Introduction
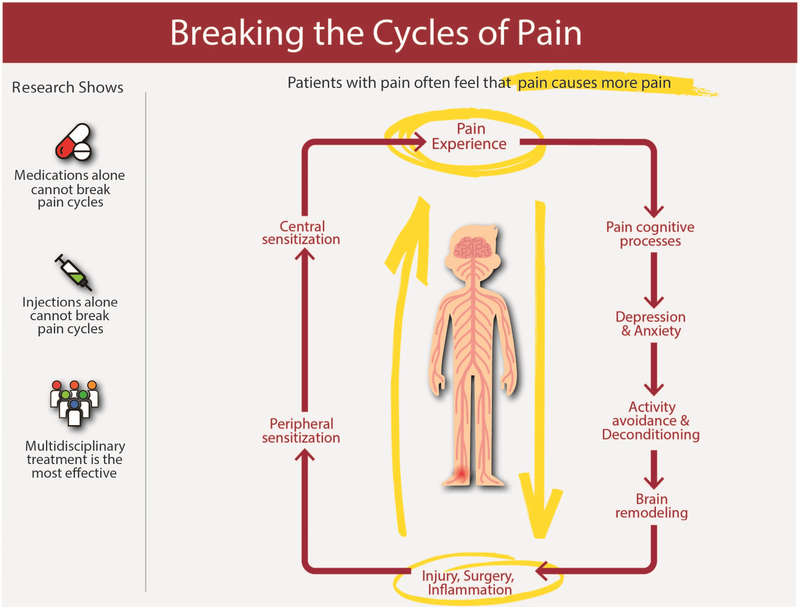
Chronic pain is one of the most common health conditions among older adults (> 65 years) and is associated with significant disability. Chronic pain in the older adult reduces mobility, is associated with depression and anxiety, and can disrupt familial and social relationships.1 Diagnosis of chronic pain in older adults has significant challenges: patient communication may be difficult due to presence of a neuromuscular or cognitive disorder, or patients may minimize their symptoms. The treatment of chronic pain in older adults is complex and should involve a multi-faceted approach that includes pharmacologic interventions, physical rehabilitation, and interventional procedures to break the pain cycle (Figure 1). It is important for healthcare providers across all specialties to develop skills to diagnose and manage chronic pain in older patients.
Figure 1. Breaking the Cycles of Pain.
Pain is a complex biopsychosocial disease that can affect all aspects of life including mood, sleep, cognition and function. This is particularly difficult for older adults who may already have co-morbid conditions contributing to problems in these areas or who may undergo surgeries that increase their risk of chronic pain. Using a multidisciplinary approach to treat chronic pain is the most likely to be effective and treatment plans should include multimodal medication options, physical therapy, pain psychology and selected interventions, as appropriate.
Courtesy of M.C. Kao, PhD, MD, CIPS, FIPP, Palo Alto, CA.
Common Causes of Chronic Pain in Older Adults
There is a common belief that chronic pain is an unavoidable consequence of getting older.2,3 Chronic pain does have a high prevalence in the older population, estimated to be over 50%, with 70% of older individuals endorsing pain in multiple sites.4 The most prevalent painful conditions affecting older adults are arthritis-related, although the incidence of chronic systemic disease that can also result in pain (i.e., diabetic complications, cancer-related pain, post-stroke pain) is also high among older individuals (Box 1).5
Box 1: Common causes of chronic pain in elderly patients.
Cancer-related pain
Central poststroke pain
Chronic post-surgical pain
Diabetic peripheral neuropathy
Fibromyalgia
Myofascial pain
Osteoarthritis
Peripheral vascular disease (ischemic pain)
Postherpetic neuralgia (shingles)
Spinal canal stenosis
Trauma-related pain (ex. Hip fracture)
Assessment
In order to treat pain effectively in the older adult, a meaningful assessment of pain is required. In general, a person’s self-reported pain level using a pain assessment tool for pain intensity remains the best indicator of pain in older adults;6 however, there are caveats to using self-reporting in this population. Many older adults will not automatically report pain due to misguided beliefs that pain with aging is expected, fears of diagnostic testing, or concerns regarding the significance of the pain and loss of independence.7 Furthermore, older adults have an increased number of comorbidities compared to the general population and are more likely to have multiple diagnoses that contribute to pain. As a result, a comprehensive history and physical is recommended, and multiple sources of pain must be considered and addressed. It should also be remembered that older adults have an increased risk of incidental findings with additional testing and diagnostic imaging, and ancillary tests should only be obtained based on clinical examination findings.8
Additionally, the assessment of pain in cognitively impaired individuals presents a unique challenge. Studies have estimated that the prevalence of persistent pain in older adults ranges from 24–50%, and seniors with and without cognitive impairment had a similar prevalence of conditions that were likely to result in pain.9 Patients with increasing amounts of cognitive impairment are less likely to self-report pain despite an equal prevalence of painful conditions. As a result, pain issues are often under-addressed among these individuals. Observation of behavior may help determine the incidence of pain in cognitively impaired older adults that are unable to adequately verbalize their symptoms. Pain may be demonstrated in a variety of ways, including changes in functional status, interactions with others, facial expressions, verbalizations, and body movements. Caregivers may also be able to provide additional information that is relevant to the pain assessment.
