Abstract
Ischemia/reperfusion (I/R)-induced inflammatory reaction is one of the most important elements in myocardial I/R injury. In addition, autophagy serves an important role in normal cardiac homeostasis, and obstructions to the autophagy process lead to severe consequences for the heart. Hydrogen exerts an effective therapeutic role in numerous diseases associated with I/R injury via its anti-inflammation, anti-apoptosis and anti-oxidative properties. Therefore, the present study investigated the effect of hydrogen on the myocardial inflammation response and apoptosis in myocardial ischemic/reperfusion (MI/R) injury, and further explored the mechanism of PTEN-induced kinase 1 (PINK1)/Parkin-induced mitophagy in the protection of hydrogen on MI/R injury. MI/R injury was performed by surgical ligation of the left coronary artery in vivo and H9C2 cell injury was performed by hypoxia/reoxygenation (H/R) in vitro. Hydrogen-rich saline was administered twice through intraperitoneal injection at a daily dose of 10 ml/kg following the operation in the in vivo model, and hydrogen-rich medium culture was used for cells instead of normal medium in vitro. The infarction size of hearts, the levels of creati-nine kinase-muscle/brain (CK-MB) and cardiac troponin I (cTnI), cardiac function, cell viability and lactate dehydrogenase (LDH) release, levels of cytokines, apoptosis and the expression of autophagy-associated proteins were detected in the different treatment groups in vivo and in vitro. The results demonstrated that treatment with hydrogen improved the myocardial infarction size of hearts, cardiac function, apoptosis and cytokine release following MI/R in rats. In vitro, hydrogen improved cell viability and LDH release following hypoxia/reoxygenation in myocardial cells. In addition, it was demonstrated that hydrogen exerted an anti-inflammatory and anti-apoptotic effect in myocardial cells induced by H/R via PINK1/Parkin mediated autophagy. These results suggested that hydrogen-rich saline alleviated the inflammation response and apoptosis induced by MI/R or H/R in vivo or in vitro, and that hydrogen-rich saline contributed to the increased expression of proteins associated with autophagy. In summary, the present study indicated that treatment with hydrogen-rich saline improved the inflammatory response and apoptosis in MI/R via PINK1/Parkin-mediated mitophagy.
Keywords: myocardial ischemia/reperfusion injury, hydrogen, autophagy, inflammation, apoptosis
Introduction
Myocardial infarction is a major public health concern, and is one of the primary causes of mortality worldwide (1). Thrombolysis, primary angioplasty, and cardiac surgery are all effective therapeutic methods to restore cardiac blood flow to the ischemic myocardium (2), which is aimed at promptly resuming blood supply to the ischemic myocardium and these methods are frequently used in clinical practice. However, reperfusion paradoxically results in insufficient protection of the myocardium and various disorders, including inflammation, apoptosis, oxidative stress injury and calcium overload. These pathological mechanisms underlie the myocardial ischemic/reperfusion (MI/R) injury (3,4), and severely influence surgical treatment and postoperative long-term recovery. Previous studies have demonstrated that hydrogen (H2) exerts an effective therapeutic role in a variety of I/R injury diseases, including MI/R (5,6), intestinal I/R injury (7), renal I/R injury (8), hepatic I/R injury (9) and retinal I/R injury (10). Hydrogen has been demonstrated to exhibit anti-inflammation, anti-apoptosis and anti-oxidative properties (5-9,11-13). Numerous studies have reported that hydrogen exerts a protective role in myocardial related diseases (6,11,14).
I/R induced-inflammatory reaction is one of the most important elements in myocardial I/R (MI/R) injury (15). The main pathological process consists of the release of inflammatory cytokines and the aggregation and infiltration of inflammatory cells upon the inflammatory response (16). During the process of inflammation, inflammatory cells are stimulated and various cytokines are released, including tumor necrosis factor α (TNF-α), interleukin (IL)-6 and IL-8 (17). In addition, myocardial apoptosis is also associated with MI/R injury (12). Myocardial apoptosis is considered to be one of the key pathological processes in MI/R injury, and may be associated with heart failure, the amount of which is determined by the severity of MI/R injury (18). During MI/R injury, myocardial apoptosis leads to myocardial contractile dysfunction, compensatory hypertrophy and reparative fibrosis, further increasing myocardial injury, which ultimately develops into cardiac dysfunction and failure (19).
Autophagy contributes to normal cardiac homeostasis, and impeding the autophagy process can lead to severe consequences for the heart, such as the accumulation of protein aggregates and dysfunctional organelles, generating cellular dysfunction and cardiac failure (20). Nakai et al (20) reported that constitutive autophagy is a homeostatic mechanism for maintaining cardiomyocyte size and normal cardiac structure and function. Of note, mitophagy was activated and served a crucial role in cardioprotection in different models of cardiac injury (21,22). The PTEN-induced kinase 1 (PINK1)/Parkin pathway exerts pivotal functions in the clearing of defective mitochondria via autophagy in cells (23). It has also been reported that Parkin functions as a regulator, and activates mitochondrial autophagy for mitochondrial degradation in cardiac myocytes (24). A recent study suggested that PINK1/Parkin-induced mitophagy regulated mitochondrial dynamics and function in myocytes (25). The absence of Parkin resulted in accumulated dysfunctional mitochondria in the myocardium with age, which led to oxidative damage and mitochondrial respiration dysfunction (26,27).
Consequently, it was hypothesized that PINK1/Parkin-mediated autophagy participated in the myocardial inflammation response and apoptosis in MI/R injury, and that hydrogen may alleviate the MI/R injury via PINK1/Parkin-mediated autophagy. To examine this hypothesis, the present study investigated the effect of hydrogen on myocardial inflammation response and apoptosis in MI/R injury in vivo and in vitro. In addition, the role of PINK1/Parkin-induced mitophagy was explored in the hydrogen-mediated myocardial protection.
Materials and methods
Animals
A total of 72 Wistar male adult rats (age, 8-10 weeks; weight, 200-250 g) were obtained from the Laboratory Animal Center of the Academy of Military Medical Sciences (Beijing, China). Rats were acclimated for 1 week prior to experiments. The rats were housed at a temperature-controlled (25°C) room under a 12-h light/dark cycle to mimic the normal physiological day-night cycle. Standard chow and water were freely available to rats following sterilization. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University and were conducted in strict accordance with the National Institutes of Health guidelines for the use of experimental animals.
Rat MI/R model
The MI/R injury model was induced according to previous research with some minor modifications (28). The rats were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneally) and the thoracic cavity was opened by left thoracotomy. After the pericardium was incised to expose the left anterior descending coronary artery (LAD), a 6-0 ligature was passed underneath the LAD and was tied to produce an occlusion. Thereafter, the artery was occluded by applying tension to the ligature. A successful ischemia was confirmed by persistent ST segment elevation on the electrocardiogram. After 30 min of ischemia, the ligature was removed. Reperfusion was confirmed by visible restoration of color in the ischemic tissue and inversion of the T wave on the electrocardiogram. The chest and skin were closed, and the rats were resuscitated by appropriate fluid replacement and placed on a 37°C heating pad until they were awake. The sham operation included all procedures except ligation of the LAD.
Cell culture and hypoxia/reoxygenation (H/R) injury
H9C2 myocardial cells were maintained in our laboratory and obtained from the American Type Culture Collection. Cells were grown in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% heat-inactivated FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin, and cultured at 37°C with 5% CO2 in a humidified atmosphere. The medium was replaced every 2-3 days. Confluent cells were used for subsequent experiments at 80-90% confluence, between the 4 and 6th passages. The cells were seeded at a density of 1×106 cells/ml.
To mimic ischemic injury in vitro, the process of H/R injury was performed as previously published (29). Cells were cultured in serum-free DMEM (glucose-free) in a humidified environment with 95% N2 and 5% CO2 for hypoxic conditions. Following a 4-h incubation, cells were transferred to normal culture conditions with routine culture medium in a normal oxygen environment for 24 h for reoxygenation.
Hydrogen treatment for rats in vivo
According to a previous study (6), hydrogen was dissolved in 0.9% saline for 6 h under high pressure (0.4 MPa) to a supersaturated level by using a hydrogen producing apparatus. Hydrogen-rich saline was stored under an atmospheric pressure at 4°C in an aluminum bag with no dead volume, sterilized by gamma radiation, which was freshly prepared once a week to ensure that the concentration was maintained at 0.6 mmol/l. A needle-type hydrogen sensor (Unisense A/S) was used to detect the hydrogen concentration of the media, according to the protocol described in our previous study (13). The hydrogen-rich saline was administered via intraperitoneal injection at a dose of 10 ml/kg and at 5 min prior to reperfusion, as described in a previous study (30).
Hydrogen treatment for cells in vitro
According to our previously study (13), hydrogen was diluted in normal medium (or as indicated) to prepare 0.6 mmol/l hydrogen-rich culture medium, using a hydrogen producing apparatus in our department, following the same protocols as for the preparation of hydrogen-rich saline. To ensure a 0.6 mmol/l concentration, hydrogen-rich medium was freshly prepared each week. In the H2 group, hydrogen-rich medium was used to culture the cells instead of normal medium.
