Abstract
Therapeutic agents used to treat sepsis-induced cardiac dysfunction are designed to suppress tumor necrosis factor (TNF)-α release and inhibit cell apoptosis. Exogenous administration of growth arrest-specific 6 (Gas6) exerts several biological and pharmacological effects; however, the role of Gas6 in sepsis-induced myocardial dysfunction remains unclear. In this study, H9C2 cardiomyocytes were stimulated with LPS (10 µg/ml) to mimic septic cardiac dysfunction and Gas6 (100 ng/ml) was applied exogenously. Subsequently, mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB activation, TNF-α expression, and apoptosis in the presence or absence of TP-0903 (15 nM) and Wortmannin (3 nM) were evaluated. The morphological alterations of H9C2 cells were visualized by phase-contrast microscopy. Cell viability was determined using the Cell Counting kit 8 assay and lactate dehydrogenase release, and TNF-α release was analyzed by ELISA analysis. Cell apoptosis was analyzed by flow cytometry and TUNEL assay. Nuclear morphological alterations were detected by Hoechst staining and caspase-3 activity was measured using biochemical methods. The expression levels of Bax and Bcl-2, and the phosphorylation and expression levels of Axl, Akt, IκB-α, p65, c-Jun N-terminal protein kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 were determined by western blotting. Furthermore, immunofluorescence analysis was performed to visualize translocation of NF-κB p65. The results demonstrated that Gas6 suppressed TNF-α release and inhibited cell apoptosis, and attenuated nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) activation via the Axl/PI3K/Akt pathway. Furthermore, the cardioprotective properties of Gas6 on the suppression of LPS-induced TNF-α release and apoptosis were abolished by treatment with TP-0903 (an Axl inhibitor) and Wortmannin (a PI3K inhibitor). Pretreatment with TP-0903 and Wortmannin abrogated the effects of Gas6 on phosphorylated-IκB-α, IκB-α, NF-κB, ERK1/2, JNK and p38 MAPK. These findings suggested that activation of Axl/PI3K/Akt signaling by Gas6 may inhibit LPS-induced TNF-α expression and apoptosis, as well as MAPK and NF-κB activation.
Keywords: apoptosis, Axl/PI3K/Akt, H9C2, lipopolysaccharide, growth arrest-specific 6, tumor necrosis factor-α
Introduction
Cardiac dysfunction is a major contributor to the significantly increased mortality rate in patients with sepsis compared with in septic patients without cardiac dysfunction (1,2). Previous studies have demonstrated that the mechanisms underlying sepsis-induced myocardial dysfunction include inflammatory mediators, structural alterations, dysfunctional cardiomyocyte contractility, reduced energy metabolism and cell death (3-5). However, the precise cause and molecular mechanism underlying the pathogenesis of septic cardiomyopathy are not completely understood. Lipopolysaccharide (LPS) from Gram-negative bacteria uses the Toll-like receptor 4 (TLR4) signaling pathway to mediate pro-inflammatory and pro-apoptotic activities and is recognized as the best characterized activator of sepsis; it has been reported that cardiomyocytes express TLR4 (6). Tumor necrosis factor (TNF)-α is an inflammatory cytokine present in the circulation that can be produced locally by cardiomyocytes (7). Evidence has indicated that modulation of TNF-α levels, either directly (8,9) or by downregulating TNF-α (10), has therapeutic significance in sepsis-induced myocardial dysfunction. Additionally, TNF-α, as a death ligand, is responsible for death receptor-induced apoptosis, which also contributes to sepsis-induced cardiac dysfunction. Previous studies have reported that endotoxin injection activates apoptotic and survival pathways in the hearts of rats. Apoptotic regulatory factors are activated in the hearts of rats treated with endotoxin, and caspase inhibition (11) or Bcl-2 overexpression (12) prevents myocardial dysfunction and cardiac apoptosis in rodent models of sepsis. It is well established that nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) serve critical roles in the inflammatory response and apoptosis regulation, and participate in the pathogenesis of sepsis-induced cardiac dysfunction.
The Tyro3, Axl and Mer (TAM) family comprises receptor tyrosine kinases, which initiate intracellular signaling cascades by binding to ligands, such as growth arrest-specific 6 (Gas6) and protein S (13). Among the TAM family, Axl is expressed ubiquitously and has the highest affinity to Gas6 (14,15). Numerous studies have reported that the Gas6/Axl pathway exerts anti-inflammatory and anti-apoptotic effects (16,17). Specifically, Axl is expressed in the heart (18) and Gas6/Axl signaling exerts a protective effect against myocardial ischemia (19). However, the role of Gas6/Axl in cardiac myocytes treated with endotoxin remains unknown. Furthermore, the Gas6/Axl/PI3K/Akt pathway is well known for its anti-apoptotic effects (20,21). Previous studies have demonstrated that the PI3K/Akt-dependent pathway, which regulates cell proliferation and survival, is protective against sepsis-induced myocardial injury in vitro and in vivo (22,23). In addition, PI3K/Akt signaling activation ameliorates sepsis-induced cardiac dysfunction and improves survival rates (24,25). Therefore, it is reasonable to speculate that modulation of the Gas6/Axl/PI3K/Akt pathway may benefit sepsis-induced myocardial dysfunction.
Gas6, which is structurally similar to protein S, is a vitamin K-dependent protein. The levels of plasma Gas6 are increased in patients with sepsis (26,27); however, its biological effects may be hindered by increased Axl (28). Recent studies have demonstrated that exogenous administration of Gas6 arrests TNF-α and interleukin-1 in monocytes/macrophages stimulated with LPS (29), and exerts protective effects on the lung (30) and kidney (31) in rodent models of sepsis, which indicates a practical role for the exogenous use of Gas6.
This study hypothesized that Gas6 may protect against sepsis-induced myocardial dysfunction via Axl/PI3K/Akt signaling, which is associated with the MAPK and NF-κB pathways. H9C2 cells were stimulated with LPS to mimic sepsis-induced cardiac dysfunction and Gas6 was applied exogenously. MAPK and NF-κB activation, TNF-α expression and apoptosis were then evaluated in the presence or absence of TP-0903 (an Axl inhibitor) and Wortmannin (a PI3K inhibitor).
Materials and methods
Cell culture and treatments
Cardiomyoblasts (H9C2 cells) obtained from the Chinese Academy of Sciences Cell Bank were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin and 100 mg/ml streptomycin at 37°C in an atmosphere containing in 5% CO2. Cells were seeded onto 6-well plates at a density of 1.5×105 cells/well. Recombinant mouse Gas6 (100 ng/ml) (R&D Systems, Inc.) was added 2 h prior to stimulation with LPS (10 µg/ml; from Escherichia coli; Sigma-Aldrich; Merck KGaA). Inhibitors TP-0903 (15 nM) and Wortmannin (3 nM) (Selleck Chemicals, LLC) were administered 15 min prior to Gas6 administration. Cells were incubated for 15 min-24 h with LPS at 37°C and were then harvested for analysis.
Cell counting kit 8 (CCK8) cell viability assay
Cells were seeded in a 96-well plate (5×103 cells/well) and exposed to various treatments after reaching 50% confluence. CCK8 reagent (10 µl; Dojindo Molecular Technologies, Inc.) and DMEM (100 µl) were then added and the plates were incubated at 37°C for 3 h. The absorbance was determined using a microplate reader (Tecan Group, Ltd.) at 450 nm.
Lactate dehydrogenase (LDH) assay
Cells were seeded in a 96-well plate (5×103 cells/well) and were exposed to various treatments after reaching 50% confluence. LDH release measurements were analyzed using the relative LDH assay kit (cat. no. A020-2; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol. Culture medium was retained to perform TNF-α analysis.
