Abstract
The evidence that diel patterns of physiology and behaviour in mammals are governed by circadian ‘clocks’ is based almost entirely on studies of nocturnal rodents. The emergent circadian paradigm, however, neglects the roles of energy metabolism and alimentary function (feeding and digestion) as determinants of activity pattern. The temporal control of activity varies widely across taxa, and ungulates, microtine rodents, and insectivores provide examples in which circadian timekeeping is vestigial. The nocturnal rodent/human paradigm of circadian organisation is unhelpful when considering the broader manifestation of activity patterns in mammals.
The evidence that daily patterns of physiology and behaviour in mammals are governed by circadian ‘clocks’ is based almost entirely on studies of nocturnal rodents. This Essay proposes that the nocturnal rodent/human paradigm of circadian rhythms is unhelpful when considering the broader manifestation of temporal organisation of activity in mammals.
It is widely held that daily patterns of physiology and behaviour in mammals are governed by the cell-autonomous rhythms of gene transcription that constitute circadian ‘clocks’ [1]. Circadian clocks have been identified and characterised in species ranging from cyanobacteria to humans, and circadian organisation is generally considered a ubiquitous controlling feature [2–5].
The empirical basis for this view, however, is surprisingly weak. Knowledge of circadian mechanisms stems from studies in model organisms in which the phenotype is prominent and, in mammals, is based almost entirely on studies in rats, mice, and hamsters. This is no coincidence: these small nocturnal rodents are cheap to maintain, perform well in the laboratory, and above all, display strong circadian organisation. Had this not been the case, they would not have been studied: they were selected as models of circadian function, not of their taxa.
The ascendancy of the circadian model has led to uncritical use of the term ‘circadian’. Identification of circadian organisation (sensu stricto) requires evidence of persistence—i.e., the demonstration that rhythms are expressed in the absence of external synchronising input (the so-called zeitgeber). Such evidence is normally sought by observing organisms such as humans and mice under constant conditions―most often continuous darkness or dim red light. The term ‘circadian’ is nevertheless frequently ascribed in scientific literature to rhythms recorded under daily cycles of light intensity. Such usage without evidence of endogenous drive renders the term ‘circadian’ synonymous with ‘24 h’ or ‘diel’ and therefore redundant.
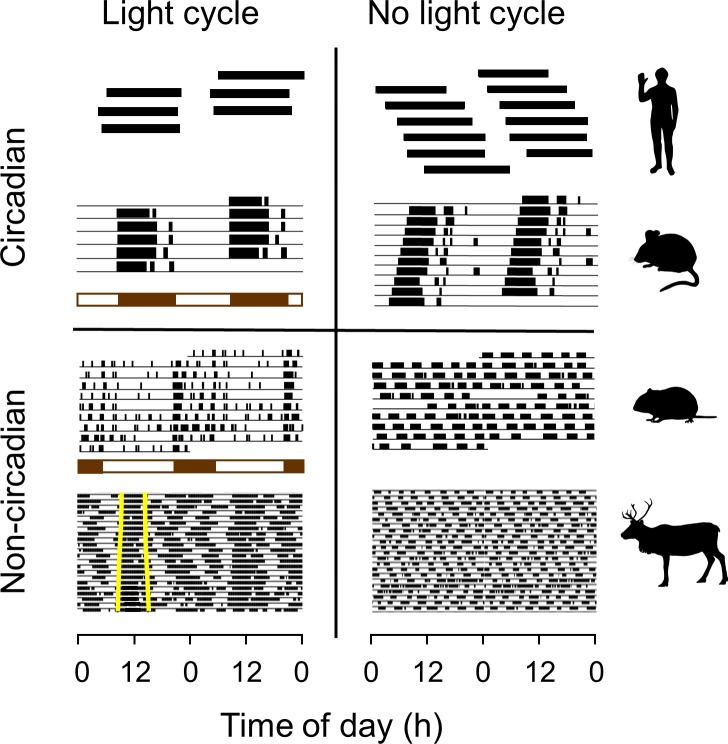
Fig 1 shows examples of two species from distinct mammalian taxa (common vole [Microtus arvalis] and reindeer/caribou [Rangifer tarandus L.], hereafter Rangifer) in which circadian organisation is not evident. Occurrence of noncircadian temporal organisation like this should make us wonder what circumstances have promoted circadian dominance in some species—notably, those upon which the canon is founded.
Fig 1. Circadian organisation is not ubiquitous in mammals.
