Abstract
It was shown as long as half a century ago that bone marrow is a source of not only hematopoietic stem cells, but also stem cells of mesenchymal tissues. Then the term of mesenchymal stem cells (MSCs) has been coined in early 1990s and over a decade later the criteria for defining MSCs have been released by International Society for Cellular Therapy. The easy derivation from a variety of fetal and adult tissues and not demanding cell culture conditions made MSCs an attractive research object. It was followed by the avalanche of reports from preclinical studies on potentially therapeutic properties of MSCs such as immunomodulation, trophic support and capability for a spontaneous differentiation into connective tissue cells, and differentiation into majority of cell types upon specific inductive conditions. While ontogenesis, niche and heterogeneity of MSCs are still under investigation, there is a rapid boost of attempts in clinical applications of MSCs, especially for a flood of civilization-driven conditions in so quickly aging societies in not only developed countries, but also very populous developing world. The fields of regenerative medicine and oncology are particularly extensively addressed by MSC applications, in part due to paucity of traditional therapeutic options for these highly demanding and costly conditions. There are currently almost 1000 clinical trials from entire world registered at clinicaltrials.gov and it seems that we are starting to witness the snowball effect with MSCs becoming a powerful global industry, however spectacular effects of MSCs in clinic still need to be shown.
Keywords: Mesenchymal stem cells, clinical, differentiation, immunomodulation, paracrine activity, history
Graphical Abstract
Introduction: MSC roots
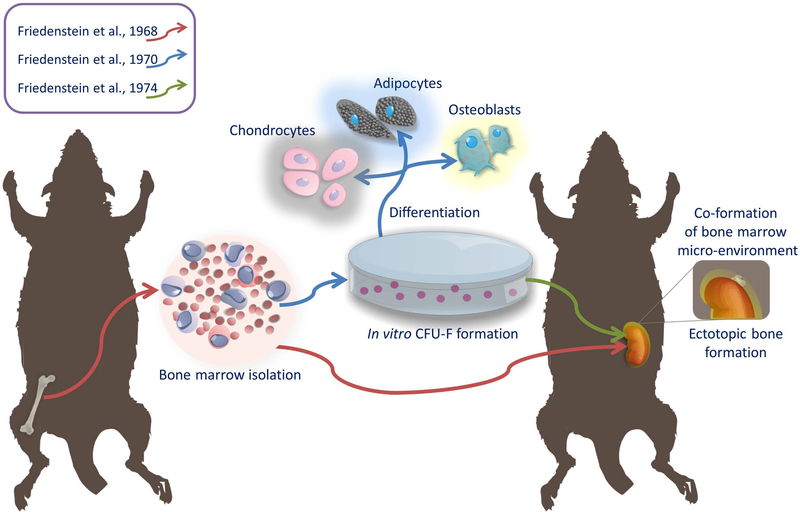
Friedenstein was one of the pioneers of the theory that bone marrow is a reservoir of stem cells of mesenchymal tissues in adult organisms. It was based on his observation at the turn of the 1960s and 1970s., that ectopic transplantation of bone marrow into the kidney capsule, results not only the proliferation of bone marrow cells, but also the formation of bone [1] (Figure 1). This indicated the existence in the bone marrow of a second, in addition to hematopoietic cells, stem cell population giving rise to bone precursors. Due to the ability of these cells to create osteoblasts, Friedenstein gave them the name of osteogenic stem cells. Friedenstein was also the first to isolate from bone marrow adherent fibroblast-like cells with the ability to grow rapidly in vitro in the form of clonogenic colonies (CFU-F; colony forming unit-fibroblast). These cells derived from CFU-F colonies were characterized by the ability to differentiate in vitro not only to osteocytes, but also to chondrocytes and adipocytes. After transplantation of CFU-F colonies into the recipient, they were capable of co-formation of the bone marrow micro-environment [2,3]. The term “mesenchymal stem cells” has been proposed by Caplan in 1991 because of their ability to differentiate into more than one type of cells that form connective tissue in many organs [4]. This name has become very popular and is currently the most commonly used, even though it raised doubts about the degree of their stemness [5]. Today, there are many substitutes in the literature for the abbreviation of MSCs, including Multipotent Stromal Cells, Marrow Stromal Cells, Mesodermal Stem Cells, Mesenchymal Stromal Cells and many more. In its latest work, Caplan recommends renaming these cells to “Medicinal Signaling Cells” due to the emphasis on the mechanism of their therapeutic effects after transplantation, which is believed to be based mainly on the secretion of factors facilitating regenerative processes [6].
Figure 1:
The roots of research on bone marrow-derived stem cells of connective tissue, which has been then named: mesenchymal stem cells
Criteria for MSCs
Due to the growing controversy regarding the nomenclature, the degree of stemness and the characteristics of the cells discovered by Friedenstein, the International Society for Cellular Therapy (ISCT) in 2006 published its position specifying the criteria defining the population of MSCs, which was accepted by the global scientific community. These guidelines recommend the use of the name multipotent mesenchymal stromal cells, however, the name mesenchymal stem cells still remains the most-used. The condition for the identification of MSCs is the growth of cells in vitro as a population adhering to the substrate, as well as in the case of cells of human origin, a phenotype characterized by the presence of CD73, CD90, CD105 surface antigens and the lack of expression of proteins such as: CD45, CD34, CD14, CD11b, CD79a or CD19 or class II histocompatibility complex antigens (HLA II, human leukocyte antigens class II). Moreover, these cells must have the ability to differentiate towards osteoblasts, adipocytes and chondroblasts [7,8]. In addition to the markers mentioned in the ISCT guidelines, the following antigens turned out to be useful in isolating the human MSCs from the bone marrow: STRO-1 (antigen of the bone marrow stromal-1 antigen, cell surface antigen expressed by stromal elements in human bone marrow-1), VCAM / CD106 (vascular cell adhesion molecule 1) and MCAM / CD146 (melanoma cell adhesion molecule), which characterizes cells growing in vitro in a adherent form, with a high degree of clonogenicity and multidirectional differentiation ability [9–11].
