value below 0.05 is described as a distinct or marked difference in the text.
Figure 3.
Spike detection flow chart for automated electromyography (EMG) spike detection. Automated EMG spike detection was computed with analysis of the absolute amplitude. A 30 Hz filter was applied and baseline cutoff values were calculated to reach a level of less than 6 spikes per minute with application of the same cutoff values for the subsequent spike detection in the post-stimulatory periods.
Gastrointestinal Electrical Activity Heat Map
The calculated data were summarized graphically in a GI electrical activity heat map using Microsoft Excel 2011 version 14.7.3. Differences between the average baseline and post-stimulatory spike activities are visualized for each post-stimulatory minute, ES parameter set (500 microseconds or 1000 microseconds, 30 Hz or 130 Hz with 30 seconds and 25 mA), EMG electrode type (hook needle or hook-wire), and electrically stimulated part of the GI tract (stomach, duodenum, jejunum, ileum, or colon). Modification of post-stimulatory compared to baseline spike activity is characterized by a background color (red: > 13 spm, orange: 5–13 spm, yellow: 0–5 spm, green: < 0 spm; Table 1). Table 2 is the statistical data table representing the Poisson P-values corresponding to Table 1.
Table 1.
Electromyography Spike Activity Heat Map After 5-fold Gastrointestinal Electrical Stimulation
| Location | Electrode type | Post-stimulatory minute 1 | Post-stimulatory minute 2 | Post-stimulatory minute 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| 500 μsec | 1000 μsec | 500 μsec | 1000 μsec | 500 μsec | 1000 μsec | ||||||||
|
|
|
|
|
|
|
||||||||
| 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | ||
| Stomach | Hook needle | 4.87 | 3.87 | 4.27 | 2.87 | 4.47 | 5.87 | 6.07 | 5.27 | 2.67 | 3.47 | 4.07 | 5.67 |
| Hook-wire | 3.53 | 4.13 | 4.73 | 9.13 | 3.73 | 4.13 | 1.53 | 8.93 | 4.13 | 0.73 | 3.33 | 8.13 | |
| Duodenum | Hook needle | 5.48 | 6.28 | 4.98 | 0.08 | 4.18 | 6.58 | 4.98 | −1.93 | 1.38 | 3.18 | 3.88 | 2.00 |
| Hook-wire | 13.13 | 17.63 | 15.38 | 8.88 | 11.25 | 8.50 | 7.88 | 3.00 | 6.75 | 10.13 | 5.50 | 4.13 | |
| Jejunum | Hook needle | 52.95 | 48.22 | 33.65 | 30.63 | 15.76 | 28.38 | 63.61 | 18.50 | 9.22 | 19.18 | 21.45 | 27.15 |
| Hook-wire | 9.23 | 15.93 | 14.96 | 16.03 | 7.64 | 14.73 | 15.14 | 15.50 | 6.68 | 14.85 | 14.44 | 24.03 | |
| Ileum | Hook needle | 5.07 | 27.20 | 17.70 | 28.57 | 8.45 | 14.95 | 15.82 | 19.70 | 13.70 | 17.95 | 11.82 | 8.70 |
| Hook-wire | 7.78 | 19.40 | 5.40 | 5.70 | 11.62 | 9.20 | 7.90 | 2.97 | 7.03 | 9.10 | 4.60 | 0.06 | |
| Colon | Hook needle | −0.45 | −1.02 | −0.38 | −1.38 | 0.33 | −1.31 | −1.67 | 0.62 | 0.41 | 0.05 | −0.88 | 3.12 |
| Hook-wire | 11.00 | 7.61 | 20.28 | 6.61 | 12.00 | 9.72 | 5.89 | 3.22 | 12.22 | 6.89 | 2.11 | 5.72 | |
Multiple subserosal electrodes were applied in the stomach, duodenum, jejunum, ileum, and colon of pigs. Electrical stimulation with 4 technical parameter sets was performed. Pre- and post-stimulatory multi-channel electromyography was recorded and spikes computed. Differences between average baseline and post-stimulatory spike activities were calculated for each gastrointestinal part, electrode type (hook needle or hook-wire), and electrical stimulation parameter (500 microseconds (μsec) or 1000 μsec, 30 Hz or 130 Hz with 30 seconds and 25 mA) and worked into a heat map (numbers). Modification of post-stimulatory compared to baseline spike activity is characterized by a background color (red: > 13 spikes per minute [spm], orange: 5–13 spm, yellow: 0–5 spm, green: < 0 spm).