PINK1 small interfering (si) RNA transfection
The PINK1-targeting siRNA and the scramble siRNA were designed and synthesized by Santa Cruz Biotechnology, Inc. Transfection was performed according to the manufacturer's protocol. The sequences were: Scramble siRNA, 5′-UUC UCC GAA CGU GUC ACG UTT-3′; and PINK1 siRNA, 5-GCC AUC UUG AAC ACA AUG ATT-3. H9C2 cells were seeded in 6-well plates overnight, and then transfected with PINK1 siRNA (50 nM) and scramble siRNA (50 nM) using siRNA Lipofectamine™ 2000 transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Experiment 1: Effect of hydrogen on infarction size of hearts and cardiac function in rats induced by MI/R
A total of 24 rats were randomly divided into four groups (n=6 rats per group): Control (Con), control + hydrogen (Con+H2), MI/R (I/R), and MI/R + hydrogen (I/R+H2) groups. MI/R injury was performed by surgical ligation of the left coronary artery. At 24 h following reperfusion, infarction size of hearts and cardiac function [maximum rate of increase of left ventricular pressure (±dp/dtmax), left ventricular ejection fraction (LVEF), left ventricular end-diastolic pressure (LVEDP), heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure (MAP)] were detected, and blood samples were collected for the detection of creatinine kinase-muscle/brain (CK-MB) and cardiac troponin I (cTnI) levels in the four groups.
Experiment 2: Effect of hydrogen on cytokines and autophagy in rats induced by MI/R
Another 24 rats were randomly divided into four groups (n=6 rats per group). The grouping method and experimental protocols were the same as experiment 1. At 24 h following reperfusion, heart tissues were collected for the detection of cytokine levels [TNF-α, IL-1β, IL-6 and high mobility group box 1 (HMGB1)] by ELISA. Apoptosis-related proteins (cleaved caspase-3, Bcl-2 and Bax) and autophagy-associated proteins [microtubule-associated protein 1 light chain 3α (LC3), autophagy-related protein (ATG) 5, ATG12, Beclin1, PINK1 and Parkin] were detected by western blotting.
Experiment 3: Effect of hydrogen on cell viability and lactate dehydrogenase (LDH) release in H9C2 cells stimulated by H/R in vitro
H9C2 cells were divided into four groups (n=6): Control (Con), control + hydrogen (Con+H2), H/R treatment (H/R), and H/R + hydrogen (H/R+H2) groups. H9C2 cell injury was performed by hypoxia and reoxygenation, as aforementioned. The C+H2 and I/R+H2 groups were cultured using hydrogen-rich medium throughout both the hypoxia and the reoxygenation. At 4 h following hypoxia and 24 h of reoxygenation, cells were harvested to detect cell viability by the MTT assay and LDH activity.
Experiment 4: Effect of hydrogen on autophagy-related proteins in H9C2 cells stimulated by H/R in vitro
H9C2 cells were divided into four groups (n=6). The grouping method and experimental protocols were the same as described in experiment 3. At 4 h following hypoxia and 24 h of reoxygenation, cells were collected to detect the protein expression levels of LC3, ATG5, ATG12, Beclin1, PINK1 and Parkin by western blot analysis.
Experiment 5: Effect of autophagy on cytokine levels and apoptosis in H9C2 cells treated with hydrogen and H/R in vitro
H9C2 cells were divided into eight groups (n=6): Control (Con), control + hydrogen (Con+H2), H/R treatment (H/R), H/R + hydrogen (H/R+H2), H/R treatment + autophagy inducer rapamycin (H/R+Rap), H/R treatment + autophagy inhibitor 3-MA (H/R+3-MA), H/R treatment + hydrogen + autophagy inducer rapamycin (H/R+H2+Rap), and H/R treatment + hydrogen+ autophagy inhibitor 3-MA (H/R+H2+3-MA) groups. Rap (20 µM; Sigma-Aldrich; Merck KGaA) and 3-MA (1 mM; Sigma-Aldrich; Merck KGaA) were added to the medium 2 h prior to the experiment. Then, hydrogen, 3-MA and/or Rap were administered throughout the hypoxia and the reoxygenation phases. At 4 h following hypoxia and 24 h of reoxygenation, the cells were collected to detect cytokine levels (TNF-α, IL-1β, IL-6 and HMGB1) by ELISA and expression levels of apoptosis-related proteins (cleaved caspase-3, Bcl-2, Bax) by western blotting.
Experiment 6: Effect of PINK silencing on cytokine levels and apoptosis in H9C2 cells treated with hydrogen and H/R in vitro
H9C2 cells were divided into eight groups (n=6): Control (C), control + hydrogen (C+H2), H/R treatment (H/R), H/R treatment + hydrogen (H/R+H2), H/R treatment + scramble siRNA (H/R+scramble siRNA), H/R treatment + hydrogen + scramble siRNA (H/R+H2+scramble siRNA), H/R treatment + PINK siRNA (H/R+PINK1 siRNA), and H/R treatment + hydrogen + PINK siRNA (H/R+H2+PINK1 siRNA) groups. At 4 h following hypoxia and 24 h reoxygenation, cells were collected to detect cytokine levels (TNF-α, IL-1β, IL-6 and HMGB1) by ELISA and expression levels of apoptosis-related proteins (cleaved caspase-3, Bcl-2 and Bax) by western blotting.
Detection of cardiac function and hemodynamic change
Following 1 h reperfusion, a fluid-filled latex balloon connected to a Millar transducer (pressure sensor) was inserted into the left ventricle as described previously (14), and ±dp/dtmax, LVEF, LVEDP, HR, SBP, DBP and MAP were measured.
Determination of myocardial infarct size
The hearts were rapidly excised and frozen at −20°C. The left ventricles were cut into 5 transverse slices, which were incubated in 2% triphenyltetrazolium chloride (TTC) solution in phosphate buffer (pH 7.4, at 37°C) in the dark for 20 min. The slices were subsequently photographed and measured to delineate the area of infarct size (IS; TTC-negative) and area at risk (AAR; TTC-stained). The images were captured using a light microscope (magnification, ×4; Leica Microsystems GmbH). Myocardial infarct sizes (IS/AARx100%) were calculated by Image-Pro Plus software (Media Cybernetics, Inc.).
Detection of apoptosis by TUNEL
After the experiments were performed, the heart tissue was collected to detect apoptosis by using an in situ Cell Death Detection kit (Roche Diagnostics), according to the manufacturer's protocol. The heart tissue was perfused with 10% formalin and placed in 10% formalin for 24 h in room temperature, embedded in paraffin, and sectioned into 5-µm thickness slices. Paraffin sections of heart tissue were stained with the TUNEL kit, which results in the nuclei of apoptotic cells to be stained red. Total DNA in all cell nuclei was counterstained blue with DAPI. The apoptosis rate was calculated as the ratio of red to blue stained nuclei and relative to the control group.
Detection of CK-MB and cTnI release in serum
After the measurement of hemodynamics and cardiac function parameters, blood samples were collected from the heart. The serum was separated by centrifugation at 3,000 × g for 15 min at 4°C, aliquoted, and stored at −20°C until subsequent experimentation. The levels of CK-MB and cTnI were detected using commercially available ELISA kits on a microplate reader (cat. no. AKC0305 for CK-MA; cat. no. MA1-20112. for cTnI; Thermo Fisher Scientific, Inc.). All procedures were performed in accordance with the manufacturer's protocol.
Cytokine detection by ELISA
Following 1 h of reperfusion or 24 h of reoxygenation, heart tissues and culture media were collected for the detection of cytokine levels by ELISA. Heart tissues were homogenized and centrifuged at 10,000 × g for 20 min at 4°C, and the cell culture media was collected and centrifuged at 3,000 × g for 10 min at 4°C. The supernatants were harvested and stored at −20°C until detection of the cytokines TNF-α (cat. no. RTA00; R&D Systems, Inc.), IL-1β (cat. no. RLB00; R&D Systems, Inc.), IL-6 (cat. no. R6000B; R&D Systems, Inc.) and HMGB1 (cat. no. ST51011; IBL International GmbH) by ELISA, according to the manufacturer's protocol.
Analysis of cell viability and LDH activity
H9C2 cells were seeded in a 96-well plate for at least 12 h at a density of 1×104 cells/well and then were subjected to the different treatments. Cell viability was determined by the MTT assay, and cytotoxicity was detected by an LDH assay. Briefly, after 24 h of reoxygenation, MTT (5 mg/ml) was added to the medium supplemented with 10% FBS for 4 h at 37°C, then the medium was discarded, and formazan blue was dissolved in 100 µl of DMSO. The absorbance was detected at 490 nm using a microplate reader. Relative cell viability was quantified relative to the control group. The cells in the control group were considered 100% viable.
Cardiomyocyte injury was measured by LDH release. At 24 h following reoxygenation, the supernatant was harvested to detect LDH activity, according to the manufacturer's protocol (cat. no. 04744926001; Roche Diagnostics GmbH). LDH activity was expressed as a percentage relative to the control cell cultures.
