Abstract
Background
Hand, foot, and mouth disease (HFMD) has become a major public health concern in the Asia-Pacific region. Knowledge of its economic burden is essential for policy makers in prioritizing the development and implementation of interventions.
Methods
A multi-hospital-based study was prospectively conducted at 3 major hospitals in Ho Chi Minh City, Vietnam, during 2016–2017. Data on direct and productivity costs were collected alongside clinical information and samples and demographic information from study participants.
Results
A total of 466 patients were enrolled. Two hundred three of 466 (43.6%) patients lived in Ho Chi Minh City, and 72/466 (15.5%) had severe HFMD. An enterovirus was identified in 74% of 466 patients, with EV-A71, CV-A6, CV-A10, and CV-A16 being the most common viruses identified (236/466, 50.6%). The mean economic burden per case was estimated at US$400.80 (95% confidence interval [CI], $353.80–$448.90), of which the total direct (medical) costs accounted for 69.7%. There were considerable differences in direct medical costs between groups of patients with different clinical severities and pathogens (ie, EV-A71 vs non-EV-A71). In Vietnam, during 2016–2017, the economic burden posed by HFMD was US$90 761 749 (95% CI, $79 033 973–$103 009 756).
Conclusions
Our findings are of public health significance because for the first time we demonstrate that HFMD causes a substantial economic burden in Vietnam, and although multivalent vaccines are required to control HFMD, effective EV-A71 vaccine could substantially reduce the burden posed by severe HFMD. The results will be helpful for health policy makers in prioritizing resources for the development and implementation of intervention strategies to reduce the burden of HFMD.
Keywords: Hand; Foot, and Mouth Disease; Economic Burden; Illness Costs; Vietnam
Hand, foot, and mouth disease (HFMD) is an infectious disease that mainly affects children ≤5 years of age and is caused by various serotypes of Enterovirus A, especially enterovirus A71 (EV-A71), coxsackievirus A6 (CV-A6), CV-A10, and CV-A16 [1]. Although the majority of the infections are uncomplicated, severe central nervous system and/or cardiovascular complications may occur, which can be fatal and are often associated with EV-A71 infection [2, 3]. Since 1997, HFMD has been recognized as a public heath threat because it has caused several large severe outbreaks across the Asia-Pacific region, including Cambodia, China, Malaysia, Singapore, Taiwan, and Vietnam, with an estimated incidence of >2 million recorded cases in these countries annually in recent years [4–8]. In Vietnam, since 2011, the annual incidence has averaged around 80 000 cases, with an epidemic peak occurring between 2011 and 2012 with >200 000 cases and >200 deaths [9]. However, like other diseses in Vietnam (such as dengue [8]), the number of recorded HFMD cases is considered to be under-reported because it is common that mild HFMD cases either visit private clinics for treatment or are managed at home by parents, and as such are not reported to the Ministry of Health. Undoubtedly, HFMD outbreaks have caused substantial economic burden in the communities and health care systems of the affected countries. The detailed data on economic loss posed by HFMD outbreaks, however, remain unknown. In addition to hospitalization-associated costs, especially in case of severe illness, HFMD causes additional burden for the affected families, especially nuclear families, and the societies of the endemic countries as a whole. This is because parents of affected children have to take off time from work to care for their children due to hospital admission and/or school closure. Yet, the effectiveness of current public health measures (eg, school closures) remains unknown [10].
Due to its burden, much attention has been focused on the development of HFMD vaccines [11]. While such efforts, especially multivalent vaccine development, are still ongoing, inactivated EV-A71 vaccines have been successfully developed in China [12–14] and were licensed in December 2015 [11]. The use of these vaccines is currently voluntary and has only been restricted within Mainland China.
In view of recent advances in HFMD vaccine development and the success of EV-A71 vaccine research as well as its potential benefit [11], it is anticipated that HFMD vaccines will be widely implemented in the near future. Therefore, knowledge about the economic burden of HFMD in Vietnam is essential to inform local policy makers in planning and prioritizing resources for vaccine development and implementation and outbreak response. However, such data are currently unavailable for Vietnam. Here, we report the results of a prospective descriptive hospital-based study aimed at estimating costs attributed to HFMD for all clinical severities, specific pathogens, and geographic locations in Vietnam.
METHODS
Settings
The present study was conducted at Children’s Hospital 1 (CH1), CH2, and Hospital for Tropical Diseases (HTD) in Ho Chi Minh City, Vietnam. These are tertiary referral centers for children with HFMD in Ho Chi Minh City and southern Vietnam with a catchment population of >40 million.