Activity patterning in (from the top) humans, mice (Mus musculus), voles (M. arvalis), and reindeer (R. tarandus) under 24-h LCs and NLCs. All four species display pronounced 24-h rhythms of activity under LC. These rhythms persist under NLC in humans and mice but not in voles and reindeer. Data for humans are from bunker experiments in which subjects were initially exposed to changes in light intensity synchronised to the solar day (LC) and then allowed to free-run with only self-imposed changes in light level (NLC [6]). For mice and voles, experimental light and dark phases are represented by horizontal white and brown bars, respectively. For the reindeer, free-living in their natural environment, natural photoperiod (onset and offset of civil twilight) is indicated by vertical yellow lines on the first day of each actogram, and the NLC regime was the polar night at 78° north latitude. Data for one individual of each species under each light regime are presented as double-plotted actograms. Black bars represent activity. LC, light–dark cycle; NLC, no-light cycle. Redrawn from [6–9].
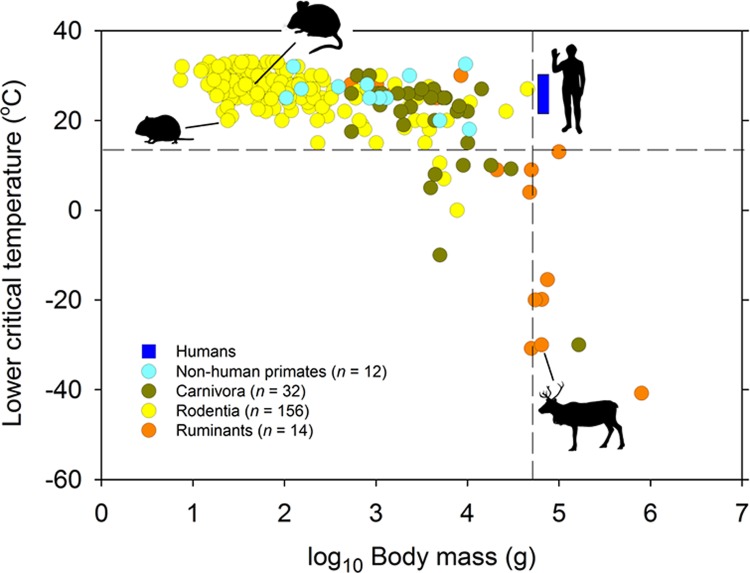
Maintenance of thermal balance is a major determinant of temporal activity patterns in small mammals [10], for which, moreover, the world is generally a cold place. Thus, although hyperthermia may be a problem in some environments (e.g., hot deserts), the mean surface temperature of the earth (14°C [11]) is substantially lower than the lower critical temperature (Tlc) of small mammals (<100 g; median Tlc = 29°C, range = 20 to 36, n = 218 species [12,13], Fig 2). Such creatures are obliged to sustain a high metabolic rate simply to maintain body temperature (Tb; median Tb = 37°C, range = 31 to 40, n = 312 species [14]), and the temporal pattern of their activity is consequently dominated by the conflicting objectives of minimising heat loss and obtaining fuel (food) to service their metabolic requirement [15].
Fig 2. Relationship between Tlc (°C) and body mass (g) in mammals.
The Tlc is defined as the ambient temperature below which the rate of metabolic heat production must be increased in order to maintain homeothermy. Of all species with Tlc above the global mean surface temperature (14°C, horizontal dashed line; [11]), humans (Tlc range from 23 to 33°C; [16,17]) are the most massive, and of all species with a body mass above 50 kg (vertical dashed line), humans have the highest Tlc. For clarity, the figure includes only data for a limited number of taxa (humans, Carnivora, nonhuman primates, Rodentia, ruminants). However, the shape of the relationship between body mass and Tlc does not change when data for other groups are included (Chiroptera, Cingulata, Dasyuromorphia, Diprotodontia, Erinaceomorpha, Eulipotyphla, Hyracoidea, Lagomorpha, Macroscelidea, Monotremata, Peramelemorphia, and Soricomorpha). Species data indicated by silhouettes are, clockwise from the left, for voles (M. arvalis), mice (M. musculus), humans, and reindeer (R. tarandus). Data from [12,13,18,19]. Tlc, lower critical temperature.
Small mammals commonly respond to thermal challenge (hot or cold) by withdrawing into a nest, burrow, or other place of concealment, in which they remain during the inhospitable phase of the day before reemerging and resuming activity [10]. The strategy of ‘avoidance through withdrawal’, however, presents a problem: the better a shelter insulates an animal from inhospitable environment, the less information the animal receives about the current state of that environment and, hence, about when to emerge and resume its activity. The situation is exacerbated when the animal, protected in its shelter, enters torpor or sleep. The solution has been evolution of an internal representation of the passage of time (i.e., the circadian clock), which renders the timing of reemergence independent of environmental cues. We suggest that avoidance through withdrawal has been a major factor in the evolution and maintenance of strong circadian organisation in small mammals.