Western blot analysis
Following 1 h of reperfusion or 24 h of reoxygenation, heart tissue and H9C2 cells were collected for the detection of caspase-3, Bcl-2, Bax, ATG5, ATG12, Beclin1, PINK1 and Parkin by western blotting. Heart tissue or H9C2 cells were resuspended in radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology) on ice for 30 min. The samples were centrifuged at 15,000 × g for 20 min at 4°C, and the supernatants were collected and boiled for 5 min. Protein concentrations were quantified using the Bradford protein assay (Thermo Fisher Scientific, Inc.). Denatured proteins (15 µg) were separated by 12% SDS-PAGE and then electrotransferred onto polyvinylidene difluoride membranes (EMD Millipore). Following blocking with 5% non-fat milk blocking buffer for 1 h at room temperature, the membranes were incubated with primary antibodies (all from Abcam) against cleaved caspase-3 (cat. no. ab13847; 1:500), Bcl-2 (cat. no. ab196495; 1:1,000), Bax (cat. no. ab32503; 1:2,000), LC3 (cat. no. ab48394; 1:1,000), ATG5 (cat. no. ab108327; 1:2,000), ATG12 (cat. no. ab155589; 1:1,000), Beclin1 (cat. no. ab62557; 1:1,000), PINK1 (cat. no. ab23707; 1:500) and Parkin (cat. no. ab77924; 1:500) and β-actin (cat. no. ab8227; 1:1,000) overnight at 4°C. The membranes were subsequently washed with PBS three times and incubated with secondary antibodies (cat. nos. ab6721 and ab6728; 1:5,000; Abcam) at room temperature for 1 h. The labeled bands were visualized with an enhanced chemiluminescence reagent using the Bio-Rad Gel Doc 2000 system (Bio-Rad Laboratories, Inc.), and quantified with the QuantityOne software (v4.6; Bio-Rad Laboratories, Inc.). Protein expressions were normalized to β-actin.
Statistical analysis
All data are presented as the mean ± standard error of the mean. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc.). Differences between groups were evaluated by one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Hydrogen alleviates myocardial infarction size of hearts
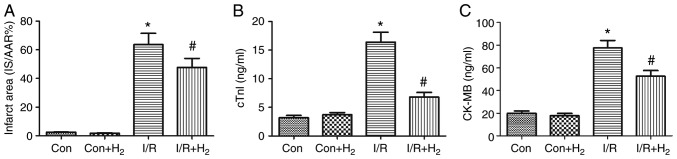
MI/R leads to myocardial cell injury (31). To investigate the effect of hydrogen on myocardial injury, TTC staining was performed to analyze the infarct area, and cTnI and CKMB levels were detected in the hearts of MI/R-treated rats. Compared with the control group, myocardial infarct, cTnI and CKMB were significantly elevated in the I/R group (P<0.05; Fig. 1A-C). Compared with the I/R group, these indicators of myocardial injury were significantly attenuated in the IR+H2 group (P<0.05; Fig. 1A-C). These results indicated that hydrogen alleviated myocardial injury induced by I/R.
Figure 1.
Hydrogen alleviates myocardial infarction size and cTnI and CK-MB in rats induced by myocardial I/R. Myocardial injury was performed by 30 min of ischemia and 24 h of reperfusion. Following ischemia, rats were treated by intraperitoneal injection of H2 saline at 5 min prior to left anterior descending coronary artery reperfusion. Heart tissue and blood samples were collected for detection of (A) infarct area, (B) cTnI and (C) CK-MB. Values are presented as mean ± standard error of the mean (n=6 rats per group). *P<0.05 vs. control group; and #P<0.05 vs. I/R group. cTnI, cardiac troponin I; CK-MB, creatinine kinase-muscle/brain; I/R, ischemia/reperfusion; Con, control.
Effect of hydrogen-rich saline on cardiac function
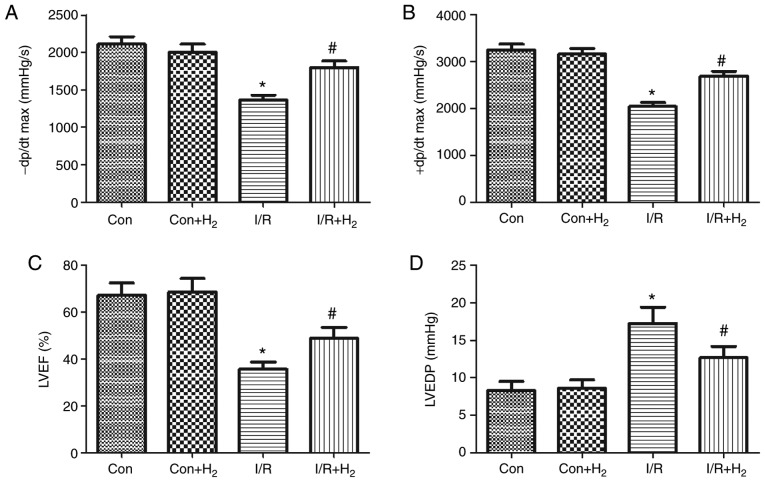
To investigate the effect of hydrogen on cardiac function, the present study measured cardiac function and hemodynamic indexes in MI/R-treated rats, namely ±dp/dtmax, LVEF, LVEDP, HR, SBP, DBP and MAP. Compared with the control group, ±dp/dtmax and LVEF at the end of the reperfusion period were significantly decreased, and LVEDP was increased, in the I/R group (P<0.05; Fig. 2A-D). Treatment with hydrogen significantly increased the value of ±dp/dtmax and LVEF, and decreased LVEDP at the end of the reperfusion period in the I/R+H2 group (P<0.05; Fig. 2A-D).
Figure 2.
Hydrogen improves cardiac dysfunction in rats induced by myocardial I/R. Myocardial injury and hydrogen treatment was performed as outlined in Fig. 1. (A) -dp/dtmax, (B) +dp/dtmax, (C) LVEF and (D) LVEDP were measured. Values are presented as mean ± standard error of the mean (n=6 rats per group). *P<0.05 vs. control group; and #P<0.05 vs. I/R group. I/R, ischemia/reperfusion; -dp/dtmax, maximal rate of the decrease of left ventricular pressure; +dp/dtmax, maximal rate of the increase of left ventricular pressure; LVEF, left ventricular ejection fraction; LVEDP, left ventricular ejection fraction; Con, control.
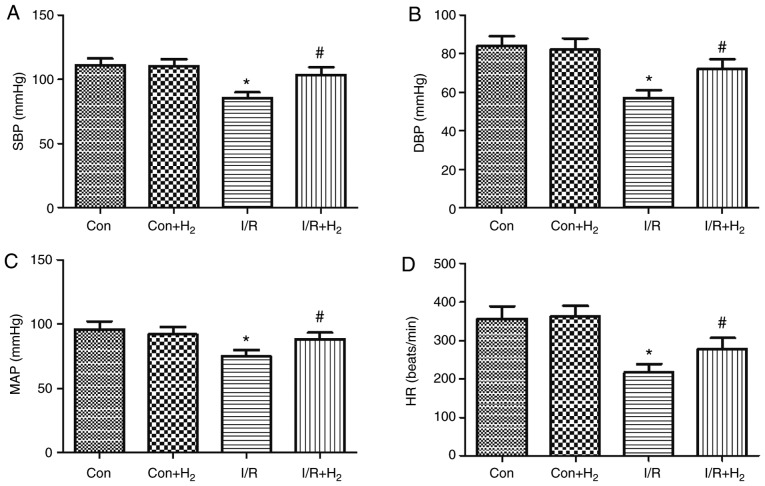
MI/R induced deterioration of the hemodynamic indexes (HR, SBP, DBP and MAP) in the I/R group compared with the control group (P<0.05; Fig. 3A-D). Compared with the I/R group, hydrogen-rich saline administration improved the absolute values of HR, SBP, DBP and MAP in the I/R+H2 group (P<0.05; Fig. 3A-D). Taken together, these results indicated that hydrogen-rich saline contributed to the recovery of cardiac function and hemodynamic change and improved the tolerance of hearts against I/R injury.
Figure 3.
Hydrogen improves cardiac dysfunction in rats induced by myocardial I/R. Myocardial injury and hydrogen treatment was performed as outlined in Fig. 1. (A) SBP, (B) DBP, (C) MAP and (D) HR were measured. Values are presented as the mean ± standard error of the mean (n=6 rats per group). *P<0.05 vs. control group; and #P<0.05 vs. I/R group. I/R, ischemia/reperfusion; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate; Con, control.
Hydrogen attenuates the expression of cytokines and apoptosis-related proteins following MI/R in rats
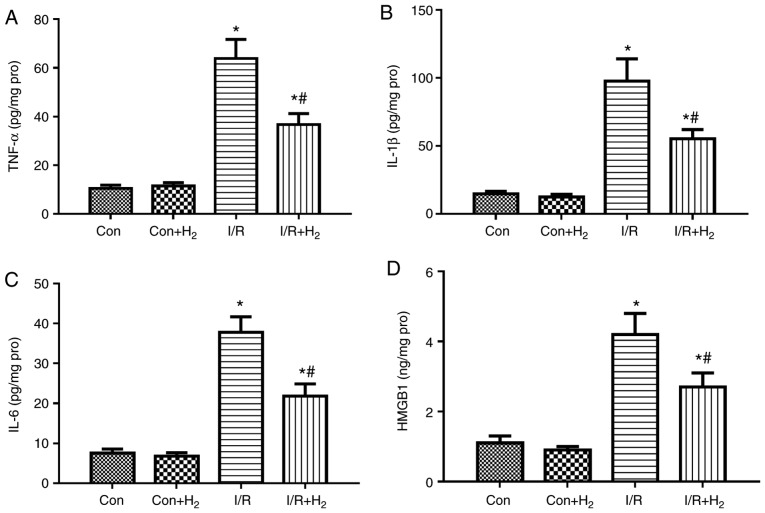
Inflammatory reaction is an integral part of the immune response to myocardial I/R injury (31). Markers of inflammation, such as TNF-α and IL-6, are usually present at undetected levels in the normal heart; however, they are upregulated under stressful conditions, such as MI/R injury (32). To examine the effect of hydrogen on cytokine production in MI/R rats, the present study detected the levels of TNF-α, IL-1β, IL-6 and HMGB1 in the hearts of the rats at the end of reperfusion. The results demonstrated that the release of TNF-α, IL-1β IL-6 and HMGB1 was significantly increased in the I/R group compared with the control group, while treatment with hydrogen-rich saline significantly downregulated the levels of the inflammatory markers in the I/R+H2 group (P<0.05; Fig. 4A-D).
