Abstract
OBJECTIVE—
To determine autism spectrum disorder (ASD) prevalence within our pediatric type 1 diabetes (T1D) clinic population and determine clinical characteristics and technology use by individuals with both ASD and T1D compared to matched controls with T1D alone and compared to our overall pediatric T1D clinic.
RESEARCH DESIGN AND METHODS—
Medical chart review revealed 30 individuals with both ASD and type 1 diabetes (ASD+T1D). Controls (n=90) were matched for age, sex, race/ethnicity, and T1D duration. ASD+T1D was compared to both matched controls and the pediatric T1D clinic population.
RESULTS—
ASD prevalence in the pediatric T1D population was 1.16% (CI 0.96–1.26). Compared to the T1D clinic, ASD+T1D had more males (93% vs 52%; p<0.0001), lower hemoglobin A1c (HbA1c) (8.2% vs 8.9%; 66 vs 74 mmol/mol; p=0.006), and lower insulin pump (CSII) use (37% vs 56%; p<0.0001). No differences were found between ASD+T1D and matched controls in HbA1c or blood glucose checks per day. The ASD+T1D group was less likely to use CSII than matched controls (37% vs 61%; p=0.03). HbA1c did not change after CSII initiation in ASD+T1D, but increased for matched controls.
CONCLUSIONS—
Prevalence of ASD in the pediatric T1D population is comparable to the general population in Colorado. Individuals with ASD may experience barriers limiting CSII use, but achieve equivalent glycemic control compared to those without ASD. CSII may be more effective in maintaining lower HbA1c over time in those with ASD than in those without ASD.
Keywords: Autistic Disorder, Diabetes Mellitus, Type 1, Pediatrics, Disease Management
Type 1 Diabetes (T1D) is one of the most common chronic diseases of childhood (1,2), requiring complex management and multiple daily interventions to administer insulin and monitor blood sugars. Psychological comorbidities such as depression have been extensively studied in T1D. However, existing research on T1D and developmental disorders, specifically autism spectrum disorder (ASD), is extremely sparse.
Impairments in social behavior and reciprocal social interactions are hallmark features of ASD (3). Individuals with ASD demonstrate delayed language development, deficits in social-emotional reciprocity, eye contact avoidance, and repetitive or restrictive behaviors and preferences (3). An ASD diagnosis now encompasses several diagnoses including autistic disorder, Asperger’s syndrome, and pervasive developmental disorder (3). Reported prevalence of ASD is high, although it is lower than the prevalence of T1D (4,5). Recent data from the Autism and Developmental Disabilities Monitoring Project found that 14.6 per 1,000 children are diagnosed with ASD in the United States (4) with a male-to-female ratio of 4.5, but ranging from 2.7 to 7.2 by state (4,6,7). In Colorado, the prevalence of ASD is 10.8 per 1,000 children (95% CI 9.8–19.0), or 1.08%, with a male-to-female ratio of 4.1 (95% CI 3.2–5.2) (4).
Whether there is an increased risk of ASD in individuals with T1D compared to the general population is unclear, as existing study findings are mixed (8–10). For example, studies in Canada and peninsular Italy found a greater prevalence of ASD in children with T1D (9). In contrast, studies in Finland and Sardinia have found no differences (8,10).
Several studies demonstrate differences in glycemic management, use of T1D technology, and adherence to T1D management in individuals with comorbid psychological disorders, such as anxiety, attention-deficit/hyperactivity disorder, intellectual disabilities, and Down Syndrome (11–16). Generally, glycemic management is worse in those with mental health conditions (11–13). Meanwhile those with Down Syndrome and T1D had a less intensive insulin regimen, but similar HbA1c compared to controls with T1D alone (14). Overall, there is a distinct lack of research on the management of diabetes in individuals with intellectual disability and developmental delays specifically. In a review of diabetes in people with an intellectual disability, the authors recommend further research to inform medical practices in treating diabetes and intellectual disability (15).
Few studies have examined the potential differences in management of chronic diseases in those with ASD. One study investigated the connection between ASD and asthma, which revealed better asthma-related outcomes in those with ASD, including lower odds of exacerbations and airway obstruction, and better force expiratory volume, but higher controller treatment levels (17). The Autism Treatment Network comments that communication difficulties (i.e. being nonverbal) could hinder the diagnosis and treatment of other physical conditions (18). No studies have examined demographic, clinical, and adherence characteristics of individuals diagnosed with T1D and comorbid ASD in the United States; nor have any studies made comparisons to age-matched controls. It is also unclear whether having comorbid ASD affects uptake of technology, whether due to potential lack of provider recommendation or potential parental or patient concerns of sensory issues or safety.