Table 2.
Statistical Poisson Analysis of Post-stimulatory Spike Activities After 5-fold Gastrointestinal Electrical Stimulation
| Location | Electrode type | Post-stimulatory minute 1 | Post-stimulatory minute 2 | Post-stimulatory minute 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| 500 μsec | 1000 μsec | 500 μsec | 1000 μsec | 500 μsec | 1000 μsec | ||||||||
|
|
|
|
|
|
|
||||||||
| 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | 30 Hz | 130 Hz | ||
| Stomach | Hook needle | 0.060 | 0.129 | 0.095 | 0.268 | 0.113 | 0.046 | 0.041 | 0.068 | 0.240 | 0.140 | 0.092 | 0.030 |
| Hook-wire | 0.093 | 0.741 | 0.572 | 0.051 | 0.019 | 0.016 | 0.065 | 0.002 | 0.072 | 0.617 | 0.123 | 0.007 | |
| Duodenum | Hook needle | 0.117 | 0.084 | 0.143 | 0.889 | 0.360 | 0.143 | 0.267 | 0.291 | 0.657 | 0.369 | 0.291 | 0.110 |
| Hook-wire | 0.001 | 0.000 | 0.000 | 0.005 | 0.007 | 0.022 | 0.028 | 0.263 | 0.214 | 0.082 | 0.307 | 0.033 | |
| Jejunum | Hook needle | 0.005 | 0.007 | 0.024 | 0.018 | 0.019 | 0.004 | 0.000 | 0.008 | 0.050 | 0.003 | 0.002 | 0.001 |
| Hook-wire | 0.009 | 0.000 | 0.002 | 0.001 | 0.013 | 0.000 | 0.000 | 0.000 | 0.015 | 0.001 | 0.001 | 0.000 | |
| Ileum | Hook needle | 0.020 | 0.001 | 0.006 | 0.001 | 0.172 | 0.021 | 0.016 | 0.005 | 0.004 | 0.001 | 0.009 | 0.034 |
| Hook-wire | 0.039 | 0.002 | 0.154 | 0.013 | 0.010 | 0.042 | 0.066 | 0.074 | 0.001 | 0.001 | 0.016 | 0.054 | |
| Colon | Hook needle | 0.716 | 0.872 | 0.716 | 0.788 | 1.000 | 0.113 | 0.267 | 0.967 | 0.970 | 0.640 | 0.583 | 0.133 |
| Hook-wire | 0.034 | 0.095 | 0.003 | 0.129 | 0.008 | 0.021 | 0.113 | 0.346 | 0.006 | 0.064 | 0.491 | 0.107 | |
The effects of multilocular electrical stimulation on the gastrointestinal spike counts were assessed by Poisson analysis. Differences between average baseline and post-stimulatory spike activity was tested for each gastrointestinal part, electrode type (hook needle or hook-wire), and electrical stimulation parameter (500 microseconds (μsec) or 1000 μsec, 30 Hz or 130 Hz with 30 seconds and 25 mA). Numbers represent P-values in Poisson analysis. A P < 0.05 was considered statistically significant.
Results
No anesthesia- or surgery-related complications occurred in any of the pigs. Vital signs were stabilized within acceptable ranges. The GI filling status was appropriate after overnight fasting and electrode placement was safe without perforation or major blood loss. Five-fold ES of the porcine GI tract with pre- and post-stimulatory multi-channel EMG recordings was performed successfully in all cases. The calculated data were visualized in a GI electrical activity heat map with corresponding Poisson P-values (Tables 1 and 2). Most commonly, the registered post-stimulatory spike activities were increased compared to the baseline electrical activity before local ES (Table 1). In the post-stimulatory period, 61 of 120 registered spike activities were distinctly different from the baseline electrical activity. No significant reactions occurred with decreases in the post-stimulatory spike count compared to baseline spike activity in the examined parts of the GI tract (Table 2).