Ontogenesis of MSCs
The common “mesenchymal” core in both versions of MSC abbreviation comes from the term mesenchyme, which is synonymous with mesenchymal tissue or embryonic connective tissue. It is used to refer to a group of cells present only in the developing embryo derived mainly from the third germ layer - mesoderm. During the development these cells migrate and diffuse throughout the body of the embryo. They give rise to cells that build connective tissue in adult organisms, such as bones, cartilage, tendons, ligaments, muscles and bone marrow. The view about the differentiation of MSCs during embryonic development from mesenchymal cells is widely spread [4]. This is due, inter alia, to the observed convergence in the expression of markers such as: vimentin, laminin β1, fibronectin and osteopontin, which are typical for mesoderm cells during embryonic development, as well as characteristic for in vitro adherent bone marrow stroma cells [12]. However, the true origin of MSCs is unknown. In the literature, we can find also reports indicating that they are ontogenetically associated with a group of cells derived from ectoderm, which originate from Sox1 + cells (SRY - sex determining region Y) that appear during the development of embryonic neuroectoderm and neural crest. These cells inhabit newborn bone marrow and meet the criteria corresponding to their designation as MSCs. However, with the development of animals, the population of these cells disappears and is replaced by cells with a different, unidentified origin [13]. It has also been shown that in the bone marrow of the developing mouse embryo, at least two MSCs populations with distinct expression of the nestin protein and the intensity of cell divisions can be distinguished. The former one originates from mesoderm that does not express nestin, and is characterized by intense proliferation and is involved in the process of creating the embryo skeleton. The latter one is derived from the cells of the neural crest, which expresses nestin and is non-dividing and remains passive during bone formation while in the adult organism contributes to a niche of hematopoietic cells [14]. It seems, therefore, that the ontogenesis of MSCs is associated with cells belonging to different germ layers and their original source determines the role and functions that they play in the adult body.
The niche of MSCs in the adult body
In 1978, the concept of a niche was defined as a place in the body that is settled by stem cells and whose environment allows them to be maintained in an undifferentiated state [15]. MSCs were first obtained from the bone marrow stroma where they constitute an element of stromal cells, participating in the production of signals modulating the maturation of hematopoietic cells. However, the precise location of the niche for MSCs has not been known so far. In the context of research results indicating that MSCs can be isolated from many mesoderm-derived tissues during embryonic development, a common element was sought for all sources from which MSCs can be isolated and a theory was proposed about the existence of their niche within the blood vessels that are present in all structures from which these cells were isolated.
Crisan and colleagues have shown that cells inhabiting the perivascular space of blood vessels, isolated from human tissues such as skeletal muscle, pancreas, adipose tissue and placenta, with the phenotype CD146 +, NG2 + (neuroglycan-2), PDGF-Rβ + (β-type platelet-derived growth factor receptor), ALP + expressing endothelial, hematopoietic and muscle cell markers described as pericytes were precursors for cells that after in vitro expansion meet the criteria for determining them as MSCs [16]. Analogously to the described by Friedenstein MSCs, CD146 + cells colonizing the perivascular space of sinusoidal sinus vessels, are responsible for the production of signals allowing the reconstruction of the bone marrow microenvironment after transplantation to heterotopic location [11]. What’s more, tracing the fate of pericytes in the process of rebuilding a damaged tooth in rodents has shown that they are transforming into odontoblasts, which arise from MSCs found in the pulp. However, the same studies showed that in the process of reconstruction of incisors in mice, a different population of odontoblasts, which is not formed from pericytes, but from MSCs of different origin migrating to the area of damage, prevailed quantitatively [17]. The second cell population associated with blood vessels, proposed as a counterpart of MSCs in the body is advent building cells with the CD34+ CD31- CD146- phenotype, which after isolation and in vitro culture meet the criteria defining the population as MSCs. However, these cells also have the ability to differentiate into pericytes [18,19]. Although pericytes and MSCs have a very similar gene expression profile as well as an analogical capacity for differentiation, it has been shown that the functionality of these cells varies. In vitro studies of endothelial cell interactions in co-culture with MSCs or pericytes have shown that only pericytes are able to form highly branched, dense, cylindrical structures with large diameter, typical for well-organized blood vessels, while isolated from the bone marrow MSCs do not have such abilities. Currently, it is believed that there is a link between pericytes and MSCs, but their mutual relations are not well defined. There are speculations that MSCs are an intermediate form of pericytes or their subpopulation, but there is still no conclusive evidence confirming this hypothesis [20,21].
Heterogeneity of MSC populations
While the cells fulfilling criteria for MSCs can be harvested from various tissues at all developmental stages (fetal, young, adult and aged) using their plastic adherence property, there are profound differences between obtained MSC populations [22,23]. Bone marrow was historically the first source from which MSCs were obtained, however, over time, there have been reports of the possibility of isolation from other sources of cells with similar properties. Mesenchymal cells are obtained from both tissues and secretions of the adult body, such as adipose tissue, peripheral blood, dental pulp, yellow ligament, menstrual blood, endometrium, milk from mothers, as well as fetal tissues: amniotic fluid, membranes, chorionic villi, placenta, umbilical cord, Wharton jelly, and umbilical cord blood [24–37]. MSCs of fetal origin as compared to cells isolated from tissues of adult organisms are characterized by a faster rate of proliferation as well as a greater number of in vitro passages until senescence [38]. However, MSCs derived from bone marrow and adipose tissue are able to create a larger number of CFU-F colonies, which indirectly indicates a higher degree of their stemness. The comparison of gene expression typical for pluripotent cells shows that only in cells isolated from the bone marrow we can observe the expression of the SOX2 gene, the activation of which is associated with the self-renewal process of stem cells as well as with neurogenesis during embryonic development [39]. Discrepancies in the ability of MSCs obtained from various sources to differentiate have also been described. The lack of differentiation of MSCs derived from umbilical cord blood towards adipocytes as well as the greater tendency of MSCs from bone marrow and adipose tissue to differentiate towards osteoblasts were observed [39,40].