Figure 4.
Effect of hydrogen on cytokine levels after myocardial injury induced by I/R. Myocardial injury and hydrogen treatment was performed as outlined in Fig. 1. Heart tissues were collected to detect the levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) HMGB1 by ELISA. Values are presented as the mean ± standard error of the mean (n=6 rats per group). *P<0.05 vs. control group; and #P<0.05 vs. I/R group. I/R, ischemia/reperfusion; TNF, tumor necrosis factor; IL, interleukin; HMGB1, high mobility group box 1; Con, control.
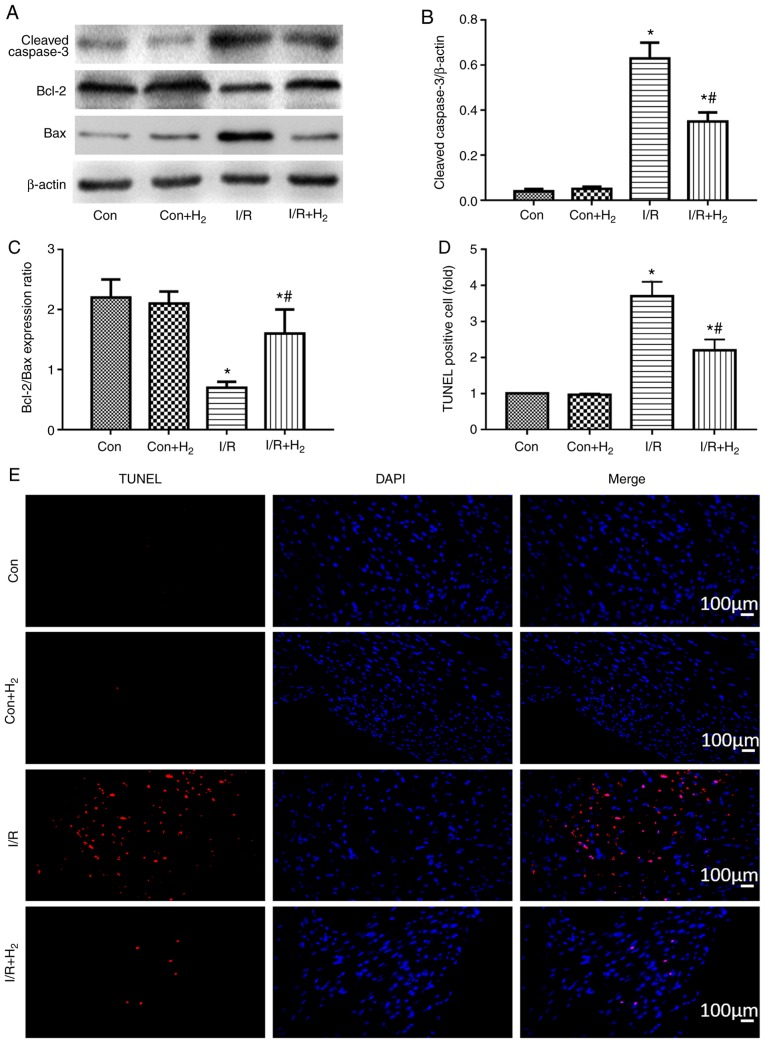
The present study then investigated whether hydrogen regulated myocardial apoptosis induced by I/R injury, by detecting the expression levels of the cleaved caspase-3, Bcl-2 and Bax proteins in myocardial rat tissues using western blotting. Caspase-3 and Bax are proapoptotic proteins, while Bcl-2 is anti-apoptotic (33). Caspase-3 levels were higher and Bcl-2/bax ratio was lower in the I/R group, compared with the control group (P<0.05; Fig. 5A-C). Compared with the I/R group, caspase-3 expression was reduced, while the Bcl-2/bax ratio was increased, in the I/R+H2 group (P<0.05; Fig. 5A-C). Apoptosis rates were further confirmed in the myocardial tissues by TUNEL staining. I/R treatment increased the number of apoptotic cells in the heart sections compared with the control group, while administration of hydrogen-rich saline markedly reversed this I/R-induced effect (Fig. 5D and E). These results indicated that hydrogen administration improved myocardial cell apoptosis following reperfusion.
Figure 5.
Effect of hydrogen on apoptosis after myocardial injury induced by I/R. Myocardial injury and hydrogen treatment was performed as outlined in Fig. 1. Heart tissues were collected to detect the expression of apoptosis-related proteins and apoptosis rates by TUNEL staining. (A) Representative images from western blot analysis. (B) Quantification of cleaved caspase-3 levels. (C) Ratio of Bcl-2/Bax expression. (D) Quantification of TUNEL staining and (E) representative images. Values are presented as the mean ± standard error of the mean (n=6 rats per group). *P<0.05 vs. control group; #P<0.05 vs. I/R group. I/R, ischemia/reperfusion; Con, control.
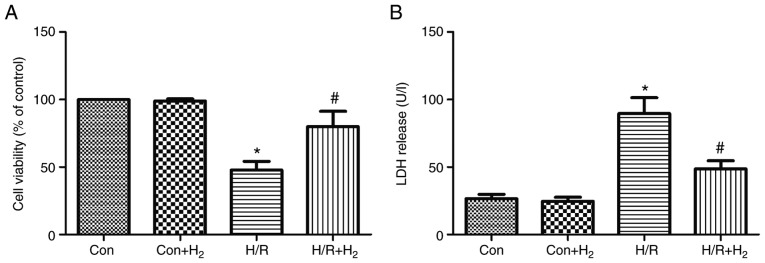
Effect of hydrogen on cell viability and LDH release following hypoxia and reoxygenation in myocardial cells
The present study used the MTT assay to assess the effect of hydrogen on cell viability following H/R in myocardial cells. The results revealed that cell viability was reduced following H/R in myocardial cells compared with the control group (P<0.05; Fig. 6A); however, hydrogen significantly increased the cell viability in the H/R+H2 group compared with the H/R group (P<0.05; Fig. 6A). The present study also investigated the cell toxicity by measuring the release of LDH. H/R induced LDH increase in the H/R group compared with the control group (P<0.05; Fig. 6B). LDH release was significantly decreased in the H/R+H2 group compared with the H/R group (P<0.05; Fig. 6B).
Figure 6.
Effect of hydrogen on cell viability and cell injury induced by H/R in H9C2 cells. Myocardial injury of H9C2 cells in vitro was induced by hypoxia followed by reoxygenation. Following a 4 h hypoxia, normal medium was replaced with hydrogen-rich medium in the Con+H2 and H/R +H2 groups. At 24 h following reoxygenation, the cells and medium were harvested to detect (A) cell viability by MTT assay and (B) LDH release in the four groups. Values are presented as the mean ± standard error of the mean (n=6). *P<0.05 vs. control group; and #P<0.05 vs. H/R group. H/R, hypoxia/reoxygenation; Con, control; LDH, lactate dehydrogenase.
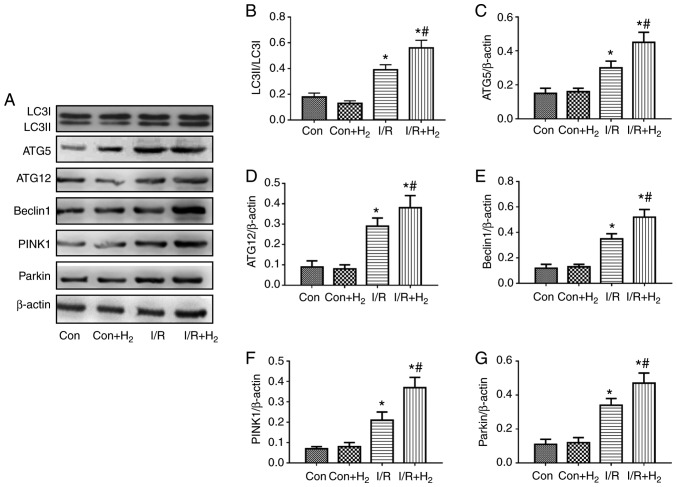
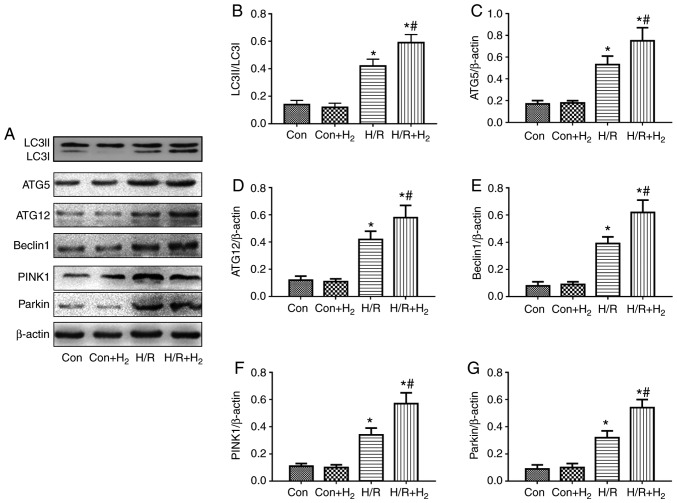
Expression of autophagy-related proteins following treatment with hydrogen in injury induced by I/R and H/R in vivo and in vitro
To examine the effects in autophagy in myocardial cells, the present study measured the protein expression levels of ATG5, ATG12 and Beclin1 by western blotting in injured myocardial cells induced by I/R in vivo and H/R in vitro. The results demonstrated that the ratio of LC3II/LC3I, ATG5, ATG12 and Beclin1 expression levels were increased in the I/R group compared with the control group, while hydrogen further augmented these effects in the I/R+H2 group when compared with the I/R group (P<0.05; Fig. 7A-E). The results from the in vitro experiments were consisted with the in vivo protein expression; H/R induced an increase in the ratio of LC3II/LC3I, and in ATG5, ATG12 and Beclin1 protein expression compared with the control group, and hydrogen further increased these effects (Fig. 8A-E).