The aims of this study were to 1) determine the prevalence of ASD in the T1D pediatric population in a large Colorado diabetes clinic compared to the prevalence of ASD in the general Colorado pediatric population; 2) compare demographic, clinical, and T1D characteristics in those diagnosed with comorbid ASD and T1D compared to the T1D clinic population and matched controls; 3) determine differences in use of T1D technology, such as insulin pumps (CSII) and continuous glucose monitors (CGM), in ASD versus the T1D clinic population and matched controls; and 4) compare changes in HbA1c before and after initiation of CSII in those with ASD and matched controls.
RESEARCH DESIGN AND METHODS:
Recruitment of Individuals/Data Collection:
Procedures.
This study comprised three groups of participants that were identified through retrospective chart review.
ASD+Type 1 Diabetes:
The ASD+T1D group included individuals with medical record comorbid diagnoses of ASD and T1D (n=30; 28 male). This study was conducted after DSM-5 was released, but before changes were made in ICD codes. Thus, the electronic medical system still used ICD-9 codes and DSM-IV criteria to code ASD diagnoses. ICD-9 codes were used to identify individuals with ASD from all those with T1D who received care at the Barbara Davis center between June 2014 and June 2015. Autistic disorder, Asperger’s syndrome, pervasive developmental disorder, childhood disintegrative disorder were considered separate disorders according to the DSM-IV diagnostic criteria. Thus, we included all four diagnoses as well as autism spectrum disorder in our inclusion criteria. Consistent with DSM-IV classifications, autistic disorder (n=13), Asperger’s syndrome (n=10), pervasive developmental disorder (n=1), and childhood disintegrative disorder were included in the definition of ASD (3); six had a diagnosis of ASD, which has the same ICD-9 code as autistic disorder. Caregivers were contacted by phone or in clinic to confirm the ASD diagnosis and rank the severity of their child’s condition and 13 responded, confirming the diagnosis and qualifying the severity of ASD. The other 17 did not respond, but the ASD diagnosis was consistently recorded in their medical record. Of those who responded, 53.8% reported mild ASD symptoms and 46.2% reported moderate ASD symptoms. None reported severe ASD symptoms.
General Type 1 Diabetes Clinic Population:
Individuals with a diagnosis of T1D, ages 18 months to 18 years were identified from all individuals who received care at the Barbara Davis Center for Diabetes between June 2014 and June 2015 (N=2597). Eighteen months was used as the lower age range as ASD is not considered diagnosable before 18 months of age.
Matched Controls:
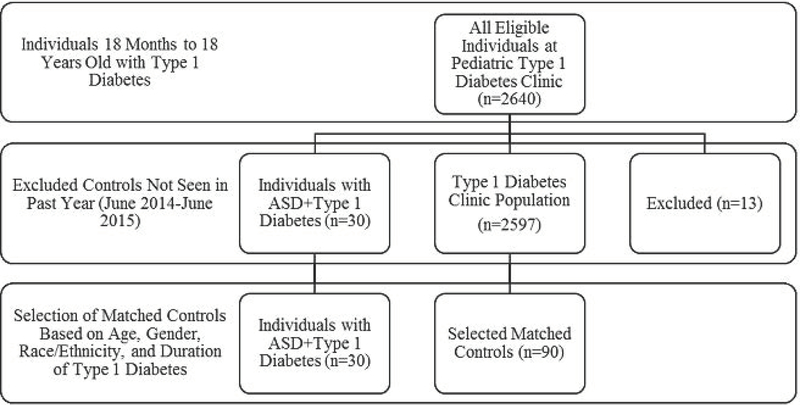
Participants with ASD+T1D were matched to individuals without cognitive impairment from the general population with T1D at the Barbara Davis Center clinic according to age, sex, race and ethnicity, and T1D duration in order to control for factors that are known to be associated with study outcomes (Figure 1). Controls were matched 3:1 to participants (n=90). Due to small sample size of the population with both T1D and ASD, a 3:1 control size was chosen to increase sample size and power.
Figure 1.