Tracking functional status as an outcome measure in addition to pain level is important in the treatment of pain in older individuals. This includes mood, mobility, activities of daily living, sleep, appetite, cognitive impairment and weight changes. Improved management of pain is expected to improve one or more elements of functional status, and untreated pain may result in worsening functional status.10
Perioperative Management
The older population has unique risks associated with their perioperative management, comprehensively addressed in other sections of this issue.
Chronic Pain Management
General Considerations
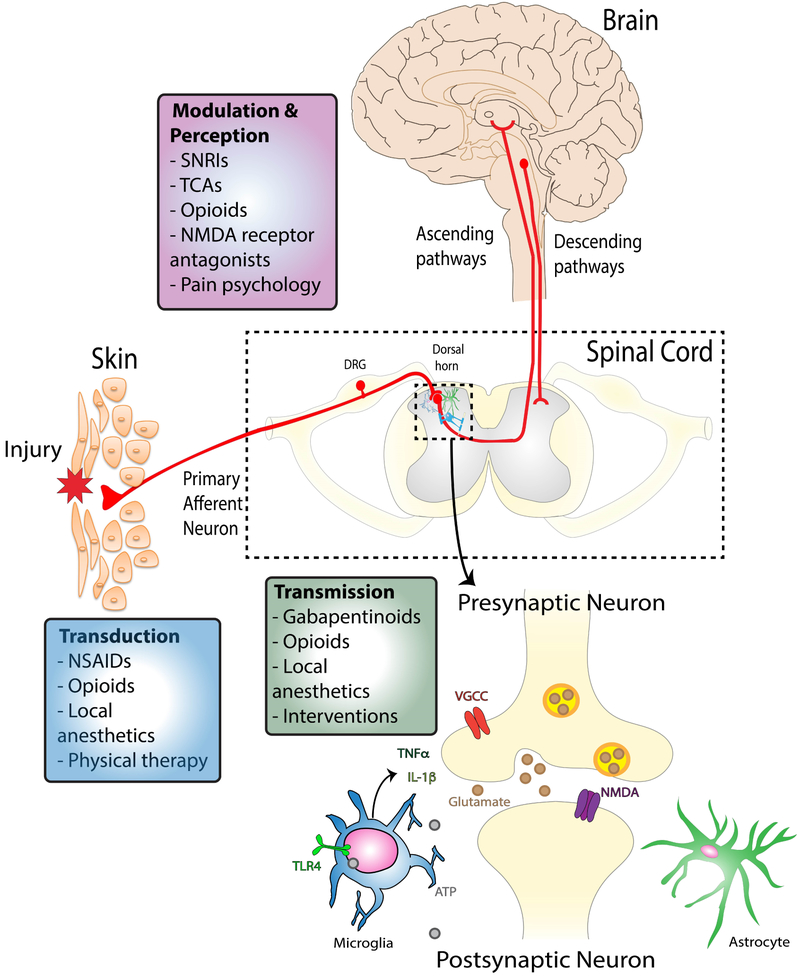
Chronic pain management in the older adult can be accomplished through a multidisciplinary approach that includes pharmacologic treatments, physical and psychological rehabilitation, and interventional approaches (Figure 1). With respect to the selection of pharmacologic agents, multimodal treatment using medications with varying mechanisms of action (Figure 2) may allow for synergistic effects but may also contribute further to polypharmacy and therefore must be undertaken with caution. Prescribers must also account for the narrower therapeutic index of most medications in older adults compared with younger individuals, and advancing age increases the risk of adverse drug reactions.11 There are both pharmacokinetic and pharmacodynamic considerations for older adults that must be taken into account when prescribing medications for pain. Pharmacokinetic changes include decreased absorption, variability in volume of distribution depending on lipophilicity of the drug, and heightened therapeutic response to protein-bound drugs due to hypo-albuminemia, decreased hepatic metabolism, and decreased renal elimination.6,12 In terms of pharmacodynamics, changes in the peripheral and central nervous system including pre-existing cognitive deficits, decreased myelination of nerves and decreased receptor density may all predispose older adults to increased side effects from commonly prescribed medications.13
Figure 2. Sites of action of multidisciplinary treatments for pain management.
In most cases pain is initiated in the periphery where primary afferent neuron terminals may be activated by local inflammatory mediators. Transduction of this signal from the peripheral to the central nervous system can be blocked by certain categories of drugs (NSAIDs, opioids, local anesthetics) and by increasing strength and mobility through physical therapy. Painful signals are then transmitted to the dorsal horn of the spinal cord where the central terminals of the primary afferent neurons form synapses with intrinsic spinal cord neurons. Gabapentinoids, opioids, local anesthetics and certain interventions can block this peripheral-to-central transmission. Finally, the painful signal is carried to the brain through ascending pathways for perception to occur and descending pathways can also be activated to modulate inputs in the spinal cord. Many medications can act on these systems including serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), N-methyl-D-aspartate (NMDA) receptor antagonists and opioids. Importantly, psychological interventions can engage descending inhibitory pathways to suppress painful signal transmission. Using multidisciplinary approaches that target different areas of the peripheral and central nervous system may limit side effects and improve efficacy of treatments.