Figure 7.
Effect of hydrogen on autophagy-associated protein expression following myocardial injury induced by I/R in vivo. Myocardial injury and hydrogen treatment were performed as outlined in Fig. 1. Heart tissues were collected to detect the protein expression levels of autophagy-associated proteins by western blotting. (A) Representative blot images. (B) Quantification of LC3, (C) ATG5, (D) ATG2, (E) Beclin1, (F) PINK1 and (G) Parkin levels. Values are presented as the mean ± standard error of the mean (n=6). *P<0.05 vs. control group; and #P<0.05 vs. I/R group. I/R, ischemia/reperfusion; LC3, microtubule-associated protein 1 light chain 3α; ATG, autophagy-related protein; PINK1, PTEN-induced kinase 1; Con, control.
Figure 8.
Effect of hydrogen on autophagy-associated protein expression following cell injury induced by H/R in H9C2 cells. Myocardial injury in vitro was induced by hypoxia followed by reoxygenation. Following a 4 h hypoxia, normal medium was replaced with hydrogen-rich medium in the Con+H2 and H/R+H2 groups. At 24 h following reoxygenation, cells were harvested to detect the expression levels of autophagy-associated proteins by western blotting. (A) Representative blot images. (B) Quantification of LC3, (C) ATG5, (D) ATG2, (E) Beclin1, (F) PINK1 and (G) Parkin levels. Values are presented as the mean ± standard error of the mean (n=6). *P<0.05 vs. control group; and #P<0.05 vs. H/R group. H/R, hypoxia/reoxygenation; Con, control; LC3, microtu-bule-associated protein 1 light chain 3α; ATG, autophagy-related protein; PINK1, PTEN-induced kinase 1.
Mitophagy mediated by PINK1/Parkin exerts a crucial function in the degradation process of injured mitochondria (34). The results of the present study demonstrated that PINK1 and Parkin were increased in the I/R group in vivo compared with the control group, and treatment with hydrogen enhanced the expressions of PINK1 and Parkin in myocardial tissue in the I/R+H2 group (P<0.05; Fig. 7A,F and G). These results were also observed in vitro. Compared with the control group, H/R induced the expressions of PINK1 and Parkin in myocardial cells, while a further increase was observed following hydrogen treatment (P<0.05; Fig. 8A,F and G).
Hydrogen treatment improves cytokines release and apoptosis via autophagy in H/R-induced myocardial cell injury in vitro
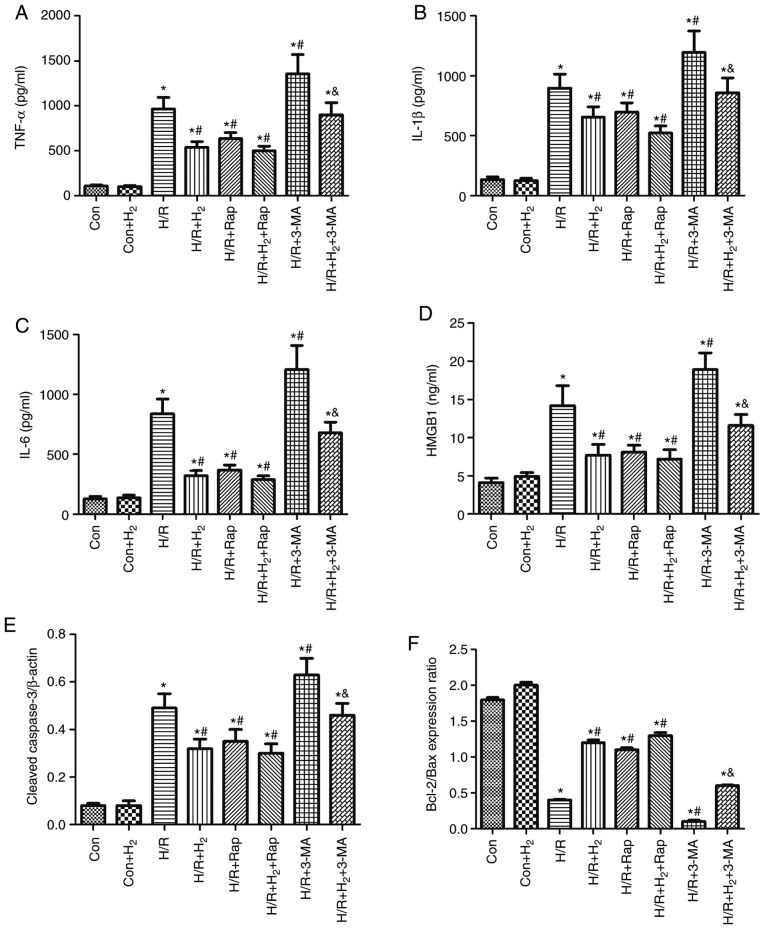
The aforementioned results demonstrated that hydrogen exerted an anti-inflammatory response via decreasing the level of cytokines and an anti-apoptotic effect via increasing the Bcl-2/bax ratio and reducing caspase-3 in rats with I/R injury. In addition, it was observed that treatment with hydrogen improved the expression of proteins associated with autophagy in I/R rats. In order to investigate whether hydrogen regulated inflammation and apoptosis via autophagy in myocardial cells, the present study investigated the effect of autophagy, using rapamycin or 3-MA to induce or inhibit the autophagy process, respectively.
The present results demonstrated that H/R induced the release of the cytokines TNF-α, IL-1β, IL-6 and HMGB1, and enhanced the expression of caspase-3 in H9C2 cells (P<0.05; Fig. 9A-E). In addition, the Bcl-2/Bax ratio was decreased in myocardial cells stimulated by H/R compared with the control group (P<0.05; Fig. 9F). Treatment with hydrogen alleviated the excessive release of the cytokines, increased the expression of caspase-3, and decreased the Bcl-2/Bax ratio in the H/R+H2 group, compared with the H/R group (P<0.05; Fig. 9A-F). Similarly, in comparison to the H/R group, there was a decrease in cytokine and caspase-3 expression levels, and an increase in the Bcl-2/Bax ratio, in the H/R+Rap and H/R+H2+Rap groups (P<0.05; Fig. 9A-F). Treatment with 3-MA partly reversed the inhibitory effect of hydrogen and Rap on cytokine expression, caspase-3 and promotion of the Bcl-2/Bax ratio in H/R myocardial cells (Fig. 9A-F).
Figure 9.
Effect of hydrogen on cytokine levels and apoptosis induced by H/R in H9C2 cells. Rap and 3-MA were added to the medium 2 h prior to the experiment. Cell injury and hydrogen treatment were described in Fig. 6. At 24 h following reoxygenation, cells were harvested to detect the levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) HMGB1 by ELISA. (E) The protein expression levels of cleaved caspase-3 and (F) the Bcl-2/Bax ratio were detected by western blotting. Values are presented as the mean ± standard error of the mean (n=6). *P<0.05 vs. control group; #P<0.05 vs. H/R group; and &P<0.05 vs. H/R+H2 group. H/R, hypoxia/reoxygenation; Rap, rapamycin; TNF, tumor necrosis factor; IL, interleukin; HMGB1, high mobility group box 1; Con, control.