Five Post-stimulatory Electrical Activity Reaction Patterns
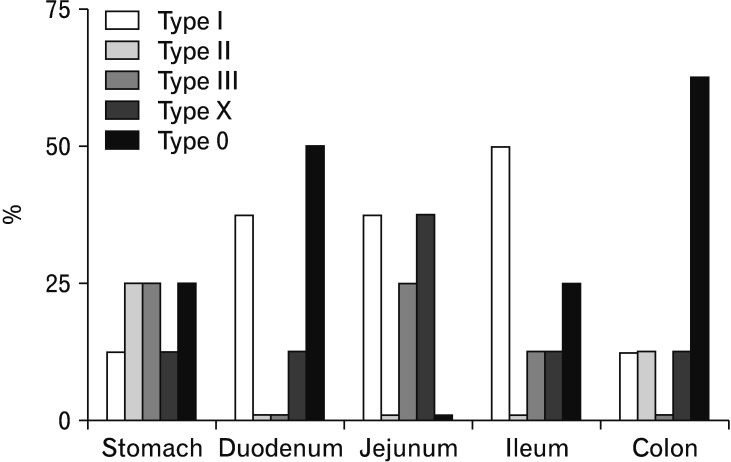
According to the analysis of our pooled data, 5 electrical activity reaction types could be differentiated by the spike count patterns in the post-stimulatory period. Absolute (fa) and relative frequencies (fr) of the reaction types were calculated with the 40 data sets from the whole GI tract. The distribution of the reaction patterns along the 5 parts of the GI tract that were examined was not uniform. Therefore, the percentages of reaction patterns were calculated separately for the gastric, duodenal, jejunal, ileal, and colonic groups (Fig. 4).
Figure 4.
Distribution of 5 electrical activity reaction types along the examined gastrointestinal (GI) parts. Multilocular electrical stimulation was performed in the GI tract. According to the electromyography analysis of 3 minutes post-stimulation, 5 electrical activity reaction types could be differentiated by the spike count patterns. Type I is a fast response to ES with a significant increase in the first minute, followed by decreasing spike activity from 2 to 3 minutes. Type II is a slow response pattern with a distinct increase in the first minute and further increase in spike frequency from 2 to 3 minutes. Type III is an intermediate short response with a maximum spike count in the second minute and decreasing spike activity in the third minute. Mixed type response patterns, classified as type X reaction, include all changes in spike count patterns in the post-stimulatory period that do not fit into type I to III reactions. Reaction patterns without significant differences from baseline electrical activity are considered type 0 reaction types. The percentages of reaction patterns were calculated separately for the gastric, duodenal, jejunal, ileal, and colonic groups.
Type I (fa = 12, fr = 30.0%) describes a fast response to ES with a significant increase in the first minute, followed by decreased electrical activity from 2 to 3 minutes as seen, for example, in the duodenum hook-wire electrode signals after DES with 30 Hz and 500 microseconds for 30 seconds. This reaction pattern was found in all parts of the GI but was more common in the small intestine reactions. In the duodenal and jejunal group, 37.5% of all reactions were type I, and 50.0% in the ileal group were type I, compared to 12.5% in the gastric and colonic groups.
Type II (fa = 4, fr = 10.0%) expresses a slow response pattern to ES with a significant increase in the first minute and further increased spike frequency from 2 to 3 minutes. This can be seen in the stomach hook needle electrodes after GES with 130 Hz and 1000 microseconds for 30 seconds. Type II reactions were found in the gastric (25.0%), ileal, and colonic groups (12.5% each), without appearance in the jejunal and duodenal groups (0.0%).
Type III (fa = 6, fr = 15.0%) is an intermediate short response with a maximum spike count in the second minute and decreasing spike activity in the 3rd minute. This is exemplified by both the jejunum hook-wire and hook needle electrodes after JES with 30 Hz and 1000 microseconds for 30 seconds. Type III ES response patterns were found in 25.0% of both the gastric and jejunal group, and 12.5% in each of the ileal and colonic groups. No type III reaction patterns were found in the duodenal group (0.0%).
A number of mixed type response patterns, classified as type X reaction (fa = 7, fr = 17.5%), were observed. This includes all significant changes in spike count patterns in the post-stimulatory period that do not fit into the type I to III reaction types. For example, the reaction pattern of the ileum hook needle electrode after IES with 130 Hz and 500 microseconds for 30 seconds, with a significant increase in spike activity in the first post-stimulatory minute, was followed by a decrease in spike activity in the second minute and late increase in the third minute. Type X patterns were registered in all examined parts of the GI tract. In the jejunal group, 37.5% were type X reactions, and 12.5% in each of the gastric, duodenal, ileal, and colonic groups.