Patient Enrollment and Data Collection
Patient enrollment was carried out at the aforementioned hospitals during April 2016–December 2017. We screened any patient ≤16 years of age presenting to outpatient departments or admitted to the inpatient wards of CH1, CH2, or HTD with a clinical diagnosis of HFMD and, if outpatients, an illness duration of ≤3 days for enrollment in our study.
We collected information regarding demographics, clinical signs/symptoms, clinical grades, treatments, laboratory tests, length of hospital stay, outcomes, and associated economic costs from the participants. For enterovirus serotype determination, we sampled acute throat and rectal swabs at enrollment.
Information about associated economic burden was captured through hospital invoices and face-to-face interviews of the accompanying relatives. The former included information about hospital fees attributed to hospitalization, including both costs covered by health insurance and patients’ relative out-of-pocket payments. The remaining information was collected for the period before hospital admission, during hospitalization, and 7 days after discharge, including costs associated with medicine, transportation (eg, visiting private clinics and hospital admission), and accommodation for relatives during the course of their child being hospitalized, as well as work loss of relatives due to caregiving.
HFMD Clinical Grade Classification
According to the Vietnamese Ministry of Health, HFMD is clinically divided into 4 major grades: Grade 1 is assigned to patients presenting with mouth ulcers or vesicles/papules on the hands, feet, or buttocks, with or without mild fever (<39°C); Grade 2 is further divided into Grade 2A (central nervous system [CNS] involvement, myoclonus reported by parents or caregivers only, fever >39°C or ataxia), Grade 2B1 (myoclonus observed by medical staff or history of myoclonus and lethargy or pulse >130 bpm), and Grade 2B2 (ataxia, cranial nerve palsies, limb weakness, nystagmus, persistent high fever, or pulse >150 bpm); Grade 3 involves autonomic dysfunction with sweating, hypertension, tachycardia, and tachypnoea; and Grade 4 is for disease with additional cardiopulmonary compromise with pulmonary edema or shock syndrome [5, 9]. Patients with Grade 2B1 or above are considered to have severe HFMD.
Enterovirus Detection and Serotype Determination
A combination of polymerase chain reaction (PCR) and sequencing was employed to identify enterovirus serotypes causing HFMD. The PCRs and sequencing procedure were carried out as previously described [15]. In brief, a specimen from a throat swab was analyzed so as to extract viral RNA using the QIAamp viral RNA kit (QIAgen GmbH, Hilden, Germany), and then 1-step multiplex real-time reverse transcription (RT)–PCR assay was carried out to simultaneously detect enterovirus (EV) and EV-A71. All specimens infected with EV were then tested to further identify the specific serotype using VP1 PCR and sequencing analysis [16]. If 1-step multiplex real-time RT-PCR did not detect EV or EV-A71, then rectal swabs were analyzed using the same procedure applied for throat swabs [15].
Components of Economic Costs and Data Analysis
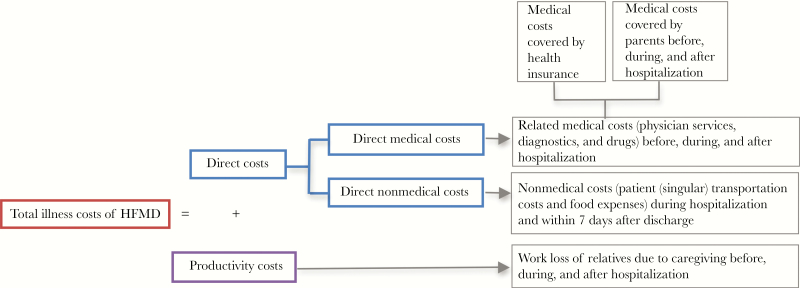
An overview of the economic burden components of HFMD is presented in Figure 1. The total illness costs consist of direct and productivity costs (Figure 1). The former includes direct medical and nonmedical costs. Direct medical costs consist of the costs associated with the goods and resources directly related to medical services over the course of the illness (eg, before hospital admission, during hospitalization, and 7 days after discharge), and direct nonmedical costs consist of the costs associated with nonmedical resources (such as transportation, food, and accommodation). The productivity costs (also known as indirect costs) include the costs attributed to work loss of relatives due to caregiving before hospital admission, during hospitalization, and 7 days after discharge. Productivity costs were estimated based on the numbers of work-off days, collected during the face-to-face interview, and average salary for those with paid work or average minimum income for those with unpaid work [17, 18]. We retrieved the average salary and minimum income of Vietnamese people for the period of the study duration from Tan et al. and Kroneman et al. [19, 20]. The productivity loss of children (such as from missed school days) was not valued.