Hydrogen exerts anti-inflammatory and anti-apoptotic effects in myocardial cells induced by H/R via PINK/Parkin-mediated autophagy
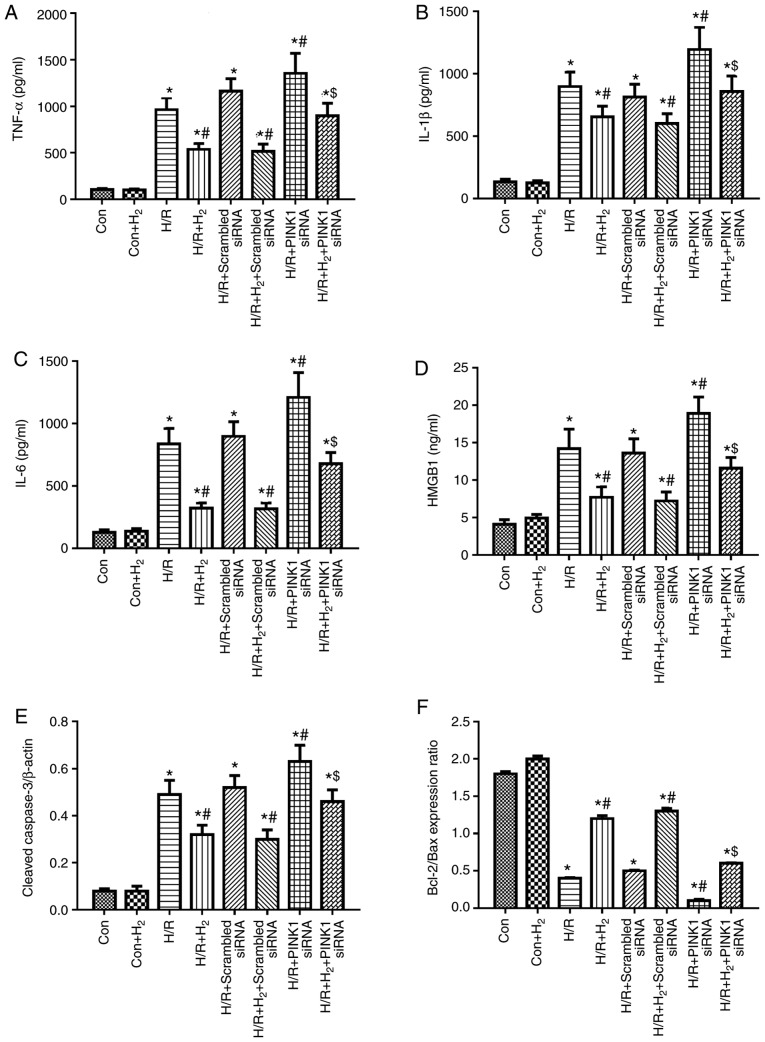
Autophagy, especially mitophagy, is a critical process of elimination of damaged organelles in the cells process during MI/R injury (35,36). PINK1/Parkin serve a key regulatory role in autophagy and mitophagy (24). Our previous research demonstrated that hydrogen could regulate PINK1 and Parkin in myocardial cells induced by H/R. The present study used a PINK1-specific siRNA to knock down its expression in myocardial cells, and successful knockdown was confirmed (Fig. S1). Compared with the H/R or H/R+H2 groups, there was not a significant difference in TNF-α, IL-1β, IL-6 and HMGB1, caspase-3, Bcl-2/Bax ratio in H/R+scramble siRNA group or H/R+H2+scramble siRNA group, respectively (P<0.05; Fig. 10A-F). However, compared with the H/R or H/R+H2 groups, levels of TNF-α, IL-1β, IL-6 and HMGB1 were increased, caspase-3 expression was enhanced and the Bcl-2/Bax ratio was decreased in the H/R+PINK1siRNA or HR+H2+PINK1siRNA groups, respectively (P<0.05; Fig. 10A-F). These results indicated that PINK silencing reversed the anti-inflammatory and anti-apoptotic effects of hydrogen in myocardial cells induced by H/R.
Figure 10.
Effect of hydrogen on cytokine levels and apoptosis induced by H/R in H9C2 cells. Prior to the experiment, scramble control siRNA and PINK1-specific siRNA were transfected into H9C2 cells. Myocardial injury and hydrogen treatment were described in Fig. 6. At 24 h following reoxygenation, cells were harvested to detect the levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) HMGB1 by ELISA. (E) The expression levels of cleaved caspase-3 and (F) the Bcl-2/Bax ratio were detected by western blotting. Values are presented as the mean ± standard error of the mean (n=6). *P<0.05 vs. control group; #P<0.05 vs. H/R group; and $P<0.05 vs. H/R+H2 group. H/R, hypoxia/reoxygenation; siRNA, small interfering RNA; PINK1, PTEN-induced kinase 1; TNF, tumor necrosis factor; IL, interleukin; HMGB1, high mobility group box 1; Con, control.
Discussion
Myocardial infarction is one of the main causes of mortality, and its incidence continues to increase worldwide, while the early restoration of coronary blood flow is conducive to attenuate myocardial tissue injury (1). However, reperfusion may lead to further myocardial injury, including inflammation response (37), apoptosis (38) and oxidative damage (39). Hydrogen has been observed to exert an anti-inflammatory, anti-apoptotic and anti-oxidative injury effect, and has a protective role in heart diseases (6). In the present study, the effect of hydrogen on MI/R injury was investigated, and the mechanism associated with I/R injury in myocardial cells in vivo and in vitro was discussed. The results demonstrated that hydrogen-rich saline attenuated the myocardial infarction size and improved cardiac dysfunction, and exerted anti-inflammatory and anti-apoptotic effects in the heart tissues in vivo. Treatment with hydrogen-rich media also reduced myocardial cell injury in vitro. In addition, myocardial cell injury contributed to the activation of autophagy, which was evidenced by an increase in the protein expression levels of LC3, ATG-5, ATG12, Beclin1, PINK1 and Parkin in vivo and in vitro; hydrogen could affect the expression of these proteins in vivo and in vitro, via the PINK1/Parkin pathway. These findings indicated that hydrogen regulated the process of autophagy and further alleviated the inflammation response and apoptosis in myocardial cell injury.
Therapy for myocardial ischemic disease depends on myocardial reperfusion treatment; however, this process not only has complex obstacles for effective treatment, but also induces cardiac dysfunction (40). For the recovery of cardiac function, myocardial enzyme release and infarct size measurement have been considered as endpoints for I/R injury evaluation (41). Nandi et al (42) reported that MI/R led to cardiac dysfunction and NaHS had a protective role. It has previously been reported that hydrogen exerts myocardial protection from heart diseases via inhibiting oxidative injury, apoptosis and cytokines release (6,11,43,44). In addition, hydrogen protected from MI/R injury via regulating the glycogen synthase kinase 3β (GSK3β) and autophagy signaling pathways (45,46). The present study predominantly focused on hydrogen treatment on MI/R injury and the mechanism of PINK1-mediated autophagy. The present results demonstrated that MI/R deteriorated the myocardial infarction size, and the markers ±dp/dtmax, LVEF and LVEDP and HR, SBP, DBP and MAP in MI/R injured rats. According to previously published research (47,48), 10 ml/kg hydrogen-rich saline was safe and had a positive therapeutic effect on MI/R injury. Similarly, the present study found that hydrogen improved myocardial infarction size, cardiac function and hemodynamic changes following reperfusion of cardiac blood flow. In addition, in the in vitro experiments, hydrogen ameliorated the LDH release and increased the cell viability in myocardial cell injury stimulated by H/R. These results were consistent with the finding that hydrogen-rich saline improved the hemodynamic parameters LVSP, +(dP/dt)max, and -(dP/dt)max and infarct size in MI/R-treated rats (11).
Apoptosis is a major cause of tissue damage, second only to reperfusion injury following ischemia (47). It has recently been reported that myocardial apoptosis is initiated by ischemia and amplified by reperfusion, which partially contributes to myocardial dysfunction and cardiomyocyte death and even heart failure (48,49). Caspase-3 exerts a pivotal role in the process of cell apoptosis, which is downstream of the Bcl-2 family (50). The Bcl-2 family includes the anti-apoptotic Bcl-2 and the pro-apoptotic Bax, and an increased Bcl-2/Bax ratio represents a protective effect of cells against apoptosis (50). In the process of apoptosis, Bcl-2 is capable of forming a heterodimer with Bax, thereby preventing Bax homodimerization and the sequential activation of caspase-3 (51). Numerous studies have demonstrated that MI/R or H/R induce myocardial apoptosis in in vivo and in vitro experiments (52,53). Sun et al (11) reported that I/R led to myocardial cell apoptosis by detection of caspase-3 and TUNEL and that hydrogen attenuated apoptosis in myocardial cells. This was corroborated by the findings of the present study. Treatment with hydrogen obviously inhibited apoptosis, by mitigating caspase-3 expression and alleviating the Bcl-2/bax ratio in MI/R-treated heart tissues, and hydrogen administration in vitro attenuated apoptosis in myocardial cell injury induced by H/R. Myocardial apoptosis triggers the inflammatory response and the release of excessive cytokines from the infarcted myocardium after acute myocardial infarction, consequently the secreted cytokine TNF-α further stimulates infiltrating leukocytes and endothelial cells to release proinflammatory cytokines, such as IL-1β and IL-6, which then initiates the inflammation cascade, inflammation response, and the subsequent myocardial dysfunction (54-57). Downregulation of HMGB1 was reported to partially reduce myocardial I/R injury (58). In the present study, I/R or H/R injury caused the excessive release of TNF-α, IL-1β, IL-6 and HMGB1 in myocardium tissues and cells, and treatment with hydrogen significantly inhibited these effects.
