Abstract
Background:
Embryonal tumors arise typically in infants and young children and are often massive at presentation. Operative resection is a cornerstone in the multimodal treatment of embryonal tumors but potentially disrupts therapeutic timelines. When used appropriately, minimally invasive surgery can minimize treatment delays. The oncologic integrity and safety attainable with minimally invasive resection of embryonal tumors, however, remains controversial.
Methods:
Query of the Vanderbilt Cancer Registry identified all children treated for intracavitary, embryonal tumors during a 15-year period. Tumors were assessed radiographically to measure volume (mL) and image-defined risk factors (neuroblastic tumors only) at time of diagnosis, and at preresection and postresection. Patient and tumor characteristics, perioperative details, and oncologic outcomes were compared between minimally invasive surgery and open resection of tumors of comparable size.
Results:
A total of 202 patients were treated for 206 intracavitary embryonal tumors, of which 178 were resected either open (n = 152, 85%) or with minimally invasive surgery (n = 26, 15%). The 5-year, relapse-free, and overall survival were not significantly different after minimally invasive surgery or open resection of tumors having a volume less than 100 mL, corresponding to the largest resected with minimally invasive surgery (P = .249 and P = .124, respectively). No difference in margin status or lymph node sampling between the 2 operative approaches was detected (p = .333 and p = .070, respectively). Advantages associated with minimally invasive surgery were decreased blood loss (P < .001), decreased operating time (P = .002), and shorter hospital stay (P < .001). Characteristically, minimally invasive surgery was used for smaller volume and earlier stage neuroblastic tumors without image-defined risk factors.
Conclusion:
When selected appropriately, minimally invasive resection of pediatric embryonal tumors, particularly neuroblastic tumors, provides acceptable oncologic integrity. Large tumor volume, small patient size, and image-defined risk factors may limit the broader applicability of minimally invasive surgery.
Introduction
Operative resection is the fundamental component of a multimodal treatment strategy implemented to cure children who have embryonal tumors. Traditionally, intracavitary embryonal tumors have been resected through an open operative approach. But laparotomies and thoracotomies are potentially morbid procedures, particularly in patients already debilitated from the disease burden of cancer and its toxic therapies. Minimally invasive surgery (MIS) potentially offers a less morbid approach and is being used increasingly to resect various malignancies in adult patients. Among adult patients having solid tumors amenable to MIS, oncologic integrity and safety appear equivalent to traditional open approaches.1–3 Until recently, the use of MIS for pediatric solid tumors was limited to biopsy, staging, evaluation of resectability, and management of therapeutic complications, such as infection, but the indications for MIS have expanded during the past 20 years now to include definitive resections.4,5
The controversy surrounding MIS for oncologic resection concerns whether MIS affords adequate visualization and access to achieve complete tumor resection without spillage and to sample lymph nodes for appropriate staging. As an emerging option for pediatric solid tumors, several institutions have reported rudimentary experience with MIS to resect solid malignancies in pediatric patients, but these studies were limited in scope and critical evaluation of the oncologic integrity of less invasive approaches.6–11 Consideration of tumor location, size, and image-defined risk factors has been proposed to assess candidacy for MIS, yet patient selection criteria remain incompletely defined.7,8,12 The challenges and limitations associated with MIS resection of pediatric intracavitary embryonal tumors include the necessary mobilization of often large masses that occupy small spaces and involve tactile constraints, risk of tumor spillage, vascular encasement and other image-defined risk factors, and individual surgeon experience with MIS.13 If these challenges can be overcome, MIS may theoretically confer decreases in postoperative pain, intraoperative blood and insensible fluid loss, the incidence of postoperative intestinal ileus and obstruction, and hospital stays. Moreover, earlier return to activity, less disruption to strict therapy timelines, and improved cosmesis are additional advantages.5,14,15 Indeed, progress in the efficacy of neoadjuvant therapy to decrease massive embryonal tumor volumes, often substantially, has rendered MIS resection more appealing in appropriate scenarios. Furthermore, strict screening guidelines for children genetically predisposed to develop embryonal tumors have helped identify smaller, earlier stage, and more biologically favorable lesions that also might be amenable to MIS.16
The overarching question to answer then is whether MIS resection of different embryonal tumors when feasible can achieve the multiple goals expected of a high-fidelity and curative cancer operation: negative margins without tumor spill, adequate lymph node sampling, organ preservation when indicated, and survival. Therefore, we conducted this retrospective analysis of all pediatric, intracavitary, embryonal tumors treated at our institution during a 15-year period to: (1) assess candidacy for MIS resection of embryonal tumors, (2) examine operative outcomes associated with minimally invasive and open approaches, and (3) assess whether equivalent oncologic integrity is maintained through MIS techniques. We hypothesized that, among pediatric patients having embryonal tumors amenable to a minimally invasive approach, an MIS resection can maintain oncologic integrity while minimizing interruptions to therapy.
Methods
Patient selection
The comprehensive Vanderbilt Cancer Registry (VCR) was queried, using ICD-O-3 morphologic and topographic codes to identify pediatric patients younger than 18 years of age at diagnosis who were treated for an intracavitary, embryonal tumor between January 1, 2002, and September 1, 2017 (n = 236). Patients were excluded if definitive resection was performed at an outside institution (n = 34). The Vanderbilt Institutional Review Board approved this study (#100734).
Radiographic analysis
To describe patients amenable to MIS, tumor volumes (TVs) were measured radiographically on the computed tomography (CT) at diagnosis and immediately before resection. When a CT (n = 196) was not available, measurements were obtained from magnetic resonance imaging (MRI) (n = 4) or ultrasonography (n = 2). Maximum diameter was measured along the axis of the tumor in the anteroposterior (a, cm), transverse (b, cm), and craniocaudal planes (c, cm), and an ellipsoid approximation was used to estimate tumor volume (cm3 = mL): . For neuroblastic tumors only, image-defined risk factors (IDRF) were evaluated to account for additional features of the tumor that might influence feasibility of an MIS resection, such as vascular encasement, infiltration into adjacent organs, and intraspinal tumor extension.17 Additionally, for neuroblastic cases only, to document fidelity of a given procedural approach to achieve the oncologic goal of gross total resection ([GTR] >98% resection of primary tumor), residual TV at the time of first CT after resection was measured and correlated with metaiodobenzylguanidine (MIBG) avidity, when available, to distinguish residual tumor from postoperative change. For patients undergoing resection of multiple, distinct tumors in either one or a staged operation, each mass was measured separately for volume, but the patient was considered once in all other analyses.
Data collection
Demographic data abstracted from the VCR and electronic medical record included sex, race, ethnicity, body surface area, and age at diagnosis. Oncologic and treatment data included Children’s Oncology Group (COG) stage and risk stratification, pathology, and pretreatment with neoadjuvant chemotherapy. Operative approach, operating time, estimated blood loss (EBL), duration of hospitalization, and delay to the next administration of chemotherapy were evaluated. Duration of hospitalization was measured from date of resection to discharge or to transfer from pediatric surgery to another hospital service (eg, pediatric hematology-oncology). Oncologic integrity was assessed by comparing resection margin status, lymph node dissection, relapse-free survival (RFS), and overall survival (OS). MIS was defined as resection via laparoscopy, thoracoscopy, or transuretheral cystoscopy.
Statistical analysis
Data were summarized using the median and interquartile range (IQR). The Wilcoxon rank sums test was applied to two-group continuous outcomes (Kruskal-Wallis for more than 2 groups), and the Pearson χ2 test was applied to categorical outcomes. Survival was calculated from the date of diagnosis to the date of death from any cause or to the date of last known contact with the patient. Similarly, RFS was calculated from date of diagnosis to the date that new local or metastatic disease was identified or to the date of last known contact with the patient. The distributions of RFS and OS were estimated using the method of Kaplan-Meier. The log-rank test was applied to test equality of survival distributions between patient groups. Missing data were excluded from the relevant analyses. All analyses were performed using Stata 15 (StataCorp, LLC, College Station, TX). All tests were 2 sided.
