ABSTRACT
Esophageal cancer is an aggressive and highly lethal malignancy that has a high death-to-incidence ratio approaching 0.90. We present a 60-year-old man with a history of Barrett's esophagus, presented with dysphagia. An upper endoscopy with biopsy confirmed invasive esophageal adenocarcinoma (EAC). Workup, including positron emission tomography scan, showed no evidence of metastasis. A preoperative colonoscopy showed a nodule in the ascending colon that was proven later to be a metastatic lesion from the esophageal primary tumor. Esophageal adenocarcinoma with an isolated colonic metastasis is an extremely rare presentation of esophageal metastasis. These metastatic lesions may not be detected by the positron emission tomography scan.
INTRODUCTION
Esophageal cancer is the eighth most common cancer and the sixth most common cause of cancer-related deaths worldwide.1 The incidence of esophageal cancer in the United States between 2001 and 2015 was 4.7 new cases per 100,000 people per year, regardless of gender.2 Esophageal adenocarcinoma (EAC) composes up to 80% of esophageal cancer in the United States, mainly affecting the non-Hispanic white population.1 Between 1975 and 2004, the incidence of esophageal cancer in white men increased from 5.76 to 8.34 per 100,000 people per year, mainly because of a 463% increase in EAC.3 Since the 1970s, the incidence of esophageal squamous cell carcinoma in the United States has been declining, whereas the incidence of EAC has been increasing.4 According to the National Cancer Institute, EAC is the fastest growing cancer in the United States (rate increased from 4 people per million in 1975 to 23 people per million in 2001).1
Esophageal cancer has an overall poor prognosis because of the high rate of advanced stage at the time of diagnosis and the lack of effective curative treatment for patients with advanced stage.5 The average survival rate of esophageal cancer is 10.2 months, with a mortality approaching 90%.6,7 Esophageal cancer has a 5-year survival rate of less than 20%, which further drops to 4% when complicated with distal metastasis.6 Currently, the only curative treatment is complete resection, making early diagnosis and proper staging highly important in the management of this malignancy. The typical pattern of disease progression once the primary cancer has invaded through the mucosal layer into the deeper esophageal tissue is local regional lymph node metastasis and eventually the tumor spreads to distant sites.
CASE REPORT
A 60-year-old man with a history of Barrett's esophagus presented with dysphagia and weight loss of a few months duration. His physical examination was unremarkable. An upper esophagogastroduodenoscopy demonstrated a fungating obstructive mass in the distal esophagus. A biopsy of the mass showed intestinal-type invasive adenocarcinoma, with foci of signet ring cell features. Staging with endoscopic ultrasound (EUS) and chest/abdomen computed tomography (CT) scan was consistent with T3N2M0. The patient was treated with chemotherapy that initially comprised taxotere, cisplatin, and 5-fluorouracil, which was then switched to a single-agent irinotecan after developing neutropenic sepsis. He also received 5,040 Gy of radiation treatment to the esophageal tumor with the long-term plan for surgical resection. The patient developed atrial fibrillation and stroke, resulting in a delay in his surgical treatment.
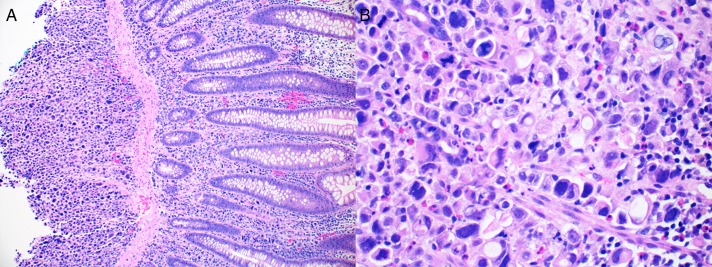
One year after presentation, restaging was performed using positron emission tomography (PET) scan that showed focal hypermetabolic activity at and just above the gastroesophageal junction focally, standardized uptake value (SUV) max 7.45 with no evidence of regional lymph nodes or distant metastasis. Esophagectomy with colonic interposition was considered; therefore, a preoperative colonoscopy was performed, which demonstrated a colonic nodule with central ulceration in the ascending colon (Figure 1). Biopsies of the ascending colon ulcer showed poorly defined sheets of malignant cells infiltrating through the lamina propria and submucosa of otherwise unremarkable colonic mucosa (Figure 2). The malignant cells are characterized by marked nuclear pleomorphism, dark condensed chromatic with variable amounts of pale amphophilic to clear cytoplasm and focal signet ring features (Figure 2). The initial diagnostic biopsies of the patient's esophageal primary tumor were found to be morphologically identical to the isolated colonic metastasis.