Evaluation of TNF-α release
The levels of TNF-α in the culture medium were evaluated using a TNF-α enzyme-linked immunosorbent assay kit (cat. no. BPE10374-09R; Shanghai Boyun Biotech Co., Ltd.) in accordance with the manufacturer's protocol.
Measurement of caspase-3 activity
Caspase-3 activity was determined using a commercial Caspase Activity kit (cat. no. BC3830-50T; Beijing Solarbio Science & Technology Co., Ltd.) according to the manufacturer's protocol.
Annexin V-FITC/propidium iodide (PI) staining for phosphatidylserine translocation
Annexin V-FITC/PI staining kit (BD Biosciences) was used to detect apoptosis, according to the manufacturer's protocol. Cells were harvested and resuspended in 150 ml binding buffer, followed by staining with 5 µl FITC-conjugated Annexin V and 5 µl PI in the dark for 15 min at room temperature. The mixture was detected by flow cytometry (FACSCalibur BD Biosciences). In the early stage of apoptosis, cell membranes were stained with FITC-conjugated Annexin V, whereas nuclei were not stained with PI. In the late stage of apoptosis, cells were stained with both FITC-conjugated Annexin V and PI. The results were analyzed by FlowJo vX.0.7 (FlowJo LLC).
TUNEL assay
Cell apoptosis was also detected using the TUNEL method. The assay was carried out with a commercial Cell Death Detection kit (cat. no. 11684817910; Roche Molecular Diagnostics) in accordance with the manufacturer's protocols. Images of the cells were captured using a fluorescence microscope.
Hoechst staining for nuclear morphology
Cells were fixed with 4% paraformaldehyde for 30 min at room temperature and washed twice with PBS. Cells were incubated with Hoechst 33342 (Beijing Solarbio Science & Technology Co., Ltd.) at room temperature for 5 min and washed with PBS three times. Images of the cells were captured using a fluorescence microscope.
Immunofluorescence analysis
Cells from the various treatment groups were fixed with 4% paraformaldehyde at 4°C for 1 h and were permeabilized with 0.5% Triton X-100 for 10 min. After blocking with 1% bovine serum albumin (BSA; Beyotime Institute of Biotechnology) at 4°C for 30 min, the cells were incubated with anti-P65 (cat. no. 8242S; 1:100; Cell Signaling Technology, Inc.) at 4°C overnight. Subsequently, samples were incubated with DyLightFluor® 488-conjugated donkey anti-rabbit IgG secondary antibodies (1:400; cat. no. BYE026; Shanghai Boyun Biotech Co., Ltd.) for 1 h at room temperature and were stained with DAPI for 7 min in the dark. The representative images were captured using an Olympus BX51 microscope (Olympus Corporation).
Western blotting
Cells were harvested with RIPA lysis buffer (Beyotime Institute of Biotechnology) on ice once cell treatment was completed. The protein concentration was measured using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology). Equal amounts of protein (5 µg/µl, 10 µl per lane) were separated by 8-12% SDS-PAGE and were transferred onto PVDF membranes using Bio-Rad western blot analysis apparatus (Bio-Rad Laboratories, Inc.). The membranes were then blocked with 5% BSA at room temperature for 1 h and incubated at 4°C overnight with antibodies against Axl (cat. no. BZ12291), phosphorylated (p)-Axl (cat. no. BS64021), Akt (cat. no. BS1810), p-Akt (cat. no. BS4006), IκB-α (BS3601; Bioworld Technology, Inc.), p-IκB-a (cat. no. 2859T), P65 (cat. no. 8242S), p-P65 (cat. no. 3033S), extracellular signal-regulated kinase 1/2 (ERK1/2) (cat. no. 4695T), p-ERK1/2 (cat. no. 4370T), c-Jun N-terminal protein kinase (JNK) (cat. no. 9252T), p-JNK (cat. no. 4668T), p38 MAPK (cat. no. 8690T), p-p38 MAPK (cat. no. 4511T), Bcl-2 (cat. no. 2870T) (Cell Signaling Technology, Inc.), Bax (cat. no. ab32503; Abcam) (dilutions, 1:1,000) or GAPDH (cat. no. BS60630; 1:8,000; Bioworld Technology, Inc.), followed by incubation at room temperature for 1 h with corresponding secondary antibodies (1:5,000; cat. no. BL003A; Biosharp Life Science, Inc.). Protein bands were detected with an enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.) and were semi-quantified using Quantity One v4.6.6 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
The results are presented as the mean ± standard deviation from at least three independent experiments. Statistical comparisons were analyzed by one-way analysis of variance and Tukey's test using GraphPad Prism 5 software (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Gas6 attenuates LPS-induced cytotoxicity in H9C2 cardiomyocytes
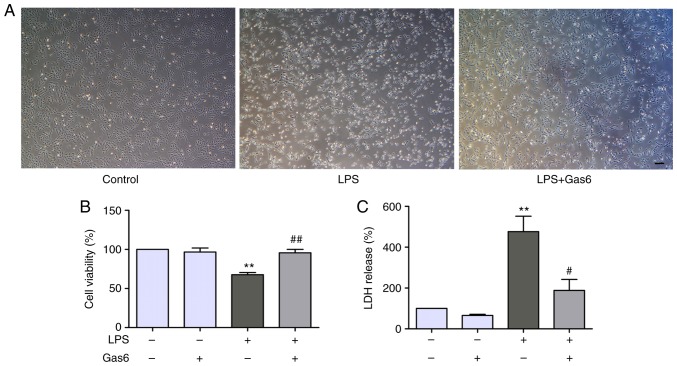
The present study determined the effects of Gas6 on LPS-stimulated H9C2 cells using phase-contrast microscopy. Notably, H9C2 cells treated with LPS for 24 h were markedly shrunk in size and decreased in number compared with untreated cells (Fig. 1A). Treatment with Gas6 (100 ng/ml) induced a significant improvement in cell morphology and decreased cell death compared with in the LPS-treated group. To further investigate the role of Gas6 in H9C2 cells challenged with LPS, CCK8 and LDH assays were performed as indicators of cytotoxicity. Treatment with LPS (10 µg/ml) decreased cell viability by 32.3% compared with the control group (P<0.01). Pretreatment with Gas6 induced a marked increase (41.3%) in the viability of cells compared with the LPS group (P<0.01; Fig. 1B). Furthermore, treatment of H9C2 cells with LPS increased LDH release by 476.1% compared with the control cells (P<0.01), which was reduced by 60.4% with co-treatment with Gas6 (P<0.05; Fig. 1C).
Figure 1.
Gas6 suppresses LPS-induced cytotoxicity in H9C2 cardiomyocytes. H9C2 cells were pretreated with Gas6 for 2 h and were then stimulated with LPS for 24 h. After LPS administration, H9C2 cells were harvested for detection and analysis. (A) Morphological alterations in H9C2 cells were visualized using phase-contrast microscopy (scale bar, 200 µm). Cell viability was determined using the (B) CCK8 assay and (C) LDH release assay. Data are presented as the mean ± standard deviation. **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the LPS group. Gas6, growth arrest-specific 6; LDH, lactate dehydrogenase; LPS, lipopolysaccharide.