Pharmacologic agents
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDs)
Mechanism of Action & Place in therapy
NSAIDs are antipyretic and anti-inflammatory medications that function by inhibiting the synthesis of prostaglandins. This inhibition is achieved by blocking the metabolism of arachidonic acid via the COX pathway.14 Older NSAIDs (aspirin, ibuprofen, naproxen) are non-selective inhibitors of both COX-1 and COX-2. Newer NSAIDs (rofecoxib, celecoxib, and valdecoxib) selectively inhibit COX-2 and have fewer adverse effects. These agents have analgesic, anti-inflammatory and anti-pyretic effects; but do not have antiplatelet activity, do not affect bleeding time, and are not as toxic to the GI system. NSAIDs are effective in the treatment of mild-to-moderate chronic pain, particularly in conditions with an inflammatory component. However, side effects must be considered before they are prescribed to an older adult.
Adverse effects and precautions
NSAIDs can cause a range of GI toxicities including nausea, diarrhea and mucosal damage (GI erosions, ulcers, perforations, bleeding) that are responsible for significant morbidity and mortality in the US. Thirty percent of patients will complain of dyspepsia on NSAID therapy, and 15–30% of NSAID users show evidence of a gastric or duodenal ulcer.15 Cytoprotective therapy can be initiated with the NSAID (an H2 antagonist or proton pump inhibitor, PPI) to prevent these symptoms, or the use of a COX-2-specific NSAID can reduce the incidence of these side effects. PPIs have been found to be the most effective cytoprotective therapy.16
NSAIDs are also associated with renal toxicity, which occurs in 5% of patients taking these agents.17 Older adults may be at greater risk of renal toxicity than younger patients. Both non-selective and selective COX-2 inhibitors have been shown to cause renal dysfunction,18 and it is recommended to avoid NSAIDs in patients with a creatinine clearance of less than 30 mL/minute.19
NSAIDs are also associated with cardiovascular risks. Studies have shown that both selective and non-selective NSAIDs increase the risk of heart failure and exacerbate heart failure symptoms.20 There is also evidence that COX-2 selective NSAIDs may have pro-thrombotic activity in patients at risk for major vascular events.21 There is further evidence that the concomitant administration of either class of NSAID may negate the cardioprotective effects of low-dose aspirin.22,23 Therefore, the cardiac comorbidities of an older patient must be carefully considered before NSAIDs are initiated.
ANTI-DEPRESSANTS
Mechanism of Action & Place in Therapy
Anti-depressants indicated in the use of chronic pain include tricyclic antidepressants (TCAs), and serotonin and norepinephrine reuptake inhibitors (SNRIs) that provide pain relief separate from their antidepressant effects.24 The mechanism of action of both classes of medications is through inhibiting reuptake of serotonin and norepinephrine resulting in increased amounts of the neurotransmitters in the synaptic cleft. Selective serotonin reuptake inhibitors (SSRIs) have shown limited efficacy in the treatment of pain,25 suggesting that the synaptic increases in norepinephrine are required for analgesic compared to anti-depressant effects. Specifically, the number needed to treat (NNT) for TCAs is reported as 2–3 depending on the pain condition while it is >6 for SSRIs. Importantly, however, many patients present to clinic already taking SSRIs. In such cases, discussion with the patient and their prescriber about a potential switch to a TCA or SNRI may be warranted to obtain both analgesic and anti-depressant effects with a single agent, thus simplifying medication regimens in a population at high risk of polypharmacy.26 In addition, it is important to be aware of all serotonergic medications a patient is taking in order to avoid combination effects leading to serotonin syndrome.13,27
Adverse Effects and Precautions
TCAs, SSRIs, and SNRIs have all been known to have increased side effects in older adults. TCAs are highly anticholinergic, and can lead to cognitive dysfunction, sedation and orthostatic hypotension. All TCAs are included on the Beers list of potentially inappropriate medications in older adults, with the exception of low-dose doxepin.28 SSRIs and SNRIs have fewer cardiovascular and anticholinergic adverse effects than TCAs, but may be associated with a higher fall risk in older adults.29
ANTI-CONVULSANTS
Mechanism of Action & Place in Therapy
Multiple classes of anticonvulsants are commonly used for chronic pain. Older anticonvulsants (carbamazepine, phenytoin, and valproic acid) are sodium channel blockers that suppress nerve hyper-excitability by increasing membrane stability.14 These are indicated in neuropathic pain including trigeminal neuralgia in which carbamazepine and oxcarbazepine remain first line drugs30 in spite of only third tier evidence of efficacy from data involving small numbers of participants with risk of bias according to a recent Cochrane review.31 Gabapentinoids are alpha-2-delta calcium channel blockers, and also work by modulating primary afferent excitability.32 Gabapentinoids have become increasingly popular in treating neuropathic pain because they are efficacious with fewer adverse side effects than older anticonvulsants.33
Adverse Effects and Precautions
Older anticonvulsants such as carbamazepine should be avoided in older adults because they increase the risk of hyponatremia and SIADH.19 In cases where it is the first line therapy indicated (i.e. for trigeminal neuralgia) the lowest effective dose should be used to decrease the incidence of side effects.