Results
Tumor volume and effect of neoadjuvant therapy
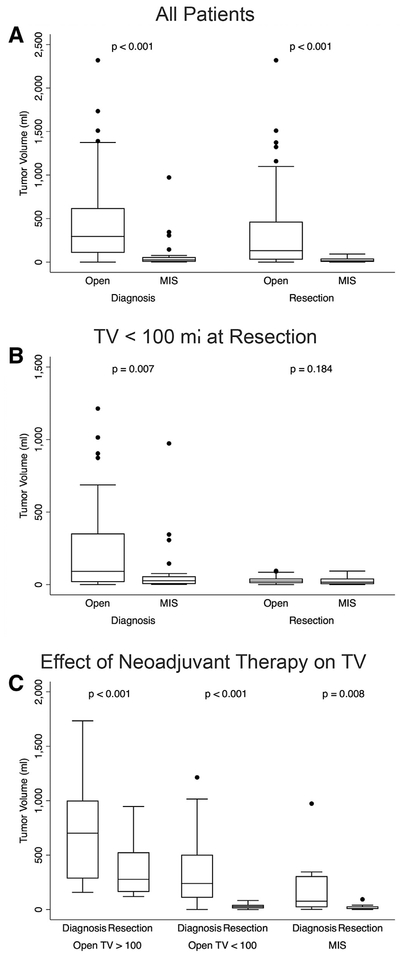
Between the years 2002 and 2017, 202 patients were treated for 206 intracavitary, embryonal tumors, including neuroblastic tumors ([NBL] n = 102), Wilms tumor ([WT] n = 70), rhabdomyosarcoma ([RMS] n = 10), hepatoblastoma ([HBL] n = 23) and pancreatoblastoma (n = 1) (Table 1). Given the sole case, pancreatoblastoma was excluded from the comparative analysis. Within this cohort, 178 embryonal tumors were resected either open (n = 152, 85%) or using MIS (n = 26, 15%). Of the 174 patients undergoing any resection, 150 (86%) had open surgery and 24 (14%) had MIS. Among all patients, the median TV at diagnosis was 215.0 mL (IQR 42.9, 546.0), and the median TV at resection was 84.0 mL (20.4, 372.5). As expected, tumors that were resected with an open approach were larger than those resected with MIS (median open TV 131.7 mL [28.6, 465] and median MIS TV 16.9 mL [3.1, 42.1]; P < .001; Table 2, Fig 1, A).
Table 1.
Patient characteristics and outcomes according to embryonal tumor type.
| n | NBL n = 101 | WT n = 67 | RMS n = 10 | HBL n = 23 | Combined n = 201 | P value | |
|---|---|---|---|---|---|---|---|
| Age at diagnosis (months) | 201 | 21 [8, 51] | 42 [20, 53] | 30.5 [19, 64] | 18 [7, 25] | 28 [12, 51] | < .001 |
| Sex | |||||||
| Male | 201 | 52% (52) | 36% (24) | 50% (5) | 577% (13) | 47% (94) | .168 |
| Female | 49% (49) | 64% (43) | 50% (5) | 44% (10) | 53% (107) | ||
| Race | |||||||
| White, non-Hispanic | 201 | 77% (78) | 69% (46) | 90% (9) | 74% (17) | 75% (150) | .708 |
| White, Hispanic | 3% (3) | 8% (5) | 10% (1) | (1) | 5% (10) | ||
| Black | 13% (13) | 16% (11) | (0) | (3) | 13% (27) | ||
| Asian | 3% (3) | (0) | (0) | (1) | (4) | ||
| Other | 4% (4) | 8% (5) | (0) | (1) | 5% (10) | ||
| Tumor volume | |||||||
| Diagnosis (mL) | 200 | 140.1 [30.5, 326.2] | 503.4 [240, 746] | 59.2 [30.2, 289.3] | 252.2 [133.9, 532] | 215.0 [42.9, 546.0] | < .001 |
| Resection (mL)* | 174 | 40.1 [11.3, 124.8] | 438.0 [115.2, 705.8] | 18.9 [4.5, 29.1] | 43.1 [34.6, 153.8] | 84.0 [20.4, 372.5] | < .001 |
| Timing of resection* | |||||||
| Upfront | 174 | 48% (40) | 82% (54) | (1) | 56% (10) | 60% (105) | < .001 |
| After neoadjuvant | 52% (44) | 18% (12) | 83% (5) | 44% (8) | 40% (69) | ||
| Resection approach | |||||||
| Open TV >100 mL | 201 | 23% (23) | 75% (50) | (1) | 35% (8) | 41% (82) | < .001 |
| Open TV <100 mL | 41% (41) | 21% (14) | 30% (3) | 44% (10) | 34% (68) | ||
| MIS | 20% (20) | (2) | 20% (2) | (0) | 12% (24) | ||
| No resection | 17% (17) | (1) | 40% (4) | 22% (5) | 13% (27) | ||
| Margin status* | |||||||
| Negative | 158 | 36% (25) | 77% (50) | 40% (2) | 94% (17) | 60% (94) | < .001 |
| Positive | 64% (45) | 23% (15) | 60% (3) | (1) | 41% (64) | ||
| Relapse at 5 years | |||||||
| No | 108 | 67% (37) | 83% (30) | 86% (6) | 100% (10) | 77% (83) | .072 |
| Yes | 33% (18) | 17% (6) | (1) | (0) | 23% (25) | ||
| Survival at 5 years | |||||||
| Alive | 117 | 70% (41) | 94% (34) | 75% (6) | 72% (10) | 78% (91) | .037 |
| Dead | 31% (18) | (2) | 25% (2) | 29% (4) | 22% (26) |
NBL, neuroblastoma; WT, Wilms tumor; RMS, rhabdomyosarcoma; HBL, hepatoblastoma; TV, tumor volume.
Includes resected tumors only (84 NBL, 66 WT, 6 RMS, 18 HBL).
Table 2.
Patient and tumor characteristics according to resection approach.
| n | Open TV >100 mL n = 82 | Open TV <100 mL n = 68 | MIS* n = 24 | No resection n = 27 | Combined n = 201 | P value | |
|---|---|---|---|---|---|---|---|
| Embryonal tumor types | |||||||
| NBL | 201 | 28% (23) | 60% (41) | 83% (20) | 63% (17) | 50% (101) | < .001 |
| WT | 61% (50) | 21% (14) | (2) | (1) | 33% (67) | ||
| RMS | (1) | (3) | (2) | 15% (4) | 5% (10) | ||
| HBL | 10% (8) | 15% (10) | (0) | 19% (5) | 11% (23) | ||
| Sex | |||||||
| Male | 201 | 44% (36) | 43% (29) | 58% (14) | 56% (15) | 47% (94) | .414 |
| Female | 56% (46) | 57% (39) | 42% (10) | 44% (12) | 53% (107) | ||
| Race/Ethnicity | |||||||
| White, non-Hispanic | 201 | 71% (58) | 71% (48) | 89% (21) | 85% (23) | 75% (150) | .201 |
| White, Hispanic | (3) | 10% (7) | (0) | (0) | 5% (10) | ||
| Black | 18% (15) | 13% (9) | (0) | (3) | 13% (27) | ||
| Asian | (1) | (2) | (1) | (0) | 2% (4) | ||
| Other | 6% (5) | (2) | (2) | (1) | 5% (10) | ||
| Age at diagnosis | 201 | 41.5 [18, 53] | 20 [9, 39.5] | 35.5 [16, 66.5] | 13 [3, 30] | 28 [12, 51] | < .001 |
| Body surface area | 196 | 0.64 [0.5, 0.74] | 0.54 [0.44, 0.67] | 0.62 [0.47, 0.79] | 0.46 [0.36, 0.54] | 0.58 [0.45, 0.71] | < .001 |
| Stage | |||||||
| Stage I/II | 189 | 45% (35) | 39% (26) | 68% (13) | (2) | 40% (76) | < .001 |
| Stage III/IV | 55% (43) | 61% (41) | 32% (6) | 92% (23) | 60% (113) | ||
| COG risk group | |||||||
| Low | 189 | 42% (33) | 38% (24) | 57% (12) | 20% (5) | 39% (74) | .016 |
| Intermediate | 38% (30) | 22% (14) | (3) | 36% (9) | 30% (56) | ||
| High | 20% (16) | 41% (26) | 29% (6) | 44% (11) | 31% (59) | ||
| Tumor volume (TV) | |||||||
| TV at diagnosis | 200 | 481.8 [263.9, 760] | 91.1 [17.6, 352.4] | 26.3 [4.2, 57.8] | 141.1 [34.8, 445.2] | 215.0 [42.9, 546.0] | < .001 |
| TV at resection | 174 | 416.6 [231.2, 678.2] | 24.3 [10.4,42.1] | 16.9 [3.1,42.1] | - | 84.0 [20.4, 372.5] | < .001 |
| Upfront | 105 | 461.0 [258.1, 733.1] | 17.7 [11.5, 37.5] | 24.7 [2.3, 46.8] | - | 220.5 [34.6, 540.4] | † |
| After neoadjuvant | 69 | 277.1 [161.5, 525.5] | 26.4 [10.2, 42.4] | 10.3 [5.5, 29.1] | - | 39.1 [12, 119.2] | |
| Timing of resection | |||||||
| Upfront | 174 | 78% (64) | 38% (26) | 63% (15) | - | 60% (105) | < .001 |
| After neoadjuvant | 22% (18) | 62% (42) | 38% (9) | - | 40% (69) | ||
| Fold decrease in TV after neoadjuvant | 68 | 1.5 [1.3, 2.9] | 9.1 [4.4, 20.5] | 7.7 [3.7, 10.4] | - | 5.5 [1.8, 15.4] | < .001 |
All TV <100 mL for MIS resection.