Gas6 activates the Axl/PI3K/Akt pathway in LPS-challenged H9C2 cardiomyocytes
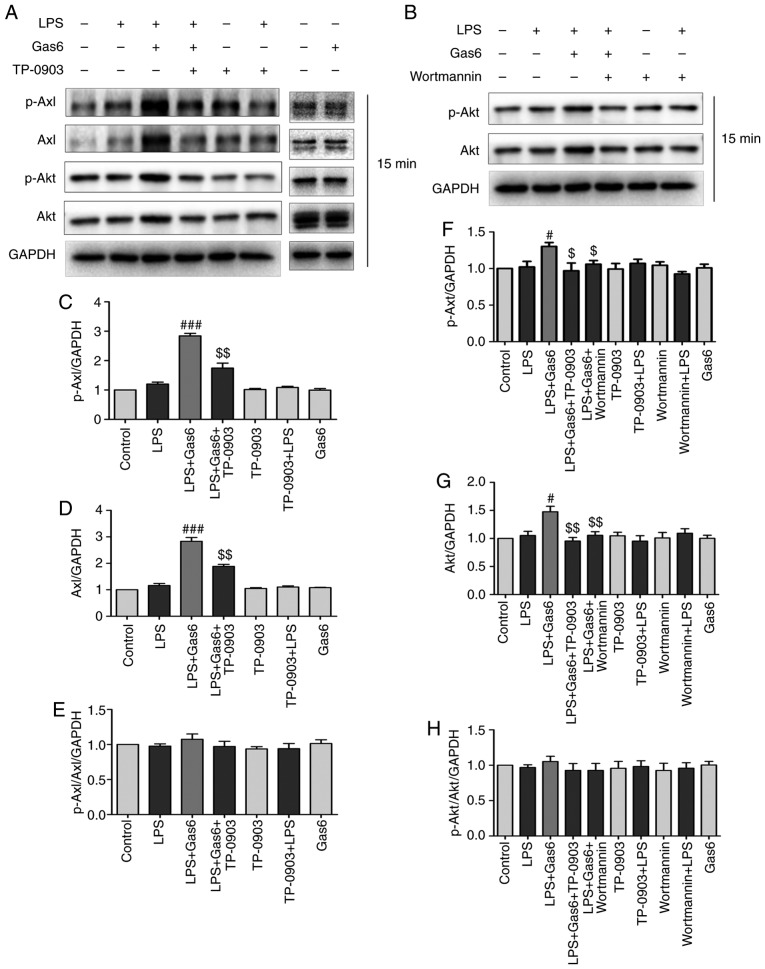
The association between Gas6/Axl and PI3K activation in is well known various cell types (20,21). To identify the signaling pathway associated with the protective effects of Gas6 on LPS-treated H9C2 cells, this study investigated whether Gas6 activated the Axl/PI3K/Akt pathway. Western blotting results demonstrated that Gas6 alone had no effect on the phosphorylation and expression of Axl and Akt. However, Gas6 enhanced the phosphorylation and expression of Axl and Akt in the presence of LPS. To determine whether Gas6-activated PI3K/Akt signaling was mediated by Axl, TP-0903, an Axl inhibitor, was administered. Pretreatment with TP-0903 abolished the enhanced phosphorylation and expression of Axl and Akt induced by Gas6 (Fig. 2A and C-H). These results suggested that Axl may mediate Gas6-induced activation of the PI3K/Akt signaling pathway. To determine the effects of Wortmannin (PI3K inhibitor) on the phosphorylation and expression of Akt, cells were treated with Wortmannin prior to Gas6. Wortmannin decreased the phosphorylation and expression of Akt induced by Gas6 treatment (Fig. 2B and F-H).
Figure 2.
Gas6 activates the Axl/PI3K/Akt signaling pathway in LPS-stimulated H9C2 cells. After pretreatment with or without TP-0903 or Wortmannin for 15 min, the cells were incubated with Gas6 for 2 h, followed by treatment with LPS for 15 min. After LPS administration, H9C2 cells were harvested for analysis. (A and B) Representative western blots and (C-H) semi-quantification of p-Axl, Axl, p-Akt and Akt in each group. Data are presented as the mean ± standard deviation. #P<0.05, ###P<0.001 vs. the LPS group; $P<0.05, $$P<0.01 vs. the LPS + Gas6 group. Gas6, growth arrest-specific 6; LPS, lipopoly-saccharide; p-, phosphorylated.
Gas6 suppresses the release of TNF-α via the Axl/PI3K/Akt pathway in LPS-challenged H9C2 cells
TNF-α (death receptor ligand) binds to TNF-receptor 1 (TNF-R1; membrane-bound death receptor) and triggers the death receptor-mediated apoptotic pathway, which has been reported to be involved in the pathogenesis of sepsis-induced myocardial dysfunction (32). As shown in Fig. 3, LPS significantly increased the release of TNF-α by 193.4% compared with in the control group (P<0.01). Conversely, Gas6 treatment decreased the release of TNF-α by 43.8% in H9C2 cells stimulated with LPS (P<0.01). To confirm the relevance of the Axl/PI3K/Akt pathway on the ability of Gas6 to decrease the production of TNF-α in response to LPS, H9C2 cells were treated with TP-0903 or Wortmannin in the presence of Gas6. TP-0903 and Wortmannin eliminated the attenuating effects of Gas6 on TNF-α release by 83.6 and 58.9%, respectively (P<0.01).
Figure 3.
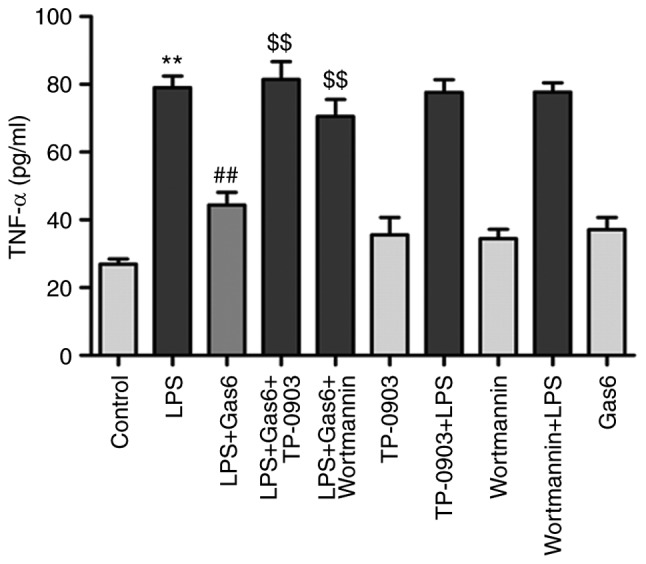
The Axl/PI3K/Akt pathway is involved in Gas9-induced suppression of TNF-α in LPS-stimulated H9C2 cardiomyocytes. After pretreatment with TP-0903 or Wortmannin for 15 min, the cells were incubated with Gas6 for 2 h, followed by LPS incubation for 24 h. After LPS administration, H9C2 cells were harvested for detection and analysis. TNF-α release was determined using ELISA. Data are presented as the mean ± standard deviation. **P<0.01 vs. the control group; ##P<0.01 vs. the LPS group; $$P<0.01 vs. the LPS + Gas6 group. Gas6, growth arrest-specific 6; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α.