Figure 1.
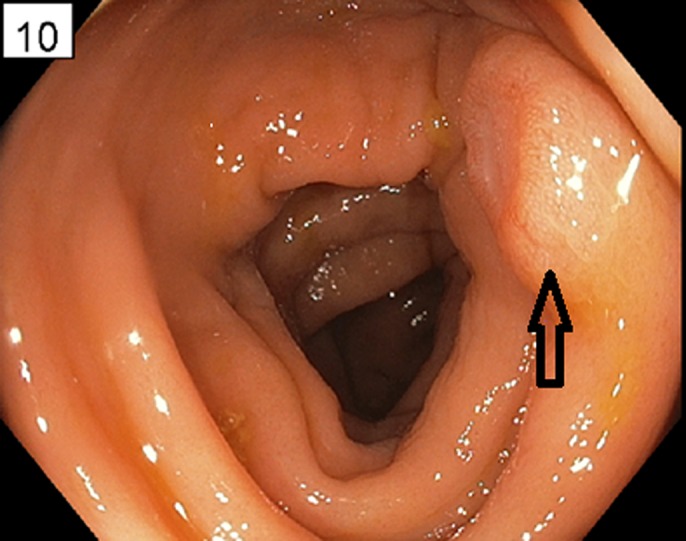
Submucosal ulcer in the ascending colon visualized by endoscopy. Note the intact mucosa and ulcerated appearance.
Figure 2.
Histopathologic findings of ulcer in the ascending colon showing (A) sheets of poorly differentiated malignant epithelial cells infiltrating through the lamina propria and submucosa of colonic mucosa with unremarkable colon epithelium (100×). (B) View of the malignant cells with marked nuclear pleomorphism, disordered microarchitecture, vacuolated cytoplasm, and focal signet ring cell features. The previous esophageal primary had many morphologic similarities to the colonic ulcer (400×).
The patient with stage IV EAC was treated with palliative chemotherapy and intermittent cryotherapy for dysphagia palliation. He survived 14 months after the diagnosis of his distal metastasis.
DISCUSSION
Esophageal cancer is one of the most highly lethal malignancies worldwide. Patients with esophageal cancer often present with advanced noncurable disease and subsequent high mortality. The usual pattern of esophageal cancer metastasis starts with regional lymph nodes and subsequent distant spreading to the liver, lungs, and adrenal glands. The spreading of metastasis to uncommon sites has been reported, which consequently influenced the diagnosis, staging, and management.
Understanding esophageal cancer and its distant metastatic pattern is crucial because these metastases are predominantly responsible for the high mortality of esophageal cancer. Shaheen et al discussed the esophageal cancer metastasis to unexpected sites in a systematic review that included 10,049 articles.6 The authors found that esophageal cancers are synchronous in 42% (survival rate of 13 months), metachronous in 58% (survival rate of 6.1 months), isolated in 53.5%, and multiple in 46.5%.6
Staging is crucial to identify patients with potential for surgical resection, the only curative treatment currently available. The current standard of care is staging with CT chest, abdomen, and pelvis, and if there is no evidence of distant metastasis, EUS is performed for local regional staging. EUS limitations include difficulty in passing the endoscope beyond the obstructive tumor mass and an inability to detect distant metastatic lesions. CT limitations include a relatively low staging sensitivity.7
Fluorodeoxyglucose-PET (FDG-PET) scan has a higher sensitivity for detecting metastatic esophageal cancer than EUS and CT combined. For diagnosing stage IV esophageal cancer, FDG-PET scan is superior to a combination of CT and EUS in accuracy (82% with FDG-PET vs 64% with EUS and CT together).7 Using PET scan in esophageal cancer can result in restaging and reclassification of many patients with EAC.5 However, the relatively good sensitivity of PET scan diminishes with the smaller size metastatic lesion. Furthermore, larger metastatic tumors with low or no uptake can also be missed by the PET scan.7
In this case, the colonic metastatic lesion was missed by the PET scan. This may raise a question about the sensitivity of PET scan in detecting isolated distant metastases and whether alternative modalities may be needed in this regard.