When gabapentinoids are initiated in older adults, they should be started at a low dose (we suggest 100 mg qHS with uptitration by 100 mg every 3–4 days as tolerated to standard TID dosing) and monitored carefully for side effects. The most common side effects of gabapentinoids are dizziness, somnolence, fatigue and weight changes.34 It should be noted, however, that prescription rates of gabapentinoids have increased three-fold between 2002 and 2015 with a particularly skewed increase in use by adults over the age of 64 and those with multiple comorbidities.35 This is of particular concern as new data from the FDA Adverse Event Reporting System indicates that these medications may have additive effects on respiratory depression when used with other CNS depressant drugs, including opioids.36
OTHER ANALGESICS
Cannabinoids
Cannabinoids have been found to be effective in a few clinical trials regarding treatment of chronic pain.37–39 They should be used with caution in older patients, because these patients are at higher risk for a dysphoric response to treatment.40
Muscle Relaxants
Muscle relaxants should be used with caution in adults 65 and older. They are often used in the treatment of acute low back pain, but are associated with side effects such as sedation, dizziness, anti-cholinergic effects and weakness.41
Low-dose Naltrexone
Low-dose Naltrexone (LDN) has been demonstrated to be effective in chronic pain conditions such as fibromyalgia and complex regional pain syndrome.42 LDN is thought to be a potent anti-inflammatory agent through antagonism of Toll-like receptor 4 (TLR4) found on myeloid-lineage cells such as microglia (central nervous system immune cells).43 Although no formal studies have been done on LDN use in the elderly, side effects of LDN are generally very mild, with the most common side effect observed being vivid dreams.43
Memantine
Memantine is an NMDA antagonist that has been found to be effective in treating neuropathic pain44 although limited studies evaluated in a recent Cochrane review showed no effect of memantine specifically in phantom limb pain.45 In general patients tolerate it well, but it should be used with caution in older patients as it can cause dizziness.46
OPIOIDS
In older adults that are carefully selected and monitored, opioids may help provide effective pain relief as part of a multimodal pain management plan.47 The effects of opioid medications are mediated through opioid receptors, located in periaqueductal gray as well as throughout the spinal cord, joint synovium and intestinal mucosa.14 The analgesic effect is primarily attributed to the mu and kappa receptors. It is important to note that there are pharmacokinetic changes relevant to opioid dosing in older adults, with significant variability among patients. This is in part due to an increased fat to lean-body-mass ratio as well as reduced clearance of renal metabolites.48 Studies have shown older patients have greater pain relief from opioids for a longer duration compared to younger adults receiving the same dose.49,50 Central side effects of opioids, including drowsiness and dizziness, may be associated with increased incidence of falls and fractures, so it is recommended that dose titration is done slowly and with caution in older adults.51
Tramadol
Tramadol is considered a weak opioid agonist as well as a monoamine uptake inhibitor.27 It reduces the seizure threshold, and should be used cautiously in patients with a history of seizures or those taking other serotonergic drugs.52 There are few studies on the use of tramadol in older adults, though one study surprisingly showed that its pharmacokinetics are only minimally affected by age, as long as renal and hepatic function are well-maintained.53
Oxycodone Oral Solution
In frail older adults, where the effects of accidental opioid overdose could be catastrophic, the authors of this paper have had success titrating oral oxycodone solution. This formulation is easier for patients with swallowing difficulties and is more amenable to titration when small doses of opioids are preferred (i.e. starting dose of less than 2.5 mg).
Transdermal Buprenorphine
A longitudinal study of nursing home residents found that the use of long-acting opioids improved functional status and social engagement compared with short acting opioids.54 Recently, transdermal buprenorphine has been advocated for use in older adults.55 Buprenorphine is a partial μ agonist and a κ and δopioid antagonist.56 Transdermal buprenorphine is associated with a slow onset and long duration of action (onset of 12–24 hours, duration of action of 3 days).57 It has a better side effect profile than most other opioids: there is a ceiling to the side-effect of respiratory depression, as well as less profound effect on decreasing gastrointestinal transit times that other opioids.58 It is also safe for use in renal impairment, which is a major advantage in the elderly.59
Opioid Addiction in the Elderly
It is estimated that 6–9% of community-dwelling older adults use opioids, and up to 70% of nursing home residents with chronic non-cancer pain receive regularly-scheduled opioids.60–62 Although rates of abuse and misuse are lower than in the younger population, it is estimated that 1–3% of older adults use opioids inappropriately.63 The potential for opioid abuse should be recognized in the older adult population, and screening for abuse and misuse should be done regularly in all older adults that are prescribed opioid medication.