Figure 1.
Diagram showing the individual components of the total costs attributed to hand, foot, and mouth disease. Abbreviation: HFMD, hand, foot, and mouth disease.
The analysis took the societal perspective, which quantifies all of the costs associated with an intervention/health care, regardless of who incurs them. It was not possible to stratify the costs depending on if they were incurred by the health care provider or the patients themselves. No excess mortality was considered in the calculation of productivity costs.
We summarized all values of illness costs as means and 95% confidence intervals (CIs) in US dollars with an average conversion rate of US$1 equivalent to 22 000 Vietnamese Dong for the duration of the study period (2016–2017; https://freecurrencyrates.com, accessed April 2019).
All statistical analyses were done using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY).
Estimation of Total Economic Burden of HFMD at the Nationwide Level During 2016–2017
To estimate the economic burden of HFMD at the nationwide level in Vietnam during the study period, we first calculated the number HFMD outpatients seeking medical treatment at CH1 during 2016–2017 using the formula:
where 2 is the average number of outpatient department visits per HFMD disease episode, as per the medical practice in Vietnam [21]; α is the proportion of outpatients progressing to inpatients, calculated based on the data collected from the present clinical study; and the number of outpatient visits included the data for 2016–2017 period, which were retrieved from the database record of CH1.
We then utilized the numbers of hospitalized HFMD cases at CH1 during 2016–2017, which we retrieved from the hospital database record, and the number of estimated outpatients at CH1, which was obtained using the above formula to calculate the ratios of inpatients and outpatients (β). With this obtained β value, we extrapolated the number of outpatients at the nationwide level during 2016–2017 by dividing the number of hospitalized cases across Vietnam by β. Finally, the total economic burden of HFMD in Vietnam is the sum of illness costs posed by inpatients and outpatients, which were calculated using the estimated illness costs for respective patient groups obtained in the present study and the estimated number of inpatients and outpatients across Vietnam.
Ethical Statement
The Institutional Review Boards of CH1, CH2, and HTD and the Oxford Tropical Research Ethics Committee (OxTREC) approved the study. Written informed consent was obtained from a parent or guardian of each enrolled patient.
RESULTS
Baseline Characteristics of the Patients
During April 2016–December 2017, we enrolled a total of 466 patients, including 427 in- and 39 outpatients. The baseline characteristics and clinical outcome are presented in Table 1. The majority (203/466, 43.6%) of the patients lived in Ho Chi Minh City, and only 25/466 (5.4%) were transferred from other hospitals. Four hundred thirty-six patients (93.6%) had between 2 and 4 caregivers during the course of illness. The number of patients with severe HFMD (ie, Grade 2B1 or above) accounted for 15.5%. Of 39 outpatients (Grade 1), 2 were subsequently progressed to Grade 2A and were then admitted to the hospital without additional deterioration. These 2 patients were treated as inpatients in subsequent analyses. Compared with mild patients, patients with severe HFMD were admitted to the hospitals later (Table 1) and had a longer duration of hospitalization: median number of days (range), 2 (0–7) vs 1 (0–7); P = .009; and 5 (1–11) vs 3 (1–31); P < .001; respectively (Table 1). In terms of clinical outcome, 463 (99.4%) patients recovered without complication, whereas unfavorable outcomes were recorded in 3, including pneumonia (n = 1), paralysis (n = 1), and death (n = 1). The fatal case had no enterovirus found in the collected swabs by the diagnostic workup of the present study.
Table 1.