Gas6 suppresses apoptosis via the Axl/PI3K/Akt pathway in LPS-challenged H9C2 cells
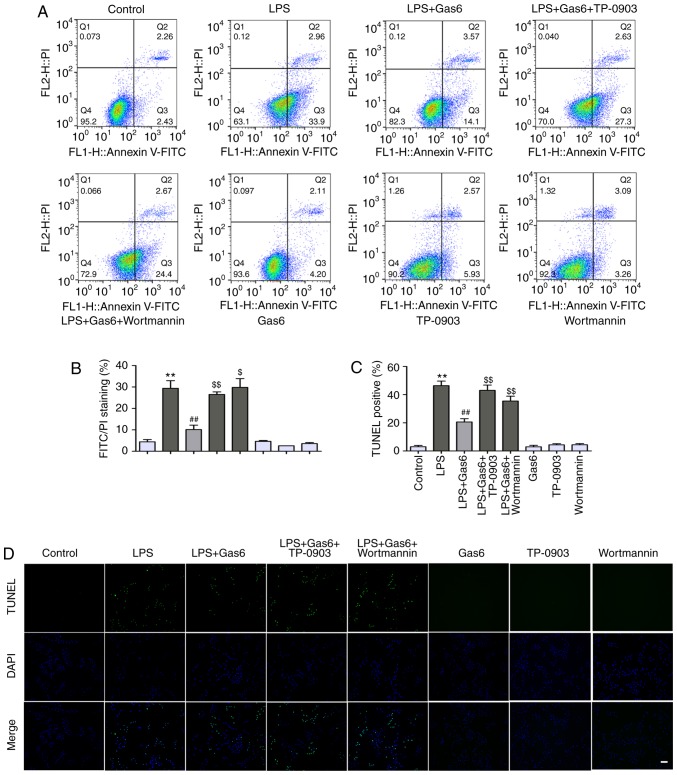
The present study examined the protective effects of Gas6 on H9C2 cells exposed to LPS. Annexin V-PI staining and TUNEL assay were applied to assess the early and late stages of apoptosis in H9C2 cells, respectively. LPS exposure led to an incremental increase in the percentage of early stage apoptotic cells from 4.4 to 29.4% compared with in the control group (P<0.01); Gas6 attenuated the percentage of early stage apoptotic cells to 10.1% (P<0.01; Fig. 4A and B). Similar results were observed using the TUNEL assay. As shown in Fig. 4C and D, LPS stimulation significantly increased the number of apoptotic H9C2 cells to 46.4% (P<0.01), whereas pretreatment with Gas6 decreased the percentage of apoptotic cells to 20.6% (P<0.01). To further assess the role of Axl/PI3K/Akt signaling in the protective effect of Gas6 on H9C2 cells stimulated with LPS, Hoechst staining, Annexin V-PI staining, and TUNEL assay were performed in LPS-treated cells incubated with Gas6 in the presence or absence of TP-0903 and Wortmannin. As shown in Fig. 5A, TP-0903 and Wortmannin reversed the amelioration of nuclear morphological alterations by Gas6 in H9C2 cells challenged with LPS. Axl and PI3K inhibitors also abolished the protective effect of Gas6 and increased the percentage of apoptotic cells to 29.8% (P<0.01) and 32.4% (P<0.05), respectively, as assessed by Annexin V-PI staining (Fig. 4A and B). TUNEL analysis also revealed that the protective effects of Gas6 on H9C2 cells treated with LPS were abrogated by treatment with Axl and PI3K inhibitors; these inhibitors enhanced the percentage of apoptotic cells to 43.1 and 37.7%, respectively (P<0.01; Fig. 4C and D). Hoechst staining, which detects nuclear condensation and fragmentation, revealed that apoptosis was markedly increased following treatment with LPS, which was ameliorated by pretreatment with Gas6 (Fig. 5A).
Figure 4.
The Axl/PI3K/Akt pathway is involved in the suppression of apoptosis by Gas6 in LPS-stimulated H9C2 cardiomyocytes. After pretreatment with TP-0903 or Wortmannin for 15 min, the cells were incubated with Gas6 for 2 h, followed by LPS incubation for (A and B) 18 h or (C and D) 24 h. Subsequently, H9C2 cells were harvested for analysis. The protective effects of Gas6 against LPS-induced apoptosis were determined by (A and B) flow cytometry and (C and D) TUNEL assay (scale bar, 100 µm). Data are presented as the mean ± standard deviation. **P<0.01 vs. the control group; ##P<0.01 vs. the LPS group; $P<0.05, $$P<0.01 vs. the LPS + Gas6 group. Gas6, growth arrest-specific 6; LPS, lipopolysaccharide; PI, propidium iodide.
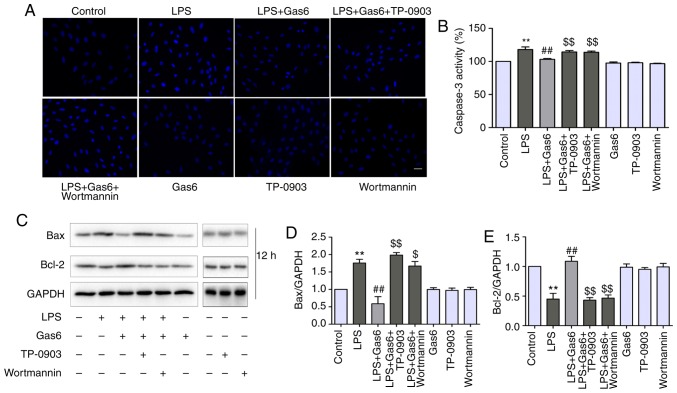
Figure 5.
The Axl/PI3K/Akt pathway is involved in suppression of alterations in nuclear morphology, attenuation of Bax expression and caspase-3 activation, and enhancement of Bcl-2 by Gas6 in LPS-stimulated H9C2 cardiomyocytes. After pretreatment with TP-0903 or Wortmannin for 15 min, cells were incubated with Gas6 for 2 h, followed by treatment with LPS for (A) 24 h or (B-E) 12 h. (A) Cells were harvested for Hoechst staining (scale bar, 50 µm), (B) caspase-3 activity determination and (C-E) western blotting. (C) Representative western blots and (D and E) semi-quantification of Bax and Bcl-2. Data are presented as the mean ± standard deviation. **P<0.01 vs. the control group; ##P<0.01 vs. the LPS group; $P<0.05, $$P<0.01 vs. the LPS + Gas6 group. Gas6, growth arrest-specific 6; LPS, lipopolysaccharide.
Axl/PI3K/Akt inhibition reverses the effects of Gas6 on caspase-3 activity, and Bax and Bcl-2 expression
Since caspase-3 is considered a central regulator of apoptosis (33), caspase-3 activity was examined to confirm apoptosis. In addition, Bax and Bcl-2 expression levels were determined, as these are Bcl-2 family members that are involved in the intrinsic apoptotic pathway (34). As shown in Fig. 5B-D, caspase-3 activity and Bax expression were increased by LPS treatment compared with the control, whereas they were decreased by Gas6 pretreatment when compared with cells stimulated with LPS. Conversely, Gas6 elevated the expression levels of the anti-apoptotic protein Bcl-2, which were decreased by LPS. To determine the role of Axl/PI3K/Akt inhibition and the effects of Gas6 on caspase-3 activity, and Bax and Bcl-2 expression, TP-0903 and Wortmannin were used to treat cells. Caspase-3 activity was attenuated by Gas6 from 117.9 to 103.2% (P<0.01); this effect was reversed with the addition of TP-0903 and Wortmannin, and caspase-3 activity was increased to 114.3 and 113.8%, respectively (both P<0.01). TP-0903 and Wortmannin also reversed the effects of Gas6 on the proapoptotic protein Bax and the anti-apoptotic protein Bcl-2 (Fig. 5C-E).