We also noticed post-stimulatory signals without significant differences from the baseline electrical activity, such as in the colon hook needle electrode after CES with 30 Hz and 500 microseconds. These patterns were considered to be type 0 reaction types (fa = 11, fr = 27.5%). Without consideration of the applied stimulation parameters, there were type 0 reactions in all GI parts, except for the jejunal group (0.0%); 50.0% in each of the duodenal and colonic groups, 25.0% in the gastric group, and 12.5% in the ileal group.
Two Electrode Types were Successfully Tested for Electromyography Spike Recording
Before bipolar EMG recordings, short circuits or incorrect tissue positioning could be excluded by impedance measurements in all pairs of hook needle and hook-wire electrodes. Adequate impedance ranges were achieved in all electrodes before data recording with lower than 5 kΩ impedance for hook needle and 100 kΩ for hook-wire electrodes, respectively. Implantation sites for hook needle and hook-wire electrodes in the 5 parts of the GI tract were comparable, but not identical, because each pair of electrodes required an approximately 1 cm2 area of the intestinal wall. At times we registered minor bleeding after electrode placement with spontaneous hemostasis. But due to the finer caliber of hook-wire electrodes, local bleeding occurred even less frequently than with hook needle electrodes. However, hook-wire electrode application through a cannula was technically more demanding, and displacement in the course of the experiment happened more often than with hook needle electrodes.
Analysis of pooled data revealed that significantly modified spike activities occurred more frequently in the hook-wire electrode group (37 of 60 test results) than in the hook needle electrode group (24 of 60 test results). No significant differences were found between baseline and post-stimulatory spike activities in the hook needle electrodes of the duodenum and colon (Table 2). According to our analysis, both electrode types seem to be appropriate for GI smooth muscle EMG.
Identification of Different Electrical Excitabilities
To assess the GI electrical excitability, we classified the pooled data for each part of the GI tract according to the frequency of significant post-stimulatory reactions (Tables 1 and 2). The investigated part of the jejunum had the highest electrical excitability. After JES, nearly all electrical responses in the jejunum hook needle and hook-wire electrodes were increased in Poisson analysis. The second highest electrical excitability was found in the ileum with 18 of 24 distinct mean changes in spike frequencies compared to baseline. Based on our pooled data, the duodenum is considered to have greater electrical excitability compared to the stomach. Distinct changes in post-stimulatory activities were found in 8 of 12 post-stimulatory data sets of the duodenal hook-wire electrode group after DES, but only in 4 of 12 data sets of the gastric hook-wire electrode group after GES. However, pooled data revealed no significant changes in the data sets of the duodenal hook needle electrode group, but 3 of 12 distinct changes in the gastric hook needle electrode group. The investigated part of the colon had the lowest electrical excitability, with only 5 distinct changes of overall 24 data sets after CES. No changes were found in the pooled data for colonic hook needle electrode group.
Optimization of Electrical Stimulation Parameters
Four different ES parameter sets with a relatively wide spectrum of electrical energy were applied to the smooth muscle of 5 parts of the GI tract. To optimize the ES parameters, the pooled data were analyzed for the greatest modifications in post-stimulatory electrical reactions. For each part of the GI tract, the most suitable ES parameter set was defined according to the criteria for highest post-stimulatory electrical spike activity with a Poisson P-value below 0.05, independent of electrode type (Tables 1 and 2).
In the gastric group, we recorded the highest difference between baseline and post-stimulatory spike activity of 8.93 spm after GES for 30 seconds with 25 mA intensity, 1000 microseconds pulse width, and 130 Hz frequency. After DES for 30 seconds with 500 microseconds and 25 mA intensity and 130 Hz frequency, we recorded a maximum significant spike count of 17.63 spm in the duodenal group.
The highest difference between baseline and post-stimulatory spike activity was 63.61 after JES for 30 seconds with 1000 microseconds and 30 Hz in the jejunal group. In the ileal group, according to our criteria, the best suitable ES parameter was found with IES for 30 seconds with 1000 microseconds and 130 Hz resulting in a spike count of 28.57 spm.