The situation for large mammals (>10 kg) is quite different. By virtue of their size, these animals escape the thermal imperative to isolate themselves from the surface environment during periods of inactivity. The median Tlc of large mammals (10–800 kg) is 10 (range from −41 to +28) °C, n = 26; [12,13,19]), which is 4°C below global mean surface temperature (Fig 2). Moreover, most large mammals spend their entire lives above ground, where they are continuously exposed to temporal information contained in the daily cycle of light intensity: light levels increasing after a period of darkness or decreasing after a period of daylight are reliable indicators of the approach of day or night, respectively. There is therefore no reason to infer selective pressure on such animals to maintain internal representation of the passage of solar time in order to schedule the 24-h pattern of their activity. Consistent with this, there is increasing evidence that large ungulates possess weak circadian mechanisms (Rangifer [20–23], red deer [Cervus elaphus] [24], horse [Equus ferus caballus] [25]).
Absence of strong circadian organisation does not, however, imply absence of strong temporal organisation. Most ungulates display highly organised patterns of behaviour consisting of alternate ultradian bouts of activity (chiefly foraging) and inactivity (chiefly digestion and sleep) that persist in continuous sequence across the 24-h cycle. This is evident in boreal species under continuous photic conditions (Fig 1; see also [26]) and also in equatorial species exposed to a strong daily cycle of light intensity (wildebeest [Connochaetes taurinus] [27]; elephant [Loxodonta africana] [28]; buffalo [Syncerus caffer] [29]). This behaviour has been modelled in cattle as a rumen oscillator from which derives a suite of predictable relationships between the dimensions of the gut, forage digestibility, and period of the feeding rhythm [30,31].
Predominantly ultradian organisation of activity is not, however, unique to large ungulates. Voles, too, depend on microbial fermentation to extract energy from the cell walls of the plants they eat, and the resulting low rate of energy uptake likewise obliges them to engage in frequent bouts of feeding to meet their metabolic requirements [32]. These small (<30 g) animals display clear ultradian organisation in the wild, with short bouts of activity spaced at intervals of 2–4 h across the 24-h cycle (M. arvalis [33], Microtus agrestis [34]), and they continue to display free-running ultradian rhythms when maintained in continuous darkness (Fig 1).
Nor is ultradian organisation unique to herbivores. Shrews (Insectivora) eat invertebrates [35] and display ultradian organisation both in the wild (Neomys fodiens, live body mass [LBM] 17 g, [36]) and in the laboratory (Blarina brevicauda, LBM 20 g [37]; see also [38,39]). The small size of these creatures means that they have both a high mass-specific metabolic rate and a very small stomach [40]. The energy gained from each small meal is therefore quickly consumed, and consequently, shrews (like voles) have to replenish with frequent short (0.1–3.0 h) bouts of feeding distributed more or less evenly across the 24-h cycle [41].
These examples illustrate ways in which metabolism and alimentary function constrain temporal organisation of activity. This is not to say that the temporal pattern of activity of such species is independent of the daily cycle of light intensity. Rather, the influence of light on activity depends on the current ecological and physiological settings. Thus, the prominent peaks of crepuscular activity in ruminants are sustained by the transitions in light level at dawn and dusk and vanish when the amplitude of the light-intensity cycle is reduced naturally around the solstices (Rangifer [9,21]) or experimentally in the laboratory (sheep [Ovis aries] [42]). The coupling of activity to the light-intensity cycle evident during equinoctial periods has been attributed in Rangifer to direct effects of light that act through a photoperiod-dependent trade-off between predation hazard and energy balance [43]. The ultradian pattern of activity in voles, likewise, is coupled to the daily cycle of light intensity. Increasing light levels at dawn directly suppress nocturnal activity and synchronise the first daytime bout between individuals [41,44]. This is considered part of an antipredator strategy [33], and for this purpose, voles (like Rangifer) seem to exploit conscious assessment of risk, directly reliant on visual cues, rather than circadian entrainment [43,45]. Hence, it seems that a range of interacting factors (thermo-energetics, alimentary function, hazard) militate for or against expression of circadian rhythmicity, and the relative influence of each varies within and between species.
The question therefore arises whether strong circadian organisation should be interpreted as an ancestral feature that has become vestigial in some groups or as a derived specialisation. Modern eutherian mammals are believed to have descended from nocturnal ancestors [46] in which exploitation of darkness, possibly in response to predation by diurnal sauropsids, was facilitated by the evolution of photoreceptive systems adapted to low light and of endothermy [47–50]. Ancestral mammals were generally rather small [51,52] and so would presumably have experienced the same thermo-energetic constraints as modern small mammals. Indeed, daily torpor is an energy conservation strategy seen predominantly in ancient mammalian lineages [53]. We suggest that the adoption of a nocturnal lifestyle and avoidance through withdrawal together favoured the evolution of circadian dominance. This ancestral characteristic has been lost for those mammals in which changes in physiological and ecological constraints have removed the need for avoidance through withdrawal (large ungulates) or, alternatively, have intensified the need to feed at an ultradian frequency (voles and shrews).