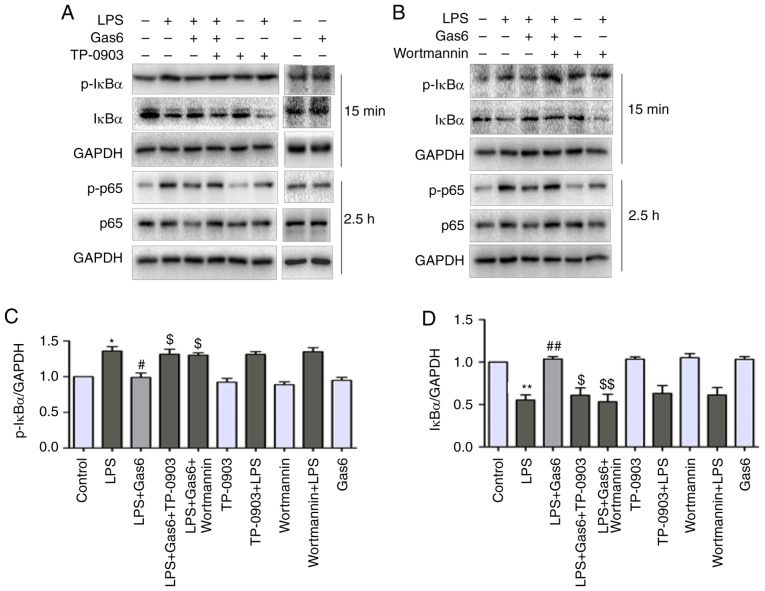
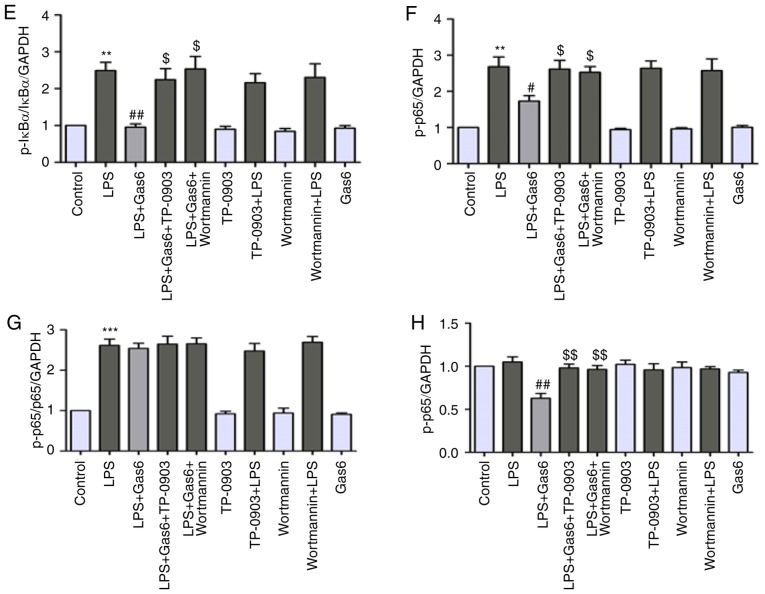
Axl/PI3K/Akt inhibition abolishes the inhibitory effects of Gas6 on activation of NF-κB in LPS-challenged H9C2 cells
Activation of NF-κB signaling is thought to be a key event in the pathogenesis of sepsis and sepsis-induced myocardial dysfunction (35). Therefore, this study investigated the role of Gas6 in activation of the NF-κB pathway in the presence of LPS. Pretreatment of cells with Gas6 resulted in inhibition of the phosphorylation and degradation of IκB-α at 15 min (Fig. 6A-D). Additionally, Gas6 suppressed the phosphorylation and expression of P65 at 2.5 h (Fig. 6A, B and E-H). Furthermore, the prominently enhanced nuclear translocation of P65 caused by LPS was inhibited by Gas6 at 1 h (Fig. 6I). Gas6 alone did not affect the phosphorylation and expression of P65 or IκB-α, or the localization of P65. In addition, to examine whether Gas6 blocked the activation of NF-κB signaling via the Axl/PI3K/Akt pathway, H9C2 cells were pretreated with TP-0903 or Wortmannin in the presence or absence of Gas6. The effects of Gas6 on LPS-induced phosphorylation and degradation of IκB-α were blocked by TP-0903 and Wortmannin (Fig. 6A-D). Similarly, the effects of Gas6 on the phosphorylation, expression and translocation of P65 were reversed by TP-0903 and Wortmannin treatment (Fig. 6A, B and E-I).
Figure 6.
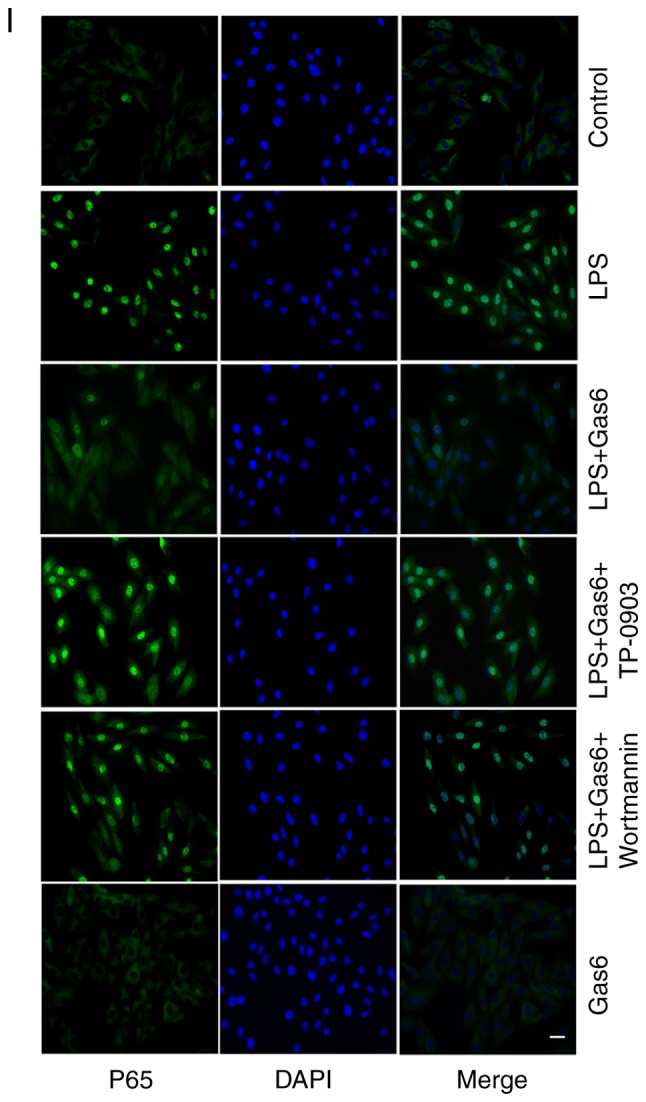
Inhibition of NF-κB signaling by Gas6 is Axl/PI3K/Akt-dependent in LPS-treated H9C2 cells. After pretreatment with TP-0903 or Wortmannin for 15 min, the cells were incubated with Gas6 for 2 h, followed by treatment with LPS for the appropriate times. Following LPS administration, H9C2 cells were harvested for analysis. (A and B) Representative western blots and (C and D) semi-quantification of p-IκBα, IκBα, p-P65 and P65. (E-H) Semi-quantification of p-IκBα, IκBα, p-P65 and P65. (I) Translocation of NF-κB P65 was visualized by immunofluorescence analysis (scale bar, 50 µm). Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01 vs. the LPS group; $P<0.05, $$P<0.01 vs. the LPS + Gas6 group. Gas6, growth arrest-specific 6; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; p-, phosphorylated.
Axl/PI3K/Akt inhibition eliminates the suppressive effects of Gas6 on activation of MAPKs in LPS-challenged H9C2 cells
To further investigate the cardioprotective mechanism underlying the effects of Gas6, the role of MAPKs in H9C2 cells pretreated with Gas6 in the presence of LPS was evaluated. Western blotting results demonstrated that Gas6 significantly decreased the phosphorylation of JNK and p38 MAPK induced by LPS stimulation at 15 min and 2 h, respectively, whereas it did not affect the expression of total JNK and p38 MAPK (Fig. 7A-D). Conversely, Gas6 increased the phosphorylation and expression of ERK at 15 min (Fig. 7A, B and E-G). Gas6 alone had no effect on the phosphorylation of JNK, ERK or p38 MAPK. To determine whether Gas6 inhibited activation of MAPKs via the Axl/PI3K/Akt pathway, LPS-stimulated cells were incubated with TP-0903 and Wortmannin in the presence or absence of Gas6. The results revealed that inhibition of Gas6/Axl signaling by TP-0903 and inhibition of PI3K/Akt signaling by Wortmannin modified Gas6-induced suppression of JNK and p38 MAPK phosphorylation in cells stimulated with LPS, and reversed the effect of Gas6 on ERK phosphorylation and expression.