CES for 30 seconds with 1000 microseconds and 30 Hz provoked the highest post-stimulatory electrical spike activity with a significant difference from baseline of 20.28 spm in the colonic group. Therefore, based on our experimental setup, it is regarded as the best suitable ES parameters for the colon. These maximal spike activities are all confirmed by small Poisson P-values.
Discussion
This experimental study exposes the significant effects of direct GES, DES, JES, IES, and CES with 4 stimulation parameters on the local electrical activity in an acute porcine model. To the best of our knowledge, no previous study has evaluated the effects of ES in 5 parts of the GI tract on the locally recorded EMG spike activity.
As the porcine intestinal tract possesses functional and pathological similarities to the human digestive tract,16 our results could have clinical relevance.
Based on our experimental protocol, the best suitable ES parameter was found for the maximum significant post-stimulatory effect in each part of the GI tract. For the highest significant post-stimulatory spike activity, 80.0% of the GI parts required 1000 microseconds (all but the duodenum) and 60.0% required 130 Hz (all but the jejunum and colon).
All of the applied ES parameters in our study included short pulse widths (500–1000 microseconds). The 4 ES parameters and post-stimulatory recording length used in our study were within the range of previously published data (300–3000 microseconds, 5–130 Hz, 7–30 mA, 2–30 seconds).17–20 Other studies applied long-pulse stimulation in the order of hundreds of milliseconds with low frequencies (< 1 Hz) and amplitudes (5–10 mA).2,12,21 For entrainment of electrical activity of the small intestine and stomach, long pulse-widths of several hundred milliseconds were reported.11,22
GI motility may be evaluated objectively by multiple techniques, such as manometry, video image tracking, transit tests, and EMG analysis. Several animal studies have combined direct ES with subserosal electrodes at up to 4 GI locations with motility analysis by intraluminal manometric techniques.12,23 However, manometry causes luminal obstruction, and even triggering of peristaltic reflexes.24 GI EMG recording with spike analysis is a well-established technique for investigating myoelectric activity and the corresponding contractile activity. Compared to questionnaires, manometry and transit tests, EMG analysis can be automated and thus delivers quick and objective results.3,7,25–27
Lychkova et al26 found an approximate spike activity of 2.6 to 3.8 spm in the human stomach and 2.4 spm in the human duodenum. In the porcine jejunum and ileum, the maximum mean spike frequencies have been reported to be 13.3 spm and 11.7 spm, respectively.27 Shafik et al7 reported a mean colon spike frequency of 4.8 spm in pigs. Due to the different technical setups and analysis methods, the reported data are not quantitatively comparable to our study results. Nevertheless, the described electrical activity distributions match our reported data. Considerably higher spike frequencies and electrical excitabilities occur in the small intestine compared to the stomach or colon.
Five electrical activity reaction types were classified in an early period after GES, DES, JES, IES, and CES. The significant electrical reactions of the stomach after GES were intermediate or relatively slow by means of type II or type III reactions. One out of 4 post-stimulatory reactions was not significantly different from baseline in the gastric group. Gastric electrical activity was described recently in patients with medically refractory diabetic gastroparesis. No significant changes were found in slow wave frequencies within an early period of implant-based high-frequency GES with up to 20 mA amplitude, 450 microseconds pulse-width, and 130 Hz frequency.20 A human study with temporary GES in patients with gastroparesis reported symptom relief after 3 to 4 days.28
Analysis of the investigated parts of the small intestine revealed that electrical reactions after DES, JES, and IES were mostly significant and fast (type I). Few studies have performed smooth muscle EMG-based activity analysis within minutes after ES. A chronic canine study evaluated JES for pacing of jejunal electrical slow wave activity. JES was performed for an average of 25 seconds until intestinal slow waves were entrained, and baseline activity was reconstituted within one minute after JES.22 This is consistent with our findings of early and quick responses to ES in the small intestine. However, no spike activities were recorded in the study for more direct assessment of smooth muscle contractions.