Interventional Therapies
Interventional techniques in pain management offer older adults treatments with fewer systemic side effects than pharmacologic interventions.64 The most common interventional therapies include epidural steroid injections, lumbar facet injections, percutaneous vertebral augmentation, sacroiliac joint injections, and hip and knee joint injections. In general, these procedures are low risk with few side effects. These procedures can be included as part of a multidisciplinary strategy for chronic pain therapy, and can help reduce pharmacologic interventions (with potentially more systemic side effects) as well as the need for larger surgeries that carry higher risk and have a longer recovery time.64 It should be noted that many of these procedures are typically performed at an outpatient surgery center, which may not be appropriate for many older adults. The anticoagulation status and comorbidities of each patient must be taken into consideration prior to performing any interventional therapy, and the procedure should be moved to a hospital setting with appropriate monitoring if the patient is determined to be high risk.65
Role of Rehabilitation/Physical Therapy in Managing Pain in Older Adults
The impact of natural senescence on multiple physiologic systems should be considered during the evaluation of older individuals with longstanding chronic pain states. Among the most prominent changes in normal aging is the loss of muscular mass and force generation through the process of sarcopenia.66 As a result, fast-twitch (2a) fibers disproportionately atrophy secondary to decreased myosin heavy-chain protein synthesis, which results in a 3.5% decrease in muscle power per year after the age of 60.67,68 Multiple other changes to the musculoskeletal system have been described with aging including; functional decline of the mitochondria (decreased endurance), increased co-activation of agonist-antagonist muscle groups (decreased peak force), decreased motor neuron excitability within the spinal cord, and decreased transmission across the neuromuscular junction.69 All of these functional declines lead to instability and require compensatory gait adjustments, such as stance-widening, increased double support time, and variability in stride to stride distance. Other important physiologic changes to consider with patients of advanced age include decreased joint range of motion from degenerative joint disease, osseous fragility from osteoarthritis, decreased cardio-pulmonary compliance, and decreased sensory acuity. Thus, a complete physical evaluation is reasonable to request prior to initiation of a physical therapy program to rule out contraindications in this population.
The overall therapeutic goal of treatment is an important consideration when caring for patients of advanced age. The primary objective of rehabilitation is to improve impairment (loss of physiologic or anatomical structure or function), which is typically accomplished through modalities that address the underlying pathophysiologic etiology (e.g. core strengthening and stabilization exercises for degenerative lumbar spondylosis). However, when improving impairment is unlikely, rehabilitation should focus instead on improving patient disability (restriction in an ability to perform an activity resulting from impairment). Occupational therapists are particularly skilled in recommending adaptations and environmental modifications to decrease patient disability and should be readily consulted to assist in teaching the patient independent living skills when impairments are unlikely to improve.
When impairment is amenable to improvement, multiple modalities of therapy have been shown to improve musculoskeletal function and improve outcomes. Strength-training focused physical therapy programs are particularly effective in improving overall mobility, balance, and physical function in the elderly population.70–73 For example, resistance-based strengthening therapies have significantly improved patient-reported pain outcomes in older individuals with a primary diagnosis of hip or knee osteoarthritis.74–76 Similar functional improvement has been reported across a diverse spectrum of active therapeutic modalities in the elderly population. Regimens focused on high-intensity strengthening (8 repetitions at 80% of single repetition maximum) and low-intensity strengthening (13 repetitions at 50% of single repetition maximum) demonstrated similar improvement in endurance and function in one study of individuals ages 60 years or older.77 Additionally, low-impact modalities such as T’ai Chi and Aqua-aerobic regimens may modestly improve balance and musculoskeletal function when performed on a regular, consistent basis.69
Direct supervision with encouragement to properly increase exercise intensity is a key determinant of program adherence for older individuals, regardless of what type of regimen is recommended. Multiple studies have demonstrated that without supervision, elderly individuals are reluctant to progress their routine and may even exhibit increased confusion or anger.78,79 Encouraging older patients to attend sessions at an exercise facility or community facility with a qualified and attentive instructor may decrease these unwanted outcomes and simultaneously have a positive effect on mood through the development of positive social interactions.69
Psychological interventions
Chronic pain is best explained by a biopsychosocial model and its treatment must include interventions aimed at co-morbid depression, anxiety and poor coping skills (Figure 1). It has been shown, for example, that patients with high levels of catastrophizing, defined as feelings of hopelessness and helplessness with respect to their pain, report higher pain intensity, decreased level of function and depression.80 Catastrophizing was also identified as a predictor of persistent pain after total knee replacement.81 Importantly, participation in just a one-day perioperative acceptance and commitment therapy workshop results in greater pain reduction at 3 months post-orthopedic surgery, and opioid cessation 9 days earlier, compared to patients receiving standard of care.82 Such studies highlight the importance of psychological management, which carries little risk but high potential for benefit, as part of a multidisciplinary approach to pain management.
Coordination of Multidisciplinary Care & Concluding remarks
The complex medical conditions of older adults put them at high risk for polypharmacy and medication mismanagement.83 It is important for primary care physicians, geriatricians and pain specialists to work together to form a patient-specific health plan that maximizes quality of life while minimizing risks of adverse events and side effects. Since older adults are often not managing their own medications, physicians must also coordinate with patients’ caretakers or long-term care facility. Overall, effective pain relief can be obtained for older adults but must involve a multidisciplinary approach that includes physical rehabilitation, occupational therapy and management of co-morbid depression and anxiety through psychological interventions. Finally, self-management strategies that target clearly defined goals for improved function will allow the patient to feel engaged in their care and have been shown to improve pain-related disability.84
KEY POINTS.
Pain management in the elderly should involve a multidisciplinary approach including multimodal medications, selected interventions, physical therapy, and rehabilitation and psychological treatments.
There are unique considerations to selecting medications in older adults including changes in pharmacokinetics, pharmacodynamics, polypharmacy and likelihood of side effects.
Physical therapy and psychological approaches should be tailored to the individual and use self-management methods for success.