Figure 7.
Inhibition of MAPK signaling by Gas6 is Axl/PI3K/Akt-dependent in LPS-treated H9C2 cells. After pretreatment with TP-0903 or Wortmannin for 15 min, the cells were incubated with Gas6 for 2 h, followed by LPS for the appropriate times. Following LPS administration, H9C2 cells were harvested for analysis. (A and B) Representative western blots and (C-G) semi-quantification of p-JNK, JNK, p-ERK, ERK, p38 and p-p38. Data are presented as the mean ± standard deviation. *P<0.05, **P<0.01 vs. the control group; #P<0.05 vs. the LPS group; $P<0.05 vs. the LPS + Gas6 group. ERK, extracellular signal-regulated kinase; Gas6, growth arrest-specific 6; JNK, c-Jun N-terminal protein kinase; LPS, lipopolysaccharide; p-, phosphorylated.
Discussion
The present study demonstrated that Gas6 suppressed TNF-α release and apoptosis in LPS-treated H9C2 cells, and inhibited their related regulators NF-κB and MAPKs. TP-0903 and Wortmannin abrogated the inhibitory effects of Gas6 on LPS-challenged H9C2 cells. Therefore, these findings indicated that Gas6-attenuated LPS-induced TNF-α release and apoptosis in H9C2 cells may involve inhibition of NF-κB and MAPK activation via the Axl/PI3K/Akt signaling pathway.
Previous studies have demonstrated that apoptosis (36) and TNF-α (37) produced by cardiomyocytes contribute to septic cardiomyopathy, and an increased Gas6 concentration is observed in the circulation of patients with sepsis (27). TNF-α, as a death receptor ligand, participates in the extrinsic apoptotic pathway. After binding with the death receptor TNF-R1, TNF-α increases the expression of caspase-8 and TNF receptor-related death domain protein, which leads to formation of the death-inducing signaling complex, followed by activation of the apoptotic cascade (32). In the intrinsic pathway, Bcl-2 family proteins (Bax, Bak and Bid) are first activated and combine with each other, and are inserted into the outer membrane, leading to the release of key enzymes (such as cytochrome c), followed by the formation of apoptotic bodies, amplification of the death signal, and apoptosis (38). Additionally, previous studies have demonstrated that exogenous administration of Gas6 prevents the release of inflammatory cytokines and exerts anti-apoptotic effects on models of sepsis (29-31). Therefore, this study investigated the effects of Gas6 on TNF-α release and apoptosis, and analyzed the underlying mechanism, in order to further improve existing therapies and treatments for septic cardiomyopathy. In the human circulatory system, the concentration of Gas6 is subnanomolar (20-50 ng/ml or 0.25 nM) and increases ~2-fold in patients with sepsis (28,39). This study confirmed the effects of 100 ng/ml Gas6 on TNF-α release and apoptosis in LPS-challenged H9C2 cells. The present study provided evidence to suggest that Gas6 may attenuate production of the proinflammatory cytokine TNF-α as well as apoptosis.
The Axl/PI3K/Akt pathway is known to be involved in the pro-survival and anti-apoptotic effects of Gas6 (21,40). Axl is a TAM receptor, which is mainly expressed on the cell surface. After binding with its ligands (e.g. Gas6), Axl is activated and autophosphorylated to initiate signaling (41). Among the signaling cascades, the PI3K/Akt pathway is necessary for the Gas6/Axl-dependent anti-apoptotic effect (21). The PI3K/Akt pathway serves a central role in cell growth and survival. Activation of Akt directly regulates apoptotic regulatory factors, such as Bax, Bad and caspase-9 (42,43), or facilitates its interaction with transcription factors such as NF-κB. To determine whether Axl/PI3K/Akt participated in the action of Gas6 in LPS-induced H9C2 cells, an Axl selective inhibitor (TP-0903) and a PI3K inhibitor (Wortmannin) were used; the results demonstrated that these inhibitors abrogated the inhibitory effects of Gas6 on TNF-α production and apoptosis in LPS-stimulated H9C2 cells. The present study revealed that Gas6 enhanced the phosphorylation and expression of Axl and Akt, whereas TP-0903 and Wortmannin reversed the phosphorylation and expression of Axl and Akt, respectively. In other studies, only the phosphorylation of Axl and Akt were affected, whereas the expression of Axl and Akt remained unaffected by inflammatory injury or drugs. However, Gas6 increased both the phosphorylation and expression of Axl and downstream signaling molecules in a model of neuroinflammation (17). TP-0903 has also been shown to inhibit both the phosphorylation and expression of Axl and Akt in lymphocytic leukemia B cells (44). Various cell origins and stimulations may explain the distinctions in the expression of Axl and Akt in different studies. In the present study, the ratios of p-Axl/Axl, p-Akt/Akt, p-p65/p65 and p-ERK/ERK were almost the same in the different groups, which indicated that alterations in the expression of phosphorylated proteins were due to alterations in the expression of the total proteins. Taken together, the findings indicated that Gas6 exerted its protective effect via the Axl/PI3K/Akt pathway in LPS-challenged H9C2 cells.
Accumulating evidence has indicated that activation of NF-κB (45) and MAPK (22) contributes to enhanced cardiomyocyte TNF-α production and apoptosis in the presence of LPS. Generally, the transcription factor NF-κB is inactive due to its binding with the inhibitory protein IκB, which exists in the cytoplasm. Once activated by LPS, NF-κB translocates to the nucleus, subsequently triggering the transcription of various inflammatory- and apoptosis-associated genes (46). In the present study, NF-κB was activated in the presence of LPS, whereas Gas6 reversed the phosphorylation and expression of NF-κB in response to LPS treatment in cardiomyocytes. The MAPK family consists of at least three members: ERK1/2, JNK1/2 and p38 MAPK. JNK and p38 MAPK contribute to endotoxin-induced enhanced cardiomyocyte TNF-α production and apoptosis. A previous study reported that ERK has the opposite effect of JNK and p38 MAPK (47). The present findings indicated that LPS induced an increase in JNK and p38 MAPK phosphorylation, but marginally decreased the phosphorylation and expression of ERK. Administration of Gas6 reversed the effects of LPS on H9C2 cells at different time-points. However, other studies have demonstrated that LPS activates ERK, JNK and p38 MAPK phosphorylation (48). The cell origin and time-point may explain this discrepancy.
Numerous studies have revealed that Gas6/Axl activation suppresses inflammation by inhibiting NF-κB (17,49). Gas6 also promotes cardiac hypertrophy via ERK1/2, which indicates a relationship between Gas6/Axl and MAPK (50). Additionally, compelling evidence has revealed cross talk between PI3K/Akt and NF-κB and MAPK in LPS-challenged cardiomyocytes (25,48). To further investigate the mechanism underlying the protective effects of Gas6 on LPS-treated H9C2 cells, Axl and PI3K inhibitors were applied. TP-0903 and Wortmannin reversed the effects of Gas6 on MAPK and NF-κB in LPS-stimulated cardiomyocytes, suggesting that Gas6 may attenuate MAPK and NF-κB in LPS-stimulated cardiomyocytes via the Axl/PI3K/Akt pathway.