In addition to the diverseness observed between MSCs from different sources, there are also differences associated with obtaining them from individual donors. Among the cells isolated from the bone marrow from donors of different ages and sexes, up to 12-fold differences in the rate of their proliferation and osteogenesis were found, combined with a 40-fold difference in the level of bone remodeling marker activity - ALP (alkaline phosphatase). At the same time, no correlations were found resulting from differences in the sex or age of donors [41]. However, the results of studies by other authors indicate that the properties of MSCs isolated from the bone marrow are strongly associated with the age of the donor. Cells collected from older donors are characterized by an increased percentage of apoptotic cells and slower rate of proliferation, which is associated with an increased population doubling time. There is also a weakened ability of MSCs from older donors to differentiate towards osteoblasts [42]. Heo in his work shows the different ability of MSCs to osteogenesis combining it with different levels of DLX5 gene expression (transcription factor with the homeodomain 5 motif) in individual donors, however independent of the type of tissue from which the cells were isolated [39].
The next stage in which we can observe diversity among the MSCs population is in vitro culture. The morphology of cultured cells that originate from the same isolation allows for differentiation into three sub-populations. There are observed spindle-shaped proliferating cells resembling fibroblasts (type I); large, flat cells with a clearly marked cytoskeleton structure, containing a number of granules (type II) and small, round cells with high self-renewal capacity [43,44]. The original hypothesis assumed that all cells that make up the MSCs population are multipotent, and each colony of CFU is capable of differentiating into adipocytes, chondrocytes and osteoblasts, as confirmed by appropriate studies [45]. However, in the literature we can find reports that cell lines derived from a common colony of CFU-F differ in their properties, characterized by uni-, di- or multipotence [46]. Some of the authors showed the division of clonogenic MSCs colonies into as much as eight groups distinct in their potential for differentiation. At the same time, it is suggested that there is a hierarchy within which cells subordinate to each other are increasingly directed towards osteo- chondro- or adipocytes and gradually lose their multipotential properties to di- and unipotential ones. This transformation may also be associated with a decrease in the rate of cell proliferation and the level of CD146 protein expression (CD; cluster of differentiation) - proposed as a marker of multipotency [47].
Immunomodulatory properties of MSCs
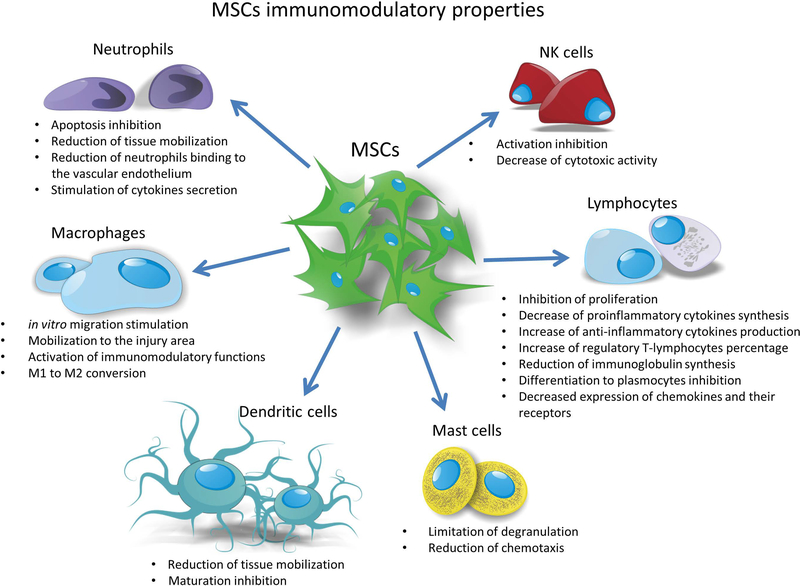
One of the main advantages of MSCs are their immunomodulatory properties. MSCs grown in vitro have the ability to interact and regulate the function of the majority of effector cells involved in the processes of primary and acquired immune response (Figure 2) [48]. They exert their immunomodulatory effects by inhibiting the complement-mediated effects of peripheral blood mononuclear cell proliferation [49,50], blocking apoptosis of native and activated neutrophils, as well as reducing the number of neutrophils binding to vascular endothelial cells, limiting the mobilization of these cells to the area of damage [51,52]. In addition, cytokines synthesized by activated MSCs stimulate neutrophil chemotaxis and secretion of pro-inflammatory chemokines involved in recruitment and stimulation of phagocytic macrophage properties [53]. Moreover MSCs limit mast cell degranulation, secretion of pro-inflammatory cytokines by these cells as well as their migration towards the chemotactic factors [54]. Native MSCs have the ability to block the proliferation of de novo-induced NK cells, but they are only able to partially inhibit the proliferation of already activated cells [55]. They also contribute to the reduction of cytotoxic activity of NK cells [56]. Moreover MSCs can block the differentiation of CD34 + cells isolated from the bone marrow or blood monocytes into mature dendritic cells both by direct contact as well as by secreted paracrine factors [57,58]. They inhibit the transformation of immature dendritic cells into mature forms and limit the mobilization of dendritic cells to the tissues [59]. Under their influence, M1 (pro-inflammatory) macrophages are transformed into M2 type cells with an anti-inflammatory phenotype, and the IL-10 (IL, interleukin) secreted by them inhibits T-cell proliferation [60,61]. In vitro studies have demonstrated a direct immunomodulatory effect of MSCs on lymphocytes. During the co-culture of MSCs with lymphocytes, suppression of activated CD4 + and CD8 + T cells and B lymphocytes was observed [62]. In addition, MSCs reduce the level of pro-inflammatory cytokines synthesized by T-lymphocytes, such as TNF-α (tumor necrosis factor α) and IFN-γ (interferon γ) [63], and increase synthesis of anti-inflammatory cytokines, e.g. IL-4. In the presence of MSCs, the inhibition of the differentiation of naive CD4 + T lymphocytes to Th17 + lymphocytes (Th; T helper cells) was observed, while the percentage of T cells differentiating towards CD4 + CD25 + regulatory T cells was found to increase [64,65]. Glennie et al. described this condition as anergy of activated T cells in the presence of MSCs [62]. MSCs also have the ability to limit the synthesis of immunoglobulins like IgM, IgG and IgA (Ig; immunoglobulin) classes secreted by activated B cells, thereby blocking the differentiation of these cells to plasma cells. They also reduce the expression of chemokines and their receptors on the surface of B lymphocytes, which probably have a negative effect on their ability to migrate [66].