Secondary loss of circadian dominance has not to date been linked to mutations at the level of the canonical ‘clock genes’, which serve as the master controllers of circadian organisation [1]. Detailed characterisation of clock genes in sheep has revealed no distinctive features in terms of DNA sequence, RNA expression cycles under a daily light-intensity cycle, protein–protein interactions, DNA binding, or transcriptional control [54]. Similarly, a survey of the genome of Rangifer [55] reveals both a full complement of clock genes and a high degree of sequence conservation between the coding and upstream promoter region in these and their homologues in rats, mice, sheep, and humans (personal communication, A. West to D. Hazlerigg). In voles, ultradian patterning of behaviour is associated with arrhythmic expression of clock genes in the liver, whereas gene expression rhythms in the suprachiasmatic nucleus (SCN) follow the light–dark cycle [56], and in blind mole rats (Spalax ehrenbergi), poorly organised activity patterns under constant light conditions are associated with low-amplitude rhythms of circadian gene expression in the brain [57]. The best-documented function of clock genes in ungulates relates to seasonal timekeeping for which measurement of day length (photoperiod) is the key attribute. Here, however, light controls the expression of clock genes directly, via the hormone melatonin and without circadian gating, and hence drives seasonal changes in physiological and behavioural function [58–60]. Furthermore, the presence of so-called clock genes in the absence of robust circadian organisation may reflect the importance of their molecular functions for biological processes that have nothing to do with timekeeping per se (e.g., casein kinase 1 role in wnt signalling [61], cryptochrome role in magnetic sensitivity [62], bmal1 role in hypoxia sensing [63]).
It is also important to distinguish between the presence and the efficacy of a trait. Demonstration of a circadian pattern of activity in an organism maintained under laboratory conditions provides no information about its temporal organisation of activity under natural conditions. Voles provide a striking example: voles living in cages furnished with running wheels normally display a strongly nocturnal pattern of activity. This pattern, moreover, may free-run in constant darkness, thus bearing the hallmark of circadian organisation [7,41,64], yet it derives specifically from the presence of the running wheel: voles living in cages without running wheels maintain their natural ultradian pattern [56]. The reasons for this effect are unknown, but running in wheels may be intrinsically rewarding [65,66]: indeed, rats, mice, shrews, and other wild creatures voluntarily climb into and run in wheels placed outside in the field [67]. Furthermore, wheel running distances—up to several kilometres in a single episode—are sensitive to energetic status [68], and making voles or mice ‘work for food’ by coupling wheel running to their food supply induces ultradian activity ([69,70] and personal communication, R.A. Hut to D. Hazlerigg). The emergent picture is one in which temporal organisation depends on complex energetic and other ecological constraints, and the running wheel is a device that artificially emphasises the circadian component.
Running wheels also corrupt our view of behavioural organisation because running rodents appeal to anthropomorphic perception of biological timekeeping. Just as voles may be considered miniature cows [71], so humans are often considered massive mice. This is essentially the precept upon which biomedical science is based, and its general worth has been proved innumerable times. Mouse models afford us insights into many aspects of human health and disease, and the analogy extends to the circadian system: despite being three orders of magnitude heavier, humans, like mice, withdraw for consolidated periods of sleep and show robust circadian rhythmicity [72]. The resolution of the paradox of strong circadian organisation in humans may lie in their unusually low thermal tolerance compared with similar-sized mammals (Fig 2). The Tlc of humans is similar to that of mice (Tlc of humans 23–33°C [range], Tlc of mouse 29°C [16,17]) and is more than 40°C higher than the median for mammals with a body mass of 50 kg or more (Fig 2). All primates have low thermal tolerance, which suggests that this is an ancestral feature (Fig 2); this and the associated need for avoidance through withdrawal during rest may at least in part account for human circadian organisation.
The temporal organisation of mammals cannot be dissociated from their ecological environment or from the trade-offs that constrain their behaviour. Metabolic and alimentary constraints can render the thermal benefit of circadian withdrawal inaccessible to a small mammal. A large mammal with unrestricted access to temporal cues may organise its behaviour directly without reference to a circadian clock. In cases like these, circadian timekeeping seems to have become vestigial. In arriving at this view, we emphasise the importance of behavioural organisation, and we see little reason to anticipate internal physiological or molecular circadian rhythmicity in organisms in which behaviour is not circadian. Unfortunately, studies addressing this issue are rare, reflecting the stultifying effect of ascribing a ‘circadian’ basis to approximately 24-h rhythms observed only under light–dark cycles. There is clearly a need to look deeper, and it is timely to do so given the ease with which gene expression can now be both monitored and manipulated in nonmodel species. Only by so doing in an unbiased way across taxa can we reach a properly nuanced view of the importance of circadian organisation in mammals.