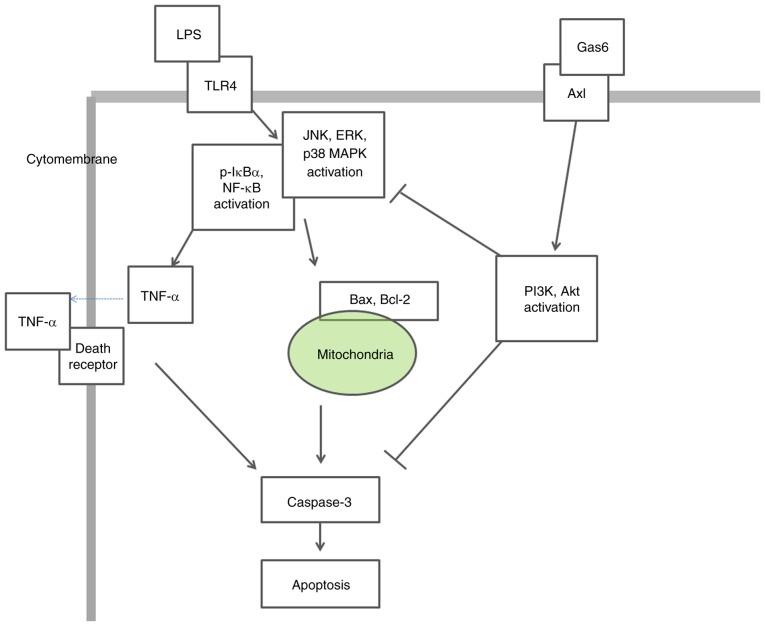
In conclusion, the present findings demonstrated that the Axl/PI3K/Akt pathway may be essential for Gas6-mediated suppression of MAPK and NF-κB activation, as well as the attenuation of TNF-α production and apoptosis, in response to LPS stimulation in H9C2 cardiomyoblasts (Fig. 8). These findings provide evidence regarding the specific molecular signaling events that participate in the protective effect of Gas6 on LPS-challenged H9C2 cardiomyocytes and support the hypothesis that Gas6 may emerge as a pharmacological therapy for the treatment of sepsis-induced myocardial dysfunction. Further studies are required to determine the protective effects of Gas6 against sepsis-induced myocardial injury in vivo and to identify its potential clinical applications.
Figure 8.
Schematic representation depicting the potential molecular mechanisms underlying the protective effects of Gas6 on LPS-stimulated cardiomyocytes via the Axl/PI3K/Akt pathway. ERK, extracellular signal-regulated kinase; Gas6, growth arrest-specific 6; JNK, c-Jun N-terminal protein kinase; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4.
Acknowledgments
Not applicable.
Funding
This study was funded by the Zhejiang Provincial Natural Science Foundation (grant no. LQ15H150003), the Zhejiang Provincial Medical and Health Science and Technology Project (grant no. 2015KYA152), the Wenzhou Municipal Science and Technology Project (grant no. Y20150182) and the Zhejiang Provincial Administration of Traditional Chinese Medicine Project (grant no. 2015ZA120). This study was supported, in part, by grants from the National Natural Science Foundation (grant nos. 81571937, 81772112 and 81871583) and the Medical Health Science and Technology Major Project of the Zhejiang Provincial Health Commission (grant no. WKJ-Z-J-1724).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
ZL, ML and JY conceived of and designed the study. ML, JY and XH performed the study. JY, KC, YW GZ and GH analyzed the data. ML, JY, GZ and GH wrote the manuscript. All authors reviewed the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 2.Landesberg G, Gilon D, Meroz Y, Georgieva M, Levin PD, Goodman S, Avidan A, Beeri R, Weissman C, Jaffe AS, Sprung CL. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33:895–903. doi: 10.1093/eurheartj/ehr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 4.Bayeva M, Sawicki KT, Butler J, Gheorghiade M, Ardehali H. Molecular and cellular basis of viable dysfunctional myocardium. Circ Heart Fail. 2014;7:680–691. doi: 10.1161/CIRCHEARTFAILURE.113.000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35:1599–1608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 6.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandel U, Fink L, Blum A, Heep M, Buerke M, Kraemer HJ, Mayer K, Bohle RM, Seeger W, Grimminger F, Sibelius U. Endotoxin-induced myocardial tumor necrosis factor-alpha synthesis depresses contractility of isolated rat hearts: Evidence for a role of sphingosine and cyclooxygenase-2-derived thromboxane production. Circulation. 2000;102:2758–2764. doi: 10.1161/01.CIR.102.22.2758. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Bakker J, Marécaux G, Schandene L, Kahn RJ, Dupont E. Administration of anti-TNF antibody improves left ventricular function in septic shock patients. Results of a pilot study Chest. 1992;101:810–815. doi: 10.1378/chest.101.3.810. [DOI] [PubMed] [Google Scholar]
- 9.Herbertson MJ, Werner HA, Goddard CM, Russell JA, Wheeler A, Coxon R, Walley KR. Anti-tumor necrosis factor-alpha prevents decreased ventricular contractility in endotoxemic pigs. Am J Respir Crit Care Med. 1995;152:480–488. doi: 10.1164/ajrccm.152.2.7633696. [DOI] [PubMed] [Google Scholar]
- 10.Peng T, Lu X, Lei M, Moe GW, Feng Q. Inhibition of p38 MAPK decreases myocardial TNF-alpha expression and improves myocardial function and survival in endotoxemia. Cardiovasc Res. 2003;59:893–900. doi: 10.1016/S0008-6363(03)00509-1. [DOI] [PubMed] [Google Scholar]
- 11.Nevière R, Fauvel H, Chopin C, Formstecher P, Marchetti P. Caspase inhibition prevents cardiac dysfunction and heart apoptosis in a rat model of sepsis. Am J Respir Crit Care Med. 2001;163:218–225. doi: 10.1164/ajrccm.163.1.2003109. [DOI] [PubMed] [Google Scholar]
- 12.Lancel S, Petillot P, Favory R, Stebach N, Lahorte C, Danze PM, Vallet B, Marchetti P, Neviere R. Expression of apoptosis regulatory factors during myocardial dysfunction in endotoxemic rats. Crit Care Med. 2005;33:492–496. doi: 10.1097/01.CCM.0000156240.31913.4A. [DOI] [PubMed] [Google Scholar]
- 13.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Knyazev PG, Clout NJ, Cheburkin Y, Göhring W, Ullrich A, Timpl R, Hohenester E. Structural basis for Gas6-Axl signalling. EMBO J. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao GJ, Zheng JY, Bian JL, Chen LW, Dong N, Yu Y, Hong GL, Chandoo A, Yao YM, Lu ZQ. Growth arrest-specific 6 enhances the suppressive function of CD4+CD25+ regulatory T cells mainly through Axl receptor. Mediators Inflamm. 2017;2017:6848430. doi: 10.1155/2017/6848430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goruppi S, Ruaro E, Schneider C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene. 1996;12:471–480. [PubMed] [Google Scholar]
- 17.Wu G, McBride DW, Zhang JH. Axl activation attenuates neuroinflammation by inhibiting the TLR/TRAF/NF-κB pathway after MCAO in rats. Neurobiol Dis. 2018;110:59–67. doi: 10.1016/j.nbd.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10:2349–2359. [PubMed] [Google Scholar]
- 19.Wu M, Lu S, Zhong J, Huang K, Zhang S. Protective effects of pterostilbene against myocardial ischemia/reperfusion injury in rats. Inflammation. 2017;40:578–588. doi: 10.1007/s10753-016-0504-2. [DOI] [PubMed] [Google Scholar]
- 20.Goruppi S, Ruaro E, Varnum B, Schneider C. Requirement of phosphatidylinositol 3-kinase-dependent pathway and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:4442–4453. doi: 10.1128/MCB.17.8.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WP, Wen Y, Varnum B, Hung MC. Akt is required for Axl-Gas6 signaling to protect cells from E1A-mediated apoptosis. Oncogene. 2002;21:329–336. doi: 10.1038/sj.onc.1205066. [DOI] [PubMed] [Google Scholar]