SYNOPSIS.
Chronic pain is extremely prevalent in older adults and is associated with significant morbidity including limited mobility, social isolation and depressed mood. Pain is defined by a biopsychosocial model highlighting the importance of a multidisciplinary approach to treatment including multimodal medications, selected interventions, physical therapy and rehabilitation and psychological treatments. In this narrative review, the authors highlight the use of these approaches in older adults with specific attention paid to considerations unique to aging including alterations in drug metabolism, avoidance of polypharmacy and physiologic changes predisposing to painful conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
None
References
- 1.Gloth FM. Handbook of pain relief in older adults: an evidence-based approach. Totowa, N.J: Humana Press; 2004. [Google Scholar]
- 2.Thielke S, Sale J, Reid MC. Aging: are these 4 pain myths complicating care? The Journal of Family Practice. 2012;61(11):666–670. [PMC free article] [PubMed] [Google Scholar]
- 3.Gignac MAM, Davis AM, Hawker G, et al. “What do you expect? You’re just getting older”: A comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis and Rheumatism. 2006;55(6):905–912. [DOI] [PubMed] [Google Scholar]
- 4.Patel KV, Guralnik JM, Dansie EJ, Turk DC. Prevalence and impact of pain among older adults in the United States: findings from the 2011 National Health and Aging Trends Study. Pain. 2013;154(12):2649–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clinic Proceedings. 2013;88(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bicket MC, Mao J. Chronic Pain in Older Adults. Anesthesiology clinics. 2015;33(3):577–590. [DOI] [PubMed] [Google Scholar]
- 7.Herr KA, Garand L. Assessment and measurement of pain in older adults. Clinics in Geriatric Medicine. 2001;17(3):457–478, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. The New England Journal of Medicine. 1994;331(2):69–73. [DOI] [PubMed] [Google Scholar]
- 9.Proctor WR, Hirdes JP. Pain and cognitive status among nursing home residents in Canada. Pain Research & Management. 2001;6(3):119–125. [DOI] [PubMed] [Google Scholar]
- 10.Hadjistavropoulos T, Herr K, Turk DC, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. The Clinical journal of pain. 2007;23(1 Suppl):S1–43. [DOI] [PubMed] [Google Scholar]
- 11.Beyth RJ, Shorr RI. Epidemiology of adverse drug reactions in the elderly by drug class. Drugs & Aging. 1999;14(3):231–239. [DOI] [PubMed] [Google Scholar]
- 12.Gagliese L, Melzack R. Chronic pain in elderly people. Pain. 1997;70(1):3–14. [DOI] [PubMed] [Google Scholar]
- 13.Spina E, Scordo MG. Clinically significant drug interactions with antidepressants in the elderly. Drugs & Aging. 2002;19(4):299–320. [DOI] [PubMed] [Google Scholar]
- 14.Goodman & Gilman’s the pharmacological basis of therapeutics. Thirteenth edition New York: McGraw Hill Medical; 2018. [Google Scholar]
- 15.Hawkey CJ. Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 2000;119(2):521–535. [DOI] [PubMed] [Google Scholar]
- 16.Yeomans ND, Tulassay Z, Juhász L, et al. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. The New England Journal of Medicine. 1998;338(11):719–726. [DOI] [PubMed] [Google Scholar]
- 17.Shimp LA. Safety issues in the pharmacologic management of chronic pain in the elderly. Pharmacotherapy. 1998;18(6):1313–1322. [PubMed] [Google Scholar]
- 18.Morales E, Mucksavage JJ. Cyclooxygenase-2 inhibitor-associated acute renal failure: case report with rofecoxib and review of the literature. Pharmacotherapy. 2002;22(10):1317–1321. [DOI] [PubMed] [Google Scholar]
- 19.Gloth FM. Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. The Journal of Pain: Official Journal of the American Pain Society. 2011;12(3 Suppl 1):S14–20. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M, Lamberts M, Olsen A-MS, et al. Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. European Heart Journal. 2016;37(13):1015–1023. [DOI] [PubMed] [Google Scholar]
- 21.Coxib and traditional NSAID Trialists’ (CNT) Collaboration, Bhala N, Emberson J, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet (London, England). 2013;382(9894):769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etminan M, Samii A. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet (London, England). 2003;361(9368):1558–1559; author reply 1559. [DOI] [PubMed] [Google Scholar]
- 23.Burnakis TG. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. The New England Journal of Medicine. 2002;346(20):1589–1590; author reply 1589–1590. [DOI] [PubMed] [Google Scholar]
- 24.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81(12):1372–1373. [DOI] [PubMed] [Google Scholar]
- 25.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83(3):389–400. [DOI] [PubMed] [Google Scholar]
- 26.Mannucci PM, Nobili A, Pasina L, REPOSI Collaborators. Polypharmacy in older people: lessons from 10 years of experience with the REPOSI register. Internal and Emergency Medicine. 2018. [DOI] [PubMed] [Google Scholar]
- 27.Beakley BD, Kaye AM, Kaye AD. Tramadol, Pharmacology, Side Effects, and Serotonin Syndrome: A Review. Pain physician. 2015;18(4):395–400. [PubMed] [Google Scholar]
- 28.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. November 2015. 2015 1532–5415. [DOI] [PubMed] [Google Scholar]