TV at resection for open with TV >100 mL by neoadjuvant status (upfront versus after neoadjuvant): P = .042; TV at resection for open with TV <100 mL by neoadjuvant status: P = .900; TV at resection for MIS by neoadjuvant status: P = .721.
MIS, minimally invasive surgery; NBL, neuroblastic tumors; WT, Wilms tumor; RMS, rhabdomyosarcoma; HBL, hepatoblastoma; COG, Children’s Oncology Group; TV, tumor volume.
Fig 1.
Tumor volumes at diagnosis and resection based on resection approach and administration of neoadjuvant chemotherapy. (A) Tumor volumes based on resection approach for all tumors at diagnosis and resection. (B) Tumor volumes at diagnosis and resection based on operative approach for all TV <100 mL at resection. (C) Reduction in TV with neoadjuvant chemotherapy from diagnosis to resection based on approach. Boxes represent interquartile range (IQR) with whiskers spanning to 1.5*IQR. Horizontal line within box represents median. Dots represent values outside of 1.5*IQR. Significance (P values) was assessed by Wilcoxon rank sum test and Wilcoxon matched-paired signed-rank test.
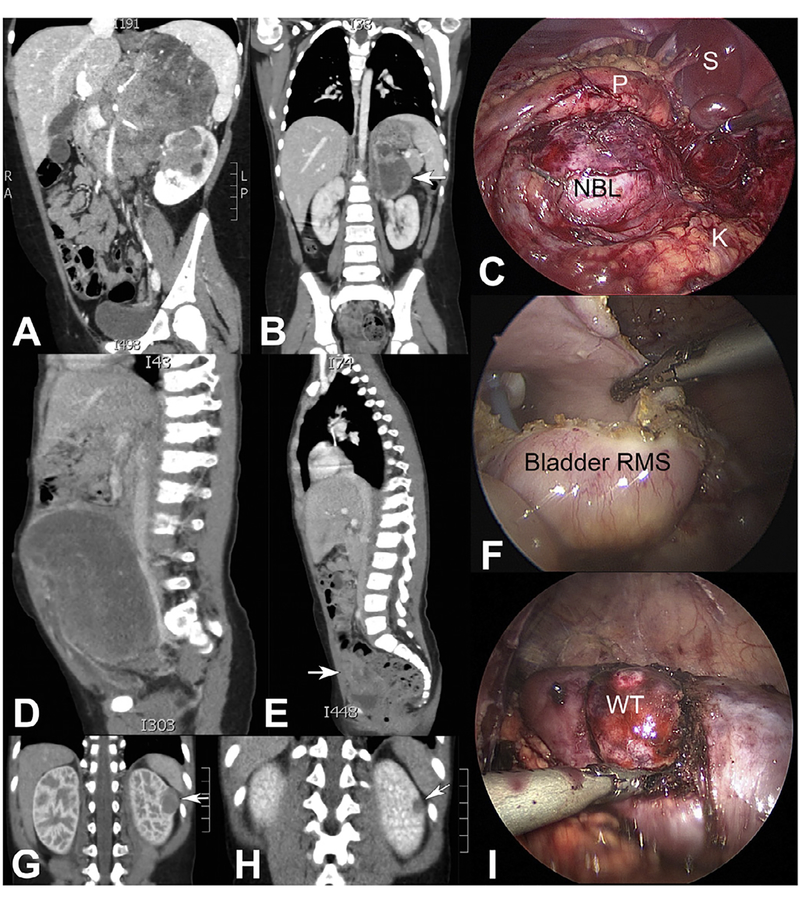
Given that tumor volume was likely the principal consideration for amenability to MIS, we chose to compare MIS and open techniques for tumors having a volume less than or equal to the largest resected tumor using an MIS approach. Therefore, corresponding to the largest tumor resected with MIS (a neoadjuvant-treated neuroblastoma measuring 93.4 mL; Fig 2, A–C), the open resection group was divided into TV >100 mL and TV <100 mL for analysis. By this definition, any tumor resected with MIS had a volume less than 100 mL. When comparing open and MIS cases performed for TV <100 mL at time of resection, the TV at diagnosis was still significantly larger for open versus MIS procedures, but TV at resection was not significantly different between operative techniques (Table 3; Fig 1, B).
Fig 2.
Effect of neoadjuvant chemotherapy to reduce tumor volume dramatically in a high-risk neuroblastoma, high-risk bladder rhabdomyosarcoma, and predisposition case of Wilms tumor. (A-C) Impact of neoadjuvant therapy to reduce a massive, high-risk neuroblastoma (NBL) of the left adrenal gland in an 8-year-old girl having multiple image-defined risk factors (vascular encasement and infiltration into adjacent left kidney; P, pancreas; S, spleen; K, kidney). Complete gross total resection was achieved using an MIS approach after 5 cycles of neoadjuvant chemotherapy (arrow in B shows tumor after neoadjuvant therapy). (D-F) A 3-year-old girl presented with a large pelvic rhabdomyosarcoma (RMS) and peritoneal studding, which responded significantly to neoadjuvant chemotherapy (arrow in E shows residual tumor) and was completely resected with negative margins using MIS. Foley catheter is visible in F. (G-J) A 1-year-old boy with Beckwith-Wiedemann Syndrome, who was discovered on routine surveillance to have a left renal Wilms tumor (WT) that regressed nicely with 4 cycles of EE-4A (arrows denote tumor before and after neoadjuvant chemotherapy). MIS was used to perform a nephron-sparing resection, and margins were negative.
Table 3.