- 22.Liu CJ, Lo JF, Kuo CH, Chu CH, Chen LM, Tsai FJ, Tsai CH, Tzang BS, Kuo WW, Huang CY. Akt mediates 17beta-estra-diol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFkappaB pathway. J Cell Mol Med. 2009;13:3655–3667. doi: 10.1111/j.1582-4934.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An R, Zhao L, Xi C, Li H, Shen G, Liu H, Zhang S, Sun L. Melatonin attenuates sepsis-induced cardiac dysfunction via a PI3K/Akt-dependent mechanism. Basic Res Cardiol. 2016;111:8. doi: 10.1007/s00395-015-0526-1. [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Ha T, Zhang X, Wang X, Liu L, Kalbfleisch J, Singh K, Williams D, Li C. The Toll-like receptor 9 ligand, CpG oligodeoxynucleotide, attenuates cardiac dysfunction in poly-microbial sepsis, involving activation of both phosphoinositide 3 kinase/Akt and extracellular-signal-related kinase signaling. J Infect Dis. 2013;207:1471–1479. doi: 10.1093/infdis/jit036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun B, Xiao J, Sun XB, Wu Y. Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: An insight into oestrogen receptor activation and PI3K/Akt signalling. Br J Pharmacol. 2013;168:1758–1770. doi: 10.1111/bph.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manfioletti G, Brancolini C, Avanzi G, Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol Cell Biol. 1993;13:4976–4985. doi: 10.1128/MCB.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibot S, Massin F, Cravoisy A, Dupays R, Barraud D, Nace L, Bollaert PE. Growth arrest-specific protein 6 plasma concentrations during septic shock. Crit Care. 2007;11:R8. doi: 10.1186/cc5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekman C, Linder A, Akesson P, Dahlbäck B. Plasma concentrations of Gas6 (growth arrest specific protein 6) and its soluble tyrosine kinase receptor sAxl in sepsis and systemic inflammatory response syndromes. Crit Care. 2010;14:R158. doi: 10.1186/cc9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alciato F, Sainaghi PP, Sola D, Castello L, Avanzi GC. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol. 2010;87:869–875. doi: 10.1189/jlb.0909610. [DOI] [PubMed] [Google Scholar]
- 30.Giangola MD, Yang WL, Rajayer SR, Nicastro J, Coppa GF, Wang P. Growth arrest-specific protein 6 attenuates neutrophil migration and acute lung injury in sepsis. Shock. 2013;40:485–491. doi: 10.1097/SHK.0b013e3182a588c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LW, Chen W, Hu ZQ, Bian JL, Ying L, Hong GL, Qiu QM, Zhao GJ, Lu ZQ. Protective effects of growth arrest-specific protein 6 (Gas6) on sepsis-induced acute kidney injury. Inflammation. 2016;39:575–582. doi: 10.1007/s10753-015-0282-2. [DOI] [PubMed] [Google Scholar]
- 32.Carlson DL, Willis MS, White DJ, Horton JW, Giroir BP. Tumor necrosis factor-alpha-induced caspase activation mediates endotoxin-related cardiac dysfunction. Crit Care Med. 2005;33:1021–1028. doi: 10.1097/01.CCM.0000163398.79679.66. [DOI] [PubMed] [Google Scholar]
- 33.Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Zhao H, Wang Y, Shao Y, Li J, Liu J, Xing M. The inflammatory responses in Cu-mediated elemental imbalance is associated with mitochondrial fission and intrinsic apoptosis in Gallus gallus heart. Chemosphere. 2017;189:489–497. doi: 10.1016/j.chemosphere.2017.09.099. [DOI] [PubMed] [Google Scholar]
- 35.Ma H, Wang X, Ha T, Gao M, Liu L, Wang R, Yu K, Kalbfleisch JH, Kao RL, Williams DL, Li C. MicroRNA-125b prevents cardiac dysfunction in polymicrobial sepsis by targeting TRAF6-mediated nuclear factor κB activation and p53-mediated apoptotic signaling. J Infect Dis. 2016;214:1773–1783. doi: 10.1093/infdis/jiw449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki J, Bayna E, Li HL, Molle ED, Lew WY. Lipopolysaccharide activates calcineurin in ventricular myocytes. J Am Coll Cardiol. 2007;49:491–499. doi: 10.1016/j.jacc.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 37.Baumgarten G, Knuefermann P, Nozaki N, Sivasubramanian N, Mann DL, Vallejo JG. In vivo expression of proinflammatory mediators in the adult heart after endotoxin administration: The role of toll-like receptor-4. J Infect Dis. 2001;183:1617–1624. doi: 10.1086/320712. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 39.Balogh I, Hafizi S, Stenhoff J, Hansson K, Dahlbäck B. Analysis of Gas6 in human platelets and plasma. Arterioscler Thromb Vasc Biol. 2005;25:1280–1286. doi: 10.1161/01.ATV.0000163845.07146.48. [DOI] [PubMed] [Google Scholar]
- 40.Ming Cao W, Murao K, Imachi H, Sato M, Nakano T, Kodama T, Sasaguri Y, Wong NC, Takahara J, Ishida T. Phosphatidylinositol 3-OH kinase-Akt/protein kinase B pathway mediates Gas6 induction of scavenger receptor a in immortalized human vascular smooth muscle cell line. Arterioscler Thromb Vasc Biol. 2001;21:1592–1597. doi: 10.1161/hq1001.097062. [DOI] [PubMed] [Google Scholar]
- 41.Mark MR, Chen J, Hammonds RG, Sadick M, Godowsk PJ. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J Biol Chem. 1996;271:9785–9789. doi: 10.1074/jbc.271.16.9785. [DOI] [PubMed] [Google Scholar]
- 42.Kamada H, Nito C, Endo H, Chan PH. Bad as a converging signaling molecule between survival PI3-K/Akt and death JNK in neurons after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:521–533. doi: 10.1038/sj.jcbfm.9600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 44.Sinha S, Boysen J, Nelson M, Secreto C, Warner SL, Bearss DJ, Lesnick C, Shanafelt TD, Kay NE, Ghosh AK. Targeted Axl inhibition primes chronic lymphocytic leukemia B cells to apop-tosis and shows synergistic/additive effects in combination with BTK inhibitors. Clin Cancer Res. 2015;21:2115–2126. doi: 10.1158/1078-0432.CCR-14-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Wang Y, Yang D, Yu X, Li H, Lv X, Lu D, Wang H. β1-adrenoceptor stimulation promotes LPS-induced cardiomyocyte apoptosis through activating PKA and enhancing CaMKII and IκBα phosphorylation. Crit Care. 2015;19:76. doi: 10.1186/s13054-015-0820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Yu X, Jia B, Wang F, Lv X, Peng X, Wang Y, Li H, Wang Y, Lu D, Wang H. α1 adrenoceptor activation by norepinephrine inhibits LPS-induced cardiomyocyte TNF-α production via modulating ERK1/2 and NF-κB pathway. J Cell Mol Med. 2014;18:263–273. doi: 10.1111/jcmm.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang P, Han Y, Gui L, Sun J, Chen YL, Song R, Guo JZ, Xie YN, Lu D, Sun L. Gastrodin attenuation of the inflammatory response in H9c2 cardiomyocytes involves inhibition of NF-κB and MAPKs activation via the phosphatidylinositol 3-kinase signaling. Biochem Pharmacol. 2013;85:1124–1133. doi: 10.1016/j.bcp.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1207–H1213. doi: 10.1152/ajpheart.00020.2004. [DOI] [PubMed] [Google Scholar]
- 50.Zhao YF, Xu DC, Zhu GF, Zhu MY, Tang K, Li WM, Xu YW. Growth arrest-specific 6 exacerbates pressure overload-induced cardiac hypertrophy. Hypertension. 2016;67:118–129. doi: 10.1161/HYPERTENSIONAHA.115.06254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.