Figure 2.
The schematic representation of immunomodulatory capabilities of MSCs
Paracrine properties of mesenchymal stem cells
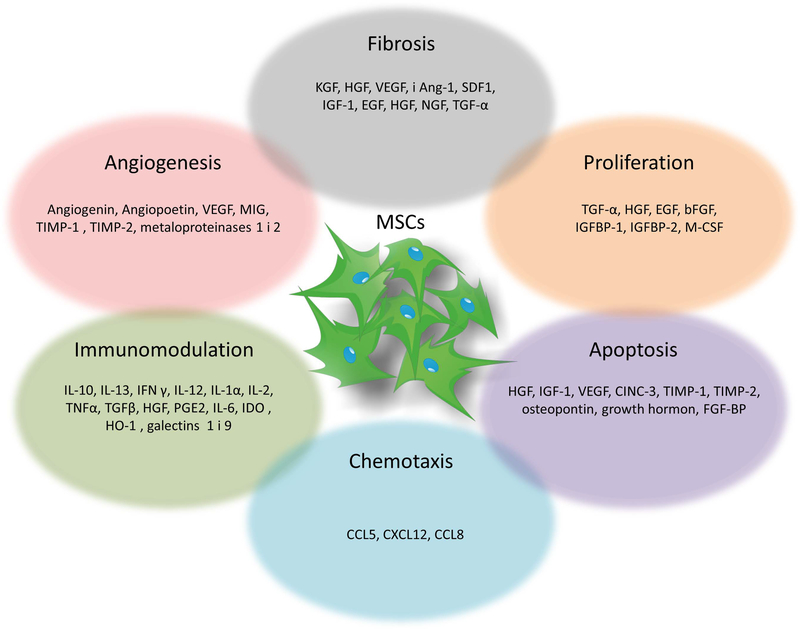
Mesenchymal stem cells secrete a wide range of paracrine factors, collectively referred to as the secretome, which support regenerative processes in damaged tissues. They comprise the components of the extracellular matrix, proteins involved in the adhesion process, enzymes as well as their activators and inhibitors, growth factors and binding proteins, cytokines and chemokines, and probably many more [67]. These factors can have distinct impact on the processes they regulate (Figure 3). MSCs secrete factors promoting angiogenesis, such as: vascular endothelial growth factor (VEGF) but they may also inhibit this process, through expression of monokine induced by interferon γ and tissue inhibitors of metalloproteinases 1 and 2 [68,69]. An important role is also played by chemokines secreted by MSCs in the process of blocking or stimulating cell chemotaxis, such as: CCL5 (RANTES, regulated by activation, expression and secretion by normal T lymphocytes), CXCL12 (SDF-1, stromal cell-derived factor 1) or CCL8 (MCP-2; monocyte chemoattractant protein 2). An essential group of factors from the point of view of regeneration processes are growth factors with an anti-apoptotic effect, including: HGF (hepatocyte growth factor), IGF-1 (insulin-like growth factor 1), VEGF, CINC-3 (cytokine induced by a chemoattractant for neutrophil chemoattractant), TIMP-1 (tissue inhibitor of metalloproteinases 1), TIMP-2 (tissue inhibitor of metalloproteinases 2), osteopontin, growth hormone, FGF-BP (bFGF binding protein), and BDNF (brain-derived growth factor; -derived neurotrophic factor) and stimulating proliferation as: TGF-α (transforming growth factor α), HGF, EGF (epidermal growth factor), NGF (nerve growth factor; nerve growth factor), bFGF (basic fibroblast growth factor), IGFBP-1, IGFBP-2 (IGFBP; insulin-like growth factor 1 binding protein, IGF-Protein-1 protein) and M-CSF (stimulant factor t molar macrophage colony; macrophage colony-stimulating factor) [68,70,71]. Growth factors secreted by MSCs have also ability to reduce fibrosis of tissues during regeneration. These include KGF (keratinocyte growth factor), HGF, VEGF, and Ang-1 (angiopoietin-1), SDF1, IGF-1, EGF, HGF, NGF, TGF-α [71,72]. There are reports about the antibacterial properties and interaction of the MSC secretome with cancer cells. Data on the impact of MSCs on neoplasia are not conclusive, however, it is assumed that both the tumor type and the origin of MSCs are of great importance for the final effect [73]. It was shown that factors enclosed within the MSCs secretome are able to reduce the proliferation, viability and migration of certain types of cancer cells (such as non-small-cell lung carcinoma) [74]. Others have shown that factors released by MSCs may increase motility, invasiveness and the ability to form metastases (including, for example, breast cancer cells) [75]. In response to bacteria, levels of cytokines such as IL- 6, IL-8, CCL5, PGE2, TNF-α, IL-1β, IL-10, VEGF and SDF-1 secreted by MSCs are subject to change [76]. MSCs contain also substances with antibacterial, anti-parasitic and antiviral activity [77].