Baseline Characteristics and Etiology of Patients Enrolled in the Study
| Characteristics | Total | Milda | Severeb | Outpatients | Inpatients | P Valuesc | P Valuesd |
|---|---|---|---|---|---|---|---|
| (n = 466) | (n = 394) | (n = 72) | (n = 37) | (n = 429) | |||
| Sex ratio, male/female | 271/195 | 226/168 | 45/27 | 16/21 | 255/174 | .42 | .055 |
| Median age (range), mo | 17.5 (4–103) | 17 (4–103) | 19 (4–58) | 22 (7–85) | 17 (4–103) | .29 | .11 |
| Transferred from other hospitals | 25 (5.4%) | 10 (2.5%) | 15 (20.8%) | 0 | 25 (5.8%) | <.001 | .25 |
| Geographic locations | |||||||
| Ho Chi Minh City, No. (%) | 203 (43.6) | 171 (43.4) | 32 (44.4) | 29 (78.4) | 174 (40.6) | .87 | .0001 |
| Other provinces, No. (%) | 263 (56.4) | 223 (56.6) | 40 (55.6) | 8 (21.6) | 255 (59.4) | ||
| Median illness days before admission (range) | 1 (0–7) | 1 (0–7) | 2 (0–7) | 1 (0–3) | 1 (0–7) | .009 | NDe |
| Median days of hospitalization (range) | 3 (1–31) | 3 (1–31) | 5 (1–11) | NA | 2 (1–30) | <.001 | NA |
| No. of caregivers per child | |||||||
| 1 | 30 (6.4) | 25 (6.3) | 5 (6.9) | 13 (35.1) | 18 (4.2) | .85 | <.001 |
| 2 | 374 (80.3) | 316 (80.2) | 58 (80.6) | 23 (62.2) | 348 (81.1) | ||
| 3 | 60 (12.9) | 51 (12.9) | 9 (12.5) | 1 (2.7) | 61 (14.2) | ||
| 4 | 2 (0.4) | 2 (0.5) | 0 | 0 | 2 (0.5) | ||
| Occupation of caregivers, No. (%) | |||||||
| Paid | 639 (66.1) | 538 (65.7) | 101 (68.2) | 34 (54.8) | 601 (66.6) | .55 | .06 |
| Unpaid | 328 (33.9) | 281 (34.3) | 47 (31.8) | 28 (35.2) | 302 (33.4) | ||
| Median No. of days off from work (range) | 12.5 (1.25–44) | 12 (1.25–44) | 15 (3.5–40) | 8 (1.25–15.5) | 13 (2.5–44) | <.001 | .004 |
| Highest clinical grade, No. (%) | |||||||
| Grade 1 | 39 (8.3) | 39 (8.3) | NA | 37 (100) | 2 (0.5) | NA | NA |
| Grade 2A | 355 (76.2) | 355 (76.2) | NA | 0 | 355 (82.8) | NA | NA |
| Grade 2B1 | 24 (5.2) | NA | 24 (5.2) | 0 | 24 (5.6) | NA | NA |
| Grade 2B2 | 12 (2.6) | NA | 12 (2.6) | 0 | 12 (2.8) | NA | NA |
| Grade 3 | 36 (7.7) | NA | 36 (7.7) | 0 | 36 (8.4) | NA | NA |
| Outcome at discharge, No. (%) | |||||||
| Full recovery | 463 (99.4) | 392 (95.5) | 71 (98.6) | 37 (100) | 426 (99.3) | .054 | .88 |
| Recovery with complicationf | 2 (0.4) | 2 (0.5) | 0 | 0 | 2 (0.5) | ||
| Deathg | 1 (0.2) | 0 | 1 (1.4) | 0 | 1 (0.2) | ||
| Etiology, No. (%) | |||||||
| EV-A71 | 90 (19.3) | 58 (4.7) | 32 (44.4) | 9 (24.3) | 81 (18.9) | <.001 | .079 |
| CV-A6 | 71 (15.2) | 67 (17.0) | 4 (5.6) | 6 (16.2) | 65 (15.2) | ||
| CV-A10 | 43 (9.2) | 40 (10.2) | 3 (4.2) | 1 (2.7) | 42 (9.8) | ||
| CV-A16 | 32 (6.9) | 28 (7.1) | 4 (5.6) | 1 (2.7) | 31 (7.2) | ||
| Other EVs | 109 (23.4) | 98 (24.9) | 11 (15.3) | 14 (37.8) | 95 (22.1) | ||
| PCR negative | 121 (26) | 103 (26.1) | 18 (25) | 6 (16.2) | 115 (26.8) |
Abbreviations: EVs, enteroviruses; ND, not done; PCR, polymerase chain reaction.
aGrade 1/2A.
bGrade 2B1 or above.
cComparison between severe and mild cases.
dComparison between out- and inpatients.
eNot done (because of enrollment bias; see “Patient Enrollment and Data Collection” for more details).
fPneumonia (n = 1) and prolonged fever and diaphragm paralysis (n = 1).
gThe fatal case was of unknown etiology.