Acknowledgments
The authors thank Hugues Dardente, Roelof Hut, Alex West, and Shona Wood for excellent critique of earlier versions of this paper.
Abbreviations
- LBM
live body mass
- LC
light-dark cycle
- NLC
no-light cycle
- SCN
suprachiasmatic nucleus
- Tb
body temperature
- Tlc
lower critical temperature
Funding Statement
DGH was supported by HFSP program grant RGP0030/2015-C301 "Evolution of seasonal timers". The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Provenance: Not commissioned; externally peer reviewed
References
- 1.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat Rev Genet. 2008;9: 764–775. 10.1038/nrg2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418: 935–941. 10.1038/nature00965 [DOI] [PubMed] [Google Scholar]
- 3.Roenneberg T, Merrow M. Circadian clocks—the fall and rise of physiology. Nat Rev Mol Cell Biol. 2005;6: 965–971. 10.1038/nrm1766 [DOI] [PubMed] [Google Scholar]
- 4.Cohen SE, Golden SS. Circadian Rhythms in Cyanobacteria. Microbiol Mol Biol Rev. 2015;79: 373–85. 10.1128/MMBR.00036-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25: 159–84. 10.1101/SQB.1960.025.01.015 [DOI] [PubMed] [Google Scholar]
- 6.Von Aschoff J, Wever R. Spontanperiodik des Menschen bei Ausschluß aller Zeitgeber. Naturwissenschaften. 1962;49: 337–342. 10.1007/BF01185109 [DOI] [Google Scholar]
- 7.Gerkema MP, Daan S. Differential Elimination of Circadian and Ultradian Rhythmicity by Hypothalamic Lesions in the Common Vole, Microtus arvalis. J Biol Rhythms. 1990;5: 81–95. 10.1177/074873049000500201 [DOI] [PubMed] [Google Scholar]
- 8.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109: 307–320. 10.1016/s0092-8674(02)00722-5 [DOI] [PubMed] [Google Scholar]
- 9.Van Oort BEH, Tyler NJC, Gerkema MP, Folkow L, Stokkan KA. Where clocks are redundant: Weak circadian mechanisms in reindeer living under polar photic conditions. Naturwissenschaften. 2007;94: 183–194. 10.1007/s00114-006-0174-2 [DOI] [PubMed] [Google Scholar]
- 10.Riede SJ, van der Vinne V, Hut RA. The flexible clock: predictive and reactive homeostasis, energy balance and the circadian regulation of sleep–wake timing. J Exp Biol. 2017;220: 738–749. 10.1242/jeb.130757 [DOI] [PubMed] [Google Scholar]
- 11.Jones PD, New M, Parker DE, Martin S, Rigor IG. Surface air temperature and its changes over the past 150 yr. Rev Geophys. 1999;37: 173–199. [Google Scholar]
- 12.Khaliq I, Hof C, Prinzinger R, Bohning-Gaese K, Pfenninger M. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc R Soc B Biol Sci. 2014;281: 20141097–20141097. 10.1098/rspb.2014.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riek A, Geiser F. Allometry of thermal variables in mammals: consequences of body size and phylogeny. Biol Rev. 2013;88: 564–572. 10.1111/brv.12016 [DOI] [PubMed] [Google Scholar]
- 14.Clarke A, Rothery P, Isaac NJB. Scaling of basal metabolic rate with body mass and temperature in mammals. J Anim Ecol. 2010;79: 610–619. 10.1111/j.1365-2656.2010.01672.x [DOI] [PubMed] [Google Scholar]
- 15.Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. In search of a temporal niche: Environmental factors. Prog Brain Res. 2012;199: 281–304. 10.1016/B978-0-444-59427-3.00017-4 [DOI] [PubMed] [Google Scholar]
- 16.Kingma BR, Frijns AJ, Schellen L, van Marken Lichtenbelt WD. Beyond the classic thermoneutral zone. Temperature. 2014;1: 142–149. 10.4161/temp.29702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallubinsky H, Schellen L, van Marken Lichtenbelt WD. Exploring the human thermoneutral zone–A dynamic approach. J Therm Biol. 2019;79: 199–208. 10.1016/j.jtherbio.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 18.Müller EF, Kamau JM, Maloiy GM. A comparative study of basal metabolism and thermoregulation in a folivorous (Colobus guereza) and an omnivorous (Cercopithecus mitis) primate species. Comp Biochem Physiol A Comp Physiol. 1983;74: 319–22. 10.1016/0300-9629(83)90608-4 [DOI] [PubMed] [Google Scholar]