Embryonal tumor characteristics and operative outcomes for all TV <100 mL at time of resection.
| n | MIS n = 24 | Open n = 68 | Combined n = 92 | P value | |
|---|---|---|---|---|---|
| Stage | |||||
| Stage I/II | 86 | 68% (13) | 39% (26) | 45% (39) | .022 |
| Stage III/IV | 32% (6) | 61% (41) | 55% (47) | ||
| COG risk group | |||||
| Low | 85 | 57% (12) | 38% (24) | 42% (36) | .286 |
| Intermediate | (3) | 22% (14) | 20% (17) | ||
| High | 29% (6) | 41% (26) | 38% (32) | ||
| TV at diagnosis (mL) | 91 | 26.3 [4.2, 57.8] | 91.1 [17.6, 352.4] | 52.3 [16.7, 279.4] | .007 |
| TV at time of resection (mL) | 92 | 16.9 [3.1, 42.1] | 24.3 [10.4, 42.1] | 23.7 [9.3, 42.1] | .184 |
| Upfront | 41 | 24.7 [2.3, 46.8] | 17.7 [11.5, 37.5] | 17.8 [6.3, 44.4] | * |
| After neoadjuvant | 51 | 10.3 [5.5, 29.1] | 26.4 [10.2, 42.4] | 24.6 [9.6, 41.8] | |
| Fold reduction in TV after neoadjuvant chemotherapy | 50 | 7.7 [3.7, 10.4] | 9.1 [4.4, 20.5] | 8.3 [4.2, 19.9] | .412 |
| IDRF at diagnosis (NBL only) | |||||
| 0 IDRF | 59 | 74% (14) | 38% (15) | 49% (29) | .009 |
| 1 + IDRF | 26% (5) | 63% (25) | 51% (30) | ||
| IDRF at resection (NBL only) | |||||
| 0 IDRF | 60 | 95% (18) | 56% (23) | 68% (41) | .003 |
| 1 + IDRF | (1) | 44% (18) | 32% (19) | ||
| Operating time (min) | 81 | 161.5 [110, 240] | 250 [176, 373] | 234 [156, 330] | .002 |
| Estimated blood loss (ml) | 81 | 10 [10, 35] | 100 [30, 240] | 70 [10, 175] | < .001 |
| Margin status | |||||
| Negative | 79 | 44% (8) | 57% (35) | 54% (43) | .333 |
| Positive | 56% (10) | 43% (26) | 46% (36) | ||
| Lymph nodes dissected | 51 | 1 [0, 3] | 2 [1, 5.5] | 2 [0, 5] | .070 |
| Duration of hospitalization (days) | 92 | 2 [1,3] | 4 [3, 5.5] | 4 [2.5, 5] | < .001 |
| Time to next chemo (days) | 58 | 12.5 [7.5, 19.5] | 19.5 [14, 26] | 18.5 [14, 25] | .051 |
| Residual TV (NBL only) | |||||
| ≥98% resection | 55 | 94% (16) | 68% (26) | 76% (42) | .038 |
| ≤98% resection | (1) | 32% (12) | 24% (13) | ||
| Relapse at 5 years | |||||
| No | 58 | 85% (11) | 69% (31) | 72% (42) | .264 |
| Yes | (2) | 31% (14) | 28% (16) | ||
| Survival at 5 years | |||||
| Alive | 56 | 100% (12) | 75% (33) | 80% (45) | .053 |
| Dead | (0) | 25% (11) | 20% (11) |
TV at resection for MIS by neoadjuvant status (upfront versus after neoadjuvant): P = .721. TV at resection for open by neoadjuvant status: P = .900.
MIS, minimally invasive surgery; TV, tumor volume; IDRF, image-defined risk factors; NBL, neuroblastic tumors.
Neoadjuvant chemotherapy preceded resection among 69 patients (40%; Table 2). For MIS and open procedures with TV <100 mL at resection, volumes were not significantly different if performed upfront (ie, without neoadjuvant therapy) versus after neoadjuvant chemotherapy. Tumors resected upfront with MIS measured 24.7 mL (2.3, 46.8), while those resected with MIS after neoadjuvant chemotherapy measured 10.3 mL (5.5, 29.1 [P = .721]; Table 3). Similarly, tumors with volumes less than 100 mL resected open upfront measured 17.7 mL (11.5, 37.5), and those resected open after neoadjuvant chemotherapy measured 26.4 (10.2, 42.4 [P = .900]; Table 3 ). The median decrease in TV for patients receiving neoadjuvant chemotherapy was not different between MIS (7.7-fold [3.7, 10.4]) or open resection of TV <100 mL (9.1-fold [4.4, 20.5]; P = .412; Fig 1, C, Table 3).
Note that the majority of MIS resections (n = 24) were for neuroblastic tumors (n = 20, 83%). Although patient demographics did not differ between resection types, a significant variation emerged regarding age at diagnosis and body surface area between procedure approaches. Specifically, MIS resections were performed more typically on children having larger body surface area (P < .001), which was expected, because they were older as well (P < .001; Table 2). COG tumor stage and risk stratification were significantly different between resection approaches, with MIS as a group tending toward earlier stage and lower risk tumors. Specifically, stage I or II tumors comprised 68% (n = 13) of MIS resections, 39% (n = 26) of open TV <100 mL resections, and 45% (n = 35) of open TV >100 mL resections (P = .001; Table 2). Low-risk tumors were resected in 57% (n = 12) of MIS cases, 38% (n = 24) of open TV <100 mL cases, and 42% (n = 33) of open TV >100 mL cases (P = .016; Table 2). Among patients with TV <100 mL, this difference in stage remained, but the difference in distribution of COG risk group between MIS and open TV <100 mL was not statistically significant (P = .022 and P = .286, respectively; Table 3). Collectively, patients undergoing MIS resection more commonly harbored lower stage tumors that were less frequently treated with neoadjuvant chemotherapy preceding resection.
Operative outcomes
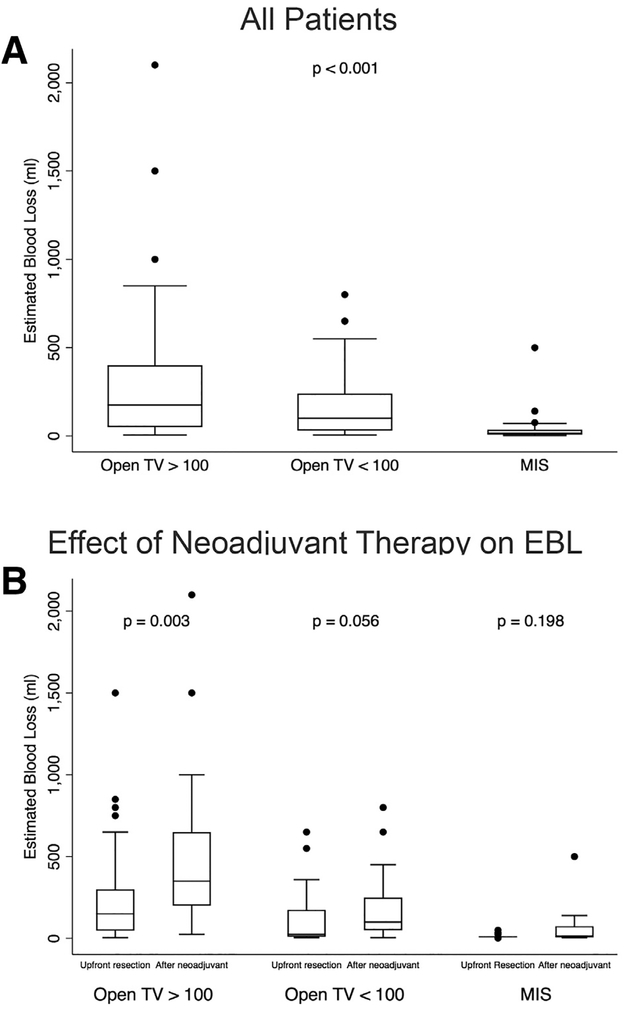
Among all TV <100 mL, procedure duration, EBL, duration of hospitalization, and time to the next cycle of chemotherapy were assessed between MIS and open resections (Table 3). Median operating time was 162 minutes (110, 240) for MIS and 250 minutes (176, 373) for open resection (P = .002). EBL for MIS was less than open resection, having a median of 10 mL (10, 35) compared with 100 mL (30, 240), respectively (P < .001). As expected, EBL was greatest among open resections having a TV >100 mL after administration of neoadjuvant chemotherapy (Fig 3).
Fig 3.
Estimated blood loss based on operative approach and pre-treatment with neoadjuvant chemotherapy. (A) Estimated blood loss (EBL) based on resection approach. (B) Differences in EBL between upfront resection or after neoadjuvant chemotherapy for each operative approach. Boxes represent interquartile range (IQR) with whiskers spanning to 1.5*IQR. Horizontal line within box represents median. Dots represent values outside of 1.5*IQR. Significance (P values) assessed by Kruskal-Wallis test and Wilcoxon rank sum test.