Numerous studies have reported that under conditions of cellular stress, autophagy is increased in the myocardium, which is initially a protective response activated by the cells (20,23,35). ATG5, ATG12, beclin1 and LC3 are key regulatory proteins of autophagy in cells (59). Decreased mitochondrial autophagy (termed mitophagy) can cause inflammation and cell death, which results in degenerative diseases (60). The PINK1/Parkin pathway is associated with marking dysfunctional mitochondria for clearance by autophagy (61). PINK1-deficiency increased the susceptibility of the heart to I/R injury ex vivo (62). PINK1-deficiency in mice contributed to heart failure more rapidly in response to pressure overload compared with wild-type mice (63). It has previously been reported that hydrogen-rich saline exerts a protective role in myocardial I/R injury via anti-oxidative, anti-apoptosis and anti-inflammation mechanisms, which inhibit endoplasmic reticulum stress, regulate Akt and GSK3β protein expression and the expressions of the autophagy-related proteins mammalian target of rapamycin (mTOR), Beclin1 and LC3, and mitochondrial-associated protein expression (30,44,64). Based on this previous literature, the present study focused on the mechanisms associated with autophagy, and then further examined the effect of PINK1/Parkin-mediated autophagy. The results of the present study indicated that MI/R or myocardial H/R induced mitophagy by increasing the expression levels of LC3, ATG5, ATG12, beclin1, PINK1 and Parkin in vitro and in vivo, which are markers of mitophagy activity in cells. To further investigate the effect PINK1/Parkin-mediated autophagy on the hydrogen-treated myocardial H/R injury, specific siRNAs were used to silence the expression PINK1 in myocardial cells. Compared with the H/R group, PINK1 silencing elevated the expression levels of the cytokines TNF-α, IL-1β, IL-6 and HMGB1, elevated caspase-3 expression, and decreased the Bcl-2/Bax ratio following H/R in myocardial cells. Therefore, PINK1 silencing partly reversed the protective effect of hydrogen on inflammation response and apoptosis in vitro.
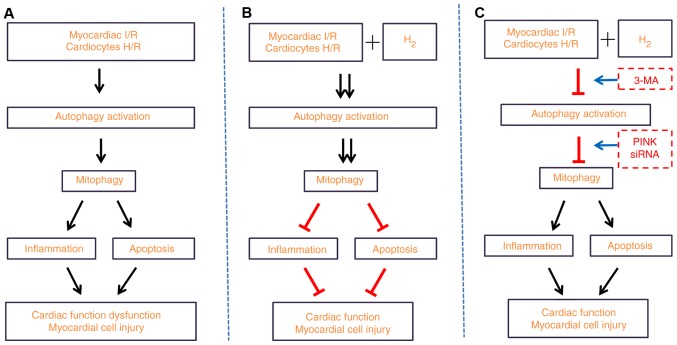
In summary, the results of the present study demonstrated that hydrogen-rich saline alleviated the inflammatory response and apoptosis induced by MI/R or H/R, and contributed to the increased expression of proteins associated with autophagy. These findings suggested that hydrogen-rich saline alleviated the inflammatory response and apoptosis via PINK1/Parkin-mediated mitophagy. The present study also elucidated the detailed mechanism by which hydrogen protected myocardial injury from the inflammation response and apoptosis (Fig. 11). Since the present study provides a novel hypothesis for the mechanisms of hydrogen therapy, further studies will be needed in the future to fully elucidate the underlying mechanisms of hydrogen treatment in disease.
Figure 11.
Hydrogen-rich saline alleviates the inflammation response and apoptosis via PINK1/Parkin-mediated mitophagy. (A) Myocardial I/R in vivo or H/R-induced injury in H9C2 cells in vitro led to autophagy and mitophagy activation, as evidenced by an increase in the expression of autophagy-associated proteins. In addition, the inflammation response and apoptosis were enhanced, thus resulting in deteriorated cardiac dysfunction. (B) Treatment with hydrogen ameliorated the aforementioned detrimental response. (C) Inhibition of autophagy by 3-MA treatment, or inhibition of mitophagy by PINK1 siRNA silencing, reversed the protection of hydrogen on cardiac function and myocardial cell injury. PINK1, PTEN-induced kinase 1; I/R, ischemia/reperfusion; H/R, hypoxia/reoxygenation; siRNA, small interfering RNA.
Supplementary Materials
Acknowledgments
Not applicable.
Funding
This study was supported by the National Nature Science Foundation of China (grant no. 81601667).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LY, KX designed the research and revised the manuscript. LY, HC and KX performed the experiments and drafted the manuscript. QW performed data analysis. LY and HC revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University and were conducted in strict accordance with the National Institutes of Health guidelines for the use of experimental animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Ding S, Yang Y, Mei J. Protective effects of L-malate against myocardial ischemia/reperfusion injury in rats. Evid Based Complement Alternat Med. 2016;2016:3803657. doi: 10.1155/2016/3803657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Wang Y, Ye J, Lu X, Cheng Y, Xiang L, Chen L, Feng W, Shi H, Yu X, et al. BFGF attenuates endoplasmic reticulum stress and mitochondrial injury on myocardial ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J Cell Mol Med. 2015;19:595–607. doi: 10.1111/jcmm.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan Z, Zhao Y, Yu H, Liu D, Xu H. Effect of hydrogen-rich saline on cardiomyocyte autophagy during myocardial ischemia-reperfusion in aged rats. Zhonghua Yi Xue Za Zhi. 2015;95:2022–2026. In Chinese. [PubMed] [Google Scholar]
- 6.Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148:91–95. doi: 10.1016/j.ijcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Shigeta T, Sakamoto S, Li XK, Cai S, Liu C, Kurokawa R, Nakazawa A, Kasahara M, Uemoto S. Luminal injection of hydrogen-rich solution attenuates intestinal ischemia-reperfusion injury in rats. Transplantation. 2015;99:500–507. doi: 10.1097/TP.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Hong Z, Liu H, Zhou J, Cui L, Yuan S, Chu X, Yu P. Hydrogen-rich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front Pharmacol. 2016;7:106. doi: 10.3389/fphar.2016.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimada S, Wakayama K, Fukai M, Shimamura T, Ishikawa T, Fukumori D, Shibata M, Yamashita K, Kimura T, Todo S, et al. Hydrogen gas ameliorates hepatic reperfusion injury after prolonged cold preservation in isolated perfused rat liver. Artif Organs. 2016;40:1128–1136. doi: 10.1111/aor.12710. [DOI] [PubMed] [Google Scholar]
- 10.Wang R, Wu J, Chen Z, Xia F, Sun Q, Liu L. Postconditioning with inhaled hydrogen promotes survival of retinal ganglion cells in a rat model of retinal ischemia/reperfusion injury. Brain Res. 2016;1632:82–90. doi: 10.1016/j.brainres.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang JH, Denoble PJ, Tao H, Sun X. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med (Maywood) 2009;234:1212–1219. doi: 10.3181/0812-RM-349. [DOI] [PubMed] [Google Scholar]
- 12.Cabigas EB, Somasuntharam I, Brown ME, Che PL, Pendergrass KD, Chiang B, Taylor WR, Davis ME. Over-expression of catalase in myeloid cells confers acute protection following myocardial infarction. Int J Mol Sci. 2014;15:9036–9050. doi: 10.3390/ijms15059036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Xie K, Han H, Li Y, Liu L, Yang T, Yu Y. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int Immunopharmacol. 2015;28:643–654. doi: 10.1016/j.intimp.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Qi L, Pan H, Li D, Fang F, Chen D, Sun H. Luteolin improves contractile function and attenuates apoptosis following ischemia-reperfusion in adult rat cardiomyocytes. Eur J Pharmacol. 2011;668:201–207. doi: 10.1016/j.ejphar.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X, Wang WL. Cholinergic anti-inflammatory pathway: A possible approach to protect against myocardial ischemia reperfusion injury. Chin Med J (Engl) 2010;123:2720–2726. [PubMed] [Google Scholar]
- 16.Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011;24:18–27. doi: 10.3109/08941939.2010.521232. [DOI] [PubMed] [Google Scholar]
- 17.Naidu BV, Farivar AS, Woolley SM, Grainger D, Verrier ED, Mulligan MS. Novel broad-spectrum chemokine inhibitor protects against lung ischemia-reperfusion injury. J Heart Lung Transplant. 2004;23:128–134. doi: 10.1016/S1053-2498(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 18.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Movassagh M, Foo RS. Simplified apoptotic cascades. Heart Fail Rev. 2008;13:111–119. doi: 10.1007/s10741-007-9070-x. [DOI] [PubMed] [Google Scholar]
- 20.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Liu B, Li T, Zhu Y, Luo G, Jiang Y, Tang F, Jian Z, Xiao Y. AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int J Mol Med. 2018;41:69–76. doi: 10.3892/ijmm.2017.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian X, Teng T, Zhao H, Qin J, Qiao Z, Sun Y, Liun Z, Xu Z. Zinc prevents mitochondrial superoxide generation by inducing mitophagy in the setting of hypoxia/reoxygenation in cardiac cells. Free Radic Res. 2018;52:80–91. doi: 10.1080/10715762.2017.1414949. [DOI] [PubMed] [Google Scholar]