Figure 3.
The mechanisms mediating MSC-dependent trophic support
Another broad and dynamically developing field in recent years which is related to paracrine MSCs activity is their ability to secrete extracellular vesicles (EVs), which include exosomes, microvesicles and apoptotic bodies. Their composition largely coincides with the components contained in the cells from which they originate. Physiologically they play an important role in the regulation of biological functions, homeostasis and the immune response of the body. It is also postulated that the biological activity of microvesicles is comparable to that of MSCs [78]. Experiments conducted using supernatant derived from in vitro culture of MSCs showed that the factors contained in their secretome are responsible for a large part of the effects exerted by MSCs during the regeneration of the damaged area including the protection of other cells against apoptosis, induction of their proliferation, prevention of excessive fibrosis of tissues, stimulation of the angiogenesis process and immunomodulatory effects, as well as the induction of endogenous stem cells differentiation [65,68,69,79–82].
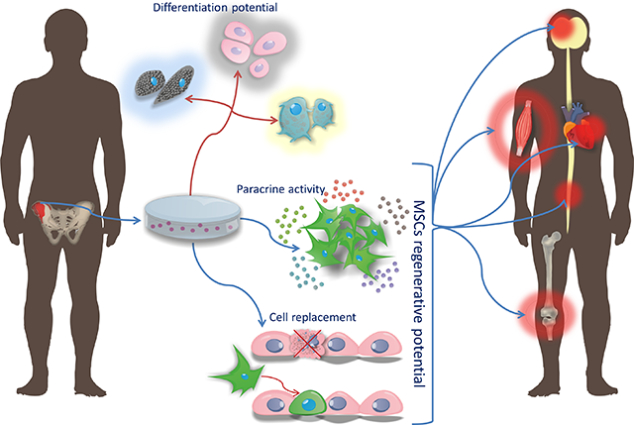
Differentiation potential of MSCs
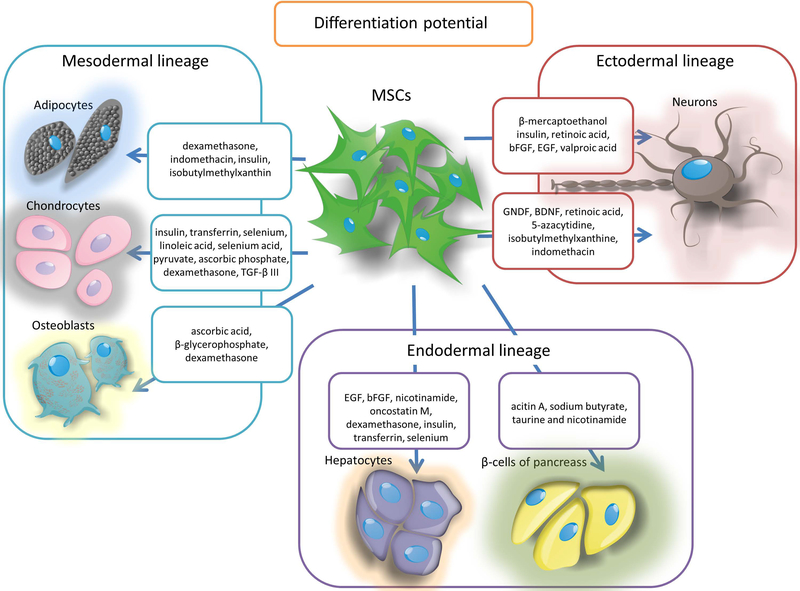
As mentioned above, the ability to differentiate into three types of cells such as: osteocytes, chondrocytes and adipocytes is one of the criterion for MSCs [8]. This phenomenon can be traced in vitro by placing MSCs in a medium containing specific supplements, for the adipogenesis process they are mainly dexamethasone, indomethacin, insulin and isobutylmethylxanthin [83], for chondrogenesis cell culture in DMEM medium (Dulbecco / Vogt Modified Eagle’s Minimal Essential Medium) supplemented with insulin, transferrin, selenium, linoleic acid, selenium acid, pyruvate, ascorbic phosphate, dexamethasone and TGF-β III [84], which may additionally be aided by the addition of IGF-1 and BMP-2 (BMP; bone morphogenetic proteins) [85]. In turn the osteogenesis is induced by the presence of ascorbic acid, β-glycerophosphate and dexamethasone [86]. Differentiation of MSCs in the appropriate cell type is assessed by identifying the production of respectively: fat droplets (adipogenesis), proteoglycans and type II collagen synthesis (chondrogenesis) or mineralization of calcium deposits and the increase of alkaline phosphatase expression (osteogenesis). However, many literature reports indicate that by the treatment with appropriate factors MSCs might be also a source of other cell types. Caplan and Dennis in their work from 2006 present a process that they call mesengenesis, in which MSCs give also rise to myoblasts, bone marrow stromal cells, fibroblasts, cells co-creating connective tissue of the body as well as ligaments and tendons [87]. Addition of 5-azacytidine to MSCs allows to obtain muscle cells, including cardiomyocytes and myoblasts having the ability to create multinucleated miotubes and expressing markers such as: β-myosin heavy chain, α-actin cardiac form and desmin [88]. In addition, in vitro studies have made it possible to obtain from MSCs at least two types of cells derived from the endoderm through their transdifferentiation into hepatocytes and β-cells of pancreatic islets. The liver cells are obtained from MSCs in two stages by culturing them in modified Dulbecco’s medium supplemented with EGF, bFGF and nicotinamide, and in the next stage with the addition of oncostatin M, dexamethasone, insulin, transferrin and selenium. The resulting cells show the presence of markers typical for hepatocytes such as albumin, α-fetoprotein and hepatocyte nuclear factor 4α (HNF-4α) [89]. By the treatment with a mixture of growth factors secreted by regenerating cells of the pancreas as well as by the use of acitin A, sodium butyrate, taurine and nicotinamide the pancreatic islets of β-cells capable of producing insulin were obtained from MSCs [90,91]. It has also been shown that stimulation with appropriate factors may result in the differentiation of MSCs into cells derived ontogenetically from ectoderm, such as neurons. The use of BME stimulation in vitro (β-mercaptoethanol) followed by NGF leads to the differentiation of MSCs into cholinergic nerve cells expressing their typical proteins such as NF-68 neurofilaments (68 kDa Neurofilament protein with 68 kDa molecular mass), NF-200 (neurofilament protein with a molecular weight 200kDa, 200kDa neurofilament protein), NF-160 (neurofilament protein molecular weight 160kDa, 160kDa neurofilament protein), choline acetyltransferase and synapsin I [92]. Other factors mentioned as compounds inducing the transformation of MSCs into nerve cells are insulin, retinoic acid, bFGF, EGF, valproic acid, BME and hydrocortisol [93]. In addition, GNDF (glial cell-derived neurotrophic factor), BDNF (brain-derived neurotrophic factor), retinoic acid, 5-azacytidine, isobutylmethylxanthine and indomethacin stimulate the transformation of MSCs into mature neurons that express markers of nervous systems cells such as: nestin, β-III tubulin, microtubule associated protein - MAP2 (microtubule associated protein 2) and neuron-specific enolase (ENO2; enolase 2) [94]. These studies show that under strictly controlled conditions prevailing during in vitro culture, in the presence of chemicals and growth factors, MSCs are able to turn into cells derived from all three embryonic germ layers (Figure 4).