Selection criteria of eligible individuals (June 2014-June 2015) for ASD+T1D cases, T1D clinic population, and matched controls.
Characteristics of Interest.
Demographic variables included age, sex, insurance type, and race/ethnicity. Clinical characteristics included BMI, ASD duration, diabetes duration, insulin regimen type (MDI vs. CSII), total daily dose of insulin, use of continuous glucose monitor (CGM), DKA at T1D onset, and total number of DKA events reported throughout duration of T1D. Total daily dose of insulin was obtained from CSII device downloads or patient report of average insulin dose taken throughout the day. The average number of blood glucose checks per day was used as a marker for adherence; glycemic control was assessed by HbA1c. The average number of blood glucose checks per day were obtained from a meter download at the most recent visit with a meter download and an HbA1c determination.
This study was approved by the Colorado Multiple Institutional Review Board.
Statistical Analyses:
Statistical analyses were performed using R version 3.1.1 software (R Foundation for Statistical Computing, Vienna, Austria). Sample size for this study was based on the primary outcome of HbA1c differences between cases and controls. Cases consisted of the entire population of patients with ASD+T1D being seen at the pediatric diabetes clinic at the time of the study. Sample size of controls was increased to 90, matching 3:1, in order to improve power. Descriptive statistics reported are means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Demographic and clinical characteristics for ASD+T1D cases and matched controls were compared using mixed effects models and conditional logistic regression for continuous and categorical variables, respectively. Duration of T1D was controlled for in each analysis. Demographic and diabetes characteristics for ASD+T1D cases and the clinic population were compared using a Satterthwaite two-sample t-test for continuous variables, and a Chi-square or Fisher’s exact test for categorical variables.
To examine changes in HbA1c from MDI (pre-CSII) to after CSII initiation, within and between the ASD+T1D cases and the controls, mixed effect modeling utilizing a knot at the first time point post-CSII initiation was used. The knot represents the CSII initiation, which helps to provide separate regression lines for comparison before and after starting CSII for both the T1D control group and those with ASD+T1D. Within each group, the difference in the slopes of HbA1c before and after CSII within each group was compared. The differences in slopes were also compared between ASD+T1D and matched controls. HbA1c values within 90 days of T1D diagnosis were excluded from this analysis.
Significance was defined as P-value <0.05. No adjustments for multiple testing were made due to the exploratory nature of the study.
RESULTS:
Prevalence of comorbid ASD in the T1D population at the Barbara Davis Center in Colorado was 11.6 per 1,000 (95% CI 9.6–12.6), or 1.16% (CI 0.96–1.26).
Demographic, Clinical, and Type 1 Diabetes Characteristics: ASD+T1D vs. Clinic Population.
The average age of participants with ASD+T1D was 12.9±3.2 years with a mean T1D duration of 4.9±3.1 years, and mean ASD duration of 4.4±3.4 years (Table 1). Age, race/ethnicity, T1D duration, and average number of blood glucose checks per day were not different between the clinic population and those with ASD+T1D (Table 1). There were significantly more males in the ASD+T1D group, compared to the clinic population (93% vs 52%; p<0.0001), with a male to female ratio of 14:1 in those with ASD. Individuals in the ASD+T1D group had significantly lower HbA1c compared to the clinic population (8.16±1.3% vs 8.86±1.8%; 66 [52–80] vs 74 [54–93] mmol/mol; p= 0.0064). For those that reported mild ASD symptoms (n=7), the mean HbA1c was 8.0±1.2% and 28.6% used CSII. Comparatively, for those who reported moderate ASD symptoms (n=6), the mean HbA1c was 8.7±1.0% and 33.3% used CSII.
Table 1.
Demographic and clinical characteristics in ASD+T1D participants, matched control participants, and the clinic population.