MIS had a median hospital stay of 2 days (1, 3), while open resection of similar volume tumors had a greater median stay of 4 days (3, 5.5) (P < .001). Finally, the median delay from MIS resection to the next administration of chemotherapy was 12.5 days (7.5, 19.5); whereas chemotherapy after open resection of TV <100 mL was delayed 19.5 days (14, 26) (P = .051; Table 3). Adjuvant chemotherapy was given after 9 of 24 (38%) MIS resections and after 50 of 68 (74%) open resections with TV <100 mL (P = .001). Patients who did not require adjuvant chemotherapy were excluded from this subanalysis of treatment delay.
Relatively few perioperative complications occurred in the entire cohort. Complications associated with open resection of tumors having a volume less than 100 mL included return to the operating room for chest tube placement after resection of a thoracic NBL, return to the operating room for bleeding after resection of a thoracic NBL, and bile leak after resection of HBL (sealed spontaneously). Most complications in the cohort occurred in patients who underwent open resection of tumors with volume > 100 mL. The only late complication after MIS was chronic bladder spasms in one patient with RMS.
Regarding MIS conversion to open resection, one abdominal ganglioneuroblastoma, having a TV of 85 mL at resection, was carried out as a planned, laparoscopic-assisted resection. For this case, most of the tumor was mobilized laparoscopically, and a small flank laparotomy was created overlying the kidney to dissect encasement of the renal vessels and deliver the tumor. Such an approach may offer some of the benefits of an MIS resection for patients who are not candidates for a complete MIS resection. No other conversions from MIS to open surgery occurred in this cohort.
Oncologic integrity and survival
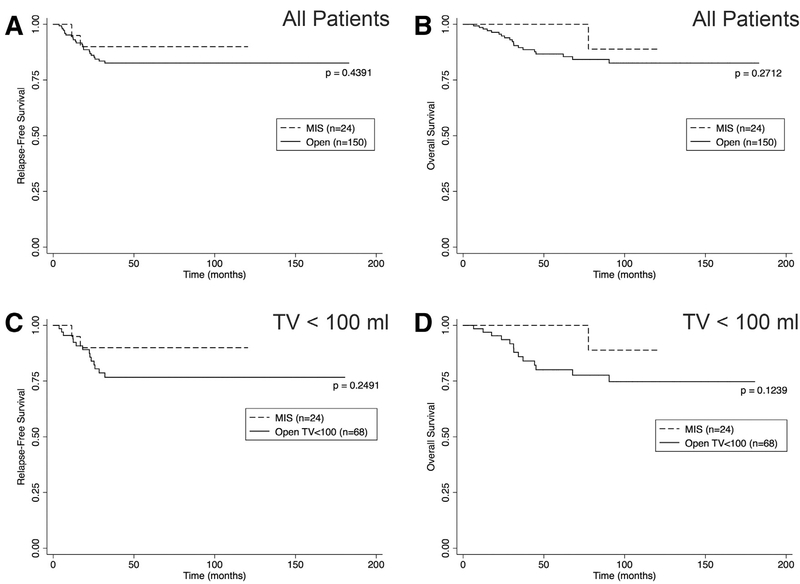
Margin status, lymph node sampling, completeness of resection, RFS, and OS were used as markers of the oncologic integrity of a resection. RFS at 5 years for all patients undergoing any resection was 0.84 (CI 0.77–0.89), and OS at 5 years was 0.88 (CI 0.82–0.93). No significant difference was detected in either RFS or OS between MIS and open resection when assessing all patients or when considering TV <100 mL only (Fig 4, A–D). Specifically, for TV <100 mL, 5-year RFS was 0.77 (CI 0.64–0.86) for open resection and 0.90 (CI 0.66–0.97) for MIS (P = .249; Fig 4, C). The 5-year OS was 0.80 (CI 0.67–0.89) for open resection of TV <100 mL and 1.00 (CI 1.00–1.00) for MIS (P = .124; Fig 4, D).
Fig 4.
Relapse-free and overall survival by operative approach. (A, B) Kaplan-Meier plots showing RFS and OS based on resection approach for all tumors regardless of TV at resection. (C, D) RFS and OS based on resection approach for tumors with volume <100 mL only. P values were assessed with the log rank test for equality of survival functions.
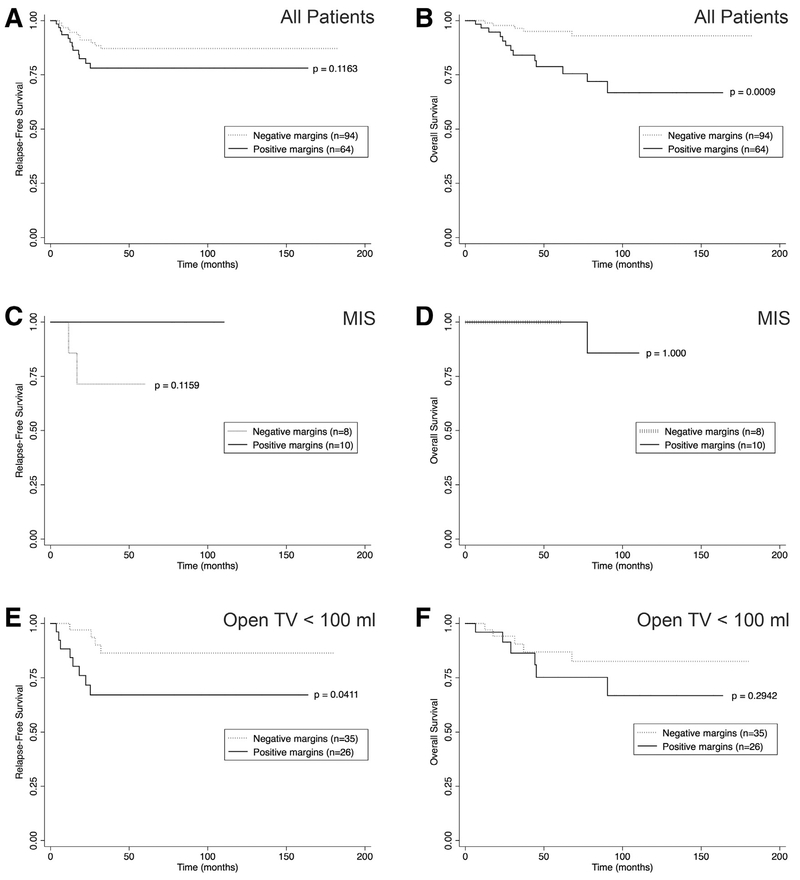
For patients who had margin status reported (n = 158), 60% (n = 94) of resections achieved negative margins. As expected, margin negative resections varied significantly by tumor type, with HBL having the highest proportion (n = 17, 94%) and NBL having the least proportion (n = 25, 36%; Table 1). When considering the entire cohort, no significant difference in RFS was observed for margin-negative or margin-positive resections ([0.87 0.78–0.93] versus 0.78 [0.64–0.87], respectively; P = .116; Fig 5, A); however, OS was poorer for margin-positive resections than margin-negative resections (0.79 [0.64–0.88] versus 0.95 [0.87–0.98], respectively; P < .001; Fig 5, B). When considering TV <100 mL only, negative margins were achieved in 44% (n = 8) of MIS cases and in 57% (n = 35) of open cases (P = .333; Table 3). For patients with open resection of TV <100 mL, positive margin status was associated with decreased RFS compared with negative margin status (0.67 [0.45–0.82] versus 0.86 [0.67–0.95], respectively; P = .041; Fig 5, C–F). Of note, the 2 relapse events in the MIS group both had negative margins at the time of resection (Fig 5, C).
Fig 5.
Resection margins and survival outcomes. Kaplan-Meier plots demonstrating (A, B) RFS and OS for all patients based on resection margins. (C, D) RFS and OS after MIS resection according to resection margins. (E, F) RFS and OS for open resection with TV <100 mL based on resection margins. P values were assessed with the log-rank test for equality of survival functions.
No significant difference was observed in the number of lymph nodes sampled based on approach for TV <100 mL. Median lymph node yield was 1 (0, 3) for MIS and 2 (1, 5.5) for open resection (P = .070; Table 3).