- 19.Nilssen KJ, Sundsfjord JA, Blix AS. Regulation of metabolic rate in Svalbard and Norwegian reindeer. Am J Physiol. 1984;247: R837–41. 10.1152/ajpregu.1984.247.5.R837 [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Meng QJ, Tyler NJC, Stokkan KA, Loudon ASI. A Circadian Clock Is Not Required in an Arctic Mammal. Curr Biol. 2010;20: 533–537. 10.1016/j.cub.2010.01.042 [DOI] [PubMed] [Google Scholar]
- 21.Van Oort BEH, Tyler NJC, Gerkema MP, Folkow L, Blix AS, Stokkan KA. Circadian organization in reindeer. Nature. 2005;438: 1095–1096. 10.1038/4381095a [DOI] [PubMed] [Google Scholar]
- 22.Arnold W, Ruf T, Loe LE, Irvine RJ, Ropstad E, Veiberg V, et al. Circadian rhythmicity persists through the Polar night and midnight sun in Svalbard reindeer. Sci Rep. 2018;8: 14466 10.1038/s41598-018-32778-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokkan KA, Van Oort BEH, Tyler NJC, Loudon ASI. Adaptations for life in the Arctic: Evidence that melatonin rhythms in reindeer are not driven by a circadian oscillator but remain acutely sensitive to environmental photoperiod. J Pineal Res. 2007;43: 289–293. 10.1111/j.1600-079X.2007.00476.x [DOI] [PubMed] [Google Scholar]
- 24.Ensing EP, Ciuti S, de Wijs FALM, Lentferink DH, ten Hoedt A, Boyce MS, et al. GPS Based Daily Activity Patterns in European Red Deer and North American Elk (Cervus elaphus): Indication for a Weak Circadian Clock in Ungulates. PLoS ONE. 2014;9: e106997 10.1371/journal.pone.0106997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy BA, Martin A-M, Furney P, Elliott JA. Absence of a serum melatonin rhythm under acutely extended darkness in the horse. J Circadian Rhythms. 2011;9: 3 10.1186/1740-3391-9-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erriksson LO, Källqvist ML, Mossing T. Seasonal development of circadian and short-term activity in captive reindeer, Rangifer tarandus L. Oecologia. 1981;48: 64–70. 10.1007/BF00346989 [DOI] [PubMed] [Google Scholar]
- 27.Berry HH, Siegfried WR, Crowe TM. Activity Patterns in a Population of Free-ranging Wildebeest Connochaetes taurinus at Etosha National Park. Z Tierpsychol. 1982;59: 229–246. 10.1111/j.1439-0310.1982.tb00340.x [DOI] [Google Scholar]
- 28.Leggett K. Daily and hourly movement of male desert-dwelling elephants. Afr J Ecol. 2010;48: 197–205. 10.1111/j.1365-2028.2009.01101.x [DOI] [Google Scholar]
- 29.Owen-Smith N, Goodall V. Coping with savanna seasonality: comparative daily activity patterns of African ungulates as revealed by GPS telemetry. J Zool. 2014;293: 181–191. 10.1111/jzo.12132 [DOI] [Google Scholar]
- 30.Gregorini P, Provenza FD, Villalba JJ, Beukes PC, Forbes MJ. Dynamics of forage ingestion, oral processing and digesta outflow from the rumen: a development in a mechanistic model of a grazing ruminant, MINDY. J Agric Sci. 2018;156: 980–995. 10.1017/S0021859618000886 [DOI] [Google Scholar]
- 31.Gregorini P, Provenza FD, Villalba JJ, Beukes PC, Forbes MJ. Diurnal patterns of urination and drinking by grazing ruminants: a development in a mechanistic model of a grazing ruminant, MINDY. J Agric Sci. 2018;156: 71–81. 10.1017/S0021859617000806 [DOI] [Google Scholar]
- 32.Hume ID, Karasov WH, Darken BW. Acetate, butyrate and proline uptake in the caecum and colon of prairie voles (Microtus ochrogaster). J Exp Biol. 1993;176. [DOI] [PubMed] [Google Scholar]
- 33.Daan S, Slopsema S. Short-term rhythms in foraging behaviour of the common vole, Microtus arvalis. J Comp Physiol—A. 1978;127: 215–227. 10.1007/BF01350112 [DOI] [Google Scholar]
- 34.Halle S, Lehmann U. Cycle-Correlated Changes in the Activity Behaviour of Field Voles, Microtus agrestis. Oikos. 1992;64: 489 10.2307/3545166 [DOI] [Google Scholar]
- 35.Rudge MR. The Food of the Common Shrew Sorex araneus L. (Insectivora: Soricidae) in Britain. J Anim Ecol. 1968;37: 565 10.2307/3075 [DOI] [Google Scholar]