- 23.Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Kubli DA, Cortez MQ, Moyzis AG, Najor RH, Lee Y, Gustafsson AB. PINK1 is dispensable for mitochondrial recruitment of parkin and activation of mitophagy in cardiac myocytes. PLoS One. 2015;10:e0130707. doi: 10.1371/journal.pone.0130707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorn GN., III Parkin-dependent mitophagy in the heart. J Mol Cell Cardiol. 2016;95:42–49. doi: 10.1016/j.yjmcc.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, Ikeda K, Ogata T, Matoba S. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. 2013;4:2308. doi: 10.1038/ncomms3308. [DOI] [PubMed] [Google Scholar]
- 27.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN, Gustafsson AB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Ma Z, Hu W, Wang D, Jiang S, Fan C, Di S, Liu D, Sun Y, Yi W. Caveolin-1/-3: Therapeutic targets for myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2016;111:45. doi: 10.1007/s00395-016-0561-6. [DOI] [PubMed] [Google Scholar]
- 29.Mao X, Wang T, Liu Y, Irwin MG, Ou JS, Liao XL, Gao X, Xu Y, Ng KF, Vanhoutte PM, Xia Z. N-acetylcysteine and allopurinol confer synergy in attenuating myocardial ischemia injury via restoring HIF-1alpha/HO-1 signaling in diabetic rats. PLoS One. 2013;8:e68949. doi: 10.1371/journal.pone.0068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Z, Zhao Y, Yu H, Liu D, Xu H. Effect of hydrogen-rich saline on cardiomyocyte autophagy during myocardial ischemia-reperfusion in aged rats. Zhonghua Yi Xue Za Zhi. 2015;95:2022–2026. In Chinese. [PubMed] [Google Scholar]
- 31.Rossello X, Lobo-Gonzalez M, Ibanez B. Pathophysiology and therapy of myocardial ischaemia/reperfusion syndrome. Eur Heart J Acute Cardiovasc Care. Jun 7, 2019. Epub ahead of print. [DOI] [PubMed]
- 32.Hinojar R, Foote L, Ucar EA, Dabir D, Schnackenburg B, Higgins DM, Schaeffter T, Nagel E, Puntmann V. Myocardial T2 mapping for improved detection of inflammatory myocardial involvement in acute and chronic myocarditis. J Cardiovasc Magn Reson. 2014;16(Suppl 1):O63. doi: 10.1186/1532-429X-16-S1-O63. [DOI] [Google Scholar]
- 33.Xu P, Cai X, Zhang W, Li Y, Qiu P, Lu D, He X. Flavonoids of Rosa roxburghii Tratt exhibit radioprotection and anti-apoptosis properties via the Bcl-2(Ca(2+))/caspase-3/PARP-1 pathway. Apoptosis. 2016;21:1125–1143. doi: 10.1007/s10495-016-1270-1. [DOI] [PubMed] [Google Scholar]
- 34.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyzis AG, Sadoshima J, Gustafsson AB. Mending a broken heart: The role of mitophagy in cardioprotection. Am J Physiol Heart Circ Physiol. 2015;308:H183–H192. doi: 10.1152/ajpheart.00708.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li T, Jiao YR, Wang LH, Zhou YH, Yao HC. Autophagy in myocardial ischemia reperfusion injury: Friend or foe? Int J Cardiol. 2017;239:10. doi: 10.1016/j.ijcard.2017.01.083. [DOI] [PubMed] [Google Scholar]
- 37.Toldo S, Marchetti C, Mauro AG, Chojnacki J, Mezzaroma E, Carbone S, Zhang S, Van Tassell B, Salloum FN, Abbate A. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. 2016;209:215–220. doi: 10.1016/j.ijcard.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 38.Yu H, Zhang H, Zhao W, Guo L, Li X, Li Y, Zhang X, Sun Y. Gypenoside protects against myocardial ischemia-reperfusion injury by inhibiting cardiomyocytes apoptosis via inhibition of chop pathway and activation of PI3K/Akt pathway in vivo and in vitro. Cell Physiol Biochem. 2016;39:123–136. doi: 10.1159/000445611. [DOI] [PubMed] [Google Scholar]
- 39.Ge M, Yao W, Yuan D, Zhou S, Chen X, Zhang Y, Li H, Xia Z, Hei Z. Brg1-mediated Nrf2/HO-1 pathway activation alleviates hepatic ischemia-reperfusion injury. Cell Death Dis. 2017;8:e2841. doi: 10.1038/cddis.2017.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma L, Liu H, Xie Z, Yang S, Xu W, Hou J, Yu B. Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-κB pathway: A mouse cardiomyocyte model. PLoS One. 2014;9:e103628. doi: 10.1371/journal.pone.0103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu J, Wang L, Akinyi M, Li Y, Duan Z, Zhu Y, Fan G. Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. Int J Clin Exp Med. 2015;8:14793–14804. [PMC free article] [PubMed] [Google Scholar]
- 42.Nandi S, Ravindran S, Kurian GA. Role of endogenous hydrogen sulfide in cardiac mitochondrial preservation during ischemia reperfusion injury. Biomed Pharmacother. 2018;97:271–279. doi: 10.1016/j.biopha.2017.10.118. [DOI] [PubMed] [Google Scholar]
- 43.Chi J, Li Z, Hong X, Zhao T, Bie Y, Zhang W, Yang J, Feng Z, Yu Z, Xu Q, et al. Inhalation of hydrogen attenuates progression of chronic heart failure via suppression of oxidative stress and P53 related to Apoptosis pathway in rats. Front Physiol. 2018;9:1026. doi: 10.3389/fphys.2018.01026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yue L, Li H, Zhao Y, Li J, Wang B. Effects of hydrogen-rich saline on Akt/GSK3beta signaling pathways and cardiac function during myocardial ischemia-reperfusion in rats. Zhonghua Yi Xue Za Zhi. 2015;95:1483–1487. In Chinese. [PubMed] [Google Scholar]
- 45.Pan Z, Zhao Y, Yu H, Liu D, Xu H. Effect of hydrogen-rich saline on cardiomyocyte autophagy during myocardial ischemia-reperfusion in aged rats. Zhonghua Yi Xue Za Zhi. 2015;95:2022–2026. In Chinese. [PubMed] [Google Scholar]
- 46.Yue L, Li H, Zhao Y, Li J, Wang B. Effects of hydrogen-rich saline on Akt/GSK3β signaling pathways and cardiac function during myocardial ischemia-reperfusion in rats. Zhonghua Yi Xue Za Zhi. 2015;95:1483–1487. In Chinese. [PubMed] [Google Scholar]
- 47.Guo J, Wang SB, Yuan TY, Wu YJ, Yan Y, Li L, Xu XN, Gong LL, Qin HL, Fang LH, Du GH. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis. 2013;231:384–391. doi: 10.1016/j.atherosclerosis.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.RES.79.5.949. [DOI] [PubMed] [Google Scholar]
- 49.Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, Gaudino M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: A review of evidence. Eur J Cardiothorac Surg. 2004;25:304–311. doi: 10.1016/j.ejcts.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Vukojevic K, Carev D, Sapunar D, Petrovic D, Saraga-Babic M. Developmental patterns of caspase-3, bax and bcl-2 proteins expression in the human spinal ganglia. J Mol Histol. 2008;39:339–349. doi: 10.1007/s10735-008-9171-4. [DOI] [PubMed] [Google Scholar]
- 51.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003;13:385–391. doi: 10.1038/sj.cr.7290184. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Geng HH, Xiao J, Qin XT, Wang F, Xing JH, Xia YF, Mao Y, Liang JW, Ji XP. MiR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1. Sci Rep. 2016;6:29082. doi: 10.1038/srep29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang YQ, Chang GL, Wang Y, Zhang DY, Cao L, Liu J. Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: Improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell Physiol Biochem. 2016;39:407–421. doi: 10.1159/000445634. [DOI] [PubMed] [Google Scholar]
- 54.Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Sun D, Huang J, Zhang Z, Gao H, Li J, Shen M, Cao F, Wang H. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS One. 2012;7:e33491. doi: 10.1371/journal.pone.0033491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn J, Kim J. Mechanisms and consequences of inflammatory signaling in the myocardium. Curr Hypertens Rep. 2012;14:510–516. doi: 10.1007/s11906-012-0309-0. [DOI] [PubMed] [Google Scholar]
- 57.Woldbaek PR, Tønnessen T, Henriksen UL, Florholmen G, Lunde PK, Lyberg T, Christensen G. Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse; a potential role in cardiac dysfunction. Cardiovasc Res. 2003;59:122–131. doi: 10.1016/S0008-6363(03)00339-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhang JJ, Peng K, Zhang J, Meng XW, Ji FH. Dexmedetomidine preconditioning may attenuate myocardial ischemia/reperfusion injury by down-regulating the HMGB1 TLR4-MyD88-NF-κB signaling pathway. PLoS One. 2017;12:e0172006. doi: 10.1371/journal.pone.0172006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao QH, Liu F, Yang ZL, Fu XH, Yang ZH, Liu Q, Wang L, Wan XB, Fan XJ. Prognostic value of autophagy related proteins ULK1, Beclin 1, ATG3, ATG5, ATG7, ATG9, ATG10, ATG12, LC3B and p62/SQSTM1 in gastric cancer. Am J Transl Res. 2016;8:3831–3847. [PMC free article] [PubMed] [Google Scholar]
- 60.de Vries RL, Przedborski S. Mitophagy and Parkinson's disease: Be eaten to stay healthy. Mol Cell Neurosci. 2013;55:37–43. doi: 10.1016/j.mcn.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1924–H931. doi: 10.1152/ajpheart.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci USA. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y, Tang Y, Suo C, Liu D, Li S, Li H. Effects of hydrogen-rich saline on endoplasmic reticulum stress during myocardial ischemia-reperfusion in rats. Zhonghua Yi Xue Za Zhi. 2014;94:3024–3028. In Chinese. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.