- 29.Coupland CaC, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technology Assessment (Winchester, England). 2011;15(28):1–202, iii-iv. [DOI] [PubMed] [Google Scholar]
- 30.Di Stefano G, Truini A, Cruccu G. Current and Innovative Pharmacological Options to Treat Typical and Atypical Trigeminal Neuralgia. Drugs. 2018;78(14):1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiffen PJ, Derry S, Moore RA, Kalso EA. Carbamazepine for chronic neuropathic pain and fibromyalgia in adults. The Cochrane database of systematic reviews. 2014(4):CD005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremont-Lukats IW, Megeff C, Backonja MM. Anticonvulsants for neuropathic pain syndromes: mechanisms of action and place in therapy. Drugs. 2000;60(5):1029–1052. [DOI] [PubMed] [Google Scholar]
- 33.Ross EL. The evolving role of antiepileptic drugs in treating neuropathic pain. Neurology. 2000;55(5 Suppl 1):S41–46; discussion S54–58. [PubMed] [Google Scholar]
- 34.Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology (Oxford, England). 2010;49(4):706–715. [DOI] [PubMed] [Google Scholar]
- 35.Johansen ME. Gabapentinoid Use in the United States 2002 Through 2015. JAMA internal medicine. 2018;178(2):292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Throckmorton DC, Gottlieb S, Woodcock J. The FDA and the Next Wave of Drug Abuse - Proactive Pharmacovigilance. The New England Journal of Medicine. 2018;379(3):205–207. [DOI] [PubMed] [Google Scholar]
- 37.Wilsey B, Marcotte T, Tsodikov A, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. The Journal of Pain: Official Journal of the American Pain Society. 2008;9(6):506–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA. 2003;290(13):1757–1762. [DOI] [PubMed] [Google Scholar]
- 39.Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Current Medical Research and Opinion. 2007;23(1):17–24. [DOI] [PubMed] [Google Scholar]
- 40.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. Pain medicine (Malden, Mass). 2009;10(6):1062–1083. [DOI] [PubMed] [Google Scholar]
- 41.Billups SJ, Delate T, Hoover B. Injury in an elderly population before and after initiating a skeletal muscle relaxant. The Annals of Pharmacotherapy. 2011;45(4):485–491. [DOI] [PubMed] [Google Scholar]
- 42.Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis and Rheumatism. 2013;65(2):529–538. [DOI] [PubMed] [Google Scholar]
- 43.Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clinical Rheumatology. 2014;33(4):451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickering G, Morel V. Memantine for the treatment of general neuropathic pain: a narrative review. Fundamental & Clinical Pharmacology. 2018;32(1):4–13. [DOI] [PubMed] [Google Scholar]
- 45.Alviar MJM, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. The Cochrane database of systematic reviews. 2016;10:CD006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olivan-Blázquez B, Herrera-Mercadal P, Puebla-Guedea M, et al. Efficacy of memantine in the treatment of fibromyalgia: A double-blind, randomised, controlled trial with 6-month follow-up. Pain. 2014;155(12):2517–2525. [DOI] [PubMed] [Google Scholar]
- 47.Opioids for persistent pain: summary of guidance on good practice from the British Pain Society. British Journal of Pain. 2012;6(1):9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cherny NI. Opioid analgesics: comparative features and prescribing guidelines. Drugs. 1996;51(5):713–737. [DOI] [PubMed] [Google Scholar]
- 49.Bellville JW, Forrest WH, Miller E, Brown BW. Influence of age on pain relief from analgesics. A study of postoperative patients. JAMA. 1971;217(13):1835–1841. [PubMed] [Google Scholar]
- 50.Kaiko RF, Wallenstein SL, Rogers AG, Grabinski PY, Houde RW. Narcotics in the elderly. The Medical Clinics of North America. 1982;66(5):1079–1089. [DOI] [PubMed] [Google Scholar]
- 51.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. Journal of Internal Medicine. 2006;260(1):76–87. [DOI] [PubMed] [Google Scholar]
- 52.Barber JB, Gibson SJ. Treatment of chronic non-malignant pain in the elderly: safety considerations. Drug Safety. 2009;32(6):457–474. [DOI] [PubMed] [Google Scholar]
- 53.Likar R, Wittels M, Molnar M, Kager I, Ziervogel G, Sittl R. Pharmacokinetic and pharmacodynamic properties of tramadol IR and SR in elderly patients: a prospective, age-group-controlled study. Clin Ther. 2006;28(12):2022–2039. [DOI] [PubMed] [Google Scholar]
- 54.Won A, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Long-term effects of analgesics in a population of elderly nursing home residents with persistent nonmalignant pain. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2006;61(2):165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vadivelu N, Hines RL. Management of chronic pain in the elderly: focus on transdermal buprenorphine. Clinical Interventions in Aging. 2008;3(3):421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Negus SS, Mello NK, Linsenmayer DC, Jones RM, Portoghese PS. Kappa opioid antagonist effects of the novel kappa antagonist 5’-guanidinonaltrindole (GNTI) in an assay of schedule-controlled behavior in rhesus monkeys. Psychopharmacology. 2002;163(3–4):412–419. [DOI] [PubMed] [Google Scholar]
- 57.Sorge J, Sittl R. Transdermal buprenorphine in the treatment of chronic pain: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Clinical Therapeutics. 2004;26(11):1808–1820. [DOI] [PubMed] [Google Scholar]