Results of Etiological Investigations
The results of the enteroviral diagnostic are presented in Table 1 and Supplementary Table 1. Enteroviruses were identified in 74% of the 466 enrolled patients with EV-A71, CV-A6, CV-A10, and CV-A16, which were the most common viruses identified, accounting for 90/466 (19.3%), 71/466 (17%), 43/466 (9.2%), and 32/466 (6.9%) patients, respectively (ie, 50.6% of total enrolled cases). Although the detection rates of these common causes were similar among inpatients and outpatients, the detection rates were statistically different between inpatients with severe and mild HFMD, with EV-A71 being more commonly detected in patients with severe clinical phenotypes (Supplementary Table 1), supporting previous reports linking EV-A71 infection to severe disease [1]. Notably, EV-A71 PCR-positive patients were older than those infected with CV-A6/10/16 and had a longer duration of hospitalization and later hospital admissions (Supplementary Table 1).
Illness Costs Attributed to HFMD: A General Overview
The detailed economic burden associated with HFMD for all enrolled patients and for different patient groups is presented in Table 2. Overall, there were considerable differences in direct medical costs between groups of patients with different clinical severities and patients infected with different pathogens (ie, EV-A71 vs non-EV-A71). In contrast, the direct nonmedical and productivity costs were relatively similar between patient groups. The mean total economic burden per case was estimated to be US$400.80 (95% CI, $353.80–$448.90), of which the total direct costs accounted for 69.7% (mean, US$279.30; 95% CI, $235.30–$328.90), whereas direct medical costs accounted for 51.1% (mean, US$204.70; 95% CI, $162.10–$253.10).
Table 2. .
Economic Burden Associated With HFMD in Vietnam
| Patient Groupsa | Direct Medical Costs, US$ | Direct Nonmedical Costs, US$ | Total Direct Costs, US$ | Productivity Costs, US$ | Total Economic Costs, US$ b | P Valuesc |
|---|---|---|---|---|---|---|
| All patients (n = 466) | 204.7 (162.1–253.1) | 74.6 (68.8–80.9) | 279.3 (235.3–328.9) | 121.5 (116.4–126.2) | 400.8 (353.8–448.9) | NA |
| Geographic locations | ||||||
| HCMC (n = 203) | 221.9 (145.5–300.0) | 46.3 (41.7–50.8) | 268.2 (190.1–346.7) | 113.5 (106.7–120.4) | 381.7 (302.9–464.5) | .50 |
| Other provinces (n = 263) | 191.4 (134.9–254.6) | 96.5 (88.0–105.6) | 287.9 (230.7–351.6) | 127.6 (121.1–134.4) | 415.5 (355.8–480.9) | |
| In/outpatients | ||||||
| Outpatients (n = 37) | 16.8 (13.5 –19.5) | 14.5 (8.2–22.3) | 31.2 (24.9–38.4) | 63.6 (54.1–73.9) | 94.8 (81.7–108.7) | <.001 |
| Inpatients (n = 429) | 220.9 (173.3–271.4) | 79.8 (73.5–86.7) | 300.7 (250.7–351.2) | 126.5 (121.8–131.6) | 427.2 (376.8–478.6) | |
| Disease severitiesd | ||||||
| Mild (n = 357) | 50.6 (41.7–63.5) | 74.8 (69.7–80.0) | 125.4 (113.9–139.9) | 120.3 (115.5–125.7) | 245.8 (231.9–261.3) | <.001 |
| Severee (n = 72) | 1065.2 (883.5–1269.4) | 104.6 (83.7–133.6) | 1169.8 (986.1–1368.0) | 157.0 (143.7–171.0) | 1326. 7 (1144.6–1528.9) | |
| Grade 2b1 (n = 24) | 342.8 (142.3–578.5) | 109.1 (71.9–152.8) | 451.9 (241.2–702.5) | 145.3 (123.3–167.2) | 597.2 (396.3–839.8) | ND |
| Grade 2b2 (n = 12) | 795.2 (521.5–1100.7) | 86.3 (66.9–104.0) | 881.4 (609.1–1185.5) | 163.3 (133.2–189.8) | 1045.0 (772.6–1341.9) | ND |
| Grade 3 (n = 36) | 1636.7 (1412.3–1865.2) | 107.7 (77.2–157.2) | 1744.5 (1539.7–1965.4) | 162.4 (140.7–183.7) | 1861.0 (1687.8–2088.9) | ND |
| Pathogens | ||||||
| EV-A71 (n = 90) | 502.2 (349.4–669.8) | 74.8 (62.1–90.7) | 577.0 (422.8–745.8) | 128.9 (117.7–140.6) | 706.0 (544.1–878.3) | <.001 |
| CV-A6/10/16 (n = 146) | 96.5 (53.3–150. 2) | 72.6 (64.6–81.6) | 169.1 (125.3–225.6) | 118.1 (109.1–125.9) | 287.1 (238.9–348.3) | |
| Other EVs (n = 109) | 118.2 (62.7–194.4) | 70.2 (56.9–87.1) | 188.4 (128.3–268.5) | 113.1 (104.1–122.7) | 301.5 (238.0–382.0) | <.001 |
Abbreviations: CI, confidence interval; EVs, enteroviruses; HFMD, hand, foot, and mouth disease; ND, not done.