Figure 4.
The differentiation potential of MSCs
Conclusion: MSC boost and their introduction on world medical market
It has been more than half a century since the curiosity has been revealed that not only hematopoietic cells, but also those capable of forming connective tissue reside in the bone marrow. Subsequent studies have begun to reveal the increasingly fascinating properties of these cells, which go far beyond forming connective tissue. This, combined with their easy derivation from various tissues, made them an attractive research object. Immunomodulatory properties, aiding repair of various tissues as well as differentiation potential to practically any types of cells stunned a whole host of scientists and established MSCs as a driving force of regenerative medicine and began also to play an increasingly important role in oncology [95]. We are currently observing a flood of clinical trials with the use of MSCs, and their number doubles every few years and currently reaches almost 1000 registered items on the clinicaltrials.gov website.
MSCs compose a negligible fraction of cells derived from in vivo tissues and there is no effective method to capture them directly. Therefore, MSCs need to be subjected to the process of in vitro expansion, which in clinical context is called biomanufacturing and biobanking and both terms are frequently used interchangeably to describe the process from procurement of cell source to deliver cells to the patients’ bed. The processing of MSCs must be performed according to current Good Manufacturing Practice (cGMP) as any other therapeutic agent and is subjected to extensive regulatory effort. Food and Drug Administration (FDA) is the main authority responsible for acceptance of medical products including those containing living cells such as MSCs in the USA. FDA has issued a perspective on MSC-based product characterization [96] and up-dated it in FDA Grand Round delivered by Steven Bauer, PhD, Chief of Cell and Tissue Therapies Branch at FDA on March 08, 2018. Both sources are an excellent overview of regulatory challenges related to the biobanking of MSCs. In general, any new product must obtain investigational new drug status (INDs) to be used in clinical trial before filing application for marketing, and there were 66 INDs submitted to FDA between 2006 and 2012. Based on that FDA engaged into regulatory research project called MSC consortium to characterize MSC based-products with an output of 16 research papers. The main organ responsible for the regulation of medical market in all Member States is European Medicines Agency (EMA) consisting of seven smaller committees. The MSCs-containing products should be classified as Advanced Therapy Medical Product (ATMP) and in detail considered as Somatic Cell Therapy Medicinal Product (CTMP) [97]. Its release on medical market has to be first accredited by Committee for Advanced Therapies (CAT) which creates the general opinion and evaluates the quality, safety and efficiency of the product. After CAT assessment the final acceptance should be then approved by Committee for the Medicinal Products for Human Use (CHMP). This type of legalization is called Centralized Marketing Authorization and it allows to use ATMP products in all European Union countries. Currently, there is a variety of protocols used for biomanufacturing and biobanking of MSCs, and once the successful stories become strong, the landscape of MSC production will probably solidify with predicted reduction of MSC production approaches due to economic and regulatory pressures.
Summing up, it seems that the MSCs are becoming a powerful global industry, ready to respond to the unmet needs of modern medicine struggling with the proper care and quality of life of rapidly aging societies, which is already affecting not only developed countries, but also very populous developing countries. In conclusion, we are beginning to observe the effect of the snowball in which ever new discoveries related to MSC are increasingly stimulating clinical applications of the MSC, which is beginning to contribute to the transformation of medical care.
Significance Statement.
The research on bone marrow-derived stem cells of connective tissue is evolving and continuously expanding with a recent boost of interest in clinical applications reflected by an avalanche of nearly 1000 registered clinical trials. While, the current name: mesenchymal stem cells (MSCs) have been coined as late as early 90-ies, it is important to commemorate of the fiftieth anniversary of research on them and provide a big picture from roots of first paper in 1968, through identification of their various potential therapeutic activities such as immunomodulation, trophic support and capability for differentiation and taking role in cell replacement strategies.
Acknowledgments
This work was funded by NCR&D grant EXPLORE ME within the “STRATEGMED I” program and by NIH R01 NS091100-01A1.