- 58.Griessinger N, Sittl R, Likar R. Transdermal buprenorphine in clinical practice--a post-marketing surveillance study in 13,179 patients. Current Medical Research and Opinion. 2005;21(8):1147–1156. [DOI] [PubMed] [Google Scholar]
- 59.Filitz J, Griessinger N, Sittl R, Likar R, Schüttler J, Koppert W. Effects of intermittent hemodialysis on buprenorphine and norbuprenorphine plasma concentrations in chronic pain patients treated with transdermal buprenorphine. European Journal of Pain (London, England). 2006;10(8):743–748. [DOI] [PubMed] [Google Scholar]
- 60.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. American Journal of Public Health. 2010;100(12):2541–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marcum ZA, Perera S, Donohue JM, et al. Analgesic use for knee and hip osteoarthritis in community-dwelling elders. Pain medicine (Malden, Mass). 2011;12(11):1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lapane KL, Quilliam BJ, Chow W, Kim MS. Pharmacologic management of non-cancer pain among nursing home residents. Journal of pain and symptom management. 2013;45(1):33–42. [DOI] [PubMed] [Google Scholar]
- 63.Papaleontiou M, Henderson CR, Turner BJ, et al. Outcomes associated with opioid use in the treatment of chronic noncancer pain in older adults: a systematic review and meta-analysis. Journal of the American Geriatrics Society. 2010;58(7):1353–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brooks AK, Udoji MA. Interventional Techniques for Management of Pain in Older Adults. Clinics in Geriatric Medicine. 2016;32(4):773–785. [DOI] [PubMed] [Google Scholar]
- 65.Mathis MR, Naughton NN, Shanks AM, et al. Patient selection for day case-eligible surgery: identifying those at high risk for major complications. Anesthesiology. 2013;119(6):1310–1321. [DOI] [PubMed] [Google Scholar]
- 66.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50(5):889–896. [DOI] [PubMed] [Google Scholar]
- 67.Barry BK, Carson RG. The consequences of resistance training for movement control in older adults. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2004;59(7):730–754. [DOI] [PubMed] [Google Scholar]
- 68.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. The Journal of Laboratory and Clinical Medicine. 2001;137(4):231–243. [DOI] [PubMed] [Google Scholar]
- 69.Braddom’s physical medicine & rehabilitation. Fifth edition Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 70.de Vries NM, van Ravensberg CD, Hobbelen JSM, Olde Rikkert MGM, Staal JB, Nijhuis-van der Sanden MWG. Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community-dwelling older adults with impaired mobility, physical disability and/or multi-morbidity: a meta-analysis. Ageing Research Reviews. 2012;11(1):136–149. [DOI] [PubMed] [Google Scholar]
- 71.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. The New England Journal of Medicine. 2002;347(14):1068–1074. [DOI] [PubMed] [Google Scholar]
- 72.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263(22):3029–3034. [PubMed] [Google Scholar]
- 73.Frontera WR, Hughes VA, Krivickas LS, Kim S-K, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle & nerve. 2003;28(5):601–608. [DOI] [PubMed] [Google Scholar]
- 74.Ettinger WH, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA. 1997;277(1):25–31. [PubMed] [Google Scholar]
- 75.Kovar PA, Allegrante JP, MacKenzie CR, Peterson MG, Gutin B, Charlson ME. Supervised fitness walking in patients with osteoarthritis of the knee. A randomized, controlled trial. Annals of Internal Medicine. 1992;116(7):529–534. [DOI] [PubMed] [Google Scholar]
- 76.van Baar ME, Dekker J, Oostendorp RA, et al. The effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a randomized clinical trial. The Journal of rheumatology. 1998;25(12):2432–2439. [PubMed] [Google Scholar]
- 77.Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to 83. Journal of the American Geriatrics Society. 2002;50(6):1100–1107. [DOI] [PubMed] [Google Scholar]
- 78.Jette AM, Rooks D, Lachman M, et al. Home-based resistance training: predictors of participation and adherence. The Gerontologist. 1998;38(4):412–421. [DOI] [PubMed] [Google Scholar]
- 79.Nelson ME, Layne JE, Bernstein MJ, et al. The effects of multidimensional home-based exercise on functional performance in elderly people. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2004;59(2):154–160. [DOI] [PubMed] [Google Scholar]
- 80.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37(1):51–56. [DOI] [PubMed] [Google Scholar]
- 81.Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Z, Katz J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. Journal of pain research. 2015;8:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dindo L, Zimmerman MB, Hadlandsmyth K, et al. Acceptance and Commitment Therapy for Prevention of Chronic Postsurgical Pain and Opioid Use in At-Risk Veterans: A Pilot Randomized Controlled Study. The journal of pain : official journal of the American Pain Society. 2018;19(10):1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opinion on Drug Safety. 2014;13(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nicholas MK, Asghari A, Blyth FM, et al. Long-term outcomes from training in self-management of chronic pain in an elderly population: a randomized controlled trial. Pain. 2017;158(1):86–95. [DOI] [PubMed] [Google Scholar]