aData are presented as mean (95% CI).
bSee Figure 1.
cComparison made for total economic costs.
dInpatients only.
eGrade 2b1 or above.
Economic Burden of HFMD by Geographic Location, Disease Severity, and Pathogen
In terms of geographic locations, although they were not statistically significant, the associated economic costs of patients coming from other provinces were slightly higher than those of patients living in HCMC (mean, US$415.50; 95% CI, $355.80–$480.90; vs mean, US$381.70; 95% CI, $302.90 –$464.50), which was attributed to the higher direct nonmedical costs (such as travel costs) of patients coming from other provinces rather than HCMC (Table 2).
As expected, patients with severe HFMD were associated with significantly higher total illness costs compared with those with mild disease (including outpatients and inpatients with Grade 2A) (Table 2), the majority of which were attributed to direct medical costs. Additionally, of the severe patients (ie, Grade 2B1 and above), the higher the clinical grade, the greater illness costs were; the estimated mean of the total illness costs (95% CI) was US$597.20 ($396.30–$839.80) for Grade 2B1, $1045.00 ($772.60–$1341.90) for Grade 2B2, and $1861.00 ($1687.80–$2088.90) for Grade 3.
In terms of enterovirus serotypes and associated costs, because EV-A71 was predominantly found in patients with severe clinical phenotypes, as a consequence the estimated mean illness costs attributed to EV-A71 were higher than those of other HFMD-causing enterovirus serotypes (including CV-A6, CV-A10, and CV-A16) (Table 2).
Total Economic Burden of HFMD at the Nationwide Level in Vietnam During 2016–2017
During the study period, Vietnam recorded a total of 94 313 hospitalized HFMD cases (47 428 cases in 2016 and 46 885 cases in 2017) [22]. Accordingly, the estimated number of outpatients in Vietnam during 2016–2017 is 532 397. This would be equivalent to a nationwide total economic burden posed by HFMD cases presenting to hospitals in Vietnam of US$90 761 749 (95% CI, $79 033 973–$103 009 756) (Table 3).
Table 3. .
Estimated Total Economic Burden of HFMD Cases Presenting to Hospitals in Vietnam During 2016–2017
| HFMD Cases | No. |
|---|---|
| Reported outpatient visits at CH1a | 95 405 |
| Estimated number of outpatients at CH1b | 45 256 |
| Hospitalized cases at CH1a | 8017 |
| Hospitalized cases in Vietnam | 94 313 |
| Estimated number of outpatients in Vietnamc | 532 397 |
| Illness costs in US$ | Mean (95% CI) |
| Outpatients | 50 471 235 (43 496 835–57 871 554) |
| Inpatients | 40 290 514 (35 537 138–45 138 202) |
| Total | 90 761 749 (79 033 973–103 009 756) |
Abbreviations: CH1, Children’s Hospital 1; CI, confidence interval; HFMD, hand, foot, and mouth disease.
aDerived from database record of CH1.
bThe proportion of outpatients progressing to inpatients (α): 2/39 = 0.05128. cThe ratio of inpatients and outpatients (β): 8017/45 256 = 0.17715.