Neuroblastic tumors only
Given the overall preponderance of neuroblastic tumors among MIS resections (n = 20, 83%), separate analyses were completed for this disease category only (Table 4; Fig 6). MIS again favored older children (P = .006) with greater body surface area (P = .003) who had stage I and II tumors (P = .005) and smaller TV at resection (P < .001). Assessment of IDRFs for neuroblastic tumors having TV <100 mL and either open (n = 64, 76%) or MIS (n = 20, 24%) resection revealed that the majority of the latter had no IDRFs at the time of operation (n = 18, 95%). A lesser proportion of patients undergoing open resection of NBL TV <100 mL (n = 23, 56%) and an even smaller proportion of patients undergoing open resection of NBL TV >100 mL (n = 8, 35%) had tumors without IDRFs (P < .001; Table 4).
Table 4.
Neuroblastic tumor characteristics and operative outcomes.
| n | Open TV >100 mL n = 23 | Open TV <100 mL n = 41 | MIS n = 20 | No resection n = 17 | Combined n = 101 | P value | |
|---|---|---|---|---|---|---|---|
| Histology | |||||||
| Neuroblastoma | 101 | 83% (19) | 88% (36) | 65% (13) | 100% (17) | 84% (85) | .050 |
| Ganglioneuroblastoma | (2) | 12% (5) | 20% (4) | (0) | 11% (11) | ||
| Ganglioneuroma | (2) | (0) | 15% (3) | (0) | 5% (5) | ||
| Age at diagnosis (months) | 101 | 40 [18, 55] | 18 [7, 38] | 44 [16, 75] | 7 [2, 27] | 21 |8, 51] | .006 |
| Body surface area | 98 | 0.65 [0.52, 0.77] | 0.50 [0.42, 0.63] | 0.62 [0.46, 0.90] | 0.39 [0.26, 0.48] | 0.53 [0.4, .68] | .003 |
| Primary NBL site | |||||||
| Thoracic | 101 | (3) | 22% (9) | 35% (7) | (3) | 22% (22) | .157 |
| Adrenal | 61% (14) | 54% (22) | 65% (13) | 47% (8) | 56% (57) | ||
| Other abdominal or pelvic | 26% (6) | 24% (10) | (0) | 35% (6) | 22% (22) | ||
| COG risk stratification | |||||||
| Low | 95 | 38% (8) | 25% (10) | 59% (10) | 24% (4) | 34% (32) | .171 |
| Intermediate | (3) | 23% (9) | (2) | 35% (6) | 21% (20) | ||
| High | 48% (10) | 53% (21) | 29% (5) | 41% (7) | 45% (43) | ||
| Stage | |||||||
| Stage I/II | 91 | 32% (6) | 28% (11) | 69% (11) | (2) | 33% (30) | .005 |
| Stage III/IV | 68% (13) | 73% (29) | 31% (5) | 88% (14) | 67% (61) | ||
| Timing of resection | |||||||
| Upfront | 84 | 52% (12) | 34% (14) | 70% (14) | - | 47% (40) | .027 |
| After neoadjuvant | 48% (11) | 66% (27) | 30% (6) | - | 52% (44) | ||
| TV at diagnosis | 100 | 311.7 [210.7, 893.5] | 91.2 [28.6, 294] | 26.3 [5.4, 57.8] | 141.1 [34.8, 184.5] | 140.1 [30.5, 326.2] | < .001 |
| TV at resection | 84 | 263.9 [166.3, 398.1] | 23 [10.5, 45.2] | 17.5 [4.2, 45.6] | - | 40.1 [11.3, 124.8] | < .001 |
| IDRF at diagnosis | |||||||
| 0 IDRF | 99 | 30% (7) | 38%(15) | 74% (14) | 24% (4) | 40% (40) | .008 |
| 1+ IDRF | 70% (16) | 63% (25) | 26% (5) | 77% (13) | 60% (59) | ||
| IDRF at resection | |||||||
| 0 IDRF | 83 | 35% (8) | 56% (23) | 95% (18) | - | 59% (49) | < .001 |
| 1+ IDRF | 65% (15) | 44% (18) | (1) | - | 41% (34) | ||
| Margins | |||||||
| Negative | 70 | 33% (7) | 35% (12) | 40% (6) | - | 36% (25) | .916 |
| Positive | 67% (14) | 65% (22) | 60% (9) | - | 64% (45) | ||
| Residual tumor | |||||||
| ≥98% resection | 78 | 74% (17) | 68% (26) | 94% (16) | - | 76% (59) | .119* |
| ≤98% resection | 26% (6) | 32%(12) | (1) | - | 24% (19) | ||
| Relapse at 5 years | |||||||
| No | 55 | 77% (10) | 54% (15) | 82% (9) | 100% (3) | 67% (37) | .141 |
| Yes | (3) | 46% (13) | (2) | (0) | 33% (18) | ||
| Survival at 5 years | |||||||
| Alive | 59 | 79% (11) | 63% (17) | 100% (10) | (3) | 70% (41) | .025 |
| Dead | (3) | 37% (10) | (0) | 63% (5) | 31% (18) |
Comparing residual TV for MIS versus open resection of TV <100 only, P =. 038.
MIS, minimally invasive surgery; TV, tumor volume; COG, Children’s Oncology Group; IDRF, image-defined risk factors
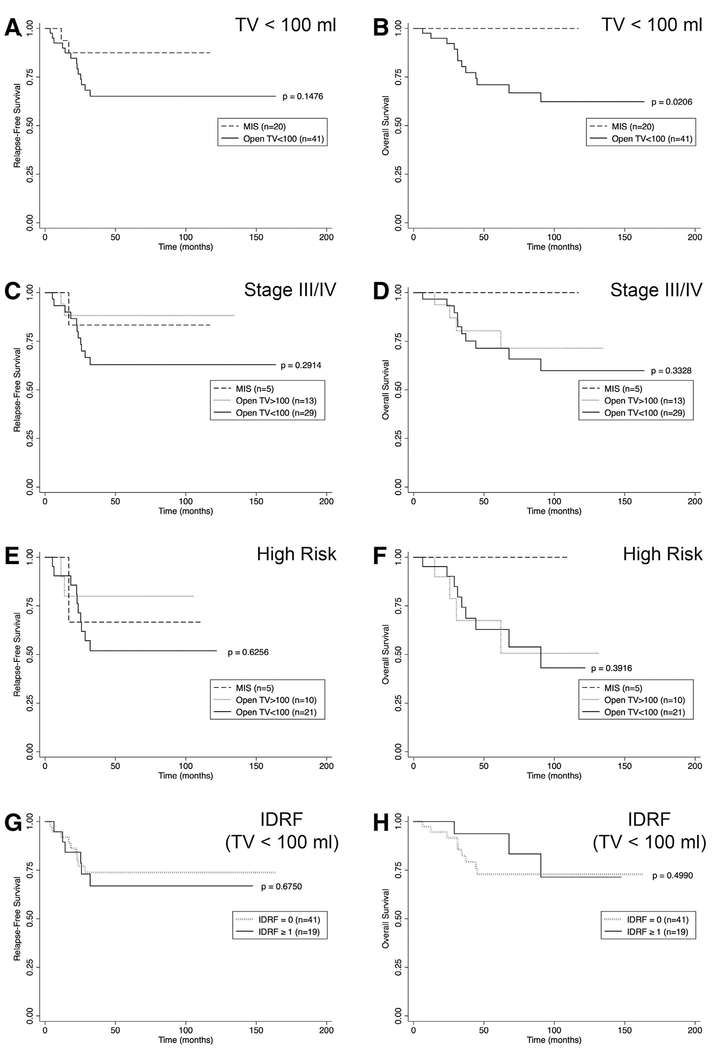
Fig. 6.
Relapse-free and overall survival for neuroblastic tumors (NBL) only based on resection approach with subgroup analyses for tumor volume < 100 mL, high stage, high risk, and IDRF. Kaplan-Meier plots showing (A, B) RFS and OS from NBL with resection of TV <100 mL based on operative approach. (C, D) RFS and OS from Stage III and IV NBL based on operative approach. (E, F) RFS and OS from high-risk NBL based on operative approach. (G, H) RFS and OS from NBL with resection of TV <100 mL based on presence or absence of IDRF. P values were assessed with the log-rank test for equality of survival functions.