| Clinic (N=2597) | ASD+T1D (n=30) | p-value§ | Matched Controls (n=90) | p-value|| | |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Age (years) | 12.4±3.8 | 12.9±3.2 | 0.420 | 13.0±2.8 | 0.763 |
| Sex (% Male) | 1356 (52%) | 28 (93%) | <0.0001‡ | 84 (93%) | NA |
| Insurance | 0.891 | ||||
| Private | -- | 23 (77%) | -- | 68 (76%) | |
| Public/Uninsured | -- | 7 (23%) | -- | 22 (24%) | |
| Race/Ethnicity | 0.076 | NA | |||
| Non-Hispanic White | 1820 (70%) | 26 (87%) | 77 (86%) | ||
| Other/Hispanic | 777 (30%) | 4 (13%) | 13 (14%) | ||
| BMI | -- | 58.5±23.7 | -- | 62.2±27.0 | 0.477 |
|
Type 1 Diabetes Clinical Characteristics | |||||
| Duration of Diabetes (years) | 5.0±3.7 | 4.9±3.1 | 0.899 | 5.6±3.6 | 0.054 |
| Insulin Regimen | <0.0001‡ | 0.032* | |||
| CSII | 959 (56%) | 11 (37%) | 55 (61%) | ||
| MDI | 740 (44%) | 19 (63%) | 35 (39%) | ||
| Total Daily Insulin Dose | -- | 40.8±28.3 | -- | 53.0±24.6 | 0.005† |
| Use of Continuous Glucose Monitor | -- | 10 (33%) | -- | 16 (18%) | 0.073 |
| DKA at presentation of Type I Diabetes | -- | 12 (40%) | -- | 38 (42%) | 0.823 |
| Number of Total DKA Cases Reported | -- | 0.5±0.6 | -- | 0.9±1.3 | 0.107 |
|
Type 1 Diabetes Glycemic Control and Adherence | |||||
| HbA1c %, mmol/mol | 8.9±1.8, 74 [54–93] |
8.2±1.3, 66 [52–80] |
0.006† | 8.7±1.6, 72 [54–88] |
0.099 |
| Blood Glucose Checks Per Day | 5.5±8.6 | 5.6±2.4 | 0.857 | 5.0±2.1 | 0.156 |
p<0.05
p<0.01
p<0.001
p-value between clinic population and ASD+T1D group
p-value between ASD+T1D group and matched controls.
Demographic, Clinical, and Type 1 Diabetes Characteristics: ASD+T1D vs. Matched Controls.
Table 1 also shows the comparison of demographic and clinical characteristics, adherence, and glycemic control between ASD+T1D and matched controls. There were no significant differences in insurance status, BMI, DKA frequency, blood glucose checks per day, or HbA1c between ASD+T1D cases and matched controls. Individuals with ASD+T1D had a significantly lower total daily dose of insulin than controls with T1D (40.8 vs 53.0 units; p=0.005).
Use of Type 1 Diabetes Technology.
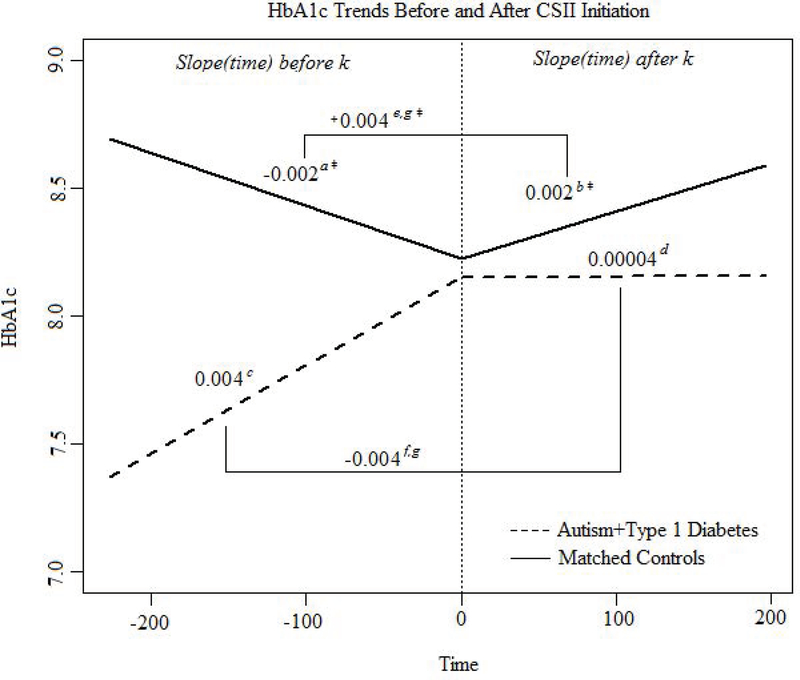
There was no significant difference in use of CGM technology in those with ASD+T1D as compared to matched controls (Table 1). In the ASD+T1D group, 37% of individuals used CSII, which was significantly lower than both the clinic population (56%; p<0.0001) and matched controls (61%; p=0.03) (Table 1). In the ASD+T1D group, those using CSII had a longer diabetes duration than those on MDI (7.2±2.9 years vs 3.6±2.5 years; p=0.003) (Table 2). Figure 2 depicts the slope of HbA1c before and after CSII initiation for both the ASD+T1D group and matched controls. For those using CSII in the ASD+T1D group, HbA1c was not statistically different before and after CSII initiation. In those using CSII in the control group, the HbA1c slope increased after initiating CSII (p<0.0001), indicating that HbA1c increased more rapidly over time for controls on CSII than when they were on MDI (Figure 2). When comparing HbA1c slope changes from MDI to CSII, HbA1c for controls increased more over time after CSII initiation than HbA1c for the ASD+T1D group after beginning CSII (p=0.01) (Figure 2).