In our study, the electrical response of the colon after CES appeared not to be reliable, with only a few significantly increased spike responses, but a high percentage of insignificant effects and even moderate decreases in spike activity compared to baseline. In contrast, Aellen et al reported reproducible and quick contractions of the cecum within 30 seconds of CES and consecutive return to basal motor activity 2 minutes after CES.17
Experimental studies with multilocular stimulation and recording are rare. Sun et al11 performed a chronic canine study for exploration of the effects of GES, DES, and CES for entrainment of local slow wave activity. Direct ES was tested at slightly higher frequencies than the intrinsic frequency of slow waves, but pacing was only achievable in the stomach and duodenum.11 This difficulty in modulating colonic electrical activity is in accordance with our findings after CES of the colon. However, slow wave data analysis was performed with static cutoff values compared to fixed slow wave frequency ranges based on previous literature without an evaluation of spike activity.
Xu et al12 evaluated the effects of GES, DES, IES, and CES on the gastric tone in canine experiments. Continuous ES for 25 minutes was able to gradually increase gastric volume without significant differences between the electrically stimulated parts of the GI tract.12 However, reactions apart from the stomach were not analyzed and electrical activity was not assessed. Liu et al conducted a study with healthy humans and reported delayed gastric emptying within 30 minutes under continuous DES. Endoluminal ring electrodes recorded baseline electrical activity in the duodenum.21 The effects of DES on post-stimulatory electrical activity were not analyzed.
An overview of previously performed studies with multilocular ES together with our experimental setup is provided (Table 3).
Table 3.
Available Studies With Electrical Stimulation of Multiple Gastrointestinal Segments
| Author, Year | Journal | Electrical stimulation | Analyzed gastrointestinal segments | Analysis method | Observed time interval | Model |
|---|---|---|---|---|---|---|
| Sun et al,11 2009 | Am J Physiol Regul Integr Comp Physiol | GES, DES, CES | Stomach, Duodenum, Colon | EMG Slow Waves | Minutes | Dog, chronic |
| Xu et al,12 2010 | Dig Dis Sci | GES, DES, IES, CES | Stomach | Manometry | Minutes | Dog, chronic |
| Agrawal et al,13 2016 | Dig Dis Sci | GES, SES | Stomach, Colon | Questionnaire | Weeks | Human, chronic |
| Schiemer et al,14 2019 | Current Directions in Biomedical Engineering | GES, DES, JES, IES, CES | Stomach, Duodenum, Jejunum, Ileum, Colon | EMG Spikes | Minutes | Pig, acute |
GES, gastric electrical stimulation; DES, duodenal electrical stimulation; CES, colonic electrical stimulation; IES, ileal electrical stimulation; SES, sacral electrical stimulation; JES, jejunal electrical stimulation; EMG, electromyography.
Previously published studies with electrical stimulation (ES) of multiple segments of the gastrointestinal (GI) tract are provided and may be compared to our current study. To the best of our knowledge we present the first study with ES of 5 GI parts: stomach, duodenum, jejunum, ileum, and colon. Furthermore there is no previous study available with smooth muscle EMG of these 5 GI segments.
In conclusion, the application of multiple electrodes in the GI tract with subsequent multi-channel EMG analysis may be a convenient clinical option for objective identification of GI motility disorders by its electrical correlate. Furthermore, direct modulation of multiple parts of the GI tract by locally applied ES is a promising option for future clinical trials in patients with therapy refractory GI motility disorders.
Multiple small communication devices distributed along the entire GI tract with integrated diagnostic and therapeutic tasks29 may meet the requirements of a complexly interacting GI system. To achieve device-based ESs that rapidly adapt to the current electrical activities in the GI segments, an automated EMG analysis is essential. We tested 4 ES parameters that differently modulated GI activity and early EMG reaction patterns within minutes after ES were characterized. Based on our data it seems possible to develop a closed-loop feedback control for the future smart devices.
Further research is needed to elucidate the reported cross-organ effects6,11,12 and possible interactions with multilocular ES. Moreover, mapping of slow wave activities in the whole GI tract after multilocular ES would lead to a more detailed perspective on GI pacesetting activity. Further technological developments are required to allow the minimally invasive implantation of multilocular theranostic devices that are safely prepared for human use.
Acknowledgements
We thank Lennart Zimniak and Christian Boedecker (Department of General, Visceral and Transplant Surgery, University Medical Center of the Johannes Gutenberg-University Mainz, Mainz, Germany) for surgical assistance and Jana Dickmann (Translational Animal Research Center, University Medical Center of the Johannes Gutenberg-University Mainz, Germany) for anesthesiological assistance during the experiments. Furthermore, we would like to thank Kornelius Lente (Department of Research and Development at inomed Medizintechnik GmbH, Emmendingen, Germany) for data computing support. We also appreciate the support of Oliver Kempski and Nadine Baumgart.