Footnotes
Disclaimers: None
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;6:230–247. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 1970;3:393–403. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974;17:331–340. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991;9:641–650. [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2008;2:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med 2017;6:1445–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005;7:393–395. [DOI] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 9.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991;78:55–62. [PubMed] [Google Scholar]
- 10.Gronthos S, Zannettino ACW, Hay SJ, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 2003;116:1827–1835. [DOI] [PubMed] [Google Scholar]
- 11.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324–336. [DOI] [PubMed] [Google Scholar]
- 12.Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells 2002;20:205–214. [DOI] [PubMed] [Google Scholar]
- 13.Takashima Y, Era T, Nakao K, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 2007;129:1377–1388. [DOI] [PubMed] [Google Scholar]
- 14.Isern J, García-García A, Martín AM, et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 2014;3:e03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978;4:7–25. [PubMed] [Google Scholar]
- 16.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 17.Feng J, Mantesso A, De Bari C, et al. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA 2011;108:6503–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol 2008;36:642–654. [DOI] [PubMed] [Google Scholar]
- 19.da Silva Meirelles L, Malta TM, de Deus Wagatsuma VM, et al. Cultured Human Adipose Tissue Pericytes and Mesenchymal Stromal Cells Display a Very Similar Gene Expression Profile. Stem Cells Dev 2015;24:2822–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blocki A, Wang Y, Koch M, et al. Not all MSCs can act as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev 2013;22:2347–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caplan AI. All MSCs are pericytes? Cell Stem Cell 2008;3:229–230. [DOI] [PubMed] [Google Scholar]
- 22.Muhammad G, Jablonska A, Rose L, et al. Effect of MRI tags: SPIO nanoparticles and 19F nanoemulsion on various populations of mouse mesenchymal stem cells. Acta Neurobiol Exp (Wars) 2015;75:144–159. [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Yu F, Sun Y, et al. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 2015;33:627–638. [DOI] [PubMed] [Google Scholar]
- 24.Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 2005;23:412–423. [DOI] [PubMed] [Google Scholar]
- 25.Kassis I, Zangi L, Rivkin R, et al. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant 2006;37:967–976. [DOI] [PubMed] [Google Scholar]
- 26.Agha-Hosseini F, Jahani M-A, Jahani M, et al. In vitro isolation of stem cells derived from human dental pulp. Clin Transplant 2010;24:E23–28. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y-T, Wei J-D, Wang J-P, et al. Isolation of mesenchymal stem cells from human ligamentum flavum: implicating etiology of ligamentum flavum hypertrophy. Spine 2011;36:E1193–1200. [DOI] [PubMed] [Google Scholar]
- 28.Meng X, Ichim TE, Zhong J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macias MI, Grande J, Moreno A, et al. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am J Obstet Gynecol 2010;203:495.e9–495.e23. [DOI] [PubMed] [Google Scholar]
- 30.Patki S, Kadam S, Chandra V, et al. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell 2010;23:35–40. [DOI] [PubMed] [Google Scholar]
- 31.Roubelakis MG, Pappa KI, Bitsika V, et al. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev 2007;16:931–952. [DOI] [PubMed] [Google Scholar]
- 32.Marongiu F, Gramignoli R, Sun Q, et al. Isolation of amniotic mesenchymal stem cells. Curr Protoc Stem Cell Biol 2010;Chapter 1:Unit 1E.5. [DOI] [PubMed] [Google Scholar]
- 33.Poloni A, Rosini V, Mondini E, et al. Characterization and expansion of mesenchymal progenitor cells from first-trimester chorionic villi of human placenta. Cytotherapy 2008;10:690–697. [DOI] [PubMed] [Google Scholar]
- 34.Miao Z, Jin J, Chen L, et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biol Int 2006;30:681–687. [DOI] [PubMed] [Google Scholar]
- 35.Girdlestone J, Limbani VA, Cutler AJ, et al. Efficient expansion of mesenchymal stromal cells from umbilical cord under low serum conditions. Cytotherapy 2009;11:738–748. [DOI] [PubMed] [Google Scholar]
- 36.Zeddou M, Briquet A, Relic B, et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int 2010;34:693–701. [DOI] [PubMed] [Google Scholar]
- 37.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000;109:235–242. [DOI] [PubMed] [Google Scholar]
- 38.Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heo JS, Choi Y, Kim H-S, et al. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med 2016;37:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24:1294–1301. [DOI] [PubMed] [Google Scholar]
- 41.Phinney DG, Kopen G, Righter W, et al. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem 1999;75:424–436. [PubMed] [Google Scholar]
- 42.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008;7:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA 2000;97:3213–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA 2001;98:7841–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 2000;113 ( Pt 7):1161–1166. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto T, Aoyama T, Nakayama T, et al. Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun 2002;295:354–361. [DOI] [PubMed] [Google Scholar]
- 47.Russell KC, Phinney DG, Lacey MR, et al. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells 2010;28:788–798. [DOI] [PubMed] [Google Scholar]
- 48.Krampera M, Galipeau J, Shi Y, et al. Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013;15:1054–1061. [DOI] [PubMed] [Google Scholar]
- 49.Tu Z, Li Q, Bu H, et al. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev 2010;19:1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moll G, Jitschin R, von Bahr L, et al. Mesenchymal stromal cells engage complement and complement receptor bearing innate effector cells to modulate immune responses. PLoS ONE 2011;6:e21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cassatella MA, Mosna F, Micheletti A, et al. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells 2011;29:1001–1011. [DOI] [PubMed] [Google Scholar]