Margin status did not differ with the resection approach for neuroblastic tumors, as negative margins were achieved in 40% (n = 6) of MIS resections, 35% (n = 12) of open resections with TV <100 mL, and 33% (n = 7) of open resections with TV >100 mL (P = .916; Table 4). For neuroblastic tumors having a TV <100 mL at resection, a greater proportion of patients undergoing an MIS approach demonstrated ≥ 98% resection based on measurement of residual tumor on postoperative imaging, perhaps owing to fewer IDRFs and more upfront resections in the MIS group (P = .038; Table 4 ).
The 5-year RFS tended to be less in the open NBL TV <100 mL group (0.65 [CI 0.47–0.78]) than the MIS group (0.88 [CI 0.59–0.97]) (P = .148; Fig 6, A), however, the 5-year OS after MIS resection of NBL was 1.00 (CI 1.00–1.00) compared with 0.71 (CI 0.53–0.83) after open resection of TV <100 mL (P = .021; Fig 6, B). To account for the greater proportion of lesser stage and risk NBL resected with MIS, we evaluated RFS and OS between MIS and open TV <100 mL for each stage and risk category. No significant difference was detected between approaches for any stage or risk group (Fig 6, C–F). Presence of IDRFs was not significantly associated with RFS or OS among neuroblastic tumors regardless of TV or operative approach (Fig 6, G–H).
Early diagnosis of embryonal tumors: Predisposition screening, paraneoplastic syndromes, and prenatal detection
Because smaller tumor volume and lesser stage appeared to be primary determinants for MIS resection, we assessed the unique contexts in which embryonal tumors present early and with lesser volumes. Circumstances leading to early detection of an embryonal tumor in this cohort included predisposition screening, paraneoplastic syndromes, and discovery on prenatal ultrasonography. Of the tumors that were ultimately resected with MIS, 30% of NBL and 100% of WT presented within one of these contexts (Table 5). Furthermore, all patients that were diagnosed with WT through predisposition screening and subsequently underwent MIS resection had an organ-sparing procedure (ie, partial nephrectomy).16 In addition to these 3 laparoscopic, nephron-sparing resections (2 of them in the same patient), 7 partial nephrectomies were performed open, 4 of which were in the context of bilateral WT, and 2 under predisposition screening.
Table 5.
Early detection of embryonal tumors.
| MIS | Open TV <100 mL | Open TV >100 mL | |
|---|---|---|---|
| Neuroblastic tumors | |||
| Opsoclonus-myoclonus ataxia | 3 | 2 | 0 |
| VIPoma | 1 | 0 | 0 |
| Prenatal ultrasound | 2 | 0 | 0 |
| Total | 6/20 (30%) | 2/41 (5%) | 0/23 (0%) |
| Wilms tumor | |||
| DHPLN | 1 | 0 | 0 |
| Beckwith-Wiedemann Syndrome | 1 | 2 | 0 |
| WAGR | 0 | 1 | 0 |
| Hemihypertrophy | 0 | 1 | 0 |
| Total | 2/2 (100%) | 4/14 (29%) | 0/50 (0%) |
| Hepatoblastoma | |||
| Hemihypertrophy | 0 | 1 | 0 |
| Beckwith-Wiedemann Syndrome | 0 | 1 | 0 |
| Total | 0/0 (n/a) | 2/10 (20%) | 0/8 (0%) |
| All tumors | |||
| Predisposition/Paraneoplastic/Prenatal | 8/24 (33%) | 8/68 (12%) | 0/82 (0%) |
Note: Category for “all tumors” includes rhabdomyosarcoma even though no special circumstances are noted in this group. In comparing proportion of early detection cases between MIS and Open TV <100 ml, P = .017.
VIP, vasoactive intestinal polypeptide; DHPLN, diffuse hyperplastic perilobar nephroblastomatosis; WAGR, Wilms tumor, aniridia, genitourinary abnormalities, and mental retardation.
Discussion
This retrospective analysis suggests that MIS, when used appropriately in highly select patients, can achieve a comparable oncologic integrity to open resection of embryonal tumors. Our single institution experience demonstrated no significant difference in margin status, lymph node sampling, RFS, and OS when comparing MIS with open resection of embryonal tumors having a volume less than 100 mL. Furthermore, when feasible, MIS was associated with shorter operating times, decreased blood loss, and lesser hospital stays. Although the numbers of patients having adjuvant therapy after MIS resections were small, a less invasive approach appeared to associate with fewer delays in resuming adjuvant chemotherapy, but additional experience is needed to establish this potential benefit more rigorously. From this analysis, the characteristic embryonal tumor amenable to definitive MIS resection appears to have a volume less than 100 mL and is most commonly a neuroblastic lesion with no IDRFs that either was remarkably responsive to neoadjuvant therapy or presented early in the context of paraneoplastic symptoms. Other scenarios in which to consider MIS resection include children under active surveillance for a cancer-predisposing syndrome, such as Beckwith-Wiedemann, hemihypertrophy, or WT1 mutations, among others.16
Concerns about how the MIS technique may adversely impact the disease process contribute to the controversy of utilizing this approach to treat an embryonal malignancy. The effect of carbon dioxide pneumoperitoneum on tumor growth and spread is a topic of investigation. In a murine model of neuroblastoma, animals inoculated with neuroblastoma cells while undergoing pneumoperitoneum experienced increased hepatic metastasis but not local peritoneal spread compared with those undergoing laparotomy.18 The mechanism driving this observation of disease progression remains unclear but may be explained partially by an observed increase in expression of C-MYC (a proto-oncogene associated with neuroblastoma) and HMGB-1 (a target of C-MYC that mediates its function) after CO2 incubation.19 Such potentially negative effects of pneumoperitoneum on embryonal tumor progression have not been demonstrated in humans nor emerged clearly in our series. An additional concern unique to MIS is the potential for disease recurrence at the endoscopic port sites. Port-site recurrence after MIS has been documented in the literature reporting about adult cancer operations20; however, this phenomenon has not been observed in pediatric patients having resection of an embryonal tumor nor in our experience.21
Without prospective randomized trials, definitive conclusions regarding the efficacy of MIS for treatment of pediatric embryonal tumors remain incomplete.22 Comparison of minimally invasive to open resection of embryonal tumors does not lend itself well to a prospective randomized study design, because MIS is indicated and feasible only in a subset of patients (15% in our experience). Indeed, small sample sizes attributable to the relative rarity of embryonal tumors challenge a retrospective review of any single- center experience. This lack of high-level data contributes to the reluctance of some surgeons to use MIS as the operative approach, given concerns of maintaining oncologic integrity. One recent study was able to assess a larger sample size by accessing the National Cancer Database.23 The analysis demonstrated no significant difference in surgical margin status or 1-year and 3-year survival when comparing MIS (n = 133) and open resection (n = 1,141) of neuroblastoma and Wilms tumors; the nature of the database, however, prohibited evaluation of tumor characteristics or selection criteria for MIS as we analyzed carefully in the current study.24 Therefore, the novelty of the current study was first to characterize the ideal candidate for MIS resection of an embryonal tumor and second to analyze critically the oncologic integrity attained through this less invasive approach.