Footnotes
Financial support: This subproject belongs to the INTAKT project and was funded by the Federal Ministry of Education and Research (BMBF; Grant No. 16SV7638).
Conflicts of interest: Jonas F Schiemer, Axel Heimann, Roman Ruff, Klaus-Peter Hoffmann, Jan Baumgart, Manfred Berres, Hauke Lang, and Werner Kneist state no conflict of interest. Karin H Somerlik-Fuchs is an employee of inomed Medizintechnik GmbH.
Author contributions: Werner Kneist and Klaus-Peter Hoffmann initiated the study and obtained funding; Jonas F Schiemer, Axel Heimann, Karin H Somerlik-Fuchs, Roman Ruff, and Werner Kneist were involved in the design of the study and experiments; Jan Baumgart and Axel Heimann handled laboratory animal issues; Axel Heimann, Karin H Somerlik-Fuchs, and Roman Ruff provided the electrotechnical setup; Karin H Somerlik-Fuch computed the data; Jonas F Schiemer and Karin H Somerlik-Fuchs performed the spike analysis; Manfred Berres and Jonas F Schiemer carried out statistical analysis; Jonas F Schiemer and Werner Kneist wrote the manuscript; and Hauke Lang reviewed the manuscript.
References
- 1.Lin Z, Eaker EY, Sarosiek I, McCallum RW. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am J Gastroenterol. 1999;94:2384–2389. doi: 10.1111/j.1572-0241.1999.01362.x. [DOI] [PubMed] [Google Scholar]
- 2.Shafik A, Shafik AA, El-Sibai O, Ahmed I. Colonic pacing: a therapeutic option for the treatment of constipation due to total colonic inertia. Arch Surg. 2004;139:775–779. doi: 10.1001/archsurg.139.7.775. [DOI] [PubMed] [Google Scholar]
- 3.Lammers WJ. Arrhythmias in the gut. Neurogastroenterol Motil. 2013;25:353–357. doi: 10.1111/nmo.12116. [DOI] [PubMed] [Google Scholar]
- 4.Jung KW, Yang DH, Yoon IJ, et al. Electrical stimulation therapy in chronic functional constipation: five years’ experience in patients refractory to biofeedback therapy and with rectal hyposensitivity. J Neurogastroenterol Motil. 2013;19:366–373. doi: 10.5056/jnm.2013.19.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keef KD, Cobine CA. Control of motility in the internal anal sphincter. J Neurogastroenterol Motil. 2019;25:189–204. doi: 10.5056/jnm18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zikos TA, Kamal AN, Neshatian L, et al. High prevalence of slow transit constipation in patients with gastroparesis. J Neurogastroenterol Motil. 2019;25:267–275. doi: 10.5056/jnm18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafik A, El-Sibai O, Ahmed A. Study of the mechanism underlying the difference in motility between the large and small intestine: the “single” and “multiple” pacemaker theory. Front Biosci. 2001;6:B1–B5. doi: 10.2741/A584. [DOI] [PubMed] [Google Scholar]
- 8.Martellucci J, Valeri A. Colonic electrical stimulation for the treatment of slow-transit constipation: a preliminary pilot study. Surg Endosc. 2014;28:691–697. doi: 10.1007/s00464-013-3192-0. [DOI] [PubMed] [Google Scholar]
- 9.Deane AM, Chapman MJ, Reintam Blaser A, McClave SA, Emmanuel A. Pathophysiology and treatment of gastrointestinal motility disorders in the acutely ill. Nutr Clin Pract. 2019;34:23–36. doi: 10.1002/ncp.10199. [DOI] [PubMed] [Google Scholar]
- 10.Avalos DJ, Sarosiek I, Loganathan P, McCallum RW. Diabetic gastroparesis: current challenges and future prospects. Clin Exp Gastroenterol. 2018;11:347–363. doi: 10.2147/CEG.S131650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Song GQ, Yin J, Lei Y, Chen JD. Effects and mechanisms of gastrointestinal electrical stimulation on slow waves: a systematic canine study. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1392–R1399. doi: 10.1152/ajpregu.00006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Lei Y, Chen JD. Effects and mechanisms of electrical stimulation of the stomach, duodenum, ileum, and colon on gastric tone in dogs. Dig Dis Sci. 2010;55:895–901. doi: 10.1007/s10620-009-0830-4. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal A, Francis SL, Deveneau NE, et al. Gastric electrical stimulation and sacral electrical stimulation: a long-term follow-up study of dual-device treatment. Dig Dis Sci. 2016;61:176–180. doi: 10.1007/s10620-015-3840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiemer JF, Heimann A, Somerlik-Fuchs KH, et al. Electrical stimulation with motility analysis of five parts of the gastrointestinal tract. Current Directions in Biomedical Engineering. 2018;4:9–11. doi: 10.1515/cdbme-2018-0003. [DOI] [Google Scholar]
- 15.Lammers WJ, Michiels B, Voeten J, Ver Donck L, Schuurkes JA. Mapping slow waves and spikes in chronically instrumented conscious dogs: automated on-line electrogram analysis. Med Biol Eng Comput. 2008;46:121–129. doi: 10.1007/s11517-007-0294-7. [DOI] [PubMed] [Google Scholar]
- 16.Brown DR, Timmermans JP. Lessons from the porcine enteric nervous system. Neurogastroenterol Motil. 2004;16:50–54. doi: 10.1111/j.1743-3150.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 17.Aellen S, Wiesel PH, Gardaz JP, et al. Electrical stimulation induces propagated colonic contractions in an experimental model. Br J Surg. 2009;96:214–220. doi: 10.1002/bjs.6455. [DOI] [PubMed] [Google Scholar]
- 18.Sevcencu C, Rijkhoff NJ, Gregersen H, Sinkjaer T. Propulsive activity induced by sequential electrical stimulation in the descending colon of the pig. Neurogastroenterol Motil. 2005;17:376–387. doi: 10.1111/j.1365-2982.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 19.Sobocki J, Nowakowski M, Herman RM, et al. Laparoscopically implanted system for stimulation of the hypogastric plexus induces colonic motility, defecation, and micturition: experimental study. Surg Innov. 2015;22:70–76. doi: 10.1177/1553350614530190. [DOI] [PubMed] [Google Scholar]
- 20.Angeli TR, Du P, Midgley D, et al. Acute slow wave responses to high-frequency gastric electrical stimulation in patients with gastroparesis defined by high-resolution mapping. Neuromodulation. 2016;19:864–871. doi: 10.1111/ner.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005;100:792–796. doi: 10.1111/j.1572-0241.2005.40511.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Hayes J, Peters LJ, Chen JD. Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Ann Biomed Eng. 2000;28:582–587. doi: 10.1114/1.294. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Chen JD. Intestinal electrical stimulation improves delayed gastric emptying and vomiting induced by duodenal distension in dogs. Neurogastroenterol Motil. 2008;20:236–242. doi: 10.1111/j.1365-2982.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-de-Juan JL, Saiz J, Meseguer M, Poncde JL. Small bowel motility: relationship between smooth muscle contraction and electroen-terogram signal. Med Eng Phys. 2000;22:189–199. doi: 10.1016/S1350-4533(00)00032-1. [DOI] [PubMed] [Google Scholar]
- 25.Latour A, Ferré JP. Computer-aided analysis of gastrointestinal myoelectric activity. J Biomed Eng. 1985;7:127–131. doi: 10.1016/0141-5425(85)90041-X. [DOI] [PubMed] [Google Scholar]
- 26.Lychkova AE. Pacemakers of the upper divisions of the digestive tract. Bull Exp Biol Med. 2014;156:518–521. doi: 10.1007/s10517-014-2388-1. [DOI] [PubMed] [Google Scholar]
- 27.Romañski KW, Rudnicki J, Slawuta P. The myoelectric activity of ileum in fasted and fed young pigs. J Physiol Pharmacol. 2001;52(4 Pt 2):851–861. [PubMed] [Google Scholar]
- 28.Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496–503. e3. doi: 10.1016/j.gie.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann KP, Olze H, Kneist W, et al. Interactive implants: vision and challenges. Current Directions in Biomedical Engineering. 2018;4:1–4. doi: 10.1515/cdbme-2018-0001. [DOI] [Google Scholar]