Table 2.
Demographic and clinical characteristics of ASD+T1D cases using CSII and ASD+T1D cases using MDI.
| CSII (n=11) | MDI (n=19) | p-value | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 11.9±3.0 | 13.5±3.3 | 0.167 |
| Sex (% Male) | 11 (100%) | 17 (89%) | 0.520 |
| Insurance | 0.215 | ||
| Private | 10 (91%) | 13 (68%) | |
| Public/Uninsured | 1 (9%) | 6 (32%) | |
| Race/Ethnicity | 0.611 | ||
| Non-Hispanic White | 9 (82%) | 17 (89%) | |
| Other/Hispanic | 2 (18%) | 2 (11%) | |
| BMI | 59.3±21.0 | 58.0±25.7 | 0.884 |
| Type 1 Diabetes Clinical Characteristics | |||
| Duration of Diabetes (years) | 7.2±2.9 | 3.6±2.5 | 0.003† |
| Total Daily Insulin Dose | 39.6±18.9 | 41.4±33.0 | 0.853 |
| Use of Continuous Glucose Monitor | 5 (50%) | 5 (26%) | 0.425 |
|
Type 1 Diabetes Glycemic Control and Adherence | |||
| HbA1c %, mmol/mol | 8.4±0.8, 68 [60–77] |
8.0±1.5, 64 [48–80] |
0.330 |
| Blood Glucose Checks Per Day | 6.3±1.9 | 5.2±2.6 | 0.184 |
p<0.05
p<0.01
p<0.001
Figure 2.
HbA1c trends before CSII versus after starting CSII for the ASD+T1D group and matched controls. A knot at time k (the first time point post pump) was used and t = 0 is time of CSII initiation. Negative time values are time before CSII and positive time points are after pump.
aMatched Control HbA1c Slope before CSII Initiation, bMatched Control HbA1c Slope after CSII Initiation, cASD+T1D HbA1c Slope before CSII Initiation, dASD+T1D HbA1c Slope after CSII Initiation, eChange in Matched Control HbA1c Slope after CSII Initiation (post-pre), fChange in ASD+T1D HbA1c Slope after CSII Initiation (post-pre), gDifference of the changes in HbA1c Slope after CSII Initiation between Matched Controls and ASD+T1D (−0.0075, p=0.0101).
‡p<0.001.
DISCUSSION:
This study is the first to investigate the prevalence of comorbid ASD and T1D in the United States. It is also the first to compare demographic and clinical characteristics, adherence, glycemic control, and T1D technology use between those with ASD and aged-matched controls without comorbid ASD. Results revealed that the prevalence of ASD in the pediatric T1D population in Colorado was comparable to the prevalence of ASD in the general Colorado pediatric population (1.16% in T1D population vs. 1.09% in general Colorado population) (4). HbA1c was lower in the ASD+T1D group than the Barbara Davis Center clinic average, and the ASD+T1D group used a lower total daily dose of insulin and used CSII less frequently than both the clinic and age-matched controls.
Prevalence of ASD.