- 52.Munir H, Rainger GE, Nash GB, et al. Analyzing the effects of stromal cells on the recruitment of leukocytes from flow. J Vis Exp 2015:e52480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandau S, Jakob M, Bruderek K, et al. Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS ONE 2014;9:e106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown JM, Nemeth K, Kushnir-Sukhov NM, et al. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy 2011;41:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107:1484–1490. [DOI] [PubMed] [Google Scholar]
- 56.Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008;111:1327–1333. [DOI] [PubMed] [Google Scholar]
- 57.Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 2006;177:2080–2087. [DOI] [PubMed] [Google Scholar]
- 58.Jiang X-X, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005;105:4120–4126. [DOI] [PubMed] [Google Scholar]
- 59.Su W-R, Zhang Q-Z, Shi S-H, et al. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells 2011;29:1849–1860. [DOI] [PubMed] [Google Scholar]
- 60.Chen P-M, Liu K-J, Hsu P-J, et al. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J Leukoc Biol 2014;96:295–303. [DOI] [PubMed] [Google Scholar]
- 61.Gao S, Mao F, Zhang B, et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood) 2014;239:366–375. [DOI] [PubMed] [Google Scholar]
- 62.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005;105:2821–2827. [DOI] [PubMed] [Google Scholar]
- 63.Yañez R, Lamana ML, García-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells 2006;24:2582–2591. [DOI] [PubMed] [Google Scholar]
- 64.Ghannam S, Pène J, Moquet-Torcy G, et al. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 2010;185:302–312. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 66.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006;107:367–372. [DOI] [PubMed] [Google Scholar]
- 67.Kupcova Skalnikova H Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 2013;95:2196–2211. [DOI] [PubMed] [Google Scholar]
- 68.Cantinieaux D, Quertainmont R, Blacher S, et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS ONE 2013;8:e69515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanotti L, Angioni R, Calì B, et al. Mouse mesenchymal stem cells inhibit high endothelial cell activation and lymphocyte homing to lymph nodes by releasing TIMP-1. Leukemia 2016;30:1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eliopoulos N, Zhao J, Bouchentouf M, et al. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol Renal Physiol 2010;299:F1288–1298. [DOI] [PubMed] [Google Scholar]
- 71.Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med 2015;30:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Álvarez D, Levine M, Rojas M. Regenerative medicine in the treatment of idiopathic pulmonary fibrosis: current position. Stem Cells Cloning 2015;8:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vizoso FJ, Eiro N, Cid S, et al. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine . Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Attar-Schneider O, Zismanov V, Drucker L, et al. Secretome of human bone marrow mesenchymal stem cells: an emerging player in lung cancer progression and mechanisms of translation initiation. Tumour Biol 2016;37:4755–4765. [DOI] [PubMed] [Google Scholar]
- 75.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449:557–563. [DOI] [PubMed] [Google Scholar]
- 76.Mezey É, Nemeth K. Mesenchymal stem cells and infectious diseases: Smarter than drugs. Immunol Lett 2015;168:208–214. [DOI] [PubMed] [Google Scholar]
- 77.Meisel R, Brockers S, Heseler K, et al. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia 2011;25:648–654. [DOI] [PubMed] [Google Scholar]
- 78.Koniusz S, Andrzejewska A, Muraca M, et al. Extracellular Vesicles in Physiology, Pathology, and Therapy of the Immune and Central Nervous System, with Focus on Extracellular Vesicles Derived from Mesenchymal Stem Cells as Therapeutic Tools. Front Cell Neurosci 2016;10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel SA, Meyer JR, Greco SJ, et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol 2010;184:5885–5894. [DOI] [PubMed] [Google Scholar]
- 80.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE 2010;5:e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Djouad F, Charbonnier L-M, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 2007;25:2025–2032. [DOI] [PubMed] [Google Scholar]
- 82.Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 2005;80:229–236; discussion 236–237. [DOI] [PubMed] [Google Scholar]
- 83.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 2006;7:885–896. [DOI] [PubMed] [Google Scholar]
- 84.Mackay AM, Beck SC, Murphy JM, et al. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 1998;4:415–428. [DOI] [PubMed] [Google Scholar]
- 85.An C, Cheng Y, Yuan Q, et al. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann Biomed Eng 2010;38:1647–1654. [DOI] [PubMed] [Google Scholar]
- 86.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–147. [DOI] [PubMed] [Google Scholar]
- 87.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–1084. [DOI] [PubMed] [Google Scholar]
- 88.Xu W, Zhang X, Qian H, et al. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–631. [DOI] [PubMed] [Google Scholar]
- 89.Lee K-D, Kuo TK-C, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004;40:1275–1284. [DOI] [PubMed] [Google Scholar]
- 90.Phadnis SM, Joglekar MV, Dalvi MP, et al. Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo. Cytotherapy 2011;13:279–293. [DOI] [PubMed] [Google Scholar]
- 91.Govindasamy V, Ronald VS, Abdullah AN, et al. Differentiation of dental pulp stem cells into islet-like aggregates. J Dent Res 2011;90:646–652. [DOI] [PubMed] [Google Scholar]
- 92.Naghdi M, Tiraihi T, Namin SAM, et al. Transdifferentiation of bone marrow stromal cells into cholinergic neuronal phenotype: a potential source for cell therapy in spinal cord injury. Cytotherapy 2009;11:137–152. [DOI] [PubMed] [Google Scholar]
- 93.Anghileri E, Marconi S, Pignatelli A, et al. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev 2008;17:909–916. [DOI] [PubMed] [Google Scholar]
- 94.Pavlova G, Lopatina T, Kalinina N, et al. In vitro neuronal induction of adipose-derived stem cells and their fate after transplantation into injured mouse brain. Curr Med Chem 2012;19:5170–5177. [DOI] [PubMed] [Google Scholar]
- 95.Nowakowski A, Drela K, Rozycka J, et al. Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse. Stem Cells Dev 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mendicino M, Bailey AM, Wonnacott K, et al. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 2014;14:141–145. [DOI] [PubMed] [Google Scholar]
- 97.Ancans J Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol 2012;3:253. [DOI] [PMC free article] [PubMed] [Google Scholar]