We believe this reported case is extremely rare and could be confused with other diagnoses. Bollschweiler et al studied the correlation between colonic polyps and EAC.8 His study showed an increased prevalence of colonic neoplasm in patients with EAC compared with age-matched control subjects, and that was explained by the similarity of risk factors. Other studies showed that colorectal cancers comprise up to 16% of esophageal secondary primary malignancies.9 However, no previous studies reported isolated EAC metastasis to the colon. There is a reported case of EAC metastasis to the colon, liver, and para-aortic lymph nodes.10 In that case, the PET scan picked the lymph nodes and liver lesions but not the colon nodules.
Colonoscopy is not usually a part of the workup of EAC patients. In this situation, a colonoscopy was requested by the surgical team because a colonic interposition was being considered. This identified the only site of distant metastasis that resulted in a significant change in the stage and management of the patient. Although colonoscopy cannot be recommended as part of the workup of esophageal cancer, this case shows this rare phenomenon of isolated colonic metastasis from EAC. Given that colon cancer is also adenocarcinoma, certain features are presented in this case to highlight the different histologic features of primary colonic adenocarcinoma and how they differ from metastasis to the colon. Features that lead to classifying this lesion as a metastasis include the submucosal location of the cancer with intact overlying mucosa and histologic features identical to the original esophageal primary such as the signet cell features. Although colonoscopy is not a part of esophageal cancer staging, age-appropriate cancer screening in patients who are being considered for major surgical resection such as esophageactomy is reasonable. In this case, the identification of the colonic metastasis led to proper staging and avoidance of a major surgical intervention that would not have been curative.
DISCLOSURES
Author contributions: A. Alshati wrote the manuscript and is the article guarantor. M. Khosla wrote the manuscript. H. Niu revised the manuscript. T. Kachaamy is the senior author and revised the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Napier KJ, Scheerer M, Misra S. Esophageal cancer: A review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6(5):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel N, Benipal B. Incidence of esophageal cancer in the United States from 2001–2015: A United States cancer statistics analysis of 50 states. Cureus. 2018;10(12):e3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. The Lancet. 2013;381(9864):P400–12. [DOI] [PubMed] [Google Scholar]
- 4.Gibson MK. Epidemiology and Pathobiology of Esophageal Cancer. UpToDate, 2018. UpToDate. Waltham, MA: UpToDate Inc. https://www.uptodate.com. Accessed on October 26, 2018. [Google Scholar]
- 5.Tanaka T, Fujita H, Matono S, et al. Outcomes of multimodality therapy for stage IVB esophageal cancer with distant organ metastasis (M1-Org). Dis Esophagus. 2010;23(8):646–51. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen O, Ghibour A, Alsaid B. Esophageal cancer metastases to unexpected sites: A systematic review. Gastroenterol Res Pract. 2017;2017:1657310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang GY, Wagner TD, Jobe BA, Thomas CR. The role of positron emission tomography in esophageal cancer. Gastrointest Cancer Res. 2008;2(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Bollschweiler E, Schloesser T, Leers J, Vallböhmer D, Schäfer H, Hölscher AH. High prevalence of colonic polyps in white males with esophageal adenocarcinoma. Dis Colon Rectum. 2009;52(2):299–304. [DOI] [PubMed] [Google Scholar]
- 9.Baeg MK, Choi MG, Jung YD, et al. Esophageal squamous cell carcinoma patients have an increased risk of Coexisting colorectal neoplasms. Gut Liver. 2016;10(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patek B, Shah HN, Shah S, for Lehigh Valley Health Network. Primary esophageal adenocarcinoma with colon metastases after esophagectomy. https://scholarlyworks.lvhn.org/cgi/viewcontent.cgi?article=1769&context=medicine. Accessed on October 26, 2018.