The prevalence of ASD in the Colorado pediatric population with T1D (1.16%) was similar to the prevalence of ASD in those with T1D in Ontario (0.9%), but higher than the prevalence of ASD in Ontario’s general population (0.7%) and higher than the ASD prevalence in T1D in other countries, such as, Finland (0.14%), and Italy (0.72%) (8–10). Recently, the German/Austrian diabetes patient follow-up registry, Diabetes-Patienten-Verlaufsdokumentation (DPV), reported the prevalence of comorbid ASD in T1D aged <20 years as 0.24%, but the prevalence of ASD in the general population was not provided (19). Differences in prevalence rates across the world may be due to differences in diagnostic criteria or in ‘diagnostic awareness’ between countries. Cultural differences and variation in the symptoms of ASD due to race, ethnicity, religion, or geographical origin could also account for the increased prevalence of ASD in US populations (20). Although the DSM-5 incorporated sensitivity to cultural differences, there is a potential that the diagnostic criteria for ASD is representative of North American culture and not other cultures. For example, symptoms may be overlooked in other cultures because of stigma and lower parental acknowledgement of symptoms, or symptoms of ASD may be more accepted in other cultures, causing ASD to be underdiagnosed. Few studies over the past four decades have investigated prevalence in ASD across different countries, and one study compared ASD symptoms in worldwide populations to evaluate the stigmas, including differences in symptom expression in verbal communication and restricted interests (21–22). Awareness and perception of symptoms in different cultures may impact the frequency of ASD diagnosis worldwide (21). Future research should continue to investigate these cultural variations in ASD.
Type 1 Diabetes Characteristics.
Blood glucose checks per day were similar in those with ASD+T1D compared to both groups, suggesting that individuals with ASD+T1D are able to achieve comparable adherence as those without ASD. When comparing ASD+T1D cases to the T1D clinic population, HbA1c was significantly lower, whereas HbA1c was similar in ASD+T1D cases and age-matched controls. Thus, it appears that individuals with ASD+T1D achieve similar glycemic control than those without comorbid ASD. This relates to the finding that those with ASD and asthma have better asthma-related outcomes than matched controls with asthma alone (17). The health outcomes of youth with both ASD and chronic illness appear similar or better compared to those with chronic illness alone, which could be explained by increased parental involvement. Parental involvement is common in the pediatric T1D population but may be even greater for patients with ASD. Given that parental involvement is known to be an important predictor of T1D-related outcomes in youth, this could be related to lower HbA1c seen in the ASD+T1D group. The DPV similarly found lower HbA1c in those with T1D and comorbid ASD, but reported a greater number of blood glucose checks per day compared to those without ASD (19). Rigid thinking associated with ASD may provide a benefit to T1D treatment, as those with ASD might have more standard routines and eat a smaller variety of foods, causing less variation in T1D management.
Similarly, the lower total daily dose of insulin seen in the ASD+T1D group compared to matched-controls, despite no difference in BMI between groups, could suggest that individuals with ASD eat more regulated meals, require less insulin, or engage in more physical activity throughout the day, which would increase their insulin sensitivity. Interestingly, those with Down Syndrome and T1D were found to achieve the same average HbA1c with less insulin shots per day than those with T1D alone, which might suggest that dependence on caregiver support might help achieve the same level or better glycemic management as their controls (14). Greater parental involvement in care may be a contributory factor in lower insulin doses, as the parent may provide a more accurate estimation of carbohydrates in mealtime doses and assist in more regulated meals. Although we did not measure daily carbohydrate intake, frequency or consistency of meals, or exercise in this study, future research is needed to determine the role that these play in achieving better glycemic control and lower total daily insulin dose in individuals with comorbid ASD and T1D.
Type 1 Diabetes Technology.
Our study demonstrated that CSII use was lower in ASD+T1D compared to matched controls, but use of CGM was the same. It is plausible that providers or caregivers are reluctant to begin CSII because the change in diabetes management might cause more distress and confusion for the individual with comorbid ASD. Also, caregivers may be concerned that their children would remove insulin pumps due to adverse sensory stimuli, such as the catheter cannula, tubing, or devices attached to the body. Behavior interventions, such as graded exposures, desensitization procedures, and counseling could potentially decrease any barriers to CSII use. Perceived limitations, such as lower intelligence or maturity raising concerns over the child’s access to unsupervised insulin dosing could unnecessarily restrict those with ASD+T1D from CSII, since caregivers can lock pumps or use insulin pumps with remote delivery, (e.g., Omnipod). When considering CGM use, individuals with T1D and comorbid ASD may have difficulty recognizing and communicating symptoms of hypoglycemia, due to the hallmark features of ASD. Therefore, caregivers of those with ASD may still use CGM technology, in spite of the adverse stimuli and risk of removal. Examination of the specific limitations in initiating CSII and assessment of fear of hypoglycemia in the caregivers of those with ASD and T1D are additional areas of needed research.