DISCUSSION
Despite the public heath threat of HFMD, scarce information about its economic burden is available to support policy makers in endemic countries in prioritizing the resources for the development and implementation of intervention strategies, especially vaccines. Likewise, no data exist for the economic burden of HFMD in Vietnam. Here, we describe the results of a prospective multi-hospital-based study during 2016–2017, aiming at estimating the economic burden of HFMD in Vietnam, where HFMD has become a major clinical problem since 2005, representing the first report about illness costs of HFMD in Vietnam. The results show that HFMD caused substantial economic burden in Vietnam, corresponding to estimated costs of US$90 761 749 (95% CI, $79 003 973–$103 009 756) for the period between 2016 and 2017, and that illness costs varied between enteroviruses and disease severities. EV-A71 caused the highest economic burden, especially if the disease was severe, and the higher costs were mostly attributed to the direct medical costs. However, in contrast to the fluctuations of the direct medical costs, the indirect medical costs and productivity losses were similar across the clinical phenotype groups. Around 50% of the HFMD economic burden is out-of-pocket payments. This is a substantial burden at the national level, especially for a developing country like Vietnam.
Although comparison of the illness costs between studies conducted in different countries has limitations due to the differences in study designs and social/economic statuses, our results are in agreement with previous reports, including those from China and Taiwan [23–25]. A recent study from China reported total costs for outpatients and inpatients with mild and severe HFMD of US$196 (95% CI, $75–$318), US$990 (95% CI, $431–$1549), and US$90 761 749 (95% CI, $79 003 973–$103 009 756). Although the data from China were retrospectively obtained through telephone interview of the participants and may therefore not accurately estimate the disease burden, the higher estimated costs for the respective disease groups in China may be due to the difference in social economic statuses between Vietnam and China (http://www.worldbank.org). Additionally, in line with a recent report from Taiwan [25], our results showed that EV-A71 infection resulted in higher illness costs than other HFMD-causing enterovirus serotypes did, attributable to the predominance of EV-A71 found among patients with severe HFMD. The productivity costs were similar between groups of patients infected with different enterovirus serotypes.
The results of our etiological investigation showed that although HFMD is diverse in etiology, EV-A71 was predominantly found in patients presenting with Grade 2B1 or above (ie, severe HFMD). As such, our findings are consistent with previous etiological studies in the region [5, 7]. Of note, existing serological data obtained from vaccine trials and in vitro experiments suggest that cross-neutralization against heterogeneous serotypes of HFMD-causing enteroviruses is absent and/or insufficient to provide long-term protection [11, 12, 14]. Collectively, our data and others’ data indicate that multivalent vaccines are needed to control HFMD [11]. However, although the development of such multivalent vaccines might be highly challenging because of the unprecedented emergence of HFMD-causing viruses [26], effective EV-A71 vaccine(s) are urgently needed, as such interventions would substantially reduce the major (~50%) burden caused by severe HFMD.
The strengths of our report include that it was prospectively conducted at major hospitals in Ho Chi Minh City, Vietnam, with a catchment population of >40 million, and that the data on illness costs were comprehensively captured from both in- and outpatients for the periods before hospital admission, during hospitalization, and 1 week after discharge. Few economic burden studies in this area have reported at this level of details [8].
Our study has some limitations. First, we only based our economic burden estimations at a nationwide level on cases presenting to hospitals for medical treatments, but we did not take into account those seeking medical treatments at private clinics or being managed at home by parents. We may therefore have underestimated the economic burden of HFMD in Vietnam. Second, we extrapolated the total costs using our estimated illness costs data obtained from major hospitals in Ho Chi Minh City, an urban setting, whereas around 50% of HFMD patients are managed at provincial hospitals, which may result in lower hospital costs. We may therefore have overestimated the economic burden of HFMD at the nationwide level. Third, despite our holistic approach, it is not always straightforward to accurately value productivity losses, which are subjected to variations in the income of the population, particularly those with unpaid work. Collectively, our results, especially the data on economic burden at the nationwide level, should be interpreted with caution.
To summarize, for the first time, we show that HFMD causes substantial annual economic burden in Vietnam. An effective EV-A71 vaccine could substantially reduce the majority of severe cases, thereby significantly reducing the illness costs from severe disease. Our results will be helpful for policy makers in Vietnam in prioritizing resources for the development and implementation of intervention strategies, including vaccines, to reduce the burden of HFMD.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Ms. Le Kim Thanh for her logistic support. We are indebted to the patients for their participation in this study.