- 36.Lardet JP. Spatial Behaviour and Activity Patterns of the Water Shrew Neomys fodiens in the Field. Acta Theriol (Warsz). 1988. [cited 2019 Jan 4];33: 293–303. Available from: http://rcin.org.pl/ibs/Content/26266/BI002_2613_Cz-40-2_Acta-T33-nr21-293-303_o.pdf. [Google Scholar]
- 37.Mann PM, Stinson RH. Activity of the short-tailed shrew. Can J Zool. 1957;35: 171–177. 10.1139/z57-012 [DOI] [Google Scholar]
- 38.Crowcroft P. The daily cycle of activity in British shrews. Proc Zool Soc London. 1954;123: 715–730. 10.1111/j.1096-3642.1954.tb00197.x [DOI] [Google Scholar]
- 39.Godfrey GK. The Activity Pattern in White-Toothed Shrews Studied With Radar. Acta Theriologica. 1978;23: 381–390. Available from: http://rcin.org.pl/ibs/Content/10512/BI002_2613_Cz-40-2_Acta-T23-nr24-381-390_o.pdf. [Google Scholar]
- 40.Clarke A, Rothery P. Scaling of body temperature in mammals and birds. Funct Ecol. 2008;22 10.1111/j.1365-2435.2007.01341.x [DOI] [Google Scholar]
- 41.Halle S. Polyphasic activity patterns in small mammals. Folia Primatol. 2006;77: 15–26. 10.1159/000089693 [DOI] [PubMed] [Google Scholar]
- 42.Ebling FJP, Lincoln GA, Wollnik F, Anderson N. Effects of Constant Darkness and Constant Light on Circadian Organization and Reproductive Responses in the Ram. J Biol Rhythms. 1988;3: 365–384. 10.1177/074873048800300406 [DOI] [PubMed] [Google Scholar]
- 43.Tyler NJC, Gregorini P, Forchhammer MC, Stokkan K-AA, Van Oort BEH, Hazlerigg DG. Behavioral Timing without Clockwork: Photoperiod-Dependent Trade-Off between Predation Hazard and Energy Balance in an Arctic Ungulate. J Biol Rhythms. 2016;31: 522–533. 10.1177/0748730416662778 [DOI] [PubMed] [Google Scholar]
- 44.Gerkema MP, Daan S, Wilbrink M, Hop MW, Van Der Leest F, Gerkema MP. Phase Control of Ultradian Feeding Rhythms in the Common Vole (Microtus arvalis): The Roles of Light and the Circadian System. J Biol Rhythms. 1993;8: 151–171. 10.1177/074873049300800205 [DOI] [PubMed] [Google Scholar]
- 45.Gerkema MP, Verhulst S. Warning against an unseen predator: a functional aspect of synchronous feeding in the common vole, Microtus arvalis. Anim Behav. 1990;40: 1169–1178. 10.1016/S0003-3472(05)80183-6 [DOI] [Google Scholar]
- 46.Walls GL. The vertebrate eye and its adaptive radiation. Bloomfield Hills MI: Cranbrook institute of science; 1942. 10.5962/bhl.title.7369. [DOI] [Google Scholar]
- 47.Kielan-Jaworowska Z, Cifelli R, Luo Z-X. Mammals from the age of dinosaurs: origins, evolution, and structure. New York, NY: Columbia University Press; 2004. [cited 2019 Jan 4]. Available from: https://books.google.no/books?hl=en&lr=&id=WgO3KyvMXUAC&oi=fnd&pg=PR2&dq=Kielan-Jaworowska+et+al.+2004&ots=mH-qhC97xg&sig=moaP-T80ZwIsyCmGtglh8Ib8C7I&redir_esc=y#v=onepage&q=Kielan-Jaworowska et al. 2004&f=false. [Google Scholar]
- 48.Hall MI, Kamilar JM, Kirk EC. Eye shape and the nocturnal bottleneck of mammals. Proc R Soc B Biol Sci. 2012;279: 4962–4968. 10.1098/rspb.2012.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerkema MP, Davies WIL, Foster RG, Menaker M, Hut RA. The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc R Soc B Biol Sci. 2013;280: 20130508–20130508. 10.1098/rspb.2013.0508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menaker M, Moreira LF, Tosini G. Evolution of circadian organization in vertebrates. Brazilian J Med Biol Res. 1997;30: 305–313. 10.1590/S0100-879X1997000300003 [DOI] [PubMed] [Google Scholar]
- 51.Jin M, Yaoming H, Chuankui L, Yuanqing W. The mammal fauna in the Early Cretaceous Jehol Biota: implications for diversity and biology of Mesozoic mammals. Geol J. 2006;41: 439–463. 10.1002/gj.1054 [DOI] [Google Scholar]
- 52.Luo Z-X. Transformation and diversification in early mammal evolution. Nature. 2007;450: 1011–1019. 10.1038/nature06277 [DOI] [PubMed] [Google Scholar]