This study suggests use of CSII may not reduce or increase HbA1c slope in those with ASD+T1D. Alternatively, HbA1c increased in the matched controls after CSII initiation, which might reflect the increase in HbA1c seen in adolescence in the general T1D population, as the average age of the matched controls was 13 years. The same change in HbA1c slope may not be present in those with T1D and ASD due to heightened caregiver involvement in the individual’s diabetes management, even into adolescence. In future studies, it would be important to examine the role of caregiver involvement in health outcomes in youth with ASD+T1D compared to matched controls. The change in the slope of HbA1c after starting CSII was significantly lower in the ASD+T1D group compared to matched controls, suggests that CSII may benefit those with ASD more than the general T1D population during the teen years. Due to more routine daily activities and rigid thinking, CSII could benefit patients with ASD through providing more customizable insulin profiles. It may be hard to predict how much a child with ASD may eat, due to decreased communication skills, so the CSII regimen would allow caregivers to give additional insulin without another shot. CSII could also benefit children with ASD and sensitivity to sensory stimuli through providing less shots per day. Given the potentials of behavioral issues in children with ASD, CSII may also reduce the need to restrain the child during insulin administration. Studies with longitudinal designs could confirm the benefit of CSII in patients with ASD compared to the general T1D population.
Strengths and Limitations.
There are several strengths of the current study. First, this study used a representative sample of Colorado, as the Barbara Davis Center treats over 80% of Colorado’s pediatric T1D population, including urban, suburban, and rural areas. The remaining 20% is seen by several other providers and data on this 20% is incomplete. Second, it provides data about T1D management in those with comorbid ASD in the United States, which until now has been lacking. Third, this study conducted novel comparisons of T1D clinical characteristics and use of T1D technology in ASD. No other study has examined changes in HbA1c over time before and after initiating CSII.
However, this study should also be considered in the context of its limitations. All data was obtained from retrospective chart review, which inherently prevents standardization of the study procedures. The initial source of ASD diagnosis was chart review via an electronic medical record query. This is a limitation, but it was combatted through attempted parental interview of all patients with an ASD diagnosis. We contacted 13 of the 30 (43.3%) and the caregivers confirmed the ASD diagnosis. The other 17 patients were confirmed through documentation of an ASD diagnosis date. About half of those who responded reported mild ASD symptoms while the other half reported moderate symptoms. Thus, children with severe ASD and T1D may exhibit different characteristics and T1D management. Future studies should be designed prospectively for greater standardization. Similarly, ASD severity was not a standardized measure, but obtained via parental report. Thus, in future studies, researchers standardized assessment tools and structured interviews should be used to confirm diagnosis and severity of ASD.
Overall, this study found no increased prevalence ASD in those with T1D compared to the general population in Colorado. This study suggests children with ASD and T1D can have similar glycemic control and T1D management. Technology uptake was lower in ASD patients, but insulin pump use may result in better glycemic management overall. Larger, prospective studies are needed to investigate these findings further, as well as to look at potential barriers in diabetes care based on severity of ASD in order to provide adequate, individualized care to all patients.
Acknowledgments:
This study was supported by a grant from the National Institutes of Health National Institute of Diabetes and Digestive Kidney Diseases (5K12DK094712). The authors would like to acknowledge the Barbara Davis Center clinic staff and research mentors for their contribution to the development of this study and its results.
Footnotes
No potential conflicts of interest relevant to this article were reported.
Prior Presentation: Parts of this study were published as abstracts and presented as posters at the 2016 and 2017 American Diabetes Association Scientific Sessions.