The authors acknowledge several limitations to the current report that temper interpretation. Foremost, we observed that MIS was performed more commonly for earlier stage and lower risk tumors (and without IDRFs in the setting of NBL), indicating that open resections may have been biased toward a more aggressive tumor biology. To overcome this selection bias, we chose to restrict our analysis to tumors having a volume less than the largest resected with MIS and to perform subanalyses of outcomes among late-stage and high-risk NBL as a proxy for other embryonal tumors. Note that doing so did not reveal less oncologic fidelity or poorer outcomes with MIS resections. Second, tumor biology is often variable between patients having the “same” disease or between embryonal tumor types. Not all embryonal tumors, even those of the same category, behave similarly, making accurate comparison of integrity and outcomes between surgical approaches challenging. Indeed, the goals of resection vary between embryonal tumor types, because a positive microscopic margin upstages a WT and HBL yet is a potential success for NBL. These oncologic goals need to be considered carefully when determining the operative approach to resect a specific embryonal tumor. Third, although our institution maintains a robust solid tumor program, a single- center experience typically will yield a small sample size that limits multivariate analysis accounting for stage, COG risk stratification, and IDRFs, among other confounders. A fourth potential limitation is variable accuracy of measurements of tumor volume. Because every tumor has a unique shape, our ellipsoid approximation potentially overestimates tumor volume in certain instances. Fifth, comparing equivalently intraoperative and postoperative data between tumors arising in different body cavities and organs and resected with thoracoscopy, laparoscopy, or endoscopy is potentially misleading and requires much larger sample sizes. Finally, differences in familiarity and comfort between 2 operative approaches add complexity to an analysis comparing MIS and open resection. During the study period, all surgeons in our group have performed oncologic resection of embryonal tumors, yet few (3 of 14) have used an MIS approach in this category of malignant diseases. This minority of surgeons then lends further to the challenge of knowing whether MIS could be a more broadly accepted approach to resect embryonal tumors even if feasible.
In conclusion, among patients carefully selected for MIS resection of pediatric embryonal tumors, high-fidelity oncologic outcomes can be achieved that are comparable to open resection of similar volume lesions. Moreover, MIS resection may be associated with the desirable outcomes of decreased postoperative hospital stays and thereby potentially fewer treatment delays in the context of multimodal therapy. Factors to consider in selecting patients for MIS are patient age and size, tumor volume at time of resection, either upfront or after neoadjuvant chemotherapy, tumor type (with neuroblastic tumors being most amenable to MIS), presence or absence of image-defined risk factors, and patients having a predisposition syndrome who are in the midst of cancer surveillance. The authors acknowledge that only a minority of embryonal tumors are amenable to an MIS approach, but, when indicated, a high-fidelity oncologic resection can be achieved.
Acknowledgments
The authors are indebted to the Vanderbilt Cancer Registry for providing the detailed list of embryonal tumor patients used to conduct this study. The authors further acknowledge the support of Monroe Carell, Jr. Children’s Hospital’s Surgical Outcomes Center for Kids.
References
- 1.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: Five-year results of a randomized prospective trial. Ann Surg, 2005;241:232–237, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, et al. Laparoscopic versus open surgery for rectal cancer: A meta-analysis. Ann Surg Oncol, 2006;13:413–424, [DOI] [PubMed] [Google Scholar]
- 3.Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms-a meta-analysis. Surgery, 2007;141:203–211. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb 3rd GW. Minimally invasive surgery for solid tumors. Semin Surg Oncol. 1999;16:184–192. [DOI] [PubMed] [Google Scholar]
- 5.Malkan AD, Loh AH, Sandoval JA. Minimally invasive surgery in the management of abdominal tumors in children. J Pediatr Surg. 2014;49:1171–1176, [DOI] [PubMed] [Google Scholar]
- 6.Malek MM, Mollen KP, Kane TD, Shah SR, Irwin C. Thoracic neuroblastoma: A retrospective review of our institutional experience with comparison of the thoracoscopic and open approaches to resection. J Pediatr Surg. 2010;45:1622–1626. [DOI] [PubMed] [Google Scholar]
- 7.Metzelder ML, Kuebler JF, Shimotakahara A, Glueer S, Grigull L, Ure BM, Role of diagnostic and ablative minimally invasive surgery for pediatric malignancies. Cancer. 2007;109:2343–2348. [DOI] [PubMed] [Google Scholar]
- 8.Irtan S, Brisse HJ, Minard-Colin V, Schleiermacher G, Canale S, Sarnacki S. Minimally invasive surgery of neuroblastic tumors in children: Indications depend on anatomical location and image-defined risk factors. Pediatr Blood Cancer, 2015;62:257–261. [DOI] [PubMed] [Google Scholar]
- 9.Leclair MD, de Lagausie P, Becmeur F, Varlet F, Thomas C, Valla JS, et al. Laparoscopic resection of abdominal neuroblastoma. Ann Surg Oncol. 2008;15:117–124. [DOI] [PubMed] [Google Scholar]
- 10.Fraga JC, Rothenberg S, Kiely E, Pierro A, Video-assisted thoracic surgery resection for pediatric mediastinal neurogenic tumors. J Pediatr Surg. 2012;47:1349–1353, [DOI] [PubMed] [Google Scholar]
- 11.Spurbeck WW, Davidoff AM, Lobe TE, Rao BN, Schropp KP, Shochat SJ, Minimally invasive surgery in pediatric cancer patients. Ann Surg Oncol, 2004;11:340–343. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher CM, Smithson L, Nguyen LL, Casadiego G, Nasr A, Irwin MS, et al. Clinical outcomes in children with adrenal neuroblastoma undergoing open versus laparoscopic adrenalectomy. J Pediatr Surg, 2013;48:1727–1732, [DOI] [PubMed] [Google Scholar]
- 13.Fuchs J, The role of minimally invasive surgery in pediatric solid tumors. Pediatr Surg Int, 2015;31:213–228, [DOI] [PubMed] [Google Scholar]
- 14.Molinaro F, Kaselas C, Lacreuse I, Moog R, Becmeur F, Postoperative intestinal obstruction after laparoscopic versus open surgery in the pediatric population: A 15-year review. Eur J Pediatr Surg, 2009;19:160–162. [DOI] [PubMed] [Google Scholar]
- 15.Heloury Y, Muthucumaru M, Panabokke G, Cheng W, Kimber C, Leclair MD, Minimally invasive adrenalectomy in children. J Pediatr Surg, 2012;47:415–421, [DOI] [PubMed] [Google Scholar]
- 16.Rauth TP, Slone J, Crane G, Correa H, Friedman DL, Lovvorn HN, Laparoscopic nephron-sparing resection of synchronous Wilms tumors in a case of hyperplastic perilobar nephroblastomatosis. J Pediatr Surg. 2011;46:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol. 2009;27:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metzelder M, Kuebler J, Shimotakahara A, Vieten G, von Wasielewski R, Ure BM. CO(2) pneumoperitoneum increases systemic but not local tumor spread after intraperitoneal murine neuroblastoma spillage in mice. Surg En-dosc. 2008;22 :2648–2653. [DOI] [PubMed] [Google Scholar]
- 19.Reismann M, Wehrmann F, Schukfeh N, Kuebler JF, Ure B, Gluer S, Carbon dioxide, hypoxia and low pH lead to overexpression of c-myc and HMGB-1 oncogenes in neuroblastoma cells. Eur J Pediatr Surg. 2009;19:224–227. [DOI] [PubMed] [Google Scholar]
- 20.Paolucci V, Schaeff B, Schneider M, Gutt C. Tumor seeding following laparoscopy: International survey. World J Surg., 1999;23:989–995 discussion 96–7. [DOI] [PubMed] [Google Scholar]
- 21.Iwanaka T, Arai M, Yamamoto H, Fukuzawa M, Kubota A, Kouchi K, et al. No incidence of port-site recurrence after endosurgical procedure for pediatric malignancies. Pediatr Surg Int, 2003;19:20 0–203, [DOI] [PubMed] [Google Scholar]
- 22.van Dalen EC, de Lijster MS, Leijssen LG, Michiels EM, Kremer LC, Caron HN, et al. Minimally invasive surgery versus open surgery for the treatment of solid abdominal and thoracic neoplasms in children. Cochrane D atabase Syst Rev, 2015;1 CD008403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College of Surgeons, American Cancer Society. National Cancer Database. American College of Surgeons Web site; https://www.facs.org/quality-programs/cancer/ncdb. [Google Scholar]
- 24.Ezekian B, Englum BR, Gulack BC, Rialon KL, Kim J, Talbot LJ, et al. Comparing oncologic outcomes after minimally invasive and open surgery for pediatric neuroblastoma and Wilms tumor. Pediatr Blood Cancer. 2018;65. doi: 10.1002/pbc.26755. [DOI] [PubMed] [Google Scholar]