- 53.Geiser F. Evolution of daily torpor and hibernation in birds and mammals: importance of body size. Clin Exp Pharmacol Physiol. 1998;25: 736–740. 10.1111/j.1440-1681.1998.tb02287.x [DOI] [PubMed] [Google Scholar]
- 54.Dardente H, Fustin J-M, Hazlerigg DG. Transcriptional feedback loops in the ovine circadian clock. Comp Biochem Physiol Part A Mol Integr Physiol. 2009;153: 391–398. [DOI] [PubMed] [Google Scholar]
- 55.Li Z, Lin Z, Ba H, Chen L, Yang Y, Wang K, et al. Draft genome of the Reindeer (Rangifer tarandus). Gigascience. 2017; 1–16. 10.1093/gigascience/gix102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Veen DR, Minh N Le, Gos P, Arneric M, Gerkema MP, Schibler U. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc Natl Acad Sci. 2006;103: 3393–3398. 10.1073/pnas.0507825103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avivi A, Oster H, Joel A, Beiles A, Albrecht U, Nevo E. Circadian Genes in a Blind Subterranean Mammal III: Molecular Cloning and Circadian Regulation of Cryptochrome Genes in the Blind Subterranean Mole Rat, Spalax ehrenbergi Superspecies. J Biol Rhythms. 2004;19: 22–34. 10.1177/0748730403260622 [DOI] [PubMed] [Google Scholar]
- 58.Johnston JD, Tournier BB, Andersson H, Masson-Pévet M, Lincoln GA, Hazlerigg DG. Multiple effects of melatonin on rhythmic clock gene expression in the mammalian pars tuberalis. Endocrinology. 2006;147: 959–965. 10.1210/en.2005-1100 [DOI] [PubMed] [Google Scholar]
- 59.Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc Natl Acad Sci. 2002;99: 13890–13895. 10.1073/pnas.212517599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hazlerigg DG, Andersson H, Johnston JD, Lincoln G. Molecular characterization of the long-day response in the Soay sheep, a seasonal mammal. Curr Biol. 2004;14: 334–339. 10.1016/j.cub.2004.01.057 [DOI] [PubMed] [Google Scholar]
- 61.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401: 345–350. 10.1038/43830 [DOI] [PubMed] [Google Scholar]
- 62.Gegear RJ, Foley LE, Casselman A, Reppert SM. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature. 2010;463: 804–807. 10.1038/nature08719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95: 5474–9. 10.1073/pnas.95.10.5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lehmann U. Short-term and circadian rhythms in the behaviour of the vole, Microtus agrestis (L.). Oecologia. 1976;23: 185–199. 10.1007/BF00361235 [DOI] [PubMed] [Google Scholar]
- 65.Albrecht U, Oster H. The circadian clock and behavior. Behav Brain Res. 2001;125: 89–91. 10.1016/S0166-4328(01)00288-1 [DOI] [PubMed] [Google Scholar]
- 66.Pendergast JS, Branecky KL, Huang R, Niswender KD, Yamazaki S. Wheel-running activity modulates circadian organization and the daily rhythm of eating behavior. Front Psychol. 2014;5: 177 10.3389/fpsyg.2014.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meijer JH, Robbers Y. Wheel running in the wild. Proc R Soc B Biol Sci. 2014;281: 20140210–20140210. 10.1098/rspb.2014.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chappell MA, Garland T, Rezende EL, Gomes FR. Voluntary running in deer mice: speed, distance, energy costs and temperature effects. J Exp Biol. 2004;207: 3839–54. 10.1242/jeb.01213 [DOI] [PubMed] [Google Scholar]
- 69.Hut RA, Pilorz V, Boerema AS, Strijkstra AM, Daan S. Working for Food Shifts Nocturnal Mouse Activity into the Day. PLoS ONE. 2011;6: e17527 10.1371/journal.pone.0017527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, et al. Cold and hunger induce diurnality in a nocturnal mammal. Proc Natl Acad Sci U S A. 2014;111: 15256–60. 10.1073/pnas.1413135111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menaker M. Circadian organization in the real world. Proc Natl Acad Sci U S A. 2006;103: 3015–6. 10.1073/pnas.0600360103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aschoff J. Circadian rhythms in Man. Science. 1965;148: 1427–1432. 10.1126/science.148.3676.1427 [DOI] [PubMed] [Google Scholar]