REFERENCES—
- 1.Dabelea D, Bell R, D’Agostino RJ, et al. Incidence of diabetes in youth in the United States. JAMA 2007;297(24):2716–24. [DOI] [PubMed] [Google Scholar]
- 2.Vehik K, Hamman RF, Lezotte D, et al. Increasing Incidence of Type 1 Diabetes in 0- to 17-Year-Old Colorado Youth. Diabetes Care 2007;30(3):503–9. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 4.Christensen DL, Baio J, Van Naarden Braun K, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002 2016;65(3):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanescu DE, Lord K, Lipman TH. The Epidemiology of Type 1 Diabetes in Children. Endocrinol Metab Clin North Am 2012;41(4):679–94. [DOI] [PubMed] [Google Scholar]
- 6.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators, Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002 2012;61(3):1–19. [PubMed] [Google Scholar]
- 7.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators, Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, six sites, United States, 2000. Morb Mortal Wkly Rep Surveill Summ Wash DC 2002 2007;56(1):1–11. [PubMed] [Google Scholar]
- 8.Freeman SJ, Roberts W, Daneman D. Type 1 Diabetes and Autism. Diabetes Care 2005;28(4):925–6. [DOI] [PubMed] [Google Scholar]
- 9.Iafusco D, Vanelli M, Songini M, et al. Type 1 Diabetes and Autism Association Seems to Be Linked to the Incidence of Diabetes. Diabetes Care 2006;29(8):1985–6. [DOI] [PubMed] [Google Scholar]
- 10.Harjutsalo V, Tuomilehto J. Type 1 Diabetes and Autism: Is there a link? Diabetes Care 2006;29(2):484–5. [DOI] [PubMed] [Google Scholar]
- 11.Majidi S, Driscoll KA, Raymond JK. Anxiety in Children and Adolescents With Type 1 Diabetes. Curr Diab Rep 2015;15(8):47–52. [DOI] [PubMed] [Google Scholar]
- 12.Hilgard D, Konrad K, Meusers M, et al. Comorbidity of attention deficit hyperactivity disorder and type 1 diabetes in children and adolescents: Analysis based on the multicentre DPV registry. Pediatr Diabetes 2017;18(8):706–13. [DOI] [PubMed] [Google Scholar]
- 13.Prinz N, Bächle C, Becker M, et al. Insulin Pumps in Type 1 Diabetes with Mental Disorders: Real-Life Clinical Data Indicate Discrepancies to Recommendations. Diabetes Technol Ther 2015;18(1):34–8. [DOI] [PubMed] [Google Scholar]
- 14.Anwar AJ, Walker JD, Frier BM. Type 1 diabetes mellitus and Down’s syndrome: prevalence, management and diabetic complications. Diabet Med 1998. Feb;15(2):160–3. [DOI] [PubMed] [Google Scholar]
- 15.McVilly K, McGillivray J, Curtis A, Lehmann J, Morrish L, Speight J. Diabetes in people with an intellectual disability: a systematic review of prevalence, incidence and impact. Diabet Med 2014;31(8):897–904. [DOI] [PubMed] [Google Scholar]
- 16.Patton SR, Driscoll KA, Clements MA. Adherence to Insulin Pump Behaviors in Young Children With Type 1 Diabetes Mellitus: Opportunities for Intervention. J Diabetes Sci Technol 2016;11(1):87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jónsdóttir U, Lang JE. How does autism spectrum disorder affect the risk and severity of childhood asthma? Ann Allergy Asthma Immunol 2017. May;118(5):570–576. [DOI] [PubMed] [Google Scholar]
- 18.Coury D, Jones NE, Klatka K, Winklosky B, Perrin JM. Healthcare for children with autism: the Autism Treatment Network. Curr Opin Pediatr 2009. Dec;21(6):828–32. [DOI] [PubMed] [Google Scholar]
- 19.Lemay J-F, Lanzinger S, Pacaud D, et al. , German/Austrian DPV Initiative. Metabolic Control of Type 1 Diabetes in Youth with Autism Spectrum Disorder: a Multicenter DPV analysis based on 61,749 patients up to 20 years of age. Pediatr Diabetes [Internet]. [cited 2018 Apr 6]; Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/pedi.12676 [DOI] [PubMed]
- 20.Substance Abuse and Mental Health Services Administration. Improving Cultural Competence. Treatment Improvement Protocol (TIP) Series No. 59. HHS Publication No. (SMA) 14–4849 Rockville, MD: Substance Abuse and Mental Health Services Administration, 2014. [PubMed] [Google Scholar]
- 21.Matsona JL, Worleya JA, Fodstada JC, Chung KM, Suh D, Jhin HK, Ben-Itzchak E, Zachor DA, Furniss F. A multinational study examining the cross cultural differences in reported symptoms of autism spectrum disorders: Israel, South Korea, the United Kingdom, and the United States of America. Research in Autism Spectrum Disorders 2011;5(4):1598–1604. [Google Scholar]
- 22.Maguire C Autism on the rise: A global perspective. Global Health Review. Harvard College 2013. April.