Financial support. This study was funded by the Wellcome Trust of Great Britain (106680/B/14/Z and 204904/Z/16/Z).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hoang MTV, Nguyen TA, Tran TT, et al. . Clinical and aetiological study of hand, foot and mouth disease in southern Vietnam, 2013-2015: inpatients and outpatients. Int J Infect Dis 2019; 80:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ooi MH, Wong SC, Lewthwaite P, et al. . Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 2010; 9:1097–105. [DOI] [PubMed] [Google Scholar]
- 3. Huang CC, Liu CC, Chang YC, et al. . Neurologic complications in children with enterovirus 71 infection. N Engl J Med 1999; 341:936–42. [DOI] [PubMed] [Google Scholar]
- 4. Sabanathan S, Tan le V, Thwaites L, et al. . Enterovirus 71 related severe hand, foot and mouth disease outbreaks in South-East Asia: current situation and ongoing challenges. J Epidemiol Community Health 2014; 68:500–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nhan LNT, Hong NTT, Nhu LNT, et al. . Severe enterovirus A71 associated hand, foot and mouth disease, Vietnam, 2018: preliminary report of an impending outbreak. Euro Surveill 2018; 23:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solomon T, Lewthwaite P, Perera D, et al. . Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778–90. [DOI] [PubMed] [Google Scholar]
- 7. Huang J, Liao Q, Ooi MH, et al. . Epidemiology of recurrent hand, foot and mouth disease, China, 2008–2015. Emerg Infect Dis 2018; 24:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hung TM, Clapham HE, Bettis AA, et al. . The estimates of the health and economic burden of dengue in Vietnam. Trends Parasitol 2018; 34:904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khanh TH, Sabanathan S, Thanh TT, et al. . Enterovirus 71-associated hand, foot, and mouth disease, Southern Vietnam, 2011. Emerg Infect Dis 2012; 18:2002–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Y, Badaruddin H, Lee VJ, et al. . The effect of school closure on hand, foot, and mouth disease transmission in Singapore: a modeling approach. Am J Trop Med Hyg 2018; 99:1625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang CY, Liu CC. Recent development of enterovirus A vaccine candidates for the prevention of hand, foot, and mouth disease. Expert Rev Vaccines 2018; 17:819–31. [DOI] [PubMed] [Google Scholar]
- 12. Zhu F, Xu W, Xia J, et al. . Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370:818–28. [DOI] [PubMed] [Google Scholar]
- 13. Li R, Liu L, Mo Z, et al. . An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370:829–37. [DOI] [PubMed] [Google Scholar]
- 14. Zhu FC, Meng FY, Li JX, et al. . Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381:2024–32. [DOI] [PubMed] [Google Scholar]
- 15. Thanh TT, Anh NT, Tham NT, et al. . Validation and utilization of an internally controlled multiplex real-time RT-PCR assay for simultaneous detection of enteroviruses and enterovirus A71 associated with hand foot and mouth disease. Virol J 2015; 12:85:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 2006; 44:2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krol M, Brouwer W. Unpaid work in health economic evaluations. Soc Sci Med 2015; 144:127–37. [DOI] [PubMed] [Google Scholar]
- 18. Krol M, Brouwer W. How to estimate productivity costs in economic evaluations. Pharmacoeconomics 2014; 32:335–44. [DOI] [PubMed] [Google Scholar]
- 19. Tan le V, Tuyen NT, Thanh TT, et al. . A generic assay for whole-genome amplification and deep sequencing of enterovirus A71. J Virol Methods 2015; 215-216:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroneman A, Vennema H, Deforche K, et al. . An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol 2011; 51:121–5. [DOI] [PubMed] [Google Scholar]
- 21. Ministry of Health of Vietnam . Guidelines for Diagnosis and Management of Hand, Foot and Mouth Disease. Ministry of Health, Vietnam. 2011. [Google Scholar]
- 22. Van HMT, Anh NT, Hong NTT, et al. . Enterovirus A71 phenotypes causing hand, foot and mouth disease, Vietnam. Emerg Infect Dis 2019; 25:788–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng Y, Jit M, Wu JT, et al. . Economic costs and health-related quality of life for hand, foot and mouth disease (HFMD) patients in China. PLoS One 2017; 12:e0184266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang ZL, Xia AM, Li YF, et al. . Socioeconomic burden of hand, foot and mouth disease in children in Shanghai, China. Epidemiol Infect 2016; 144:138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu DP, Wang TA, Huang WT, et al. . Disease burden of enterovirus infection in Taiwan: implications for vaccination policy. Vaccine 2016; 34:974–80. [DOI] [PubMed] [Google Scholar]
- 26. Anh NT, Nhu LNT, Van HMT, et al. . Emerging coxsackievirus A6 causing hand, foot and mouth disease, Vietnam. Emerg Infect Dis 2018; 24:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.